Abstract
Rats infected with the protozoan parasite Toxoplasma gondii exhibit reduced avoidance of predator odours. This behavioural change is likely to increase transmission of the parasite from rats to cats. Here, we show that infection with T. gondii increases the propensity of the infected rats to make more impulsive choices, manifested as delay aversion in an intertemporal choice task. Concomitantly, T. gondii infection causes reduction in dopamine content and neuronal spine density of the nucleus accumbens core, but not of the nucleus accumbens shell. These results are consistent with a role of the nucleus accumbens dopaminergic system in mediation of choice impulsivity and goal-directed behaviours. Our observations suggest that T. gondii infection in rats causes a syndromic shift in related behavioural constructs of innate aversion and making foraging decisions.
Keywords: behavioural manipulation, brain, delay discounting, dopamine, monoamines, parasites
1. Introduction
Toxoplasma gondii is a protozoan parasite of the rat (Rattus novergicus) and many other animals. This host–parasite association has been widely studied as an example of parasitic manipulation of host behaviour. Rats infected with T. gondii exhibit greater exploration of spaces containing cat odours [1–3]. This behavioural change is thought to increase parasite transmission because cats are the ultimate host of this parasite [4,5].
Innate aversion to predators is a flexible behaviour. Odours that are more predictive of immediate predator presence evoke a stronger aversion compared with partial cues like urine or faeces [6]. Similarly, more concentrated cat odours elicit a larger fear response [6]. Apart from conditional dependence on predator cues, innate aversion is also in contiguity with other behaviours like searching for food. For example, laboratory ant colonies (Lasius pallitarsis) unequivocally choose feeding sites offering more concentrated sugar solutions. In the presence of predators, the preference for concentrated sugar is diminished, yet the devaluation for the richer feeding site becomes more blunted as the concentration of sugar solution offered is increased [7]. This and several similar observations [8] suggest that foraging decisions and predator aversion are related behavioural constructs. Foraging decisions in the laboratory settings have often been framed in terms of an intertemporal choice between larger later and smaller sooner food receipts. In a delay discounting task where delays to larger later rewards (LLRs) are progressively varied while keeping intervals between successive trial initiations constant within a block, consistent choice of LLR represents an economically rational choice. In this setting, choice for smaller sooner rewards (SSRs) demonstrates an intolerance to delay, and thus is interpreted as an impulsive choice. In the light of these observations, we investigated whether T. gondii infection led to more impulsive delay-averse foraging decisions in the infected rats.
The midbrain dopaminergic system is critical for evaluating salience of various options [9]. This system pivots around the nucleus accumbens, which receives dense dopaminergic inputs from the ventral tegmental area. The nucleus accumbens interacts with limbic regions involved in emotional valence like the amygdala [10], and also with frontal cortical regions involved in executive functions, like the orbitofrontal, anterior cingulate, prelimbic and infralimbic cortices [11]. The nucleus accumbens also influences goal-directed behaviours through its projections to the globus pallidum and hypothalamic nuclei. Consistent with this, selective excitotoxic lesions or quinolinic acid-induced lesions of nucleus accumbens core, but not shell, enhance impulsive choice in rats [12–14]. These observations suggest that the dopaminergic signalling in the nucleus accumbens acts as the interface between salience of various challenges and opportunities, and the resultant behavioural output. In view of its central role in choice impulsivity, we also investigated changes in neuronal morphology and dopamine content of the nucleus accumbens.
2. Material and methods
Adult male Wistar rats were employed (detailed methods in electronic supplementary material). Animals were either infected with tachyzoites (5 × 106, i.p.) or mock-infected with sterile saline and tested more than eight weeks post-infection.
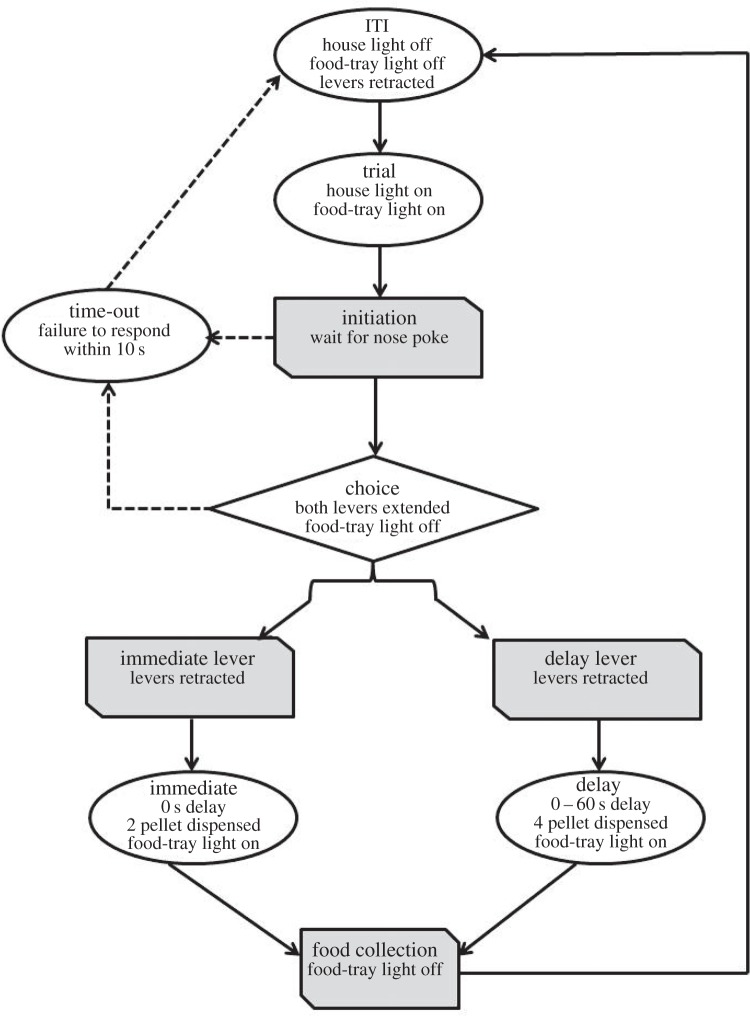
Procedure to measure delay averseness was adopted from [15] (figure 1). Animals were tested daily for 6 days per week (one session per day). Each session consisted of 60 choice trials executed at 100 s intervals, consisting of five delay blocks of 12 trials each (delays = 0, 10, 20, 40 and 60 s). One of the levers delivered the SSR (one pellet, immediate) and the alternate lever delivered the LLR (four pellets, after an appropriate delay). The number of LLR choice was used as a measure of impulsivity. Data presented depict an average of 3 day block. Subsequently, sucrose preference was measured by giving rats a choice between two bottles containing either tap water or 1% sucrose (test duration = 2 h). The consumption was measured by weighing the bottles.
