Abstract
Selective logging is one of the most common forms of forest use in the tropics. Although the effects of selective logging on biodiversity have been widely studied, there is little agreement on the relationship between life-history traits and tolerance to logging. In this study, we assessed how species traits and logging practices combine to determine species responses to selective logging, based on over 4000 observations of the responses of nearly 1000 bird species to selective logging across the tropics. Our analysis shows that species traits, such as feeding group and body mass, and logging practices, such as time since logging and logging intensity, interact to influence a species' response to logging. Frugivores and insectivores were most adversely affected by logging and declined further with increasing logging intensity. Nectarivores and granivores responded positively to selective logging for the first two decades, after which their abundances decrease below pre-logging levels. Larger species of omnivores and granivores responded more positively to selective logging than smaller species from either feeding group, whereas this effect of body size was reversed for carnivores, herbivores, frugivores and insectivores. Most importantly, species most negatively impacted by selective logging had not recovered approximately 40 years after logging cessation. We conclude that selective timber harvest has the potential to cause large and long-lasting changes in avian biodiversity. However, our results suggest that the impacts can be mitigated to a certain extent through specific forest management strategies such as lengthening the rotation cycle and implementing reduced impact logging.
Keywords: bird conservation, forest management, forest degradation, phylogeny, reduced impact logging, tropical timber harvest
1. Background
As global efforts to curb tropical deforestation continue, biodiversity in the remaining tropical forests is increasingly endangered by a more insidious threat: forest degradation. The most common cause of tropical forest degradation is logging characterized by unsustainably high harvest rates, poor road design and poor silvicultural practices [1,2]. The Food and Agriculture Organization of the United Nations (FAO) estimates that at least one-third of all tropical forests are designated as production forests, destined to be selectively logged [3,4]. However, only a small part of these forests are sustainably managed [5]. Intensive and poorly implemented selective logging can rapidly lead to forest degradation [6,7].
Forest degradation is a problematic term to define, and there is as yet no harmonized, globally accepted definition [8–10]. However, for the purpose of this study, we define forest degradation as the loss of a certain forest property or function, such as biomass accumulation, canopy cover or avian diversity, which does not fully recover in the time before the next human intervention. It should be noted that selective logging does not automatically lead to forest degradation. In terms of avian diversity, it is as yet unknown which types of selective logging lead to forest degradation and which do not. This is because the precise consequences of selective logging for tropical biodiversity are poorly understood, despite the large number of case studies. As an example, the best-known measure of biodiversity, species richness, has been found to increase, decrease or stay the same in forests that have been selectively logged [1,11,12]. This reflects, among other factors, the broad diversity of interventions that the term selective logging encompasses, including for example reduced impact logging (RIL) and other forms of sustainable forest management, high-intensity conventional logging (CL) on steep slopes, single species extraction, illegal manual logging, etc. [11].
Species composition and functional diversity have also been found to change in forests following selective logging. In addition to the above-outlined variability of what selective logging means, it is likely that species traits also play an important role in determining whether a particular species will thrive or decline in a selectively logged forest [12–15]. For example, a global level study of primates in selectively logged forests showed that the response of individual species to selective logging is highly heterogeneous, and site specific, yet can be determined to some extent by considering species biological traits [14]. Similarly, in an analysis of the response of tropical dung beetles to forest degradation, including that caused by unsustainable selective logging, researchers found that body size and life-history traits were key predictors [16].
There has not, to our knowledge been a global scale study of the responses of birds to selective logging [13], despite the fact that birds are perhaps the most intensively studied taxonomic group. Nevertheless, based on local and regional case studies, and more general studies of land-use change, it appears that the species most susceptible to logging are large-bodied insectivores that forage in the understory and use tree cavities as nesting sites [13,15,17–20]. Conversely, low logging intensities, RIL, and allowing for a long time interval between logging cycles are hypothesized to reduce the impact of logging on bird diversity and abundance [11,12,15,21].
We set out to test these hypotheses simultaneously at a pantropical scale, using an unprecedented dataset on the changes in abundance of nearly a thousand bird species (roughly 10% of the global avifauna) as a response to different types of selective logging. Particularly, we aim to: (i) rank the importance of predictors, including interactions, of bird species' response to logging; and (ii) develop a model to help managers anticipate logging impact on a species, based on its traits and logging practices. We consider the following species traits and logging variables: feeding group; diet breadth; body mass; nesting; record of being hunted; geographical range size; logging intensity; time since logging; harvest type; number of logging cycles; continental location. With this analysis, we aim to clarify how selective logging, be it sustainable or not, interacts with life-history traits to produce species responses. The results can then be used to develop guidelines for logging practice more sustainable in terms of tropical avifauna.
