Abstract
Global warming is increasing the overheating risk for many organisms, though the potential for plasticity in thermal tolerance to mitigate this risk is largely unknown. In part, this shortcoming stems from a lack of knowledge about global and taxonomic patterns of variation in tolerance plasticity. To address this critical issue, we test leading hypotheses for broad-scale variation in ectotherm tolerance plasticity using a dataset that includes vertebrate and invertebrate taxa from terrestrial, freshwater and marine habitats. Contrary to expectation, plasticity in heat tolerance was unrelated to latitude or thermal seasonality. However, plasticity in cold tolerance is associated with thermal seasonality in some habitat types. In addition, aquatic taxa have approximately twice the plasticity of terrestrial taxa. Based on the observed patterns of variation in tolerance plasticity, we propose that limited potential for behavioural plasticity (i.e. behavioural thermoregulation) favours the evolution of greater plasticity in physiological traits, consistent with the ‘Bogert effect’. Finally, we find that all ectotherms have relatively low acclimation in thermal tolerance and demonstrate that overheating risk will be minimally reduced by acclimation in even the most plastic groups. Our analysis indicates that behavioural and evolutionary mechanisms will be critical in allowing ectotherms to buffer themselves from extreme temperatures.
Keywords: plasticity, acclimation, thermal tolerance, climate change, ectotherm
1. Introduction
Climate change is increasing mean environmental temperatures and the frequency of extreme thermal events [1]. As a result, organisms across the globe will be more likely to experience temperatures beyond their physiological limits unless they can in some way buffer themselves from environmental change [2,3]. One mechanism that could greatly reduce the risk of overheating is physiological plasticity in thermal tolerance, such as the reversible changes in thermal tolerance known as acclimation (if measured in the laboratory) or acclimatization (if measured in the field) [4–7]. For example, the upper thermal tolerance limits of many organisms increase (within individuals) as mean body temperatures rise, meaning that physiological adjustments can potentially compensate for the negative consequences of rising habitat temperatures [8]. Despite the potential importance of plasticity in dictating population vulnerability to climate change, calculations of overheating risk rarely take into account plasticity in thermal tolerance (e.g. [2,9]). Ignoring plasticity can affect estimations of absolute overheating risk, and lead to errors in assessing patterns of risk that may exist among taxa from different habitats or taxonomic groups [10].
Two primary hypotheses currently exist to explain broad patterns of variation in thermal plasticity among ectotherms. First, the latitudinal hypothesis predicts a pattern of increasing thermal tolerance plasticity as one moves from the equator to the poles based on the concomitant increase in thermal seasonality [11–14]. This expected relationship is one of several reasons that tropical organisms are proposed to be particularly vulnerable to warming [15]. Second, the trade-off hypothesis predicts that organisms with the highest overall thermal tolerance, or inherent thermal tolerance [16], will have the lowest tolerance plasticity [4,17]. This hypothesis is based on observations of reduced plasticity in organisms from extreme environments (e.g. [18,19]), and leads to the somewhat counterintuitive prediction that in some cases organisms with the highest thermal tolerance may be the most vulnerable to warming owing to a lack of ability to physiologically adjust to thermal change [20]. Comprehensive tests of the latitudinal and trade-off hypotheses are currently lacking for thermal tolerance plasticity, with tests coming from a few phylogenetically restricted analyses (i.e. within single genera) that have yielded mixed results [16,20–27]. For example, multiple studies have assessed geographical patterns of thermal tolerance plasticity in Drosophila, but have produced little evidence that high-latitude species have greater plasticity [16,25,26]. Support for the trade-off hypothesis has been demonstrated in Petrolisthes porcelain crabs [20], but not within Deronectes diving beetles [21].
The lack of consensus about broad-scale drivers of tolerance plasticity prevents robust global estimates of the degree to which tolerance plasticity can mitigate the effects of warming. To address this important gap in our knowledge, we assessed the latitudinal and trade-off hypotheses using a large dataset containing 394 estimates of thermal tolerance plasticity from 232 ectotherm species, including vertebrate and invertebrate groups living in terrestrial and aquatic habitats. Taxa sampled included insects, crustaceans, fish, amphibians and reptiles. We used data on critical thermal limits (CTmax and CTmin for upper and lower thermal tolerance, respectively), defined as the body temperatures at which an organism loses muscle coordination such that it cannot escape from a harmful situation [28,29]. CTs are a particularly ecologically relevant estimate of thermal tolerance because they are measured during gradual increases in body temperature, similar to the patterns of thermal change that organisms experience in their natural environments [25,30]. Plasticity was calculated as the acclimation response ratio (ARR), the slope of the line describing the change in thermal tolerance with a given change in acclimation temperature [31–34]. In addition to determining support for the latitudinal and trade-off hypotheses, we investigated patterns of plasticity in thermal tolerance among major ectotherms groups and calculated the degree to which observed plasticity in thermal tolerance can buffer ectotherms from rising temperatures.