Figure 1.
Procedure employed for quantifying delay aversion, depicting a single trial. A progressive delay protocol was used, whereby delay across trials within a block monotonically progressed from 0 to 60 s [15].
Dopamine and 5-HT levels were measured by HPLC in brain punched obtained from 500 µm thick sections. For spine density measurements, brains were processed for rapid Golgi staining [16,17]. Spines on a continuous 80 µm of secondary dendrites were counted at 1000× magnification.
Analysis of variance (ANOVA) was used to estimate statistical significance of main effects and interactions. Spine density and neurotransmitter content within nucleus accumbens were analysed using Mann–Whitney U-test.
3. Results
(a). Infection increased delay aversion
Control (14 animals) and infected (12 animals) subjects were tested for their propensity to choose between SSR and LLR (figure 1).
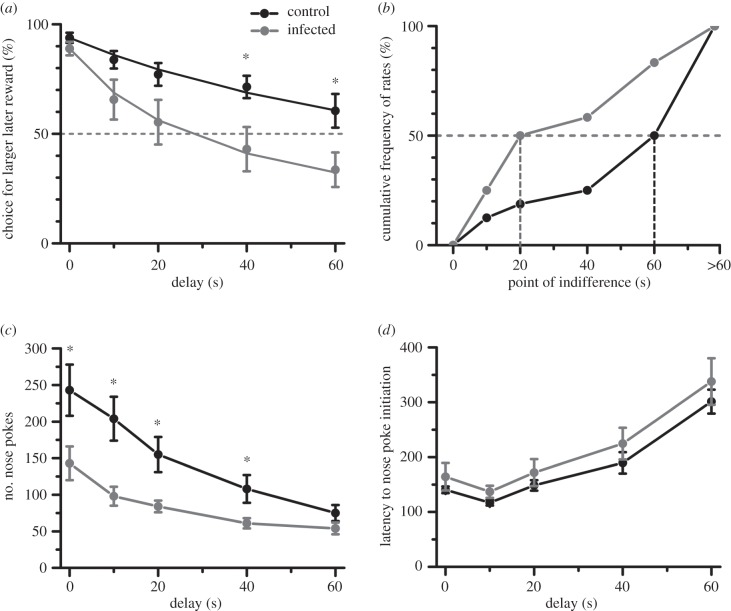
Figure 2a depicts the choice exhibited by the animals for LLR (% of total trials) over successive delays. Both control and infected animals preferred the larger reward in absence of delay (figure 2a, 0 s; one-sample t-test against chance of 50%; p < 0.0001; control: |t13| = 19.2, infected: |t11| = 12.7). Control and infected animals did not significantly differ in choice of the larger reward when the delay was set to zero (independent sample t-test; |t24| = 1.3, p > 0.2). As the delays increased, animals progressively reduced their preference for the LLR (repeated measure ANOVA; table 1). Control animals preferred LLR at all delays examined, except at 60 s (figure 2a; one-sample t-test against chance; |t13| ≥ 4.19, p ≤ 0.001, Bonferroni correction applied post-hoc to correct alpha probabilities for multiple testing of five delays). By contrast, preference for LLR was statistically insignificant at all delays greater than 0 s for infected animals (|t11| ≤ 2.07, p ≥ 0.31). Between the two experimental groups, infected animals exhibited greater intolerance to the delay of rewards (ANOVA; table 1; main effect of infection status: p = 0.024). Post-hoc analysis revealed statistical significant differences between control and infected at delays of 40 and 60 s (figure 2a; LSD: p < 0.05, Bonferroni correction applied to correct for multiple comparisons).
Figure 2.
