Abstract
Difficulties with the ability to appreciate the perspective of others (mentalizing) is central to both autism and schizophrenia spectrum disorders. While the disorders are diagnostically independent, they can co-occur in the same individual. The effect of such co-morbidity is hypothesized to worsen mentalizing abilities. The recent influential ‘diametric brain theory’, however, suggests that the disorders are etiologically and phenotypically diametrical, predicting opposing effects on one's mentalizing abilities. To test these contrasting hypotheses, we evaluated the effect of psychosis and autism tendencies on the perspective-taking (PT) abilities of 201 neurotypical adults, on the assumption that autism tendencies and psychosis proneness are heritable dimensions of normal variation. We show that while both autism tendencies and psychosis proneness induce PT errors, their interaction reduced these errors. Our study is, to our knowledge, the first to observe that co-occurring autistic and psychotic traits can exert opposing influences on performance, producing a normalizing effect possibly by way of their diametrical effects on socio-cognitive abilities. This advances the notion that some individuals may, to some extent, be buffered against developing either illness or present fewer symptoms owing to a balanced expression of autistic and psychosis liability.
Keywords: comorbidity, diametric, psychiatry, theory of mind, social cognition
1. Introduction
The relationship between schizophrenia and autism has been a contentious issue since autism was first distinguished from schizophrenia [1]. While currently conceptualized as separate disorders, several recent lines of evidence suggest that the disorders co-morbidly occur at a higher than expected rate [2–4], and can themselves be mutual risk factors [5–7]. Both disorders are also thought to exist on extended phenotypic continua [5,8–10], with overlapping diagnostic (such as deficits in social interaction and communication) and non-diagnostics traits (such as impaired attention and mentalizing). Despite evidence for such overlaps, no studies to date have examined the impact that either diagnostic or trait-level co-occurrence could have on cognition and behaviour.
Socio-cognitive difficulties, particularly understanding and using the mental perspectives of others, are a core feature of both disorders and are variably affected by the degree of their severity [11,12]. These abilities are essential for social and linguistic functioning in that they allow us to understand and predict the behaviour of others in terms of the state of their knowledge, intentions, beliefs and desires. Thus, social cognition is one central domain where the relationship between the two disorders can be evaluated [13].
On the assumption that both autistic tendencies and psychotic proneness exist on a continuum, ranging from typicality to disorder, one approach to evaluating the impact of co-occurring traits on social cognition is by examining the association of autistic tendencies and psychosis proneness among non-clinical populations. This approach allows us to study both schizophrenia- and autism-like socio-cognitive characteristics without the confounding effects of medication or active symptomatology. To this end, the socio-cognitive abilities of 201 healthy adults were examined using Apperly et al.'s [14] variant of the Keysar et al. [15] referential communication task in which participants are required to follow the instructions of ‘director’ characters. Critical trials required the participant to follow requests/instructions from a director who did not know about all of the possible objects in a grid, and participants had to take this into account when interpreting the director's instructions. Relational trials involved three critical objects varying in size or shape (e.g. three sizes of block). In these trials, only two of these three objects were visible to the director, and participants had to take this into account when following his instruction (e.g. to ‘Move the large block … ’). Ambiguous trials involved two critical objects described with homophones (e.g. a computer mouse and a rodent mouse) of which only one is visible to the director. In both cases, correct responses required participants to ignore a potential referent that was not visible from the director's view, and select a valid referent that was visible to the director (see Material and methods and figure 1). Thus, successful compliance with the director's instructions requires an understanding that the director has a different state of knowledge, and use of that information to constrain linguistic reference. As such, this task captures a critical social component of interpersonal communication that relies on efficient use of perspective-taking (PT) abilities. Psychosis proneness was assessed using the positive scale of the Community Assessment of Psychic Experiences (CAPEp) questionnaire [16], and autism tendencies were assessed using the Autism Spectrum Quotient (AQ) questionnaire [8].
Figure 1.
(a,b) Instruction grids to participants. (c) Experimental relational trial. (d) Control condition of the experimental relational trial. (Online version in colour.)
