Abstract
Ants are thought to be special among Hymenopterans in having only dichromatic colour vision based on two spectrally distinct photoreceptors. Many ants are highly visual animals, however, and use vision extensively for navigation. We show here that two congeneric day- and night-active Australian ants have three spectrally distinct photoreceptor types, potentially supporting trichromatic colour vision. Electroretinogram recordings show the presence of three spectral sensitivities with peaks (λmax) at 370, 450 and 550 nm in the night-active Myrmecia vindex and peaks at 370, 470 and 510 nm in the day-active Myrmecia croslandi. Intracellular electrophysiology on individual photoreceptors confirmed that the night-active M. vindex has three spectral sensitivities with peaks (λmax) at 370, 430 and 550 nm. A large number of the intracellular recordings in the night-active M. vindex show unusually broad-band spectral sensitivities, suggesting that photoreceptors may be coupled. Spectral measurements at different temporal frequencies revealed that the ultraviolet receptors are comparatively slow. We discuss the adaptive significance and the probability of trichromacy in Myrmecia ants in the context of dim light vision and visual navigation.
Keywords: colour vision, navigation, spectral sensitivity, photoreceptors, ants
1. Introduction
Colour vision is beneficial for reliable object discrimination, because it is not affected by shifts in the intensity or colour of the ambient light conditions [1–3]. Colour vision requires the comparisons of input signals from at least two photoreceptors with different spectral sensitivities. Trichromatic colour vision, in addition, greatly enhances the range of wavelengths over which colour discriminations are possible [4]. Based on a number of studies, ants have long been considered unique among hymenopterans for having only two spectral classes of photoreceptors with peak sensitivities in the short- (ultraviolet (UV) or violet) and long- (green) wavelength regions of the spectrum [5–10]. Ants were therefore thought to be dichromats. In dichromatic species that have a UV photoreceptor combined with a long-wavelength sensitive receptor, the ability to discriminate between colours would be limited to the UV and blue region of the spectrum because of the absence of a middle-wavelength sensitive photoreceptor. Several behavioural experiments have provided some evidence that ants indeed do have colour vision [11–14], with one behavioural study proposing tetrachromatic colour vision in Cataglyphis bicolor [11].
Some ants are highly visual animals, especially individually foraging ants that do not use pheromone trails during foraging excursions, but rely on visual cues for path integration and landmark-based navigation [15–25]. Ants such as C. bicolor and Cataglyphis fortis, which live in landmark-poor deserts and salt pans, for instance, use the pattern of polarized skylight as a compass reference for their path integration system [18,21,26,27]. Ants such as the Australian desert ant Melophorus bagoti that inhabit landmark-rich environments containing grass tussocks, shrubs and trees, rely more heavily on information provided by the visual landmark panorama to find home [22,23]. Both diurnal and nocturnal bull ants of the genus Myrmecia have been shown to be guided by the landmark panorama [24,25,28] and in the case of nocturnal ants, also by the pattern of polarized skylight [24].
UV images are highly effective for sky/horizon segmentation and the horizon contour can be used to support effective navigation [29]. It has also been argued that the ant visual system is well suited for the extraction of skyline contour information based on the presence of a UV–green opponent channel [30]. Despite an abundance of interest in their navigational abilities, the spectral organization of the visual system of ants remains disputed to this day, with little clear physiological or behavioural information [5–14]. However, an accurate understanding of their visual capabilities is crucial for the modelling of their navigational performance (e.g. [31]).
Here, we investigate the spectral sensitivity of two Australian ants, the diurnal Myrmecia croslandi and the nocturnal Myrmecia vindex, by applying an electrophysiological approach. We show the existence of three spectrally distinct photoreceptors in both ant species, providing the physiological substrate for trichromatic colour vision. The previously undiscovered middle-wavelength (blue) sensitive receptor class has the potential to significantly extend the range of functional colour vision into the green/yellow part of the spectrum. Furthermore, as even nocturnal Myrmecia ants rely exclusively on vision for navigation [24,28], our results highlight the possible importance of trichromatic colour vision in the context of visual navigation. This will therefore force a detailed analysis and initiate behavioural studies on the importance of colour vision in navigational tasks.