2. Material and methods
(a). Data collection
To collect all relevant publications on the impact of selective logging on avifauna in tropical forests, we used a database of over 200 publications collated in another study, examining the impacts of selective logging on the biodiversity of different taxonomic groups [11]. From this database, we selected studies that measured changes in abundance or density of bird species at selectively logged sites, and equivalent control (undisturbed) sites in tropical forests. Studies had to contain quantitative information on all logging variables (see the electronic supplementary material). In total, we found 26 unique studies that we included in the meta-analysis (electronic supplementary material, table S1). When selecting the studies, we faced a trade-off between the number of studies that could be included, and the number of logging variables that could be included in the analysis, as many studies did not report fully on all the logging variables that we considered crucial.
(b). Response variable: standardized difference in abundance
For each species, we extracted its abundance or density at a logged site, and at a control site from the same study, in order to calculate our response variable, the standardized difference in abundance. The survey methods encountered in the studies were mist netting, point counts and visual detection of individuals per area using transects and we standardized measures to the same unit in each category (see the electronic supplementary material). Next, to calculate standardized difference, we took the square root of abundance or density at the control site and subtracted it from the square root of abundance at the logged site. Species that decreased in abundance at logged sites therefore have a negative standardized difference in abundance, whereas those that increased in abundance have a positive value.
The standardized differences, when considered for each method separately (mist nets, point counts and area measurements) were all approximately Gaussian, with mean 0. We divided all values by the standard deviation of each method, in order to standardize the distributions across sampling methods. Despite standardization, each method may still introduce a detection bias. For example, mist nets can systematically underestimate mid-story and canopy species, and point counts are likely to miss secretive, quiet species. We account for this by including the control categorical variable ‘method’ in our model, which stands for the different sampling methods (point count, mist net and area). We believe that such bias may influence the magnitude of the response, but probably not the direction of the response (decrease or increase in abundance or density).
(c). Predictors: logging variables and species traits
We extracted the following logging variables from each study: (i) logging intensity (in m3 ha−1 of timber extracted per hectare per cycle, (ii) time since logging (the number of years that have elapsed between selective logging and sampling), (iii) continent, (iv) number of logging cycles, (v) type of logging (RIL versus CL), (vi) method used for abundance estimation (hereafter method; point counts, mist nets, encounters per area), and (vii) study (with this variable, we take into account the influence of local environmental variables, forest type, altitude, seasonality, as well as the sampling bias introduced by teams of researchers, and unknown additional disturbances, such as unreported hunting). The availability of information on each of these variables was conditional for the inclusion of a study in the meta-analysis (see the electronic supplementary material).
For each species, we also collected the following information on species traits: (i) body mass, (ii) feeding group (vertebrate-eating carnivores, granivores, nectarivores, insectivores, frugivores, omnivores and herbivores), (iii) diet breadth (narrow, medium and broad), (iv) use of trees for nesting (‘Nest1’—only uses trees for nest construction; ‘Nest2’—predominantly uses trees; ‘Nest3’—includes trees; ‘Nest4’—excludes trees; ‘Nest5’—nesting habit unknown), (v) hunting pressure (yes—species hunted for food or pet trade; no—not hunted), and (vi) geographical range size. The data on bird species came from the world bird ecology database, which is based on a comprehensive literature survey and updated regularly [22,23] (see the electronic supplementary material).
(d). Phylogenetic control
Our data points are not independent, owing to the variable degree of phylogenetic relatedness between the 992 bird species used in our analysis. We account for this by constructing a covariance matrix, indicating the proportion of the evolutionary path shared between each pair of species, in a phylogenetic generalized least square model (see the electronic supplementary material).
(e). Model selection
We constructed a full phylogenetic generalized least square model, which included the most important hypothesized relationships between logging variables, species traits and bird abundance in logged forests. This model is based on the current knowledge, described in the literature (e.g. [18]). We limited the number of interactions in the full model (expressed with ×) to the most ecologically meaningful ones:
response ∼ method + study + continent + logging cycles + harvest type + logging intensity × feeding group + time × feeding group + feeding group × body mass + diet breadth + nesting + geographical range size + hunting,
where response is the standardized difference in abundance of a bird species in a selectively logged forest, compared with a control forest that has not been logged.
Even though all of the included variables and interactions probably play some role in shaping the species' response to selective logging, some may play a more important role than others. We assessed this by selecting the most parsimonious model (see the electronic supplementary material). To do this, we used the information theoretic approach [24]. This approach relies on assessing the likelihood of obtaining a dataset, given a model. Models, fit to the same dataset, are compared using the Kullback–Leibler information, estimated by the Akaike information criterion adjusted for small sample sizes (AICc) [24–26]. The lower the AICc, the more parsimonious the model, within the given set of candidate models.
We also calculated the relative importance of each variable and interaction between pairs of variables. To do this, we summed up the Akaike weight of all models in which each variable and interaction term occurs [24] (see the electronic supplementary material).