2. Material and methods
For each population, mean thermal tolerance at each acclimation temperature was taken from text or tables of published studies (see the electronic supplementary material, table S1). In some cases, values were not reported so mean thermal tolerances were estimated from figures (sensu [35]). Negative ARRs values (i.e. thermal tolerance decreases as the thermal challenge increases) are indicative of physiologically damaging acclimation conditions. Thus, we excluded ARRs that were less than −0.15 (small negative values are expected owing to experimental error). Adult animals were targeted, so if acclimation capacity was reported for multiple ages or developmental stages in a population, we always chose data for the oldest or most developmentally advanced group. In addition, if plasticity values were measured across categories such as sex or season for a population, as a rule we always chose data for the group that demonstrated the greatest plasticity.
Support for hypotheses was assessed within an information theoretic framework [36]. For each hypothesis, an a priori set of linear mixed models was constructed that included terms and interactions that might explain variation in thermal tolerance plasticity (see below). All models included a nested, hierarchical random term representing taxonomic affinities of the taxa included (phylum/class/order/family/genus/spp) to account for non-independence of data since no phylogenetic tree is available for all taxa included in this study [37,38]. Model validation was conducted by examining diagnostic plots (e.g. histograms of standardized residuals, plots of standardized residuals versus fitted values, and plots of standardized residuals versus explanatory variables [39]). Models were run using the lme function in the nlme package in R [40]. Model comparisons were based on AICc scores given our small sample sizes relative to the number of parameters estimated in our mixed models [41]. Model comparisons were conducted with the MuMIn package in R.
Both the time that experimental animals are given to acclimate and the heating/cooling rates used during tolerance measurement are methodological factors that could influence the amount of plasticity measured [30,42]. However, data on acclimation times and heating/cooling rates were not given for all populations, so including these factors in our full models would result in a loss of data to test our hypotheses. Thus, we conducted preliminary analyses to assess effects of these variables before including them in our primary models. Acclimation time had a small but positive association with both upper and lower plasticity (see the electronic supplementary material, figure S1 and table S2), but cooling rate and heating rate had very small effects on CTmin ARR and CTmax ARR, respectively (see the electronic supplementary material, figure S1 and table S2). Thus, only acclimation time (in days) was included as a covariate in our models.
For the latitudinal hypothesis, we included latitude as a term in models including habitat type (marine, freshwater, terrestrial), hemisphere (N/S) and acclimation time. The latitudinal hypothesis is based on the general trend of increasing thermal seasonality with latitude. However, latitude is not a perfect proxy for seasonality, as factors such as ocean currents and proximity to oceans (in terrestrial habitats) can alter climate dynamics [43]. To account for this, we conducted a second set of analyses using direct estimates of thermal seasonality with the subset of our data for which we had both latitude and longitude. For the analysis of CTmin ARR and seasonality, we had small sample sizes for aquatic taxa (freshwater N = 6, marine N = 13) that precluded the estimation of interactions with habitat [44]. Thus, in that case we conducted analyses with terrestrial data only. Geospatial data for assessments of the latitudinal hypothesis were compiled in several ways. Latitudes and longitudes for collection localities were either given or could be estimated from locality descriptions. In instances where collection localities were not given, the latitude was taken to be the midpoint of the latitudinal range of the species based on collection records stored primarily in the Global Biodiversity Information Facility database (gbif.org). For observations in which latitude and longitude were available, we extracted bioclimatic data on seasonality in thermal conditions (see the electronic supplementary material, figure S2 for map of localities). Seasonality for terrestrial and freshwater taxa was the standard deviation of annual mean weekly air temperatures extracted from 0.25 × 0.25′ Bioclim climatic layers (Bioclim variable Bio4; air temperature is often used as a proxy for freshwater thermal conditions [45]). Seasonality for marine taxa was the standard deviation of mean weekly Sea Surface Temperatures from 1989 to 2014, taken from NOAAs 1° × 1° Optimum Interpolated Sea Surface Temperature dataset.
The trade-off hypothesis predicts that organisms that can tolerate the most extreme conditions will have the lowest tolerance plasticity. To assess this, we used linear mixed models as described above, including maximum measured thermal tolerance (maximum CTmax or minimum CTmin) as a term [16,22].