A natural prediction from the standard clinical conception of autism and schizophrenia as independent disorders [1] is that related characteristics in the typical population make independent negative contributions to PT performance. It follows that co-occurring high levels of these traits should be associated with worse PT than high levels of either set of traits alone. A recent influential theory, however, hypothesizes that both autism spectrum disorders (ASD) and schizophrenia spectrum disorders (SSD) are etiologically and thus largely phenotypically diametrical [17]. Central to this model is that ASD and SSD represent opposite extremes of a social cognition continuum [17,18], wherein ASD is associated with under-active mechanistic social cognition and SSD with hyper-active mentalistic social cognition, deviating in opposite directions from typical performance. Such conceptualization would predict that the relative dominance of traits for either condition would predispose individuals to increased socio-cognitive difficulties. In the event that there is a balance between the two, this model predicts that these socio-cognitive difficulties would be diametrically modulated towards typical performance by co-occurring phenotypic traits that are disorder-specific.
2. Material and methods
(a). Participants
The socio-cognitive abilities of 201 healthy adults (43 males, 158 females; mean age (s.d.) = 21.37 ± 4.32) were examined in this study. Participants were excluded from the study if they had a history of psychiatric illness, epilepsy, neurological disorders, suffered brain injury or may have current alcohol or substance abuse problems.
(b). Procedures
In a quiet room, participants first completed Apperly et al.'s [14] variant of the Keysar et al. [15] referential communication task, followed by completing the CAPEp questionnaire [16], and autism tendencies were assessed using the AQ [8].
(c). Materials
(i). The perspective-taking task
The task was based on Apperly et al. [14], Experiment 1. In this task, participants are presented with a 4 × 4 grid that contained eight cartoon images (figure 1). On the opposite side of the grid stands a male director, and on the front side a female director who shares the same view as the participant. Five slots of each grid are occluded from the view of the male director, thus creating a different perspective than that of the participant (figure 1a,b). The male director is ignorant of the content that these slots may contain. Audio instructions are played to the participant in a male voice (representing the male director) or a female voice (representing the female director). Instructions pertained to moving objects within the grid ‘up’ or ‘down’, ‘left’ or ‘right’. Participants were explicitly told to take the perspective of the male director when fulfilling his instructions.
The task consisted of 32 grids (three to five instructions per trials each) for a total of 128 trials. Of the 128 trials, 96 trials were fillers and thus are not part of the analyses. The remaining critical trials consisted of 16 experimental and 16 control trials. All the critical trials are spoken by the male director. Instructions given immediately before the critical instructions were equally often from the male and the female directors. The critical (experimental and control) trials were equally divided into ambiguous and relational trials. The eight experimental relational trials pertained to objects that are relative to each other either in size or location. Figure 1c,d presents an actual example of an experimental relational trial with the matching control. In this trial, the participant is instructed to ‘move the bottom block one slot left’. For a correct compliance with the instruction, the participant needs to ignore the distracting block (marked ‘X’ in figure 1c and which is not available from the view of the director) and move the block marked ‘Y’. The control trial contains the same information as the experimental trial except the block in the bottom row is replaced with a different object (a lipstick) (figure 1d). In the eight experimental ambiguous trials (not shown in the figure), the noun denoting the object to be moved has two potential referents. For example, ‘glasses’ in ‘move the glasses one slot to the left’ could be referring to either a pair of reading glasses or a pair of drinking glasses. Only one of these items is available from the view of the male director, as the other ‘competing’ item is in an occluded slot. In the matching control for this condition, the ‘competing’ object in the occluded slot is swapped with a different object (e.g. a toy car).
Seated approximately 60 cm from a 17″ monitor, the session started with two practice grids with non-experimental instructions. The 32 grids of the main experiment were presented in two fixed pseudo-random orders between-participant. The participant always moved the objects from their own perspective with the computer mouse. This was achieved by first clicking on the object and then dragging it with the cursor to the appropriate location. Participants were told that doing this would not actually move the object, but should act and move the mouse as if it did. Each grid appeared for 5 s of study time before the instruction was given. The instructions were given at 5 s intervals. Correct responses were recorded if the participant clicked on the object that fit the instruction and could be seen from both the director's and the participant's perspective. Incorrect responses were recorded if the participant selected the distracter object (i.e. block marked X in figure 1c) or clicked on some other cell. Timeouts were also recorded, but these were not included in the error count. Response times (RTs) were measured from the onset of the noun phrase. Following earlier work, we did not expect RTs to reveal condition differences, but they do give the opportunity to examine any trade-offs between speed and accuracy. This also allows us to examine differences between the corresponding control and experimental conditions. The experiment was run in a single block using E-prime v. 2.1.