(f). Model parametrization
To parametrize the most parsimonious model, we fitted the model to 10 000 randomly drawn subsets of our data, associated randomly with one of the 100 sampled phylogenetic trees. We present parameter estimates as a mean of the 10 000 estimates. Ninety-five per cent of the 10 000 estimates fall within the reported 95% confidence intervals (CI) for estimates. For continuous variables, we also present the percentage of times, out of the 10 000 estimate, that the slope was negative. To evaluate how well the model fits our data, we calculated adjusted R2 for each model fitting event, and we present the average adjusted R2 value in the results. The analysis was implemented with the phylogenetic package caper, and the multi-model inference package MuMIn in the statistical software R [27].
3. Results
(a). Summary findings
Our database contains information on 4283 responses of 992 species of birds to selective logging from 26 studies (electronic supplementary material, table S1). Each species has been encountered by on average 4.3 studies (min. 1, max. 12 and median 2). Overall, 2022 responses to logging were negative and 2052 responses were positive (electronic supplementary material, figure S1). In 209 cases, bird species abundance did not change with selective logging. On average, our full, phylogenetic generalized least square model explained 32% of variance in the data (mean adjusted R2 of 10 000 model fitting events = 0.32; s.d. = 0.17; max. = 0.89, min. = 0.09).
(b). Variable importance
Our analysis shows that logging variables and species traits, as well as interactions between them, are important in explaining and predicting the response of bird species to selective logging (electronic supplementary material, tables S2 and S3). The most parsimonious model (electronic supplementary material, equation S1) was equivalent to the full model, without the variable geographical range size (electronic supplementary material, table S2). The most important logging variables are time since logging and harvest type (RIL versus CL), and the most important species traits are feeding group and whether a species is subjected to hunting (electronic supplementary material, table S3).
(c). Main effects
RIL is relatively less harmful to birds than CL in terms of abundance of individual species (table 1). Two logging cycles are more harmful to birds than one logging cycle (table 1). Bird species in South America respond to selective logging overall more negatively than those in Southeast Asia. African bird species respond on average most positively.
Table 1.
Main effect estimates for the most parsimonious model of changes in abundance of bird species with selective logging (electronic supplementary material, equation S1). (The estimates are means of 10 000 model fitting events. A total of 9500 (95%) of estimates fall within the 95% CI.)
| parameter | coefficient | level of categorical variable | mean | 95% CI |
|---|---|---|---|---|
| feeding group | a0 + k0 | omnivores | −3.9966 | −6.7; −1.3 |
| a0 + k1 | frugivores | 0.3936 | −1.6; 2.2 | |
| a0 + k2 | insectivores | −0.0788 | −1.5; 1.3 | |
| a0 + k3 | nectarivores | −0.6636 | −3.1; 1.6 | |
| a0 + k4 | herbivores | 2.4196 | 0.3; 4.5 | |
| a0 + k5 | granivores | −0.5768 | −2.1; 1.1 | |
| a0 + k6 | carnivores | 1.0225 | −0.8; 3.0 | |
| continent | b0 | Africa | 0 | |
| b1 | Asia | −0.1522 | −0.7; 0.4 | |
| b2 | America | −0.2108 | −0.6; 0.3 | |
| nesting | i0 | only trees | 0 | |
| i1 | mostly trees | −0.0425 | −0.4; 0.3 | |
| i2 | includes trees | −0.0659 | −0.6; 0.5 | |
| i3 | excludes trees | 0.0301 | −0.4; 0.5 | |
| i4 | unknown | −0.1218 | −0.6; 0.4 | |
| diet breadth | j0 | narrow | 0 | 0 |
| j1 | medium | 0.1038 | −0.2; 0.4 | |
| j2 | broad | 0.2155 | −0.6; 0.9 | |
| logging cycles | c0 | cycles—1 | 0 | 0 |
| c1 | cycles—2 | −0.2436 | −0.7; 0.2 | |
| harvest type | d0 | CL | 0 | 0 |
| d1 | RIL | 0.2551 | −0.1; 0.7 | |
| hunting | e0 | not hunted | 0 | 0 |
| e1 | hunted | 0.2065 | −0.1; 0.5 |
Bird species that do not use trees for nesting respond better to selective logging than those that do need trees (table 1). Species whose nesting habit is currently unknown respond most negatively to selective logging. However, overall, the effect of nesting on abundance is relatively small, compared with other parameters (table 1).
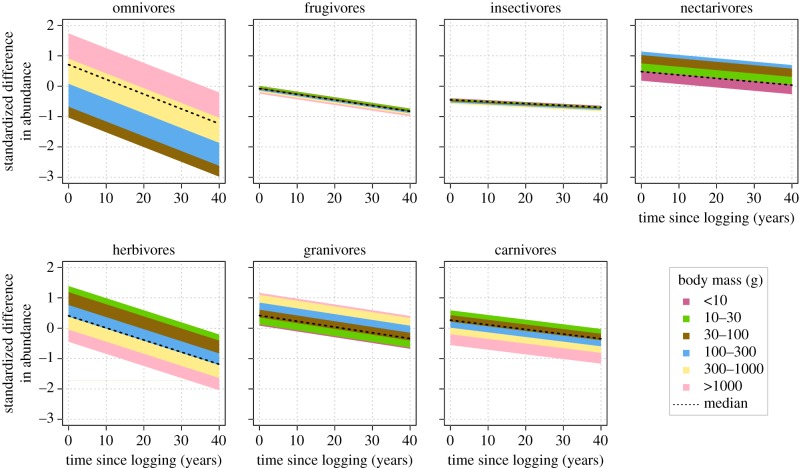
Species reported to be hunted for food or the pet trade respond on average more positively to logging than species for which no hunting is reported (table 1). The broader the diet, the more positively a species responds to selective logging (table 1). At median values of all other variables, frugivores and insectivores are the most negatively impacted by selective logging, whereas nectarivores and granivores are most positively impacted (table 1 and figure 1).