2. Experimental procedures
(a). Animals
We studied workers of both diurnal and nocturnal Myrmecia ants. The nocturnal M. vindex Smith were collected from three nests on the University of Western Australia Crawley campus, Perth (31°59′05.8″ S, 115°49′18.7″ E). The diurnal M. croslandi Taylor were collected from the Campus Field Station at the Australian National University, Canberra (35°16′50.14″ S, 149°06′42.13″ E). Solitary foragers of M. vindex forage exclusively after sunset (electronic supplementary material, figure S1), whereas activity of M. croslandi occurs strictly during the day [32–34]. The body lengths of workers are 11–25 mm in M. vindex and 10–13 mm in M. croslandi.
(b). Electrophysiology
The animals were kept on ice for 5 min. After removing its legs and gaster, an ant was fixed with the dorsal side up on a plastic stage with beeswax and then mounted in a Faraday cage. Monochromatic stimulation light and adaptation light was produced by computer-controlled TILL Polychrome V monochromators (Till Photonics GmbH, Gräfelfing, Germany) with 150 W xenon lamps. White light was provided by a high-power xenon light source (HPX-2000, Ocean Optics Inc., FL, USA). Our electrophysiological measurement design is similar to that described in detail in a previous study [35]. To investigate spectral sensitivities, a flickering light stimulus was used, alternating equally between light and dark at a set frequency of 10, 20 or 30 Hz. Every second light flash was of monochromatic light, and every other of a reference white light. At each wavelength tested, the intensity of the monochromatic light was adjusted to produce the same response amplitude as the white light. Comparing the number of photons of monochromatic light required to match the white light across different wavelengths allowed us to calculate relative spectral sensitivity for all measured wavelengths. The selective adaptation light reducing the contribution of some photoreceptor classes was applied only during each recording, for approximately for 10–20 min.
We recorded the electroretinogram (ERG) to determine the spectral sensitivity of the eye as a whole. A silver/silver-chloride wire of 100 µm diameter was inserted into the mesosoma and served as the indifferent electrode. A platinum wire of 0.254 mm diameter was attached to the surface of the compound eye with conductive gel (Livingstone International Pty Ltd., New South Wales, Australia). ERGs were recorded through a differential amplifier (DAM50, World Precision Instruments Inc., FL, USA) connected to a computer via a 16-bit data acquisition card (USB-6353, National Instruments, Austin, TX, USA). Measurements were taken across the spectrum from 340 to 650 nm in 10 nm steps.
Photoreceptor spectral sensitivities were determined by intracellular recordings. An ant was mounted at the centre of rotation of a cardan arm perimeter device. A borosilicate glass microelectrode filled with 1 M potassium chloride with a resistance of about 100 MΩ was inserted into the retina through a hole made in the cornea. The results were the same as using electrodes filled with 3 M potassium acetate. Membrane potentials were recorded through an amplifier (Getting Model 5A, Getting Instruments, San Diego, CA, USA), connected to a computer via the 16-bit data acquisition card (see above). After penetrating a photoreceptor with the electrode, the position of the optical fibre was adjusted to produce the maximal photoreceptor response. Measurements were taken across the spectrum from 340 to 650 nm in 10 nm steps. Adaptation lights were delivered through a beam splitter to achieve exact alignment with the recording light.
(c). Statistical analysis
To test whether the sensitivity of the eye at shorter wavelengths differed between conditions, such as species, time of day or the stimulation frequency, a linear mixed model (REML) was implemented in the nlme package of R [36] (electronic supplementary material, table S1, Combined model). Animal identity was used in the random effects model and stimulation frequency, species and their interaction were used as the fixed effects model. Single species models were then used to confirm the effect of stimulation frequency independently for both species and to test for day versus night effects (electronic supplementary material, table S1, M. vindex and M. croslandi). Frequency and time of day (day versus night) were entered into the model as factors. A model was constructed by sequentially fitting parameters. Only parameters that reached significance at a 5% level when added to the final model were included in the model (electronic supplementary material, table S1). The individual components of variation between ants (ant identity) were accounted for by incorporating them into the model as a random factor. A random factor in the mixed model is equivalent to the block structure in the analysis of variance.