(ii). The community assessment of psychic experiences questionnaire
This self-report questionnaire is based on the Peters et al. Delusions Inventory-21 [19] and consists of 42 items measuring the presence of positive psychotic experiences (20 items), negative psychotic experiences (14 items) and depressive experiences (eight items) that an individual may have experienced over the last 12 months ([16]; http://www.cape42.homestead.com/). The occurrence of these symptoms is reported on a likert frequency scale from 1 (never) to 4 (nearly always), and the associated distress on a scale ranging from 1 (not distressed) to 4 (very distressed). Cronbach's α for this scale in this study is 0.92, which indicates high internal consistency. For current purposes, the 20 item community assessment of psychic experiences positive scale is used as a measure of psychosis proneness. The internal consistency of this scale in this study is very good (Cronbach's α = 0.84) and falls within the range of values reported in other studies within the general population [20].
(iii). The autism spectrum quotient questionnaire
This self-report questionnaire consists of 50 items that measure the presence of traits associated with the autistic spectrum within the general population [8]. Each item is given a score of 0 or 1. Higher scores indicate the presence of greater autistic tendencies. The AQ's internal consistency in this study is good (Cronbach's α = 0.82) and is comparable to the values reported in other studies [21].
3. Results
Before the main analysis, we examined the rate of errors made in the ambiguous and relational trials. On average, participants erred (i.e. failed to appreciate the perspective of the director) on 20.6% of the ambiguous trials and 41.5% on the relational trials. These rates are similar to previous reports using this task [14,22]. An examination of the RTs showed no evidence of speed-accuracy trade-offs (see the electronic supplementary material, table S1). Finally, an examination of the association between the CAPEp and AQ scores showed a modest but a significant association (r = 0.31, p < 0.001), which is consistent with the observed phenotypic overlaps between the autism and psychosis spectra (see the electronic supplementary material, figure S1).
To examine the effect of autism tendencies and psychosis proneness, the participants' PT error counts on the ambiguous and relational trials were analysed using Poisson regression models with negative binomial distribution. Using generalized linear models, we first investigated the association of the participant's PT errors on the relational trials with the AQ scores, the CAPEp scores and their interaction. The omnibus test shows that the overall model is significant (χ32 = 13.38, p = 0.004). The model's parameter estimates (i.e. the main effects and the interaction term) are also significant (table 1). When entering gender into the model, which is regarded as a relative risk factor for autism and psychosis, the results remained unchanged (electronic supplementary material, table S2). Although ambiguous trials showed a far lower error rate, they yielded data with the same qualitative pattern we observed for the relational condition (electronic supplementary material, table S3). However, the overall model was not significant when these data were subject to the same analysis as the relational trials (χ32 = 2.91, p = 0.406).
Table 1.
Summary of coefficients with errors on the experimental relational trials as the dependent variable. (AQ, autism quotient; CAPEp, positive scale of the Community Assessment of Psychic Experiences.)
| model | ||||||
|---|---|---|---|---|---|---|
| coefficient | β | s.e. | Wald χ2 | d.f. | Exp(β) | significance |
| constant | −1.795 | 0.4299 | 17.428 | 1 | 0.166 | <0.001 |
| AQ | 0.053 | 0.0233 | 5.200 | 1 | 1.054 | =0.023 |
| CAPEp | 0.045 | 0.0156 | 8.224 | 1 | 1.046 | =0.004 |
| AQ × CAPEp | −0.002 | 0.0008 | 4.655 | 1 | 0.998 | =0.031 |
From table 1, we see that an increase in the AQ or the CAPEp resulted in an increase in PT errors. Intriguingly, however, the interaction between these two terms is negatively associated with PT errors. To probe the nature of the interaction term, we follow the method by Hayes & Matthes [23], whereby the effect of one predictor on the probability of committing PT errors (derived from the regression equation) is examined at the mean, 1 s.d. below the mean and 1 s.d. above the mean of the other predictor. Figure 2a visualizes the interaction between psychosis and PT errors by plots of simple regression lines for the participants with low AQ (AQ = 10.04), average AQ (AQ = 16.33) and high AQ (AQ = 22.63), and figure 1b visualizes the interaction between autism tendencies and PT errors for the participants with low CAPEp (CAPEp = 22.53), average CAPEp (CAPEp = 27.37) and high CAPEp (CAPEp = 32.21). The analysis presented in figure 2a suggests that the relationship between psychosis proneness and the increased probability of committing PT errors is significant when the AQ scores were low (−1 s.d.) (β = 0.023, p = 0.003) as well as when the AQ scores were at the mean (β = 0.013, p = 0.004). Conversely, when the AQ scores are high (+1 s.d.), the relationship between psychosis proneness and PT errors is non-significant (β = 0.003, p = 0.558). This suggests that individuals with higher psychosis proneness commit PT errors mainly when they have low or average levels of AQ scores. Conversely, high AQ scores seem to have an attenuating effect on the PT errors associated with an increase in psychosis proneness.