Figure 1.
The effect of time since logging on the abundance of bird species in different feeding groups and body mass categories in selectively logged tropical forests. Values are shown for species that require trees for nesting, have narrow diet breadth, are not hunted, in a forest in Asia, which has been conventionally logged one time. The width of the bands, which represent the body mass categories, corresponds to the effect size of body mass in each feeding group (table 2). Namely, wider bands signify that there are bigger differences between the response of small and large species within the feeding group. Responses are shown only for realistic body mass values within each feeding group, corresponding to the minimum, maximum and median mass for each group in our database. This results, for example, in the absence of the two largest body weight categories in the nectarivores panel, as there were no nectarivores heavier than 165 g in our database. (Online version in colour.)
(d). Interactions
The abundance of all feeding groups is negatively correlated with time elapsed since logging, however, to different extents (figure 1 and table 2; electronic supplementary material, figure S2). The effect of time is the smallest in species that on average respond negatively to logging (frugivores and insectivores). Species that on average respond positively (omnivores, nectarivores, granivores, herbivores and carnivores) decline or return to pre-logging abundance more rapidly (figure 1 and table 2; and electronic supplementary material, figure S2).
Table 2.
Interaction estimates for the most parsimonious model of changes in abundance of bird species with selective logging (electronic supplementary material, equation S1). (The estimates are means of 10 000 model fitting events. For each estimate, we indicate the percentage of times the estimate was negative.)
| intensity |
time |
body mass |
|||||
|---|---|---|---|---|---|---|---|
| z | feeding group | f0 + lz | estimate negative (%) | g0 + mz | estimate negative (%) | h0 + nz | estimate negative (%) |
| 1 | omnivores | 0.0029 | 26 | −0.0497 | 100 | 1.5849 | 0 |
| 2 | frugivores | −0.0011 | 56 | −0.0191 | 91 | −0.1011 | 62 |
| 3 | insectivores | −0.0008 | 60 | −0.0059 | 70 | −0.0805 | 60 |
| 4 | nectarivores | 0.0066 | 19 | −0.0116 | 80 | 0.5237 | 29 |
| 5 | herbivores | 0.0011 | 43 | −0.0408 | 100 | −0.8100 | 97 |
| 6 | granivores | 0.0031 | 26 | −0.0193 | 90 | 0.4884 | 10 |
| 7 | carnivores | 0.0019 | 35 | −0.0159 | 87 | −0.4139 | 79 |
The effect of logging intensity on species varies according to feeding group (table 2). Even though the interaction of logging intensity and feeding group is present in all 10 most parsimonious models (electronic supplementary material, table S2), the effect sizes are relatively large only for nectarivores, granivores and carnivores. Species that on average respond negatively to logging (frugivores and insectivores) were even more adversely affected by increasing logging intensity. By contrast, species that were on average positively affected by logging, at least early after logging, tended to increase in abundance even further at higher logging intensities (omnivores, nectarivores, herbivores, granivores and carnivores).
Body mass influences the response of species to logging in different directions, depending on the feeding group. Larger omnivores, granivores and nectarivores respond more positively than smaller members of the same feeding groups. By contrast, out of herbivores and carnivores, smaller ones respond to logging more positively than larger species. The effect of body mass on the change in abundance is also negative but small for frugivores and insectivores (figure 1).
4. Discussion
We analysed the response of 992 species of birds to different types of selective logging in tropical forests across three continents, in order to provide quantitative estimates of how species traits and logging variables affect the responses of avifauna to selective logging.
We found that all variables included in our analysis, with the exception of the species' geographical range size, play an important role in explaining the response of species to selective logging. Small geographical range has been emphasized as an important predictor of species extinctions, including in birds [28–30]. However, our findings show that, similarly to [20], in the case of birds, small geographical range might not in fact be associated with an intrinsic susceptibility of bird species to degradation, which could in turn lead to a higher likelihood of extinction. Rather, species with smaller geographical ranges may simply be more likely to have a larger proportion of their population affected by anthropogenic land use.
Of the various species traits, feeding group is the most important predictor (electronic supplementary material, table S3). This finding agrees with the qualitative findings of previous local and regional studies [13,15,17]. Of logging variables, the time that has elapsed since logging was the most important one. The interaction between feeding group and time since logging was also important (electronic supplementary material, table S3 and equation S1).