3. Results
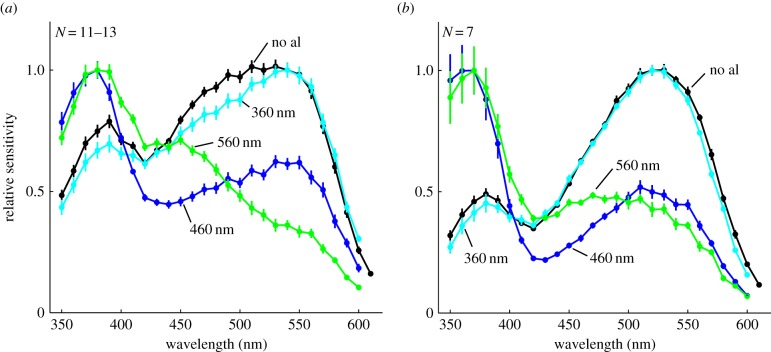
We demonstrate that congeneric day- and night-active Australian Myrmecia ants have three spectrally distinct photoreceptors using flicker ERG and intracellular recordings. The ERG recordings in the nocturnal ant M. vindex show a peak spectral sensitivity at 530 nm with a secondary peak at 390 nm (figure 1a, black line, N = 13). A 560-nm adaptation light lowers the contribution of long-wavelength sensitive receptors and shifts the sensitivity maximum towards the UV with a peak at 380 nm (figure 1a, green line, N = 12). It also uncovers a new secondary peak at 450 nm. To confirm that these peaks correspond to two additional photoreceptor types, we used two adaptation lights, at 360 and 460 nm respectively, to alter their relative contributions to the overall spectral sensitivity. As expected, a 360-nm adaptation light selectively reduces the eye's relative sensitivity at short wavelengths (figure 1a, cyan line, N = 11). By contrast, an adaptation light at 460 nm produces a peak sensitivity at 380 nm with a distinct secondary peak at 550 nm (figure 1a, blue line, N = 12). These results demonstrate clearly the existence of three distinct spectral types of photoreceptors in the retina of the nocturnal ant, M. vindex, with peak sensitivities in the UV, blue and green parts of the spectrum, respectively.
Figure 1.
Spectral responses of the entire eye in (a) the nocturnal M. vindex and (b) the diurnal M. croslandi recorded by ERG. Spectral response curves without adaptation lights (no al, black) and with 360 nm (cyan), 460 nm (blue) and 560 nm (green) adaptation lights are shown (mean ± s.e., N = sample size). See also electronic supplementary material, figures S1 and S2. (Online version in colour.)
In addition, we recorded intracellularly from photoreceptors of M. vindex and indeed found three receptor classes (figure 2a,b): a UV receptor (λmax: 370 nm), a blue receptor (λmax: 430 nm) and a green receptor (λmax: 550 nm). While eight out of 18 UV receptors were exclusively sensitive to short wavelengths, the 10 other UV cells had a broad secondary peak around 530–550 nm (figure 2b). We also found variation in the spectral profiles of blue and green receptors such as the six blue receptors with a broad secondary peak around 530–550 nm and the seven green receptors with a spectral half bandwidth (width at half maximum) of about 150 nm (figure 2b). In addition, data recorded in a previous year showed only broad spectra for all spectral types (N = 36) (data not shown). In instances where we measured broad spectral sensitivities, we applied monochromatic adaptation lights aligned to the stimulation light with a beam splitter (electronic supplementary material, figure S2) in order to reduce secondary peaks. We succeeded in reducing the secondary peak at around 530–550 nm of UV and blue receptors with a 560 nm adaptation light (electronic supplementary material, figure S2a–d). A 420 nm adaptation light was effective to reduce the shorter wavelength side of broad sensitivity of green receptors (electronic supplementary material, figure S2e,f). We therefore conclude that the broad spectral sensitivities of receptors shown in figure 2b are the result of contributions from more than one photoreceptor cell. All recordings presented here showed excellent stability (more than 30 min), consistent resting membrane potentials (50–80 mV) and large response amplitudes, measured as the response–stimulus intensity (V–log I) function over a 4 log unit intensity range at the photoreceptor's peak wavelength with flashes (20–80 mV), indicating stable and clean recordings.
Figure 2.