Figure 2.
(a) The relationship between psychosis proneness and the probability of committing PT errors, evaluated at low, average and high AQ scores. (b) The relationship between autism tendencies and the probability of committing PT errors, evaluated at low, average and high CAPEp scores. Asterisks indicate significant slopes.
By contrast, the analysis presented in figure 2b suggests that the relationship between the AQ scores and the increased probability of committing PT errors is significant only when the CAPEp scores were low (−1 s.d.) (β = 0.011, p = 0.047). Conversely, when the CAPEp scores are average or high, the relationships between AQ and PT errors are non-significant (β = 0.003, p = 0.407; β = −0.005, p = 0.394, respectively). This suggests that AQ is predictive of PT errors only in participants with low CAPEp scores and that average and high CAPEp scores seem to have an attenuating effect on the PT errors caused by an increase in the AQ scores.
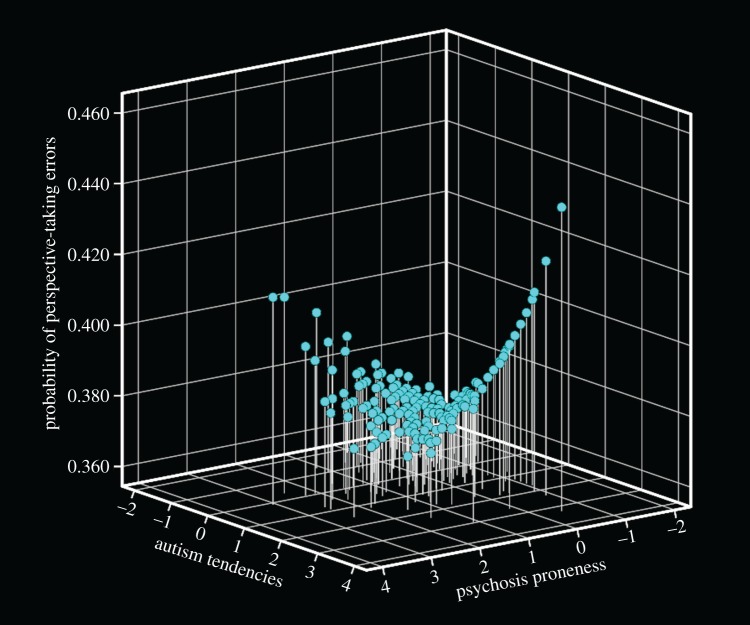
To estimate if the relative dominance of autism tendencies or psychosis proneness was associated with the occurrence of errors in these trials, the AQ and CAPEp scores were converted into Z-scores. A bias score for each participant was then derived by subtracting the CAPEp Z-values from the AQ Z-values. An inspection of the data suggested a curvilinear relationship between the bias score and the errors in the relational and ambiguous conditions. To investigate this possibility, we entered into the regression model the bias score (AQz – CAPEpz), the sum of the Z-scores of both scales (AQz + CAPEpz), the interaction term of the bias score with the sum of Z-scores and the quadratic terms of the bias score and the sum of scores. The overall model was significant (χ52 = 14.48, p = 0.013), with only the quadratic term of the bias being significant (β(±s.e.) = 0.021(0.001), Wald χ12 = 4.83, p = 0.028). Here too, gender had no effect on the model (electronic supplementary material, table S4). As can be seen from figure 3, the probability of committing PT errors is associated with the relative dominance of autism tendencies or psychosis proneness, following a U-shape pattern. That is, individuals with elevated tendencies to either autism or psychosis were equally likely to commit PT errors. Interestingly, however, individuals with either high or low tendencies to both autism and psychosis, performed at similar levels. A similar, though non-significant, pattern was also observed for errors in the ambiguous condition (electronic supplementary material, figure S2).
Figure 3.
Three-dimensional representation of the relationship between autism tendencies and psychosis proneness (represented as standardized Z-scores) and the probability of making PT errors on the relational trials. The negative scores represent low tendencies and the positive scores represent high tendencies. (Online version in colour.)