To explore the potential for plasticity in thermal tolerance to buffer ectotherms from rising temperatures, we calculated how thermal safety margins (the difference between mean body temperature and upper thermal tolerance) will change as mean body temperatures rise given the mean CTmax ARR for each major clade. This was calculated as
| 2.1 |
3. Results
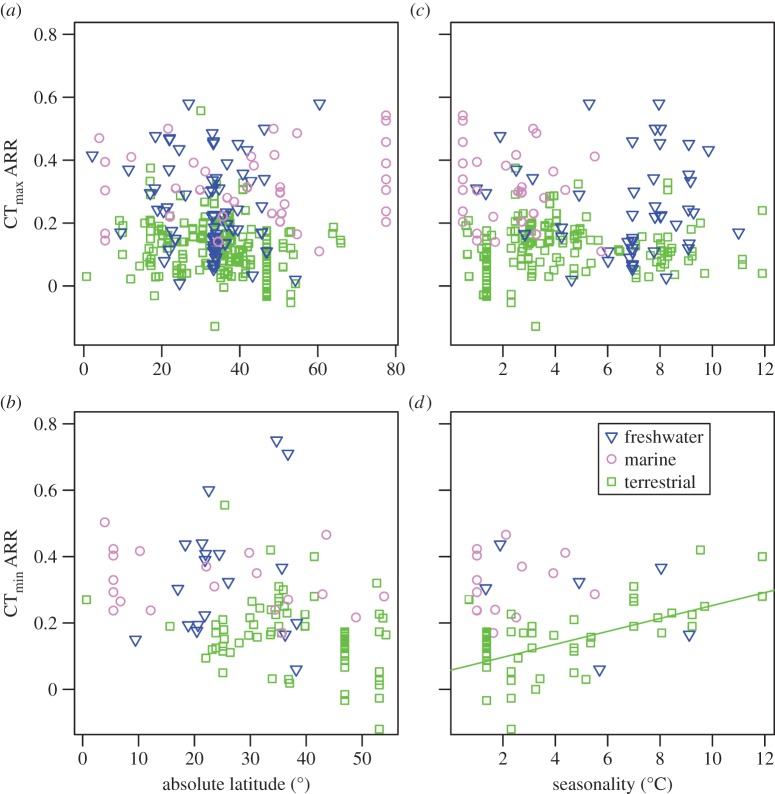
We report only the top models (Δi < 2), but summaries of all models considered can be found in the electronic supplementary material, tables S3–S7. Contrary to expectation, there was little evidence that latitude was related to plasticity in upper thermal tolerance (figure 1a; see clade-specific data in the electronic supplementary material, figure S3). Latitude was not included in the top model (table 1a), the model-averaged effect size of latitude was small (slope = 0.0003), and the highest ranked models that included latitude explained no more variation than the top model (see the electronic supplementary material, table S3). However, habitat type was included in the top model (and the top five models overall), with aquatic taxa having mean CTmax ARRs more than twice that of terrestrial taxa (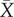 ± s.e.: terrestrial
± s.e.: terrestrial 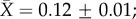 freshwater
freshwater 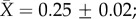 marine
marine 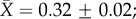 figure 1a and table 1a). Similar patterns were found with respect to CTmin ARR. An interaction between habitat and latitude was included in one of the top models but that model explained only 4% more variation than the top model with only acclimation time, habitat and hemisphere (table 1). Latitude also had a very low model-averaged effect on CTmin ARR (slope = 0.001; figure 1b). However, examination of taxon-specific plots suggests a positive relationship between CTmin ARR and latitude in reptiles (see the electronic supplementary material, figure S4). Habitat type was again retained in the top models, with aquatic taxa having higher plasticity than terrestrial taxa (terrestrial
figure 1a and table 1a). Similar patterns were found with respect to CTmin ARR. An interaction between habitat and latitude was included in one of the top models but that model explained only 4% more variation than the top model with only acclimation time, habitat and hemisphere (table 1). Latitude also had a very low model-averaged effect on CTmin ARR (slope = 0.001; figure 1b). However, examination of taxon-specific plots suggests a positive relationship between CTmin ARR and latitude in reptiles (see the electronic supplementary material, figure S4). Habitat type was again retained in the top models, with aquatic taxa having higher plasticity than terrestrial taxa (terrestrial 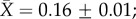 freshwater
freshwater 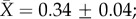 marine
marine 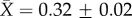 ).
).
Figure 1.
Acclimation in thermal tolerance in relation to absolute latitude, seasonality and habitat. Seasonality is the standard deviation of weekly mean environmental temperatures (see §2). (a) CTmax ARR with respect to latitude. (b) CTmin ARR with respect to latitude. (c) CTmax ARR with respect to seasonality and (d) CTmin ARR with respect to seasonality. (Online version in colour.)
Table 1.
Summary of top models for acclimation in thermal tolerance including latitude or seasonality. k, number of parameters; log(L), model log likelihood; AICc, model Akaike Information Criterion score; Δi, difference in AICc score from top model; wi, model weight; R2, marginal R2 (variance explained by fixed factors within the models).
| model | k | log(L) | AICc | Δi | wi | R2 |
|---|---|---|---|---|---|---|
| (a) CTmax ARR and latitude | ||||||
| accl. time + habitat | 11 | 255.558 | −488.1 | 0.00 | 0.428 | 0.269 |
| (b) CTmin ARR and latitude | ||||||
| accl. time + habitat | 11 | 94.303 | −163.9 | 0.00 | 0.300 | 0.288 |
| habitat | 10 | 92.944 | −163.8 | 0.15 | 0.278 | 0.267 |
| accl. time + habitat + hemisphere | 12 | 94.635 | −162.1 | 1.85 | 0.119 | 0.314 |
| accl. time + habitat × latitude | 14 | 97.162 | −161.9 | 1.99 | 0.111 | 0.352 |
| (c) CTmax ARR and seasonality | ||||||
| accl. time + habitat | 11 | 217.449 | −411.7 | 0.00 | 0.487 | 0.216 |
| (d) CTmin ARR and seasonality | ||||||
| seasonality | 9 | 72.000 | −122.4 | 0.00 | 0.731 | 0.408 |
There was little evidence that thermal seasonality is related to plasticity in upper thermal tolerance. Seasonality was not included in the top model (table 1c), the model-averaged effect size of seasonality was small (slope = 0.0007; figure 1c), and models that included seasonality did not explain more variation than those without it (see the electronic supplementary material, table S5). In contrast to the latitudinal analyses, there is support for a substantial effect of seasonality on plasticity in lower thermal tolerance in terrestrial taxa. Seasonality was retained in the top model (table 1d) and had an effect size over an order of magnitude higher than that for CTmax ARR (model-averaged slope = 0.019; figure 1d).