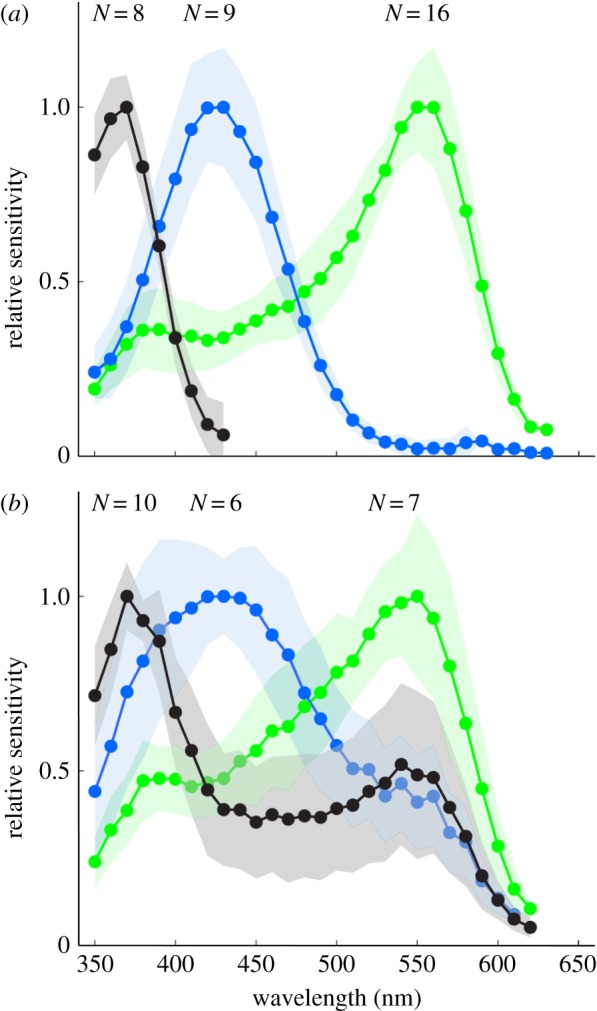
Spectral sensitivities of photoreceptors in the nocturnal M. vindex as determined by intracellular recordings (mean ± s.d.; N, sample number). Recordings were divided into (a) narrow- and (b) broad-band spectral sensitivity with a distinct secondary peak. The three types of photoreceptors have peak sensitivities (λmax) at 370 nm (UV, black curve), 450 nm (blue, blue curve) and 550 nm (green, green curve). (Online version in colour.)
ERG recordings in the day-active ant M. croslandi show the same shift in spectral sensitivities in response to monochromatic adaptation lights (figure 1b). The retina of M. croslandi, therefore, also contains three spectrally distinct photoreceptor types, with slightly shifted peak sensitivities at around 370, 470 and 510 nm, respectively. The un-adapted ERG sensitivity has a higher relative UV contribution in the night-active M. vindex compared with the day-active M. croslandi (compare black lines figure 1a versus 1b).
We modelled the photoreceptor sensitivities using the Govardovskii template [37], which describes visual pigment absorption and spectral sensitivities. The spectral sensitivity of UV receptors was accurately reproduced by a visual pigment absorption spectrum using the Govardovskii template with a λmax at 365.8 nm (R365.8) (figure 3a, magenta line). The spectral sensitivity of blue receptors was also well explained by a single rhodopsin absorption spectrum with a λmax at 430.8 nm (R430.8) (figure 3b, magenta line). The spectral sensitivity of green receptors was too narrow to be fitted by a single rhodopsin template with a peak at R543 nm (figure 3c, magenta line). The short-wavelength side of the sensitivity curve was better fitted by the linear combination of rhodopsin absorption spectra with a λmax at 542.6 nm (R542.6, 83%) and R430.8 (17%) (figure 3c, green line). The peak sensitivity, however, was still too narrow, which may be due to the coloured screening pigments in the retinular cells. Our modelling did not include self-screening effects [38], because the exact numbers of each photoreceptor type contributing to the ant's closed rhabdoms are not yet known.
Figure 3.
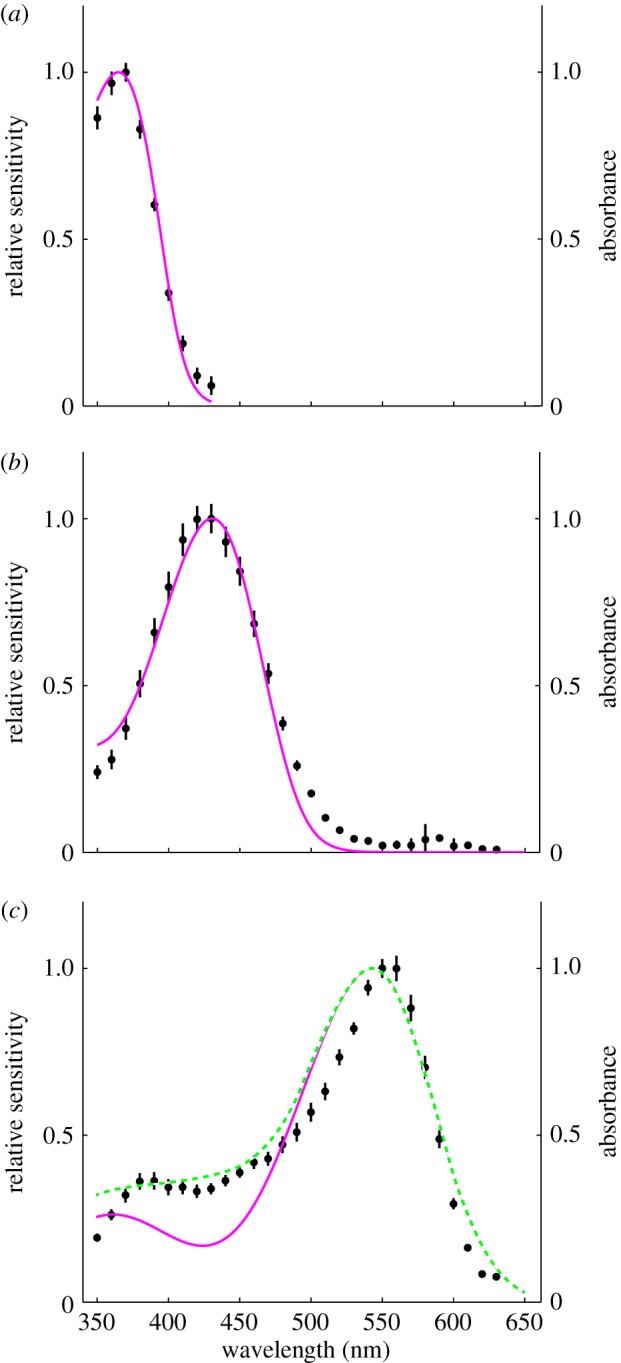
Model calculations for the three types of photoreceptors in the nocturnal M. vindex retina. The spectral sensitivities (black dots, mean ± s.e.) of the UV (a), blue (b) and green photoreceptor (c) are shown together with spectra calculated using a model (coloured lines). The spectra were modelled using three pigment templates with λmax at 365.8, 430.8 and 542.6 nm, respectively (magenta curves). The green receptor was fitted with a linear combination of two pigments templates λmax at 430.8 and 542.6 nm (dashed green curve). (Online version in colour.)