4. Discussion
Our study reveals a dose-dependent relationship between autism tendencies and psychosis proneness and mentalizing difficulties. This finding confirms earlier reports showing that both autistic tendencies [8,24] and psychosis proneness [25–27] impact PT and socio-cognitive abilities in healthy adults. Our findings thus provide further support to the continuity/dimensional models of ASD and SSD. They suggest that subclinical manifestations of core features of both disorders are detectable in a healthy population and that such sub-threshold levels can influence socio-cognitive abilities.
Surprisingly, co-occurring autism tendencies and psychosis proneness have a moderating effect on the mentalizing difficulties engendered by either disorders alone (figure 2a,b), such that the moderating effects are greatest when both tendencies are high rather when both are low. This can be clearly seen in figure 3 where the performance of participants presenting with high tendencies to both disorders is similar to participants presenting with low tendencies to both disorders. Thus, the association of the interaction between autistic tendencies and psychosis proneness with a decrease in mentalizing difficulties can be seen as support for the diametrical model [17] which posits that autism and schizophrenia have opposing effects on behaviour and cognition.
Whether the errors we observe, as would be predicted by the diametric model, are due to hypomentalism (in autism) or hypermentalism (in psychosis) is not directly discernable in the Director task. Critically, however, the fact that our data show that highly psychosis prone individuals err at similar levels to individuals with high autistic tendencies is not inconsistent with the diametric model. Both hypomentalism and hypermentalism can equally lead to deleterious effects on mentalizing abilities, albeit for different reasons, because otherwise they could not explain the fact that both disorders result in impaired social ability. With this in mind, hypermentalizing is a plausible cause of errors, and it provides a way of making sense of how psychosis proneness (leading to hypermentalizing) can compensate for autistic tendencies (which could lead to hypomentalizing) [18,28]. We speculate that, under time pressure, mentalizing places high demands on information selection whereby overly narrow information selection can lead to undermentalizing, whereas overly broad selection can lead to overmentalizing. Consequently, the efficiency of information flow and the frequency with which information is captured has an effect on the number of hypotheses generated and consequently the probability assigned to each hypothesis [29]. Information capture tends to be slow in autism owing to increased focus of attention [8,30], and fast in individuals with positive schizotypy/schizophrenia owing to overswitching [31]. Thus by considering the mechanisms behind mentalizing, it becomes apparent how these different mentalizing styles, characteristic of autism and schizophrenia, can compensate for one another. Another important difference between both conditions is that schizophrenia is characterized by a ‘jumping to conclusions’ cognitive style (which appears to be specifically associated with delusions), whereas autism is characterized by a more deliberative cognitive style [32]. The attenuating effect, observed in individuals presenting with high expressions in both autism and psychosis traits (figure 3), thus predicts the presence of a brain mechanism that can accommodate the coexistence of these contrasting cognitive styles. The anti-correlational nature of the default mode network (associated with mentalistic thinking) with the task positive network (associated with mechanistic thinking) [33] is a promising neural framework to investigating these contrasting mentalizing styles in autism and schizophrenia.
Substantial evidence has accumulated showing that psychosis and autism traits are not bound to the presence of the disorder [8,34], with clinical and non-clinical forms of these traits sharing common genetic, neurocognitive and neurobiological features [35–39], so with due caution [40], we consider the clinical relevance of the current approach and findings. First, in the search for disorder-specific phenotypic markers, it is difficult to distinguish whether the aberrant marker is a cause or consequence of the disorder. By showing that the presence of sub-threshold clinical traits in healthy adults impact functions that are deficient in patients with these disorders, we provide evidence for a mechanism by which the risk of the disorder may, at least in part, be mediated through variation in these socio-cognitive functions. Second, our findings highlight the importance of testing whether social cognition is moderated by the relative expression of autism versus psychosis within the clinical population. Such confirmation would warrant reconsideration of current practices perceiving these conditions as distinct, and consequently would facilitate the development of individualized mentalizing-based therapeutic approaches [41]. Finally, the diametric influences of autism and psychosis traits on behaviour suggest that these conditions are affected by reciprocal causes. Indeed, some phenomena are risk factors for autism but protect against schizophrenia, or vice versa. For example, duplications of 22q11.2 protect against schizophrenia but represent an autism risk factor [42], and congenital blindness causes autism traits but protects against schizophrenia [43,44]. This means that the causes of one condition might be developed into treatments for the other. As has already been pointed out by others [45], independent efforts demonstrate the potential of this suggestion. For example, mGluR5 antagonists carry a great potential for the treatment of fragile X of which about 30% have comorbid ASD [46], and its agonists are being developed for the treatment of schizophrenia [47].