Contrary to prediction, there was little evidence that inherent thermal tolerances were related to thermal tolerance plasticity. Inherent thermal tolerance was not retained in the top models (table 2a,b), had small effect sizes (model-averaged slope = −0.0001 and 0.00009 for upper and lower inherent tolerance, respectively; figure 2) and did not increase the explanatory power of models (see the electronic supplementary material, table S6). Although not linked to inherent thermal tolerance, there does appear to be a substantial positive association between plasticity in upper and lower thermal tolerance (see the electronic supplementary material, figure S5 and table S7).
Table 2.
Summary of top models for acclimation in thermal tolerance including inherent thermal tolerance. See table 1 for symbol descriptions.
| model | k | log(L) | AICc | Δi | wi | R2 |
|---|---|---|---|---|---|---|
| (a) CTmax ARR versus maximum CTmax | ||||||
| accl. time | 9 | 251.266 | −483.9 | 0.00 | 0.713 | 0.019 |
| (b) CTmin ARR versus minimum CTmin | ||||||
| accl. time | 10 | 90.870 | −159.5 | 0.00 | 0.693 | 0.074 |
Figure 2.
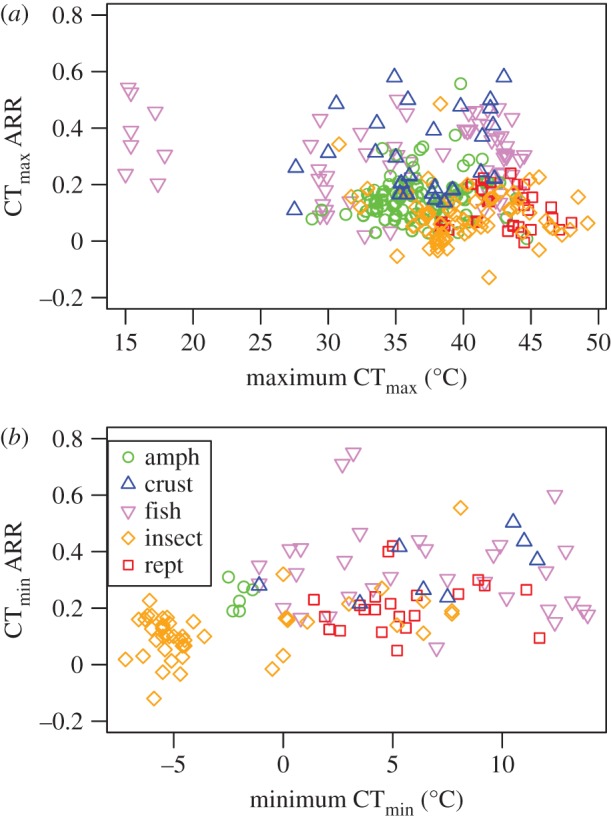
Relationship between acclimation and inherent thermal tolerances. (a) CTmax ARR with respect to maximum CTmax and (b) CTmin ARR with respect to minimum CTmin. (Online version in colour.)
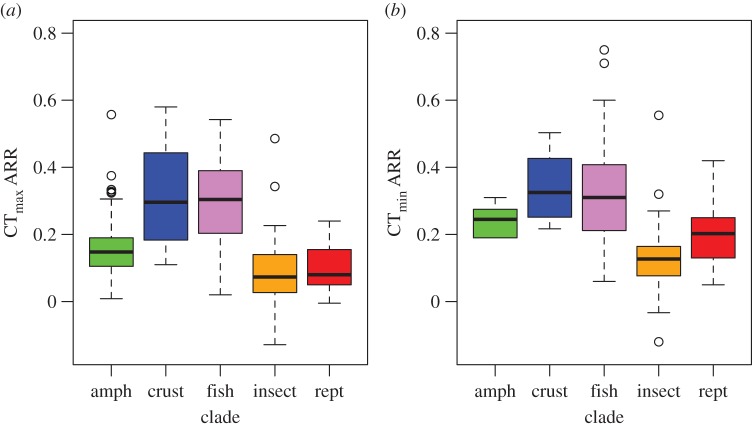
To investigate the capacity for plasticity to reduce overheating risk under rising temperatures, we used data on CTmax ARRs to calculate how much thermal safety margins (CTmax – mean body temperature) will be reduced for a given change in body temperature under warming while taking tolerance plasticity for each major clade (figure 3) into account. When the data are rescaled in this way, we find that on average fish and crustaceans will have smaller decreases in thermal safety margin than insects, reptiles and amphibians for a given rise in body temperature (figure 4), in accord with the finding that aquatic organisms have higher plasticity than terrestrial organisms. Nonetheless, warming will substantially reduce thermal safety margins for members of all of the groups that we considered, as physiological compensation will still be nowhere near complete even for aquatic taxa (figure 4).
Figure 3.
Acclimation in (a) upper and (b) lower thermal tolerance by clade. (Online version in colour.)
Figure 4.