(a). Interaction of time since logging and feeding group
It is intuitive that the abundance of bird species would change with time that elapses since logging. As forest regenerates, its vegetation structure may become more similar to the pre-logging state [31] or tend towards a different forest state, never regenerating the original vegetation structure or composition [32]. As expected in the former case, the feeding groups that increased in abundance after selective logging gradually return towards their pre-logging abundance levels, and in some cases fall below their pre-logging abundance (table 2 and figure 1).
Nectarivores, such as Thalurania furcata or Florisuga mellivora, have been hypothesized to benefit from selective logging, owing to the higher abundance of flowering plants in newly created canopy gaps [15,17]. Our results show that this effect indeed exists, but is short-lived (figure 1 and table 2; electronic supplementary material, figure S2), probably because as canopy gaps seal, the abundance of nectar sources decreases. Similarly, carnivores, such as Accipiter melanoleucus, increase in abundance, perhaps because their specific niche is maximized by the forest treatment, owing to increased visibility of animal prey in the newly created forest gaps, or owing to the higher abundance of rodents in logged forests [15,33].
Frugivores have been hypothesized to be more resilient to forest disturbance [15,17]. Overall, fruit-bearing is negatively impacted by logging. Specifically, regenerating forests are often rich in small, edible fruits, and thus might support a larger number of frugivorous organisms [15]. We found that frugivores do indeed respond positively to logging, but only at low logging intensities, and this increase is only transient (tables 1 and 2, and figure 1; electronic supplementary material, figure S2). However, in the long run and at medium to high logging intensities, frugivore abundance decreases. This could be because trees producing large fruits, such as figs, which are often removed during logging, may take several decades to regenerate to a fruit-bearing age [34]. This might harm populations of canopy species dependent on fruit, such as Treron olax.
Granivores, such as Turtur tympanistria, have been hypothesized to have a response similar to frugivores [15,17]. We found that, in the short to medium-term, granivores and herbivores increase in abundance after selective logging. However, this response is strongly dependent on time since logging. In the long run, granivores and herbivores appear to suffer from logging. Certain types of seeds, such as those from quickly growing and reproducing pioneer species, grasses and forbs, may be more abundant shortly after logging ceases, due to openings allowing more light to penetrate to the forest floor. This is however only temporary, as the canopy seals within a few months to a few years after logging [35]. Other seeds, from large trees targeted by selective logging, might be scarce for many decades after extraction ceases. Indeed, our results suggest that despite a temporary lift in abundance of certain species, selective logging may lead to an eventual decrease below pre-logging levels even in these temporarily positively responding species.
We expected the opposite trend for species that are on average negatively impacted by selective logging: as forest regenerates after logging, reduced populations of insectivores should return to their pre-logging levels. Nevertheless, insectivores, which were on average negatively influenced by logging, decreased even further as time went on (figure 1 and table 2; electronic supplementary material, figure S2).
The cause of the frequently reported hyper-sensitivity of insectivores is not known [6,15,17]. Consequently, it is also unclear why these species, such as Kenopia striata, decline even further with time since logging. A possible reason is that insectivorous species are often highly specialized, an example being army ant followers, which are known to disappear completely from logged forests [36]. Many insectivores are dependent on forest undergrowth that is often cleared during logging operations; they may also suffer higher direct mortality [13,15,17,18]. Further, opening of the forest canopy and creating gaps in the forest make the understory microclimate hotter and drier, which has further negative effects on the prey base and physiology of insectivorous birds [19]. Alternatively, species typical of river banks and forest clearings, such as Malacopteron affine, might initially benefit from the openings created by logging gaps. After some time, however, a regenerating forest might become denser than a primary forest, putting such species at a disadvantage.
The further decline of many species with time may be a sign that logged forests are actually population sinks, especially for frugivores and insectivores. Such an effect has been hypothesized, but could not be detected previously, owing to the ‘snapshot’ nature of most case studies [15]. The few case studies that assessed bird abundance at several points in time showed that bird species populations do not fully recover even after several decades [37,38].
Whereas a logging operation might cause little direct mortality to some species, the reproductive success of a species can still be impaired by the lack of food, or an increased pathogen load and stress [37]. Even species that initially benefit from logging, presumably due to a higher abundance of food source, may eventually decline because of a lack of suitable nesting sites and these impacts might become apparent only after one or several generations [7]. Perhaps even more importantly, such a population sink effect could be caused, or at least exacerbated, by increased hunting and collecting of birds in logging concessions, facilitated by road construction [39].
Regardless of the cause, our results, showing that the abundance of all feeding groups decreases with time, suggest that it is a serious concern, which must be considered when the value of logged forests in biodiversity conservation is discussed. In terms of management recommendations for logging concessions, disentangling the effects of logging and hunting on bird populations will be a crucial next step in research. See the electronic supplementary material for a brief discussion of other notable variables, including hunting.