Infection induced impulsive choice by increasing delay aversion, without affecting motor impulsivity. (a) Control animals chose LLR more frequently than infected animals. The ordinate depicts the number of choices made for LLR (mean ± s.e.m.) for a series of sequentially larger delays (depicted in abscissa). Solid lines represent a hyperbolic discount curve fitted to the data. V = D0/(1 + k * D), where D0 is preference for LLR at zero delay. *p < 0.05, post-hoc test between control and infected, Bonferroni's correction for multiple tests applied. The dotted grey line parallel to the abscissa depicts the point of indifference. (b) More of the infected animals reached the point of indifference at shorter delays to reward. The point of indifference is defined as the earliest delay when an animal chose an SSR in five or more trials (out of 10). Animals that did not reach the point of indifference at the highest delay used (60 s) were ascribed a value of more than 60 s. Median is depicted by the dotted grey line. (c) Infected animals executed fewer redundant nose pokes during the inter-trial interval, suggesting that the enhanced choice impulsivity is not a generalized phenomenon. *p < 0.05, post-hoc test. (d) Latency to initiate rewarded trials through nose poke was not different between control and infected animals. n = 14 for control and 12 for infected.
Table 1.
Analysis of variance for infection status and delay aversion. Between-subject source of variance: control or infected; within-subject: delay = 0, 10, 20, 40 or 60 s. n = 14 control and 12 infected animals.
| d.f. | F | p-value | |
|---|---|---|---|
| choice for LLR | |||
| infection status | 1,24 | 5.79 | 0.024 |
| delay | 4,96 | 38.92 | <0.0001 |
| interaction | 4,96 | 2.98 | 0.023 |
| no. nose pokes | |||
| infection status | 1,24 | 6.51 | 0.018 |
| delay | 4,96 | 52.09 | <0.0001 |
| interaction | 4,96 | 5.31 | 0.0007 |
| latency to nose-poke initiation | |||
| infection status | 1,24 | 0.21 | 0.648 |
| delay | 4,96 | 18.92 | <0.0001 |
| interaction | 4,96 | 0.09 | 0.986 |
The sensitivity of rats to the delay in reward receipt is typically time inconsistent, in that devaluation rate of the reward is not constant across proximal and distal time delays [18]. To recapitulate this, we fitted the mean number of LLR choice obtained for each group at the various delays to a hyperbolic model using nonlinear regression (figure 2a, solid lines; table 2; V = D0/(1 + k * D), where D0 is preference for LLR at zero delay). Infected animals exhibited a steeper coefficient of discounting, suggesting a greater sensitivity of the reward value to the delay in its receipt (fit model and group parameters in table 2). For the individual data, the rate of hyperbolic decay (k) was calculated. Only individuals with R2 > 0.8 were included in the analysis. Distribution of parameter k was non-normal; hence a non-parametric test was used to compare experimental groups (Shapiro–Wilk test: p < 0.001). Infected subjects exhibited greater coefficient of discounting (Mann–Whitney U-test; |Z| = 2.05, p = 0.0093; n = 8 control and 7 infected animals).
Table 2.
Hyperbolic discounting model for control and infected groups. Delay = 0, 10, 20, 40 or 60 s. n = 14 control and 12 infected animals. Hyperbolic discounting; V=D0/(1 + k * D), where D0 is preference for LLR at zero delay; d.f. = 4. Delay = 0, 10, 20, 40 or 60 s. n = 8 control and 7 infected animals. Only individuals with R2 > 0.8 were included in the analysis.
| fit for means of two group at various delay | k | 95% confidence interval of k | R2 |
|---|---|---|---|
| control | 0.00909 | 0.00745–0.01072 | 0.973 |
| infected | 0.02913 | 0.02498–0.03327 | 0.991 |
| fit for individual animal performance at various delay | D0 | k | R2 |
|---|---|---|---|
| control animals | 9.292 ± 0.353 | 0.01630 ± 0.00432 | 0.829–0.965 |
| infected animals | 9.047 ± 0.256 | 0.2976 ± 0.1933 | 0.919–0.999 |
| inter-group comparisons Mann–Whitney U-test | |Z| = 0.98, p = 0.3162 | |Z| = 2.05, p = 0.0093 |
Approximately 50% of control animals did not reach a point of indifference even during the longest delay used in the experiment (figure 2b). The mean choice of the control animals was above the point of indifference at all delays. By contrast, more of the infected animals reached the point of indifference at much shorter delays (figure 2b; two-sample Kolmogorov–Smirnov test: |Z| = 2.33, p < 0.001).
Consistent with the preference for SSR, infected animals earned fewer food pellets during the task (pellets earned during session, mean ± s.e.m.: control 189 ± 7, infected = 158 ± 11; |t24| = 2.5, p < 0.05). Despite being delay averse, the infected animals were less likely to engage in premature or persistent responding measured by the number of inter-trial interval nose pokes (figure 2c and table 1). The latency to initiate rewarded trials through nose poke did not differ significantly (figure 2d and table 1). Thus, the behaviour of infected rats in this task was guided by intertemporal choice impulsivity without a probable contribution from generic or motor impulsivity.
(b). Infection did not reduce sensitivity to the reward
We further tested whether infection altered the sensitivity of the animal to reward (n = 14 animals for control and 12 animals for infected). When provided with water and 1% sucrose simultaneously, the preference for sucrose was more pronounced in infected animals (figure 3a; independent sample t-test: |t24| = 3.15, p = 0.004). Infected animals consumed a greater amount of sucrose (figure 3b; |t24| = 3.43, p = 0.002), whereas the total consumption was not significantly affected by the infection (|t24| = 1.66, p = 0.11). Results for sucrose preference were in direct contrast to changes in intertemporal choice. Despite a greater preference for rewards, infected animals exhibited a reduced tendency to wait for larger rewards when delays were imposed. Control and infected animals gained comparable body weight during the experimental period (|t26| = 0.89; p > 0.3, independent sample t-test).