We also modelled the spectral sensitivities of the entire eye as determined by ERG recordings in both species (electronic supplementary material, figures S3 and S4). The model spectra were calculated by using three rhodopsin pigment templates fitted to all adaptation light conditions in a stepwise fashion. The short- and middle-wavelength absorbing pigment templates were estimated from recordings with 560 nm adaptation lights. The best-fitting λmax values at 378 and 447 nm in M. vindex and at 365 and 446 nm in M. croslandi were then fixed for all further fitting. The λmax values of long-wavelength absorbing pigments were estimated to be 539 nm in M. vindex and at 524 nm in M. croslandi from recordings with 460 nm adaptation lights. The results provide further evidence that both species possess three spectrally distinct types of photoreceptors. However, the λmax values of the three pigment templates did not exactly match the respective estimates from the intracellular recordings in M. vindex. Modelling of self-screening effects may eventually improve the model fits to the ERG recordings, but has no impact on our main conclusion that ants have three spectrally distinct photoreceptors.
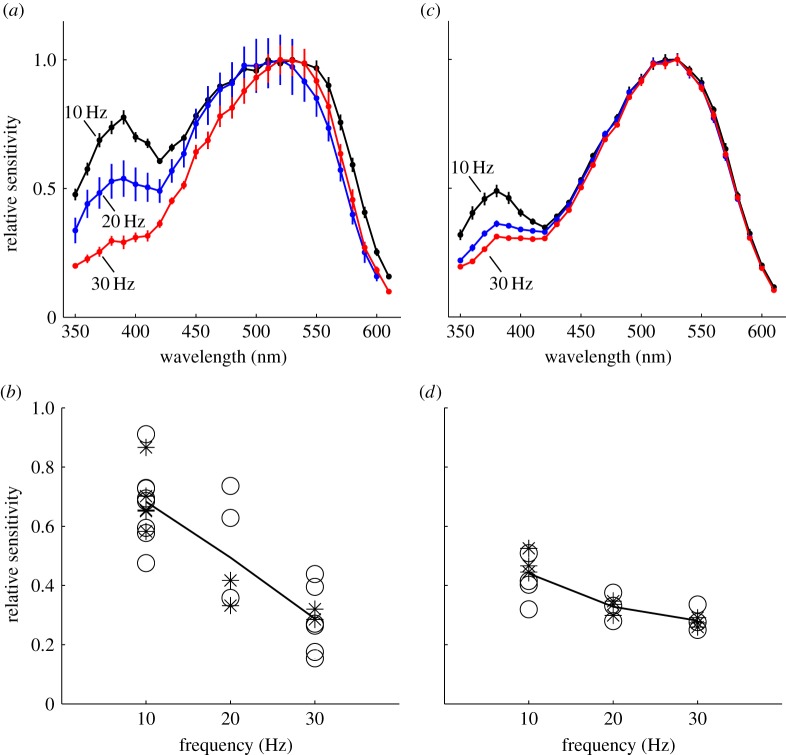
Performing spectral ERG recordings at different temporal frequencies allowed us to compare the dynamic properties of photoreceptors in the night-active M. vindex and the day-active M. croslandi. We found that the contribution of the UV receptors to the overall spectral sensitivity of the compound eye depends on the stimulation frequency (figure 4a,c). In the nocturnal ant, UV sensitivity dropped by 42.2% when the stimulation frequency increased from 10 to 30 Hz (average sensitivity between 350 and 400 nm, N = 27, p ≪ 0.001; figure 4b). This reduced sensitivity at short wavelengths in response to higher stimulation frequency was also present in the diurnal ant (N = 19, p ≪ 0.001; figure 4c,d), but the effect was much smaller. The difference in frequent-dependent sensitivity between the two species was tested in a combined model (p = 0.0049, model predictions are shown by the solid black lines in figure 4b,d). There was no effect of the time of day when the recordings were taken (day stars versus night circles in figure 4b,d, combined model, p = 0.8118). The slightly reduced sensitivity around 450 nm in response to 30 Hz stimulation suggests that the blue receptor may also be slower than the green receptor.
Figure 4.
The effects of stimulation flicker frequency on spectral sensitivity in (a,b) M. vindex and (c,d) M. croslandi. (a,c) Spectral sensitivities of the whole compound eye with flickering light at 10 (black), 20 (blue) and 30 Hz (red) were recorded in both species (mean ± s.e.). (b,d) The relative sensitivities of the compound eye of (b) M. vindex and (d) M. croslandi at 350–400 nm, recorded during the day (asterisks) and at night (open circles) depending on flicker frequency. Black lines are model predictions. (Online version in colour.)
4. Discussion
(a). Three spectrally distinct photoreceptors in ants
Evidence to date suggested ants to be rare dichromats among the trichromatic hymenopteran insects, with spectral sensitivities in the UV (or violet) and green parts of the spectrum [5–10,39,40], although one behavioural study had proposed the presence of four spectral receptors in C. bicolor [11].