(b). Comparison to forest fragmentation
Our findings mirror the main results from tropical forest fragmentation studies: diet has been found to be an important factor in explaining the susceptibility of bird species to fragmentation [40,41]. For example, nectarivores have been found to thrive in even small forest fragments, probably owing to high abundance or flowering plants, particularly along fragment edges, but also due to smaller spatial requirements of nectarivores [41–43]. Equally, insectivores, especially understory specialists have been found to be most prone to extinction from fragments [41,44]. Importantly, as tropical logged forests become increasingly surrounded by non-forest landscapes, it will be crucial to study the effects of fragmentation and degradation or habitat alteration at the same time, as movement between fragments is highly dependent on the habitat type of the surrounding matrix [42].
(c). Limitations
We were able to include only a limited selection of bird species traits, largely because many traits commonly available for temperate species are as yet unknown for many tropical species. We believe that considering fewer, ecologically meaningful traits, rather than fewer species, gives more robust results. This is because the amount of knowledge is probably non-randomly distributed between species. For example, rare, inconspicuous species might be less well known than large, common species, and this could introduce a strong bias into our analysis [45].
Nevertheless, several of the omitted traits might play an important role in influencing the abundance of bird species owing to selective logging, especially given that our best model explains on average 32% of variance. For example, large home range requirements and low dispersal abilities might render a species more sensitive to forest disturbance. However, both of these traits are to some extent correlated with body mass [19].
The responses of individual species to selective logging might be indirectly affected by the responses of other species in the community. Local extinction or population declines of one species due to logging may trigger competitive release on other species in the same community [46].
We emphasize that only a minority of the studies included in the meta-analysis examine avian populations in forests under ‘best-practice’ management, such as RIL. Indeed, the majority of the studies refer to forests without a sound sustainable forest management plan. As a result, our findings probably underestimate the potential of well-managed timber production forests for avian biodiversity conservation. Unfortunately, the relative paucity of such well-managed forests in our meta-analysis reflects the reality: as yet, the majority of tropical forests are not managed sustainably [47].
If the number of well-managed, and also well-documented examples of timber extraction accumulates in the future, it will become possible to evaluate even more specific aspects of forest management, for which there are currently not enough data points. These might include the importance of set-asides, logging on different slopes, or maintenance of ecosystem legacies [7].
(d). Implications for forest management and conclusions
Our model could be used by forest managers to predict the response of individual species, based on the planned logging practice, and on each species' traits. Such predictions may be useful in the planning of logging operations in countries where intensive logging operations will begin in the near future, such as the Democratic Republic of Congo [48]. Additionally, they may also help in assessing the impact of completed operations where biodiversity was not monitored, but mitigation efforts are required.
Current high-intensity, industrial logging operations probably cause a large-scale homogenization of the forest, or what remains of it [7]. Entire age and size classes of the harvested species are often removed, which may lead to a paucity of fruiting trees in the longer term. Similarly to primates [14], the response of bird species to selective logging is highly variable. We have shown that it depends at least in part on the logging intensity employed, the type of logging management and the length of logging cycles (tables 1 and 2, and figure 1). Worryingly, species that are mostly negatively impacted by selective logging do not appear to recover within approximately 40 years after logging ceases (figure 1). We conclude that the maintenance of heterogeneity of the logged forest stands is therefore crucial to tropical forest management [7]. We recommend that within each logging concession, some areas should always remain unlogged, and, if possible, forests should be allowed to regenerate for much longer than 40 years before they are re-logged. The more complex the remaining forest landscape that results from different logging intensities staggered in time, the higher the chances that even a species with highly specialized requirements will be able to obtain the resources essential for its survival.
Supplementary Material
Acknowledgements
We thank Jacob Socolar, Bert Harris, Robi Bagchi, Matteo Tanadini and Louis du Plessis for useful insights. We are grateful to Sherron Bullens, Debbie Fisher, David Hayes, and especially Beth Karpas, Kathleen McMullen and Jason Socci for their dedicated help with the world bird ecology database. We thank Kyle van Houtan and an anonymous reviewer for helpful suggestions that improved our manuscript.
Authors' Contributions
All authors conceived the study, advised on the analysis, helped with writing of the manuscript and gave a final approval for publication. Z.B. designed the study, collected data from literature, carried out the analysis and drafted the manuscript. T.M.L., X.G., D.W. and L.P.K. helped with study design and analysis. C.H.S. provided bird ecology data.
Competing Interests
We declare we have no competing interests.
Funding
Z.B. is funded by the Swiss National Science Foundation. C.H.S. is supported by the University of Utah. L.P.K. is supported by the Australian Research Council.