Figure 3.
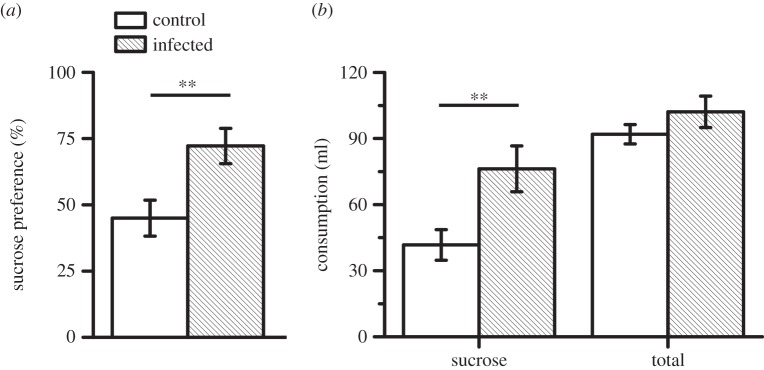
Infection increased sensitivity to rewards. (a) Infected animals exhibited greater preference for 1% sucrose reward, compared with water (%, relative to sucrose + water consumption). **p < 0.01, independent t-test. (b) Infection increased sucrose consumption, but total consumption remained unchanged. n = 14 animals for control and 12 animals for infected group.
(c). Infection reduced spine density of the neurons in the nucleus accumbens core
We quantified the number of spines over 80 µm segment for neurons of nucleus accumbens core (AcbC) and shell (AcbSh). The infection caused a marked reduction in the number of spines for AcbC neurons (figure 4; Mann–Whitney U-test: |Z| = 2.57, p = 0.01; n = 6 control and 6 infected animals). In fact, the minimum observed value of spine density for the control group was still greater than in 5 out of 6 infected animals. Similarly, the maximum observed value of the infected group was observed to be below the median of the control animals. The spine density of AcbSh neurons did not significantly differ between control and infected animals (figure 4; |Z| = 0.16, p = 0.87), suggesting that effects of the infection were specific to the core sub-region of the nucleus accumbens. Figure 4b depicts representative examples of AcbC dendrites.
Figure 4.
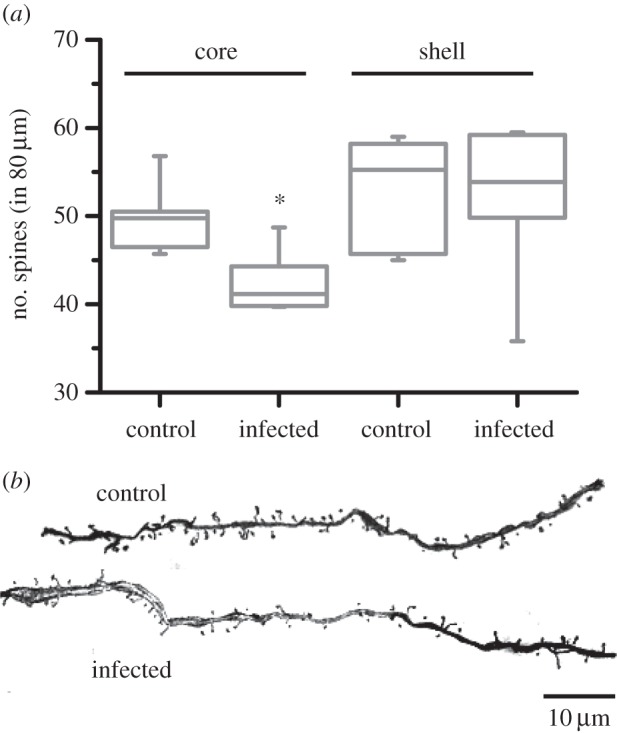
(a) Infection reduced spine density of the neurons in nucleus accumbens core, but not in shell. Bars depict medial and inter-quartile range. *p < 0.05 for comparison between control and infected animals. n = 6 animals each for control and infected groups. (b) Representative examples of AcbC dendrites.
In order to preclude a generalized change in the spines, we also quantified spine density in brain regions anatomically connected to the nucleus accumbens (basolateral amygdala, anterior cingulate cortex, orbitofrontal cortex and medial prefrontal cortex). Infection did not significantly alter spine density in these brain regions (Mann–Whitney U-test; |Z| < 1.14; p > 0.15).
(d). Infection reduced dopamine content in the nucleus accumbens core
We quantified the amount of dopamine and 5-HT in tissue micro-punches obtained from AcbC and AcbSh. Apart from causing changes in spine density measurements, the infection caused a statistically significant decrease in dopamine content of the AcbC (figure 5; Mann–Whitney U-test: |Z| = 2.07, p = 0.039; n = 8 control and 6 infected animals; control = 9.82 ± 0.70 ng mg−1, infected = 5.99 ± 1.59 ng mg−1). The dopamine content of AcbSh did not significantly differ between control and infected animals (figure 5; |Z| = 0.52, p = 0.62; control = 6.40 ± 0.95 ng mg−1, infected = 4.69 ± 1.21 ng mg−1). The infection did not cause a statistically significant difference in 5-HT content of either AcbC (control = 3.29 ± 0.41 ng mg−1, infected = 3.08 ± 0.57 ng mg−1; |Z| = 0.26, p = 0.80) or AcbSh (control = 1.74 ± 0.18 ng mg−1, infected = 1.45 ± 0.20 ng mg−1; |Z| = 0.78, p = 0.44), with the exception of BLA (control = 1.72 ± 0.19 ng mg−1, infected = 1.19 ± 0.14 ng mg−1; |Z| = 2, p = 0.0426). AcbC dopamine content was not significantly correlated with discounting constant k or preference for LLR at zero delay (p > 0.75).