Our study is, to our knowledge, the first to observe that co-occurring autistic and psychotic traits can exert opposing influences on socio-cognitive performance. Reminiscent of the ‘normality effect’ that is observed in certain co-occurring diametrical pathologies such as Parkinson's disease and hemiballismus [48,49], our findings thus raise the possibility that autism–schizophrenia comorbidity can have an attenuating effect on socio-cognitive difficulties. More broadly, this suggests that some individuals may, to some extent, be buffered against developing either illnesses or present fewer symptoms owing to a balanced expression of autistic and psychosis liability, and will only be diagnosed at the extreme state of either illness. In this regard, our analytical approach of indexing these factors in terms of bias and additive effects is potentially a useful framework to understanding the effect of common risk factors.
Supplementary Material
Ethics statement
The study was approved by the University of Birmingham Research Ethics Committee, and written informed consent was obtained from each participant.
Data accessibility
No permission was obtained from the participants to share the data publicly. In compliance with the Data Protection Act 1998, the information will be retained for 10 years by the University of Birmingham and will be accessible for the purpose of research and statistical and audit purposes.
Funding statement
No funding bodies were associated with this research.
Authors' contributions
A.M.A. designed the study, collected and analysed the data and wrote the manuscript. I.A.A. and S.J.W. designed the study. P.C.H. contributed to conception of analytical approach. All authors discussed the results and commented on the manuscript.
Conflict of interests
The authors report no competing interests.
References
- 1.Kolvin I. 1971. Studies in the childhood psychoses. I. Diagnostic criteria and classification. Br. J. Psychiatry 118, 381–384. ( 10.1192/bjp.118.545.381) [DOI] [PubMed] [Google Scholar]
- 2.Hofvander B, et al. 2009. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry 9, 35 ( 10.1186/1471-244X-9-35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nylander L, Lugnegård T, Hallerbäck MU. 2008. Autism spectrum disorders and schizophrenia spectrum disorders: is there a connection? A literature review and some suggestions for future clinical research. Clin. Neuropsychiatry 5, 43–54. [Google Scholar]
- 4.Sheitman BB, Kraus JE, Bodfish JW, Carmel H. 2004. Are the negative symptoms of schizophrenia consistent with an autistic spectrum illness? Schizophr. Res. 69, 119–120. ( 10.1016/S0920-9964(03)00177-4) [DOI] [PubMed] [Google Scholar]
- 5.Crespi B, Stead P, Elliot M. 2010. Comparative genomics of autism and schizophrenia. Proc. Natl Acad. Sci. USA 107, 1736–1741. ( 10.1073/pnas.0906080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King BH, Lord C. 2011. Is schizophrenia on the autism spectrum? Brain Res. 1380, 34–41. ( 10.1016/j.brainres.2010.11.031) [DOI] [PubMed] [Google Scholar]
- 7.Sullivan PF, et al. 2012. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch. Gen. Psychiatry 69, 1099–1103. ( 10.1001/archgenpsychiatry.2012.730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. 2001. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17. ( 10.1023/A:1005653411471) [DOI] [PubMed] [Google Scholar]
- 9.Claridge G, McCreery C, Mason O, Bentall R, Boyle G, Slade P, Popplewell D. 1996. The factor structure of ‘schizotypal‘ traits: a large replication study. Br. J. Clin. Psychol. 35, 103–115. ( 10.1111/j.2044-8260.1996.tb01166.x) [DOI] [PubMed] [Google Scholar]
- 10.Wing L. 1988. The autistic continuum. In Aspects of autism: biological research (ed. Wing L.), pp. v–viii. London, UK: Gaskell/Royal College of Psychiatrists. [Google Scholar]
- 11.Abu-Akel A. 2003. A neurobiological mapping of theory of mind. Brain Res. Rev. 43, 29–40. ( 10.1016/S0165-0173(03)00190-5) [DOI] [PubMed] [Google Scholar]