References
- 1.Putz FE, et al. 2012. Sustaining conservation values in selectively logged tropical forests: the attained and the attainable. Conserv. Lett. 5, 296–303. ( 10.1111/j.1755-263X.2012.00242.x) [DOI] [Google Scholar]
- 2.Asner GP, Knapp DE, Broadbent EN, Oliveira PJC, Keller M, Silva JN. 2005. Selective logging in the Brazilian Amazon. Science 310, 480–482. ( 10.1126/science.1118051) [DOI] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations (FAO). 2012. Global forest land-use change 1990–2005. FAO Forestry paper no. 169. Rome, Italy: FAO United Nations and European Commission Joint Research Centre. [Google Scholar]
- 4.Gaveau DLA, et al. 2013. Reconciling forest conservation and logging in Indonesian Borneo. PLoS ONE 8, e69887 ( 10.1371/journal.pone.0069887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations (FAO). 2010. Global Forest Resources Assessment 2010. FAO Forestry paper no. 163. Rome, Italy: FAO. [Google Scholar]
- 6.Fimbel RA, Grajal A, Robinson JG. 2001. The cutting edge. New York, NY: Columbia University Press. [Google Scholar]
- 7.Messier C, Puettmann KJ, Coates DK. (eds). 2013. Managing forests as complex adaptive systems, 1st edn New York, NY: Routledge. [Google Scholar]
- 8.Putz FE, Romero C. 2014. Futures of tropical forests (sensu lato). Biotropica 46, 495–505. ( 10.1111/btp.12124) [DOI] [Google Scholar]
- 9.Thompson ID, Guariguata MR, Okabe K, Bahamondez C, Nasi R, Heymell V. 2013. An operational framework for defining and monitoring forest degradation. Ecol. Soc. 18, 20 ( 10.5751/ES-05443-180220) [DOI] [Google Scholar]
- 10.Simula M. 2009. Towards defining forest degradation: comparative analysis of existing definitions. Rome, Italy: FAO. [Google Scholar]
- 11.Burivalova Z, Şekercioğlu CH, Koh LP. 2014. Thresholds of logging intensity to maintain tropical forest biodiversity. Curr. Biol. 24, 1–6. ( 10.1016/j.cub.2014.06.065) [DOI] [PubMed] [Google Scholar]
- 12.Bicknell JE, Struebig MJ, Edwards DP, Davies ZG. 2014. Improved timber harvest techniques maintain biodiversity in tropical forests. Curr. Biol. 24, 1119–1120. ( 10.1016/j.cub.2014.10.067) [DOI] [PubMed] [Google Scholar]
- 13.Cleary DFR, Boyle TJB, Setyawati T, Anggraeni CD, van Loon EE, Menken SBJ. 2007. Bird species and traits associated with logged and unlogged forest in Borneo. Ecol. Appl. 17, 1184–1197. ( 10.1890/05-0878) [DOI] [PubMed] [Google Scholar]
- 14.Cowlishaw G, Pettifor RA, Isaac NJB. 2009. High variability in patterns of population decline: the importance of local processes in species extinctions. Proc. R. Soc. B 276, 63–69. ( 10.1098/rspb.2008.0767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijaard E, et al. 2005. Life after logging, 1st edn Jakarta, Indonesia: Center for International Forestry Research. [Google Scholar]
- 16.Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K. 2007. Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol. Conserv. 137, 1–19. ( 10.1016/j.biocon.2007.01.023) [DOI] [Google Scholar]
- 17.Lambert FR, Collar NJ. 2002. The future for Sundaic lowland forest birds: long-term effects of commercial logging and fragmentation. Forktail 18, 127–146. [Google Scholar]
- 18.Sodhi NS, Liow LH, Bazzaz FA. 2004. Avian extinctions from tropical and subtropical forests. Annu. Rev. Ecol. Evol. Syst. 35, 323–345. ( 10.2307/annurev.ecolsys.35.112202.30000013) [DOI] [Google Scholar]
- 19.Sodhi NS, Şekercioğlu CH, Barlow J, Robinson SK. 2011. Conservation of tropical birds. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 20.Newbold T, Scharlemann JPW, Butchart SHM, Şekercioğlu CH, Alkemade R, Booth H, Purves DW. 2013. Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc. R. Soc. B 280, 20122131 ( 10.1098/rspb.2012.2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putz FE, Redford KH, Robinson JG, Fimber R, Blate GM. 2000. Biodiversity conservation in the context of tropical forest management. Washington, DC: The World Bank. [Google Scholar]
- 22.Şekercioğlu CH. 2012. Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J. Ornithol. 153, 153–161. ( 10.1007/s10336-012-0869-4) [DOI] [Google Scholar]
- 23.Şekercioğlu CH, Daily GC, Ehrlich PR. 2004. Ecosystem consequences of bird declines. Proc. Natl Acad. Sci. USA 101, 18 042–18 047. ( 10.1073/pnas.0408049101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnham KP, Anderson DR. 1998. Model selection and multimodel inference, 2nd edn New York, NY: Springer. [Google Scholar]