Figure 5.
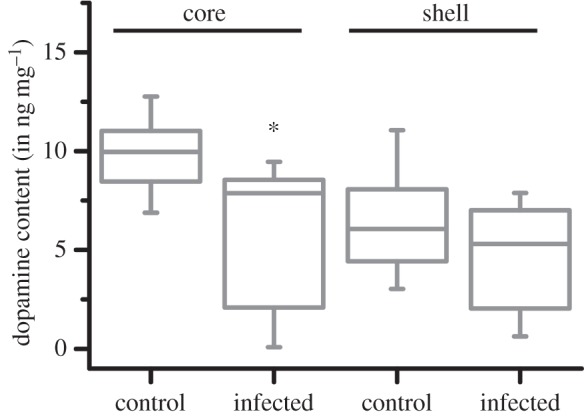
Infection reduced dopamine content in nucleus accumbens core, but not in shell. Bars depict medial and inter-quartile range. *p < 0.05 for comparison between control and infected animals. n = 8 animals for control and 6 animals for infected group.
In order to preclude a generalized change, we also quantified dopamine content in brain regions that send dopaminergic projections to and from the nucleus accumbens (ventral tegmental area, basolateral amygdala, medial amygdala, ventral pallidum, anterior cingulate cortex, posterior cingulate cortex, caudate putamen dorsal, caudate putamen ventral and medial prefrontal cortex). Infection did not significantly alter dopamine content in these brain regions (Mann–Whitney U-test; |Z| > 1.16; p > 0.245).
4. Discussion
Earlier work demonstrates that rats infected with T. gondii lose their innate aversion to cat odours. In this report, we show that infection with T. gondii creates delay aversion in male rats by increasing steepness of the discounting for receipt of larger rewards at increasing delays. This is reflected as preference for SSRs. Behavioural changes within an infected individual have often been viewed as a collection of independent phenotypes arising in isolation from each other. A contrarian view posits that multi-dimensional behavioural changes in the host reflect a syndrome arising because of inter-connected biological imperatives [19]. We propose that the host behavioural change after T. gondii infection is not an intransigent reduction of the innate fear. Instead it represents a behavioural syndrome consisting of reduced innate fear, increased sexual attractiveness and greater delay aversion; all hallmarks of a ‘carpe diem’ animal personality [20–22]. Biological imperatives that bind these behavioural changes remain presently unknown, although a plausible and untested speculation can be offered. Several studies cutting across phylogenetic boundaries show that a shortening of lifespan results in greater ‘carpe diem’ impulsivity [20,22,23]. However, metabolic investment resulting in current payoffs often exists in a trade-off with future/residual payoffs [24–26]. We speculate that delay aversion and loss of innate fear are contiguous behavioural changes reflecting an expedited life history for the host. This notion agrees with the observations that the infected host increases current metabolic investment in the form of androgen and sexual pheromone production [4,27,28]. Similarly, the presence of T. gondii cysts in mouse brains increases exploration of open and exposed regions of an arena, suggesting a change in perceived risk [29].
The concept of impulsivity has often been divided into motor impulsivity and choice impulsivity [30]. Motor impulsivity is typically characterized as a reduced ability to stop an ongoing motor response or to refrain from making a new motor response. Choice impulsivity, on the other hand, refers to cognitive decisions made under risk/uncertainty or when delays to receipt are involved. Specifically, choice impulsivity manifests itself as a reduced tolerance for delayed gratification, a characteristic similar to those exhibited by T. gondii-infected rats. Within the delay discounting task, infection reduced nose pokes during inter-trial interval when receipt of food was not possible. Nose pokes during the inter-trial interval might reflect either a failure to inhibit premature responding or persistent action in absence of reinforcement. By contrast, the latency of nose poke to initiate a trial remained unaffected. We suggest that the increase in impulsivity of T. gondii-infected rats is restricted or at least more pronounced in the domain of choice rather than motor phenotypes. This agrees with observations in human subjects, showing greater ability of the infected individual to inhibit a pre-potent motor response [31], though choice impulsivity in infected humans subjects has not yet been tested. As an important caveat, we have not explicitly tested for motor impulsivity in this report. It is possible that nose pokes during inter-trial interval may be influenced by choice in the preceding trial, and thus might not be an independent measure of motor impulsivity. A split-sample analysis conducted on our dataset indeed demonstrated that number of nose pokes at zero delay and discounting of nose poke numbers across delays could be predicted from choice made in the preceding trial.
The infection did not diminish preference for food when delays were not involved, as demonstrated by the increased preference for sucrose post-infection. This suggests that T. gondii infection did not alter overall appetite as measured by food intake (which could have explained a preference for the smaller reward outcome), nor did it bias the animals away from high-calorie food (as indicated by an increased preference for sucrose over water compared to controls). These observations agree with prior observations that animals infected with T. gondii retain comparable body weights [2], have similar food consumption after deprivation [2] and continue to perform energetically expensive behaviours [32] as compared with uninfected controls.