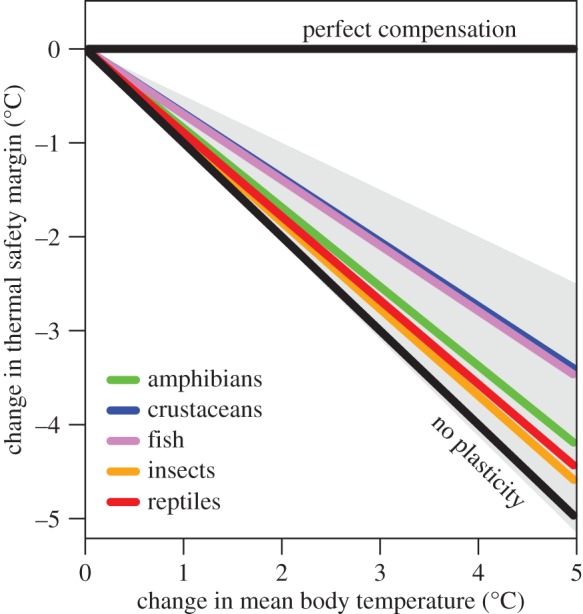
Predicted changes in thermal safety margin given changes in mean body temperature. Coloured lines are based on mean CTmax ARR values for each clade. Grey polygon bounds the central 95% of CTmax ARR measurements across all clades.
4. Discussion
We tested leading hypotheses to explain broad-scale patterns of plasticity in ectotherm thermal tolerance. A primary result from our analysis is that plasticity in lower thermal tolerance is more likely to be associated with variation in thermal environments than plasticity in upper thermal tolerance (figure 1; see the electronic supplementary material, figure S4). We hypothesize that the greater response in lower tolerance plasticity may be owing to differences in the effectiveness of behavioural thermoregulation at influencing maximum versus minimum body temperatures. The warmest temperatures occur during the day during seasons when there is the greatest spatial variability in operative thermal conditions. For example, in terrestrial habitats, exposure to sun can increase body temperature if temperatures are cold, while if air temperatures are warm, seeking out shade can reduce body temperature [46]. Similar processes can also occur in freshwater systems (e.g. [47]). Behavioural thermoregulation thus has the ability both to reduce variability in maximum body temperature and to prevent organisms from experiencing damaging extremes [48,49]. By contrast, the coldest temperatures that organisms experience occur during seasons when operative thermal conditions are less variable and when many animals are dormant and cannot behaviourally adjust to changing conditions. As a result, behavioural thermoregulation is less effective at reducing variability in the minimum temperatures experienced and in preventing exposure to extreme cold. This mechanism may also contribute to parallel geographical patterns seen in inherent thermal tolerances, where CTmin changes more quickly with latitude and elevation than CTmax [37,50,51].
The higher thermal plasticity of organisms in aquatic versus terrestrial habitats was unexpected (figures 1 and 3; but see [38]). Aquatic habitats generally have less seasonal variability in temperature than terrestrial habitats, so one might expect less plasticity in aquatic taxa. However, this pattern may again be driven by variation in the ability to effectively thermoregulate. Aquatic habitats tend to have less spatial variability in operative thermal conditions than terrestrial habitats, and thus aquatic organisms may be less able to behaviourally buffer themselves from changing thermal conditions relative to terrestrial taxa. Indeed, ranges of marine taxa are shifting more rapidly than those of terrestrial taxa per degree of warming [52], perhaps owing to the reduced potential for thermoregulation.
Based on the habitat-dependent patterns of variation in thermal tolerance plasticity that we observed, we hypothesize that the ‘Bogert effect’ may play a primary role in mediating the evolution of thermal plasticity. The Bogert effect refers to the ability of behavioural adjustments to buffer organisms from experiencing environmental (including thermal) variation, potentially reducing selection pressure on other phenotypic (including physiological) traits [48,49]. Our hypothesis is based on two primary observations: (i) plasticity in lower thermal tolerance has a greater response to thermal variation than plasticity in upper thermal tolerance in terrestrial habitats and (ii) aquatic organisms have higher plasticity overall than terrestrial organisms. In both cases, greater physiological plasticity is observed under environmental conditions in which the potential for behavioural thermoregulation is reduced (see above).
We found little support for the trade-off hypothesis, as lower and upper inherent thermal tolerances had minimal effects on CTmin ARR and CTmax ARR, respectively. Thus, although trade-offs are observed within some taxa (e.g. [20,53]), there is no overall expectation for reduced thermal plasticity in taxa that can tolerate extreme thermal environments [21,54]. However, we do find an association between plasticity in upper thermal tolerance and plasticity in lower thermal tolerance. This indicates that these traits may evolve in a correlated manner, although clearly the linkage is not perfect given the differential responses of CTmax ARR and CTmin ARR to thermal seasonality in terrestrial taxa (figure 1).