We provide here the first clear evidence for three spectrally distinct photoreceptors in two congeneric Australian ants, M. vindex and M. croslandi. In M. vindex, modelling showed that the spectral sensitivities obtained from the intra- and extracellular recordings can both be explained by the presence of three types of photoreceptors with λmax values at 370, 430 and 550 nm, respectively (figures 1a and 2, and electronic supplementary material, figure S3). While we have so far not recorded intracellularly from the photoreceptors of M. croslandi, our ERG recordings and modelling indicate very clearly that it too has three spectral types of photoreceptors with λmax values at around 370, 470 and 510–530 nm, respectively (figure 1b and electronic supplementary material, figure S4).
While many of our intracellular recordings suggest that photoreceptors in M. vindex contain only one visual opsin, we recorded an unusually high number of very broad-band spectral sensitivities, with a distinct secondary peak (figure 2b). Because their relative contributions to the overall spectral sensitivity were affected by adaptation lights (electronic supplementary material, figure S2), this secondary peak appears to be produced by input from neighbouring photoreceptors rather than by two opsins being present in the same cell. If the broad spectral sensitivities were produced by multiple pigments in the same photoreceptor, the adaptation state of the entire photoreceptor would be changed by adaptation lights, leaving its spectral sensitivity unchanged. Separate spectral populations of photoreceptors, however, would adapt independently, and therefore change their relative contributions to the recording. Only very strong adaptation lights that lead to significant bleaching of visual pigment would be able to produce a shift in the spectral sensitivity of a single photoreceptor type with multiple visual pigments. However, such strong adaptation lights would also produce a significant change of the adaptation state of this photoreceptor and strongly reduce its response strength. This was clearly not the case, as we did not have to increase the intensity of our standard white light during adaptation experiments to compensate for adaptation, even in those cases where the secondary peak almost completely disappeared (electronic supplementary material, figure S2a,d–f). We cannot exclude at this point that some of the receptors contain more than one visual pigment [41] because adaptation lights do not work completely for some receptors (electronic supplementary material, figure S2b,c). It is theoretically also possible that two visual opsins in a single photoreceptor do not share the same transduction cascade and therefore could adapt independently, mimicking the effects of separate, but coupled photoreceptor classes. However, to investigate these alternatives, molecular and histological techniques are required to identify and localize visual pigments across the eye.