The mesolimbic dopamine system is involved in mediating impulsivity in delay discounting tasks [9]. This system pivots around the nucleus accumbens, receiving dopaminergic projections from the ventral tegmental area. In rats, bilateral excitotoxic lesions of AcbC increase delay aversion in discounting tasks, while lesions of AcbSh do not affect this behaviour [12]. This is consistent with our observations that the delay aversion in the infected rats is accompanied by a reduced spine density in AcbC but not in AcbSh. A pharmacological decrease in dopaminergic transmission by receptor antagonism increases delay aversion in rats [33,34]. This is consistent with our observations that the infection-induced increase in impulsivity is concomitant with a reduction in dopamine levels in the AcbC. Interestingly, effects of the infection on innate fear can be rescued by haloperidol, an inverse agonist of dopamine receptors [35].
Thus, reduced dopamine content and spine density of the nucleus accumbens agrees well with increased delay aversion post-infection. What remains an unresolved surprise is the fact the T. gondii infection is previously suggested to increase dopamine, in contrast to the present report [36] (but see also [37]). For example, in vitro infection of mammalian dopaminergic cells by the parasite results in a robust increase of dopamine synaptic release [36]. Indeed, T. gondii genome contains two amino acid hydroxylase genes that are surprisingly similar in sequence to mammalian tyrosine hydroxylase, a rate-limiting enzyme in dopamine synthetic pathway [38]. The protein product of these parasite genes has been demonstrated in infected mouse brains, and parasitic cysts in mouse brains exhibit robust immunoreactivity to dopamine antibodies [36]. It is unknown whether the decrease in the nucleus accumbens dopamine reported by us is derived from the host or the parasite tyrosine hydroxylase.
Like other neurotransmitters, the effects of dopamine on behaviour are intricately dependent on the brain region. For example, administration of atomoxetine, resulting in increased dopamine in the prefrontal cortex but not the nucleus accumbens, decreases impulsivity in a delay discounting task and a five-choice serial reaction time task [39]. Administration of amphetamine leads to more widespread dopaminergic stimulation, resulting in increased impulsivity in the five-choice serial reaction time task [40] and decreased impulsivity in the delay discounting task [41]. Moreover, the effects of atomoxetine on a five-choice serial reaction time task can be reversed by selective dopamine antagonism in the AcbC [42]. These observations suggest that the site of dopamine change in the brain has a significant effect on the behaviour. We report a region-specific rather than a generalized alteration in dopamine content. It is presently unclear how a generalized supply of tyrosine hydroxylase-like genes from T. gondii can result in sub-region-specific changes in dopamine concentrations [43]. This is pertinent because T. gondii does not exhibit an exclusive tropism to nucleus accumbens or its sub-regions [44].
Toxoplasma gondii has earlier been reported to cause structural changes in neurons of host brain [45]. In this report, we show that the infection reduces neuronal spine density in AcbC. It is plausible that the reduced spine density of AcbC neurons results in a decrease in inward synaptic current and resultant firing rates experienced by these neurons. This could potentially result in increased impulsivity through weaker disinhibition of efferent brain regions. This possibility remains currently unstudied. In both cases of dopamine and spine density, the infection induced effects remain more pronounced in AcbC compared with AcbSh. Mechanisms of such anatomically restricted changes remain presently unknown. It has earlier been suggested that T. gondii preferentially concentrates in certain brain regions, and this tropism can explain behavioural changes post-infection through local manipulation of neuronal signalling and/or damage [2,46,47]. Two early studies in this regard reported a rather widespread occurrence of tissues cysts in a variety of brain regions [2,47]. Both of these reports suggested a mild tropism to the nucleus accumbens, ventromedial hypothalamus or amygdala. Core and shell divisions of the nucleus accumbens were not analysed separately in these studies. More recent studies have failed to reveal any substantial tropism in any of these three brain structures in mice and rats, instead reporting a rather ‘probabilistic’ spread of the parasites [29,48]. Another potential source of region-specific effects could arise because of the selective innervation from vasopressinergic fibres, rather than tropism of the T. gondii cysts themselves. Within the nucleus accumbens, most of the arginine vasopressin-containing fibres terminate in the core rather than the shell [49]. Arginine vasopressin neurons in the medial amygdala have been previously shown to be preferentially activated by cat odour [27]; an atypical event because these neurons are typically activated during sexual signalling [50]. The atypical recruitment of arginine vasopressin neurons is mediated by an epigenetic event dependent on a parasite-induced increase in testosterone synthesis [51]. It is plausible—and yet unproven—that stronger post-infection vasopressinergic inputs coupled with a tendency of these fibres to terminate in the core but not in the shell could lead to selective anatomical/neurochemical effects within the nucleus accumbens.
The concurrent changes in choice impulsivity, AcbC spine density and AcbC dopamine point towards a concerted shift in the behaviour of the infected rats. The concordance between these variables suggests a ‘conformity to a priori expectations based on purported function’ [52, p. 1373]. In other words, it is unlikely that the increase in choice impulsivity is an accidental by-product of the infection, because it is accompanied by non-generalized changes in biological substrates known a priori to be involved in the behaviour.
Finally, the data presented here provide an impetus to integrate parasitic changes in host behaviour with trade-offs that are commonly pre-existing in life-history choices. In addition, these changes provide us with a useful paradigm to better understand neuropathology in conditions characterized by increased impulsivity, such as substance abuse, gambling, attention-related disorders and high-risk behaviours [41].
Supplementary Material
Ethics
All animal procedures were approved by NTU-IACUC.
Data Accessibility
All raw data are available in Dryad repository (http://dx.doi.org/10.5061/dryad.6s5vv).