5. Implications for climate change
The ability to acclimatize to changing thermal conditions is expected to be a primary factor that dictates the vulnerability of taxa to rising temperatures [4–6,10,20]. Our broad-scale analysis of thermal tolerance plasticity across ectotherms has many implications for our understanding of the interactions between plasticity and warming. First, it has been suggested that tropical taxa may be more vulnerable to warming than temperate taxa, in part, because tropical taxa are expected to have lower plasticity in thermal physiology [12,14,15]. However, we find little support for decreased plasticity in upper thermal tolerances in taxa from low latitude/low seasonality habitats. This result serves as a complement to a recent macrophysiological analysis on plasticity in metabolic rate processes that suggests low-latitude taxa have greater plasticity in metabolic rates than high-latitude taxa [38]. Thus, for lethal (this study) and sub-lethal [38] physiological traits, low-latitude taxa are not at a disadvantage with respect to plasticity. While there may be several reasons why tropical taxa are more susceptible to warming than high-latitude taxa [2,15,55,56], a lack of thermal plasticity is unlikely to be one of them. Similarly, it has been hypothesized that taxa adapted to the warmest habitats will be highly vulnerable to warming owing to small thermal safety margins and low plasticity in thermal tolerance caused by an expected trade-off between plasticity and inherent thermal tolerance [4,20,57]. However, on the broadest taxonomic and environmental scale we find limited evidence for a trade-off between inherent upper thermal tolerance and plasticity. Therefore, there is no general expectation that a relative lack of tolerance plasticity should cause warm habitat taxa to be more vulnerable to warming than cool habitat taxa (see also [21]).
Perhaps the most striking result from our analysis is the low overall plasticity in thermal tolerance that ectotherms possess. Very few species or populations had CTmax ARRs greater than 0.5, and mean CTmax ARRs of all of the taxa included are closer to 0 than they are to 1 (figure 3). Thus, despite the fact that nearly all ectotherms possess some ability to adjust thermal tolerances in response to changing thermal conditions, in most cases that plasticity will be unable to prevent substantial decreases in thermal safety margins when body temperatures rise. This is a troubling result given that both mean environmental temperatures and the frequency of thermal extremes are increasing with climate change [1,58]. The problem of low acclimation potential is particularly acute for taxa that are primarily terrestrial (insects, amphibians and reptiles), which on average have very low CTmax ARRs. Air temperatures are increasing faster than ocean temperatures [59], meaning that terrestrial taxa face faster warming but with less acclimation capacity than their aquatic counterparts (although, as discussed above, terrestrial taxa are likely to have more potential for behavioural thermoregulation). It should be noted that ARRs do not account for nonlinearity in plastic responses and are instead akin to an average of plastic responses across a given thermal window. However, in many cases nonlinearity will make plasticity even less effective at buffering organisms from warming. This is because the degree of plasticity often decreases at relatively high acclimation temperatures (i.e. the line explaining changes in CTmax with changing acclimation temperature reaches an asymptote; e.g. [60,61]).
Our results indicate that physiological plasticity may do little to buffer ectotherms from rising temperatures. This means that an ability to undergo behavioural and evolutionary responses to climate change may be critical for population persistence, particularly in taxa that lack mobility or do not have suitable routes to shift their ranges [62,63]. The ability to thermoregulate changes geographically [2,15,56,64,65], and by habitat type (see discussion of aquatic versus terrestrial taxa above), and thus many taxa may not have behavioural mechanisms at their disposal. There is also much debate about the degree to which populations can evolve to combat changing conditions [66–71]. Plasticity is of course a trait that can evolve, and the evolution of plasticity (or canalization of plastic responses [72]) has been suggested as a means by which organisms can evade negative effects of climate change [73]. However, our analyses indicate that plasticity in thermal tolerance may be evolutionarily constrained. Among ectotherms, CTmax ARR rarely increases above 0.5, or 50% compensation, with most taxa considerably lower than that. Thus, the phenotypic space over which plasticity in thermal tolerance can evolve may be restricted to relatively low levels of compensation.
6. Conclusion
A central challenge for biologists is to generate predictive models of organismal responses to climate change. Because the phenotypes of individuals are not fixed through time, knowledge of capacities for physiological plasticity is an essential component of any forecast. Our analysis provides a comprehensive synthesis of data on tolerance plasticity and demonstrates broad patterns of geographical, habitat and taxonomic variation in this trait. These data can serve as an important foundation for the incorporation of tolerance plasticity into robust assessments of global patterns of climate change vulnerability among ectotherms.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Nathan Miller, Anne Todgham and Mike Sears for helpful discussions and comments on this project and paper. The R script used to extract ocean temperature data was a modification of a script kindly provided online by Luke Miller.
Authors' Contributions
A.R.G. and J.H.S. designed the study and wrote the paper. A.R.G. collected and analysed the data.
Funding
This project was supported by funds from San Francisco State University, the National Science Foundation (grant no. 1041225 to J.H.S), and a grant from the Gordon and Betty Moore Foundation to the Berkeley Initiative in Global Change Biology.