The frequency of occurrence and the stability of our recordings suggest that the broad sensitivities stem from inter-receptor coupling [42] rather than from simultaneous penetration of multiple cells. The maximum response amplitudes were between 20 and 80 mV at the peak sensitive wavelength for both narrow and broad spectral sensitivity recordings. We were unsuccessful in finding a correlation between the width of the spectral sensitivity profiles and the recording position across the visual field, as well as other factors such as electrode impedance (50–200 MΩ). Further research will need to investigate the mechanism behind these broad spectral sensitivities and whether they are an adaptation to a low light environment. Most importantly, behavioural experiments will be needed to clarify whether these ants use all three photoreceptors to produce trichromatic colour vision.
The slower speed of the UV photoreceptors, compared with the blue and green receptors, is most likely an adaptation to low light levels. Slow photoreceptors are commonly found in nocturnal and/or relatively slow-moving insects [43–46]. By compromising temporal resolution, slowing down a photoreceptor can increase its signal-to-noise ratio and improve contrast discrimination at lower frequencies by suppressing photon noise at frequencies too high to be reliably resolved [43–48]. In the nocturnal ants, slower UV receptors might selectively increase sensitivity to short wavelengths. Indeed, the ratio of the spectral sensitivity at short wavelengths to peak sensitivity is relatively higher in the nocturnal ants.
The adaptive significance of the increased UV sensitivity relative to other receptors is unlikely to be the need for an overall increase in sensitivity, but rather a specific need to keep the UV signal reliable at low ambient light intensities. It has recently been reported that UV contrast is highly effective for sky segmentation and the resulting binary panoramic skyline images can support effective navigation in ants [29,30]. The night-active M. vindex may be forced to slow down their UV receptors not only to ensure sufficient skyline contrast for landmark guidance [28], but also to obtain reliable compass information from the dim pattern of polarized skylight in the evening twilight [24].
(b). Are Australian ants special?
It is difficult to say at this stage whether Myrmecia ants are unique or whether all ants have three types of photoreceptors but studies so far have missed the blue receptor, because it may be rare in some species. Myrmecia ants have retained several ancestral characters from their wasp-like ancestors [49]. Having three distinct classes of photoreceptors may be one of these characters [9]. Following this hypothesis, the more ‘highly evolved’ ants, Formica and Cataglyphis, may have lost the blue receptor over evolutionary time. A similar phenomenon has been documented in red flour beetles Tribolium castaneum, which have lost the blue receptor that most other insects possess [50]. The difference between Myrmecia and other ants may reflect differences in the visual ecology of different species, in particular in the context of navigation. For instance, Cataglyphis ants rely predominantly on path integration and, as part of this, on the pattern of polarized skylight as a compass reference [21], for which colour vision would not seem to be required. Ants in landmark-rich habitats however, including the two species studied here, are guided by the visual panorama, often in preference to path integration information (e.g. [23,25,51,52]). In such cases, spectral information may improve the reliability of navigational cues. To our knowledge, the only study addressing the possible role of colour vision in landmark guidance [53], as distinct from target recognition or beacon aiming [2], unfortunately failed to control for luminance cues. The evolution of trichromacy in arthropods, including insects, predates the evolution of flowers [54] and we propose here that the most fundamental common need for colour vision may well have been in the context of landmark guidance. Targeted behavioural experiments investigating the role of colour vision in navigation are overdue.
Supplementary Material
Authors' contributions
Y.O. and J.M.H. contributed to all stages of the project, M.F. helped with the development of the preparation, equipment and recording software as well as the manuscript, A.N. and J.Z. helped identify and define the problem, provided funding and helped revise the manuscript.
Competing interests
We have no competing interests.
Funding
This study was supported by the Australian Research Council Centre of Excellence Scheme (CE0561903), an APA PhD Scholarship and St Andrew's College Post Graduate Research Residential Scholarship to M.F., an ARC Future Fellowship to J.M. (FT110100528), ARC Fellowships to A.N. (DE120100019, FT140100221) and a Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad to Y.O.