Authors' Contributions
D.T. designed and conceptualized experiments, conducted the delay discounting experiment and parts of the sucrose preference, dopamine quantification and spine density experiments, conducted data collection and statistical analysis, and wrote the paper. L.J.T.S. conducted parts of the delay discounting and spine density experiments. L.W.L. conducted parts of the sucrose preference and dopamine quantification experiments. T.C.W.D. conducted parts of the dopamine quantification experiment. X.Z. conducted parts of the dopamine quantification experiment. A.V. took part in conceptualization, conducted statistical analysis and wrote the paper. All authors commented and gave final approval for publication.
Competing Interests
We have no competing interests.
Funding
D.T., L.J.T.S., L.W.L., A.V. are funded by the Ministry of Education, Singapore; and T.C.W.D. and X.Z. are under block funding from Duke-NUS.
References
- 1.Berdoy M, Webster JP, Macdonald D. 2000. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B 267, 1591–1594. ( 10.1098/rspb.2000.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyas A, Kim S-K, Giacomini N, Boothroyd JC, Sapolsky RM. 2007. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl Acad. Sci. USA 104, 6442–6447. ( 10.1073/pnas.0608310104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram WM, Goodrich LM, Robey EA, Eisen MB. 2013. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS ONE 8, e75246 ( 10.1371/journal.pone.0075246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vyas A. 2013. Parasite-augmented mate choice and reduction in innate fear in rats infected by Toxoplasma gondii. J. Exp. Biol. 216, 120–126. ( 10.1242/jeb.072983) [DOI] [PubMed] [Google Scholar]
- 5.Webster JP, Kaushik M, Bristow GC, McConkey GA. 2013. Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour? J. Exp. Biol. 216, 99–112. ( 10.1242/jeb.074716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi LK, Nakashima BR, Hong H, Watanabe K. 2005. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci. Biobehav. Rev. 29, 1157–1167. ( 10.1016/j.neubiorev.2005.04.008) [DOI] [PubMed] [Google Scholar]
- 7.Nonacs P, Dill LM. 1990. Mortality risk versus food quality trade-offs in a common currency: ant patch preferences. Ecology 71, 1886–1892. [Google Scholar]
- 8.Lima SL, Dill LM. 1990. Behavioural decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 9.Dalley JW, Mar AC, Economidou D, Robbins TW. 2008. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 90, 250–260. ( 10.1016/j.pbb.2007.12.021) [DOI] [PubMed] [Google Scholar]
- 10.Morrison SE, Salzman CD. 2010. Re-valuing the amygdala. Curr. Opin. Neurobiol. 20, 221–230. ( 10.1016/j.conb.2010.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meredith GE, Pennartz CMA, Groenewegen HJ. 1993. The cellular framework for chemical signalling in the nucleus accumbens. In Progress in brain research (eds Arbuthnott GW, Emson PC.), pp. 3–24. Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- 12.Cardinal RN, Pennicott DR, Lakmali C, Robbins TW, Everitt BJ. 2001. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292, 2499–2501. ( 10.1126/science.1060818) [DOI] [PubMed] [Google Scholar]
- 13.Pothuizen HH, Jongen-Rêlo AL, Feldon J, Yee BK. 2005. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur. J. Neurosci. 22, 2605–2616. ( 10.1111/j.1460-9568.2005.04388.x) [DOI] [PubMed] [Google Scholar]
- 14.Bezzina G, Cheung T, Asgari K, Hampson C, Body S, Bradshaw C, Szabadi E, Deakin J, Anderson I. 2007. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on inter-temporal choice: a quantitative analysis. Psychopharmacology 195, 71–84. ( 10.1007/s00213-007-0882-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evenden JL, Ryan CN. 1996. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128, 161–170. ( 10.1007/s002130050121) [DOI] [PubMed] [Google Scholar]
- 16.Vyas A, Mitra R, Rao BS, Chattarji S. 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas A, Mitra R, Chattarji S. 2003. Enhanced anxiety and hypertrophy in basolateral amygdala neurons following chronic stress in rats. Ann. NY Acad. Sci. 985, 554–555. ( 10.1111/j.1749-6632.2003.tb07127.x) [DOI] [Google Scholar]
- 18.Mazur JE, Biondi DR. 2009. Delay-amount tradeoffs in choices by pigeons and rats: hyperbolic versus exponential discounting. J. Exp. Anal. Behav. 91, 197–211. ( 10.1901/jeab.2009.91-197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cézilly F, Perrot-Minnot MJ. 2010. Interpreting multidimensionality in parasite-induced phenotypic alterations: panselectionism versus parsimony. Oikos 119, 1224–1229. ( 10.1111/j.1600-0706.2010.18579.x) [DOI] [Google Scholar]
- 20.Carstensen LL. 2006. The influence of a sense of time on human development. Science 312, 1913–1915. ( 10.1126/science.1127488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly M, Wilson M. 2005. Carpe diem: adaptation and devaluing the future. Q. Rev. Biol. 80, 55–60. ( 10.1086/431025) [DOI] [PubMed] [Google Scholar]
- 22.Roitberg BD, Mangel M, Lalonde RG, Roitberg CA, van Alphen JJ, Vet L. 1992. Seasonal dynamic shifts in patch exploitation by parasitic wasps. Behav. Ecol. 3, 156–165. ( 10.1093/beheco/3.2.156) [DOI] [Google Scholar]
- 23.Murphy PJ. 2003. Context-dependent reproductive site choice in a Neotropical frog. Behav. Ecol. 14, 626–633. ( 10.1093/beheco/arg042) [DOI] [Google Scholar]