- 12.Chung YS, Barch D, Strube M. 2013. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr. Bull. 40, 602–616. ( 10.1093/schbul/sbt048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasson NJ, Pinkham AE, Carpenter KL, Belger A. 2011. The benefit of directly comparing autism and schizophrenia for revealing mechanisms of social cognitive impairment. J. Neurodev. Disord. 3, 87–100. ( 10.1007/s11689-010-9068-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apperly IA, Carroll DJ, Samson D, Humphreys GW, Qureshi A, Moffitt G. 2010. Why are there limits on theory of mind use? Evidence from adults’ ability to follow instructions from an ignorant speaker. Q. J. Exp. Psychol. 63, 1201–1217. ( 10.1080/17470210903281582) [DOI] [PubMed] [Google Scholar]
- 15.Keysar B, Barr DJ, Balin JA, Brauner JS. 2000. Taking perspective in conversation: the role of mutual knowledge in comprehension. Psychol. Sci. 11, 32–38. ( 10.1111/1467-9280.00211) [DOI] [PubMed] [Google Scholar]
- 16.Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, Verdoux H, van Os J. 2002. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol. Med. 32, 347–358. ( 10.1017/S0033291701005141) [DOI] [PubMed] [Google Scholar]
- 17.Crespi B, Badcock C. 2008. Psychosis and autism as diametrical disorders of the social brain. Behav. Brain Sci. 31, 241–261; discussion 61–320 ( 10.1017/S0140525X08004214) [DOI] [PubMed] [Google Scholar]
- 18.Abu-Akel A, Bailey AL. 2000. The possibility of different forms of theory of mind impairment in psychiatric and developmental disorders. Psychol. Med. 30, 735–738. ( 10.1017/S0033291799002123) [DOI] [PubMed] [Google Scholar]
- 19.Peters ER, Joseph SA, Garety PA. 1999. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory). Schizophr. Bull. 25, 553–576. ( 10.1093/oxfordjournals.schbul.a033401) [DOI] [PubMed] [Google Scholar]
- 20.Lin A, et al. 2011. The relationship between coping and subclinical psychotic experiences in adolescents from the general population: a longitudinal study. Psychol. Med. 41, 2535–2546. ( 10.1017/S0033291711000560) [DOI] [PubMed] [Google Scholar]
- 21.Austin EJ. 2005. Personality correlates of the broader autism phenotype as assessed by the autism spectrum quotient (AQ). Pers. Individual Differences 38, 451–460. ( 10.1016/j.paid.2004.04.022) [DOI] [Google Scholar]
- 22.Keysar B, Lin S, Barr DJ. 2003. Limits on theory of mind use in adults. Cognition 89, 25–41. ( 10.1016/S0010-0277(03)00064-7) [DOI] [PubMed] [Google Scholar]
- 23.Hayes AF, Matthes J. 2009. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav. Res. Methods 41, 924–936. ( 10.3758/BRM.41.3.924) [DOI] [PubMed] [Google Scholar]
- 24.Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Kolevzon A, Ochsner KN. 2010. Oxytocin selectively improves empathic accuracy. Psychol. Sci. 21, 1426–1428. ( 10.1177/0956797610383439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fyfe S, Williams C, Mason OJ, Pickup GJ. 2008. Apophenia, theory of mind and schizotypy: perceiving meaning and intentionality in randomness. Cortex 44, 1316–1325. ( 10.1016/j.cortex.2007.07.009) [DOI] [PubMed] [Google Scholar]
- 26.Gooding DC, Pflum MJ. 2012. The nature of diminished pleasure in individuals at risk for or affected by schizophrenia. Psychiatry Res. 198, 172–173; author reply 4–5 ( 10.1016/j.psychres.2011.07.029) [DOI] [PubMed] [Google Scholar]
- 27.Pickup GJ. 2006. Theory of mind and its relation to schizotypy. Cogn. Neuropsychiatry 11, 177–192. ( 10.1080/13546800444000236) [DOI] [PubMed] [Google Scholar]
- 28.Ciaramidaro A, Bolte S, Schlitt S, Hainz D, Poustka F, Weber B, Bara BG, Freitag C, Walter H. 2014. Schizophrenia and autism as contrasting minds: neural evidence for the hypo-hyper-intentionality hypothesis. Schizophr. Bull. 41, 171–179. ( 10.1093/schbul/sbu124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas R, Dougherty MR, Buttaccio DR. 2014. Memory constraints on hypothesis generation and decision making. Curr. Dir. Psychol. Sci. 23, 264–270. ( 10.1177/0963721414534853) [DOI] [Google Scholar]
- 30.Russell-Smith SN, Maybery MT, Bayliss DM. 2010. Are the autism and positive schizotypy spectra diametrically opposed in local versus global processing? J. Autism Dev. Disord. 40, 968–977. ( 10.1007/s10803-010-0945-7) [DOI] [PubMed] [Google Scholar]
- 31.Yogev H, Sirota P, Gutman Y, Hadar U. 2004. Latent inhibition and overswitching in schizophrenia. Schizophr. Bull. 30, 713–726. ( 10.1093/oxfordjournals.schbul.a007125) [DOI] [PubMed] [Google Scholar]