- 25.Akaike H. 1973. Information theory as an extension of the maximum likelihood principle. In Second Int. Symp. Information Theory (eds Petrov BN, Csaki F.), pp. 267–281. Budapest, Hungary: Akademiai Kiado. [Google Scholar]
- 26.Symonds MRE, Moussalli A. 2010. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21. ( 10.1007/s00265-010-1037-6) [DOI] [Google Scholar]
- 27.R Development Core Team R. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Harris G, Pimm SL. 2008. Range size and extinction risk in forest birds. Conserv. Biol. 22, 163–171. ( 10.1111/j.1523-1739.2007.00798.x) [DOI] [PubMed] [Google Scholar]
- 29.Manne LL, Brooks TM, Pimm SL. 1999. Relative risk of extinction of passerine birds on continents and islands. Nature 399, 258–261. ( 10.1038/20436) [DOI] [Google Scholar]
- 30.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952. ( 10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman CA, Chapman LJ. 1997. Forest regeneration in logged and unlogged forests of Kibale National Park, Uganda. Biotropica 29, 396–412. ( 10.1111/j.1744-7429.1997.tb00035.x) [DOI] [Google Scholar]
- 32.Brown KA, Gurevitch J. 2004. Long-term impacts of logging on forest diversity in Madagascar. Proc. Natl Acad. Sci. USA 101, 6045–6049. ( 10.1073/pnas.0401456101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struhsaker TT. 1997. Ecology of an African rain forest logging in Kibale and the conflict between conservation and exploitation. Gainesville, FL: University Press of Florida. [Google Scholar]
- 34.Datta A. 1998. Hornbill abundance in unlogged forest, selectively logged forest and a forest plantation in Arunachal Pradesh, India. Oryx 32, 285–294. ( 10.1017/S0030605300030088) [DOI] [Google Scholar]
- 35.Souza CM, Roberts DA, Cochrane MA. 2005. Combining spectral and spatial information to map canopy damage from selective logging and forest fires. Remote Sens. Environ. 98, 329–343. ( 10.1016/j.rse.2005.07.013) [DOI] [Google Scholar]
- 36.Thiollay J. 1997. Disturbance, selective logging and bird diversity: a Neotropical forest study. Biodivers. Conserv. 6, 1155–1173. ( 10.1023/A:1018388202698) [DOI] [Google Scholar]
- 37.Dranzoa C. 1998. The avifauna 23 years after logging in Kibale National Park, Uganda. Biodivers. Conserv. 7, 777–797. ( 10.1023/A:1008892419940) [DOI] [Google Scholar]
- 38.Johns AG. 1989. Recovery of a peninsular Malaysian rainforest avifaune following selective timber logging: the first twelve years. Forktail 4, 89–105. [Google Scholar]
- 39.Robinson JG, Redford KH, Bennett EL. 1999. Wildlife harvest in logged tropical forests. Science 284, 595–596. ( 10.1126/science.284.5414.595) [DOI] [Google Scholar]
- 40.Bregman TP, Sekercioglu CH, Tobias JA. 2014. Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation. Biol. Conserv. 169, 372–383. ( 10.1016/j.biocon.2013.11.024) [DOI] [Google Scholar]
- 41.Laurance WF, et al. 2011. The fate of Amazonian forest fragments: a 32-year investigation. Biol. Conserv. 144, 56–67. ( 10.1016/j.biocon.2010.09.021) [DOI] [Google Scholar]
- 42.van Houtan KS, Pimm SL, Halley JM, Bierregaard RO, Lovejoy TE. 2007. Dispersal of Amazonian birds in continuous and fragmented forest. Ecol. Lett. 10, 219–229. ( 10.1111/j.1461-0248.2007.01004.x) [DOI] [PubMed] [Google Scholar]
- 43.Stouffer PC, Bierregaard RO. 1995. Effects of forest fragmentation on understory hummingbirds in Amazonian Brazil. Conserv. Biol. 9, 1085–1094. ( 10.1046/j.1523-1739.1995.9051072.x-i1) [DOI] [PubMed] [Google Scholar]
- 44.Stouffer PC, Strong C, Naka LN. 2009. Twenty years of understorey bird extinctions from Amazonian rain forest fragments: consistent trends and landscape-mediated dynamics. Divers. Distrib. 15, 88–97. ( 10.1111/j.1472-4642.2008.00497.x) [DOI] [Google Scholar]
- 45.Freckleton RP. 2009. The seven deadly sins of comparative analysis. J. Evol. Biol. 22, 1367–1375. ( 10.1111/j.1420-9101.2009.01757.x) [DOI] [PubMed] [Google Scholar]
- 46.Debinski DM, Holt RD. 2000. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 14, 342–355. ( 10.1046/j.1523-1739.2000.98081.x) [DOI] [Google Scholar]
- 47.FAO. 2005. Global forest resources assessment 2005: progress towards sustainable forest management. FAO Forestry paper no. 147 Rome, Italy: FAO. [Google Scholar]
- 48.Nair CT. 2006. What is the future for African forests and forestry? Int. For. Rev. 8, 4–13. ( 10.1505/ifor.8.1.4) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.