References
- 1.Diffenbaugh NS, Field CB. 2013. Changes in ecologically critical terrestrial climate conditions. Science 341, 486–492. ( 10.1126/science.1237123) [DOI] [PubMed] [Google Scholar]
- 2.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte H, Tejedo M, Katzenberger M, Marangoni F, Baldo D, Beltrán JF, Martí DA, Richter-Boix A, Gonzalez-Voyer A. 2012. Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Glob. Change Biol. 18, 412–421. ( 10.1111/j.1365-2486.2011.02518.x) [DOI] [Google Scholar]
- 4.Somero G. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 5.Huey RB, Kearney MR, Krockenberger A, Holtum JA, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botero CA, Weissing FJ, Wright J, Rubenstein DR. 2014. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. 2014. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. ( 10.1126/science.1251336) [DOI] [PubMed] [Google Scholar]
- 8.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomanek L. 2010. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 213, 971–979. ( 10.1242/jeb.038034) [DOI] [PubMed] [Google Scholar]
- 11.Chown S, Gaston K, Robinson D. 2004. Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Funct. Ecol. 18, 159–167. ( 10.1111/j.0269-8463.2004.00825.x) [DOI] [Google Scholar]
- 12.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 13.Bozinovic F, Calosi P, Spicer JI. 2011. Physiological correlates of geographic range in animals. Annu. Rev. Ecol. Evol. Syst. 42, 155–179. ( 10.1146/annurev-ecolsys-102710-145055) [DOI] [Google Scholar]
- 14.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 15.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Pérez HJÁ, Garland T. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948. ( 10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 17.Chown SL. 2001. Physiological variation in insects: hierarchical levels and implications. J. Insect Physiol. 47, 649–660. ( 10.1016/S0022-1910(00)00163-3) [DOI] [PubMed] [Google Scholar]
- 18.Somero GN, DeVries AL. 1967. Temperature tolerance of some Antarctic fishes. Science 156, 257–258. ( 10.1126/science.156.3772.257) [DOI] [PubMed] [Google Scholar]
- 19.Gause G. 1942. The relation of adaptability to adaptation. Q. Rev. Biol. 17, 99–114. ( 10.1086/394649) [DOI] [Google Scholar]
- 20.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65 ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 21.Calosi P, Bilton DT, Spicer JI. 2008. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 4, 99–102. ( 10.1098/rsbl.2007.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyamukondiwa C, Terblanche J, Marshall K, Sinclair B. 2011. Basal cold but not heat tolerance constrains plasticity among Drosophila species (Diptera: Drosophilidae). J. Evol. Biol. 24, 1927–1938. ( 10.1111/j.1420-9101.2011.02324.x) [DOI] [PubMed] [Google Scholar]
- 23.Magozzi S, Calosi P. 2014. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob. Change Biol. 21, 181–194. ( 10.1111/gcb.12695) [DOI] [PubMed] [Google Scholar]
- 24.Berrigan D, Hoffmann AA. 1998. Correlations between measures of heat resistance and acclimation in two species of Drosophila and their hybrids. Biol. J. Linnean Soc. 64, 449–462. [Google Scholar]
- 25.Sgro CM, Overgaard J, Kristensen TN, Mitchell KA, Cockerell FE, Hoffmann AA. 2010. A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J. Evol. Biol. 23, 2484–2493. ( 10.1111/j.1420-9101.2010.02110.x) [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann AA, Watson M. 1993. Geographical variation in the acclimation responses of Drosophila to temperature extremes. Am. Nat. 142, S93–S113. ( 10.1086/285525) [DOI] [PubMed] [Google Scholar]
- 27.Boher F, Godoy-Herrera R, Bozinovic F. 2010. The interplay between thermal tolerance and life history is associated with the biogeography of Drosophila species. Evol. Ecol. Res. 12, 973–986. [Google Scholar]
- 28.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can. J. Zool. 75, 1553–1560. ( 10.1139/z97-782) [DOI] [Google Scholar]
- 29.Cowles R, Bogert C. 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Museum Nat. Hist. 83, 261–296. [Google Scholar]
- 30.Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. 2007. Critical thermal limits depend on methodological context. Proc. R. Soc. B 274, 2935–2943. ( 10.1098/rspb.2007.0985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kir M, Kumlu M. 2008. Effect of temperature and salinity on low thermal tolerance of Penaeus semisulcatus (Decapoda: Penaeidae). Aquaculture Res. 39, 1101–1106. ( 10.1111/j.1365-2109.2008.01973.x) [DOI] [Google Scholar]
- 32.Kingsolver JG, Huey RB. 1998. Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am. Zool. 38, 545–560. [Google Scholar]
- 33.Hutchison VH. 1961. Critical thermal maxima in salamanders. Physiol. Zool. 34, 92–125. [Google Scholar]
- 34.Claussen DL. 1977. Thermal acclimation in ambystomatid salamanders. Comp. Biochem. Physiol. A 58, 333–340. ( 10.1016/0300-9629(77)90150-5) [DOI] [Google Scholar]
- 35.Beitinger TL, Bennett WA, McCauley RW. 2000. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 58, 237–275. ( 10.1023/A:1007676325825) [DOI] [Google Scholar]
- 36.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information–theoretic approach. Berlin, Germany: Springer. [Google Scholar]
- 37.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 39.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer. [Google Scholar]
- 40.R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 41.Anderson DR, Link WA, Johnson DH, Burnham KP. 2001. Suggestions for presenting the results of data analyses. J. Wildl. Manag. 65, 373–378. ( 10.2307/3803088) [DOI] [Google Scholar]