- 32.Brosnan M, Chapman E, Ashwin C. 2014. Adolescents with autism spectrum disorder show a circumspect reasoning bias rather than 'jumping-to-conclusions’. J. Autism Dev. Disord. 44, 513–520. ( 10.1007/s10803-013-1897-5) [DOI] [PubMed] [Google Scholar]
- 33.Jack AI, et al. 2012. fMRI reveals reciprocal inhibition between social and physical cognitive domains. NeuroImage 66C, 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. 2009. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 39, 179–195. ( 10.1017/S0033291708003814) [DOI] [PubMed] [Google Scholar]
- 35.Corlett PR, Fletcher PC. 2012. The neurobiology of schizotypy: fronto-striatal prediction error signal correlates with delusion-like beliefs in healthy people. Neuropsychologia 50, 3612–3620. ( 10.1016/j.neuropsychologia.2012.09.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lustenberger C, et al. 2014. Sleep spindles are related to schizotypal personality traits and thalamic glutamine/glutamate in healthy subjects. Schizophr. Bull 41, 522–531. ( 10.1093/schbul/sbu109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noguchi H, Hori H, Kunugi H. 2008. Schizotypal traits and cognitive function in healthy adults. Psychiatry Res. 161, 162–169. ( 10.1016/j.psychres.2007.07.023) [DOI] [PubMed] [Google Scholar]
- 38.Stefansson H, et al. 2014. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 505, 361–366. ( 10.1038/nature12818) [DOI] [PubMed] [Google Scholar]
- 39.Vollema MG, Sitskoorn MM, Appels MC, Kahn RS. 2002. Does the schizotypal personality questionnaire reflect the biological-genetic vulnerability to schizophrenia? Schizophr. Res. 54, 39–45. ( 10.1016/S0920-9964(01)00350-4) [DOI] [PubMed] [Google Scholar]
- 40.David AS. 2010. Why we need more debate on whether psychotic symptoms lie on a continuum with normality. Psychol. Med. 40, 1935–1942. ( 10.1017/S0033291710000188) [DOI] [PubMed] [Google Scholar]
- 41.Allen JG, Fonagy P. 2006. Handbook of mentalization-based treatment. Chichester, UK: John Wiley. [Google Scholar]
- 42.Rees E, et al. 2014. Evidence that duplications of 22q11.2 protect against schizophrenia. Mol. Psychiatry 19, 37–40. ( 10.1038/mp.2013.156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobson RP, Bishop M. 2003. The pathogenesis of autism: insights from congenital blindness. Phil. Trans. R. Soc. Lond. B 358, 335–344. ( 10.1098/rstb.2002.1201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverstein SM, Wang Y, Keane BP. 2012. Cognitive and neuroplasticity mechanisms by which congenital or early blindness may confer a protective effect against schizophrenia. Front. Psychol. 3, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crespi B, Stead P, Elliot M. 2010. Evolution in health and medicine Sackler colloquium: comparative genomics of autism and schizophrenia. Proc. Natl Acad. Sci. USA 107(Suppl. 1), 1736–1741. ( 10.1073/pnas.0906080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolen G, Bear MF. 2009. Fragile X syndrome and autism: from disease model to therapeutic targets. J. Neurodev. Disord. 1, 133–140. ( 10.1007/s11689-009-9015-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conn PJ, Lindsley CW, Jones CK. 2009. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 30, 25–31. ( 10.1016/j.tips.2008.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergman H, Wichmann T, DeLong MR. 1990. Reversal of experimental Parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438. ( 10.1126/science.2402638) [DOI] [PubMed] [Google Scholar]
- 49.Mitchell IJ, Sambrook MA, Crossman AR. 1985. Subcortical changes in the regional uptake of [3H]-2-deoxyglucose in the brain of the monkey during experimental choreiform dyskinesia elicited by injection of a gamma-aminobutyric acid antagonist into the subthalamic nucleus. Brain 108, 405–422. ( 10.1093/brain/108.2.405) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No permission was obtained from the participants to share the data publicly. In compliance with the Data Protection Act 1998, the information will be retained for 10 years by the University of Birmingham and will be accessible for the purpose of research and statistical and audit purposes.