- 24.Wolf M, Van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 25.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 26.Gustafsson L, Qvarnström A, Sheldon BC. 1995. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375, 311–313. ( 10.1038/375311a0) [DOI] [Google Scholar]
- 27.Hari Dass SA, Vasudevan A, Dutta D, Soh LJT, Sapolsky RM, Vyas A. 2011. Protozoan parasite Toxoplasma gondii manipulates mate choice in rats by enhancing attractiveness of males. PLoS ONE 6, e27229 ( 10.1371/journal.pone.0027229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim A, Kumar V, Hari Dass SA, Vyas A. 2013. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol. Ecol. 22, 102–110. ( 10.1111/mec.12042) [DOI] [PubMed] [Google Scholar]
- 29.Afonso C, Paixão VB, Costa RM. 2012. Chronic Toxoplasma infection modifies the structure and the risk of host behavior. PLoS ONE 7, e32489 ( 10.1371/journal.pone.0032489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. 2010. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin. Exp. Res. 34, 1306–1318. ( 10.1111/j.1530-0277.2010.01215.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stock A-K, Heintschel von Heinegg E, Köhling H-L, Beste C. 2014. Latent Toxoplasma gondii infection leads to improved action control. Brain Behav. Immunity 37, 103–108. ( 10.1016/j.bbi.2013.11.004) [DOI] [PubMed] [Google Scholar]
- 32.Berdoy M, Webster J, Macdonald D. 1995. Parasite-altered behaviour: is the effect of Toxoplasma gondii on Rattus norvegicus specific? Parasitology 111, 403–409. ( 10.1017/S0031182000065902) [DOI] [PubMed] [Google Scholar]
- 33.Floresco SB, Maric T, Ghods-Sharifi S. 2008. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33, 1966–1979. ( 10.1038/sj.npp.1301565) [DOI] [PubMed] [Google Scholar]
- 34.Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. 2011. Effects of selective dopaminergic compounds on a delay discounting task. Behav. Pharmacol. 22, 300–311. ( 10.1097/FBP.0b013e3283473bcb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster J, Lamberton P, Donnelly C, Torrey E. 2006. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proc. R. Soc. B 273, 1023–1030. ( 10.1098/rspb.2005.3413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prandovszky E, Gaskell E, Martin H, Dubey J, Webster JP, McConkey GA. 2011. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE 6, e23866 ( 10.1371/journal.pone.0023866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang ZT, Harmon S, O'Malley KL, Sibley LD. 2014. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine levels. Infect. Immunity 83, 1039–1047. ( 10.1128/IAI.02465-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. 2009. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE 4, e4801 ( 10.1371/journal.pone.0004801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. 2008. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33, 1028–1037. ( 10.1038/sj.npp.1301487) [DOI] [PubMed] [Google Scholar]
- 40.Robbins T. 2002. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163, 362–380. ( 10.1007/s00213-002-1154-7) [DOI] [PubMed] [Google Scholar]
- 41.Winstanley CA. 2011. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br. J. Pharmacol. 164, 1301–1321. ( 10.1111/j.1476-5381.2011.01323.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole BJ, Robbins TW. 1992. Forebrain norepinephrine: role in controlled information processing in the rat. Neuropsychopharmacology 7, 129–142. [PubMed] [Google Scholar]
- 43.Vyas A, Sapolsky R. 2010. Manipulation of host behaviour by Toxoplasma gondii: what is the minimum a proposed proximate mechanism should explain? Folia Parasitol. 57, 88–94. ( 10.14411/fp.2010.011) [DOI] [PubMed] [Google Scholar]
- 44.McConkey GA, Martin HL, Bristow GC, Webster JP. 2013. Toxoplasma gondii infection and behaviour—location, location, location? J. Exp. Biol. 216, 113–119. ( 10.1242/jeb.074153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra R, Sapolsky RM, Vyas A. 2013. Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Dis. Models Mech. 6, 516–520. ( 10.1242/dmm.009928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans AK, Strassmann PS, Lee I, Sapolsky RM. 2014. Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long–Evans rats. Brain Behav. Immunity 37, 122–133. ( 10.1016/j.bbi.2013.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, Colasante C, Pino S, Hernandez L. 2007. Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats: a behavioral analysis. Behav. Brain Res. 177, 70–79. ( 10.1016/j.bbr.2006.11.012) [DOI] [PubMed] [Google Scholar]
- 48.Berenreiterova M, Flegr J, Kubena AA, Nemec P. 2011. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS ONE 6, e28925 ( 10.1371/journal.pone.0028925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rood BD, De Vries GJ. 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J. Comp. Neurol. 519, 2434–2474. ( 10.1002/cne.22635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hari Dass SA, Vyas A. 2014. Copulation or sensory cues from the female augment Fos expression in arginine vasopressin neurons of the posterodorsal medial amygdala of male rats. Front. Zool. 11, 42 ( 10.1186/1742-9994-11-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hari Dass SA, Vyas A. 2014. Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Mol. Ecol. 23, 6114–6122. ( 10.1111/mec.12888) [DOI] [PubMed] [Google Scholar]
- 52.Poulin R. 1995. ‘Adaptive’ changes in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383. ( 10.1016/0020-7519(95)00100-X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data are available in Dryad repository (http://dx.doi.org/10.5061/dryad.6s5vv).