- 42.Elliott J, Elliott J. 1995. The effect of the rate of temperature increase on the critical thermal maximum for parr of Atlantic salmon and brown trout. J. Fish Biol. 47, 917–919. ( 10.1111/j.1095-8649.1995.tb06014.x) [DOI] [Google Scholar]
- 43.Wang G, Dillon ME. 2014. Recent geographic convergence in diurnal and annual temperature cycling flattens global thermal profiles. Nat. Clim. Change 4, 988–992. ( 10.1038/nclimate2378) [DOI] [Google Scholar]
- 44.Bell BA, Morgan GB, Kromrey JD, Ferron JM. 2010. The impact of small cluster size on multilevel models: a Monte Carlo examination of two-level models with binary and continuous predictors. JSM Proc. Survey Res. Methods Section, 4057–4067. [Google Scholar]
- 45.Hein CL, Öhlund G, Englund G. 2014. Fish introductions reveal the temperature dependence of species interactions. Proc. R. Soc. B 281, 20132641 ( 10.1098/rspb.2013.2641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hertz PE, Huey RB, Stevenson R. 1993. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am. Nat. 142, 796–818. ( 10.1086/285573) [DOI] [PubMed] [Google Scholar]
- 47.Freidenburg KL, Skelly DK. 2004. Microgeographical variation in thermal preference by an amphibian. Ecol. Lett. 7, 369–373. ( 10.1111/j.1461-0248.2004.00587.x) [DOI] [Google Scholar]
- 48.Bogert CM. 1949. Thermoregulation in reptiles, a factor in evolution. Evolution 3, 195–211. ( 10.2307/2405558) [DOI] [PubMed] [Google Scholar]
- 49.Huey RB, Hertz PE, Sinervo B. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 161, 357–366. ( 10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 50.Muñoz MM, Stimola MA, Algar AC, Conover A, Rodriguez AJ, Landestoy MA, Bakken GS, Losos JB. 2014. Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proc. R. Soc. B 281, 20132433 ( 10.1098/rspb.2013.2433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bozinovic F, Orellana MJ, Martel SI, Bogdanovich JM. 2014. Testing the heat-invariant and cold-variability tolerance hypotheses across geographic gradients. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 178, 46–50. ( 10.1016/j.cbpa.2014.08.009) [DOI] [PubMed] [Google Scholar]
- 52.Burrows MT, et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655. ( 10.1126/science.1210288) [DOI] [PubMed] [Google Scholar]
- 53.Faulkner KT, Clusella-Trullas S, Peck LS, Chown SL. 2014. Lack of coherence in the warming responses of marine crustaceans. Funct. Ecol. 28, 895–903. ( 10.1111/1365-2435.12219) [DOI] [Google Scholar]
- 54.Seebacher F, Davison W, Lowe CJ, Franklin CE. 2005. A falsification of the thermal specialization paradigm: compensation for elevated temperatures in Antarctic fishes. Biol. Lett. 1, 151–154. ( 10.1098/rsbl.2004.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706. ( 10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 56.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stillman JH. 2004. A comparative analysis of plasticity of thermal limits in porcelain crabs across latitudinal and intertidal zone clines. International Congress Series, pp. 267–274. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 58.Hansen J, Sato M, Ruedy R. 2012. Perception of climate change. Proc. Natl Acad. Sci. USA 109, E2415–E2423. ( 10.1073/pnas.1205276109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.IPCC. 2014. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 60.Fangue NA, Hofmeister M, Schulte PM. 2006. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 209, 2859–2872. ( 10.1242/jeb.02260) [DOI] [PubMed] [Google Scholar]
- 61.Otto RG. 1973. Temperature tolerance of the mosquitofish, Gambmia affinis (Baird and Girard). J. Fish Biol. 5, 575–585. ( 10.1111/j.1095-8649.1973.tb04490.x) [DOI] [Google Scholar]
- 62.Buckley LB, Tewksbury JJ, Deutsch CA. 2013. Can terrestrial ectotherms escape the heat of climate change by moving? Proc. R. Soc. B 280, 20131149 ( 10.1098/rspb.2013.1149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forero-Medina G, Joppa L, Pimm SL. 2011. Constraints to species’ elevational range shifts as climate changes. Conserv. Biol. 25, 163–171. ( 10.1111/j.1523-1739.2010.01572.x) [DOI] [PubMed] [Google Scholar]
- 64.Gunderson AR, Leal M. 2012. Geographic variation in vulnerability to climate warming in a tropical Caribbean lizard. Funct. Ecol. 26, 783–793. ( 10.1111/j.1365-2435.2012.01987.x) [DOI] [Google Scholar]
- 65.Logan ML, Huynh RK, Precious RA, Calsbeek RG. 2013. The impact of climate change measured at relevant spatial scales: new hope for tropical lizards. Glob. Change Biol. 19, 3093–3102. ( 10.1111/gcb.12253) [DOI] [PubMed] [Google Scholar]
- 66.Kelly MW, Sanford E, Grosberg RK. 2011. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. R. Soc. B 279, 349–356. ( 10.1098/rspb.2011.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 68.Leal M, Gunderson AR. 2012. Rapid change in the thermal tolerance of a tropical lizard. Am. Nat. 180, 815–822. ( 10.1086/668077) [DOI] [PubMed] [Google Scholar]
- 69.Logan ML, Cox RM, Calsbeek R. 2014. Natural selection on thermal performance in a novel thermal environment. Proc. Natl Acad. Sci. USA 111, 14 165–14 169. ( 10.1073/pnas.1404885111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 71.Schou M, Kristensen T, Kellermann V, Schlötterer C, Loeschcke V. 2014. A Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. J. Evol. Biol. 27, 1859–1868. ( 10.1111/jeb.12436) [DOI] [PubMed] [Google Scholar]
- 72.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 73.Chevin L-M, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.