References
- 1.Johnsen S, Kelber A, Warrant EJ, Sweeney AM, Widder EA, Lee RL, Hernández-Andrés J. 2006. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J. Exp. Biol. 209, 789–800. ( 10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- 2.Somanathan H, Borges RM, Warrant EJ, Kelber A. 2008. Nocturnal bees learn landmark colours in starlight. Curr. Biol. 18, 996–997. ( 10.1016/j.cub.2008.08.023) [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita M, Arikawa K. 2014. Color and polarization vision in foraging Papilio. J. Comp. Physiol. A 200, 513–526. ( 10.1007/s00359-014-0903-5) [DOI] [PubMed] [Google Scholar]
- 4.Kelber A, Vorobyev M, Osorio D. 2003. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. 78, 81–118. ( 10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- 5.Menzel R, Knaut R. 1973. Pigment movement during light and chromatic adaptation in the retinula cells of Formica polyctena (Hymenoptera, Formicidae). J. Comp. Physiol. A 86, 125–138. ( 10.1007/BF00702533) [DOI] [Google Scholar]
- 6.Menzel R, Blakers M. 1975. Functional organisation of an insect ommatidium with fused rhabdom. Cytobiol. 11, 279–298. [Google Scholar]
- 7.Lieke E. 1981. Graded and discrete receptor potentials in the compound eye of the Australian bulldog-ant (Myrmecia gulosa). Biol. Cybern. 40, 151–156. ( 10.1007/BF00344293) [DOI] [Google Scholar]
- 8.Labhart T. 1986. The electrophysiology of photoreceptors in different eye regions of the desert ant, Cataglyphis bicolor. J. Comp. Physiol. A 158, 1–7. ( 10.1007/BF00614514) [DOI] [Google Scholar]
- 9.Peitsch D, Fietz A, Hertel H, de Souza J, Ventura DF, Menzel R. 1992. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 170, 23–40. ( 10.1007/BF00190398) [DOI] [PubMed] [Google Scholar]
- 10.Briscoe AD, Chittka L. 2001. The evolution of color vision in insects. Annu. Rev. Entomol 46, 471–510. ( 10.1146/annurev.ento.46.1.471) [DOI] [PubMed] [Google Scholar]
- 11.Kretz R. 1979. A behavioural analysis of colour vision in the ant Cataglyphis bicolor (Formicidae, Hymenoptera). J. Comp. Physiol. A 131, 217–233. ( 10.1007/BF00610430) [DOI] [Google Scholar]
- 12.Cammaerts M. 2007. Colour vision in the ant Myrmica sabuleti Meinert, 1861 (Hymenoptera: Formicidae). Myrmecol. News 10, 41–50. [Google Scholar]
- 13.Cammaerts M, Cammaerts D. 2009. Light thresholds for colour vision in workers of the ant Myrmica sabuleti (Hymenoptera, Formicidae). Belgian J. Entomol. 139, 40–49. [Google Scholar]
- 14.Camlitepe Y, Aksoy V. 2010. First evidence of fine colour discrimination ability in ants (Hymenoptera, Formicidae). J. Exp. Biol. 213, 72–77. ( 10.1242/jeb.037853) [DOI] [PubMed] [Google Scholar]
- 15.Wehner R, Räber F. 1979. Visual spatial memory in desert ants, Cataglyphis bicolor (Hymenoptera: Formicidae). Experientia 35, 1569–1571. ( 10.1007/BF01953197) [DOI] [Google Scholar]
- 16.Wehner R, Wehner S. 1990. Insect navigation: use of maps or Ariadne's thread? Ethol. Ecol. Evol. 2, 27–48. ( 10.1080/08927014.1990.9525492) [DOI] [Google Scholar]
- 17.Collett TS, Dillmann E, Giger A, Wehner R. 1992. Visual landmarks and route following in desert ants. J. Comp. Physiol. A 170, 435–442. ( 10.1007/BF00191460) [DOI] [Google Scholar]
- 18.Wehner R, Michel B, Antonsen P. 1996. Visual navigation in insects: coupling of egocentric and geocentric information. J. Exp. Biol. 199, 129–140. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan MV. 2001. Homing in on ant navigation. Nature 411, 752–753. ( 10.1038/35081224) [DOI] [PubMed] [Google Scholar]
- 20.Wehner R. 2003. Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579–588. ( 10.1007/s00359-003-0431-1) [DOI] [PubMed] [Google Scholar]
- 21.Wehner R, Müller M. 2006. The significance of direct sunlight and polarized skylight in the ant's celestial system of navigation. Proc. Natl Acad. Sci. USA 103, 12 575–12 579. ( 10.1073/pnas.0604430103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham P, Cheng K. 2009. Ants use the panoramic skyline as a visual cue during navigation. Curr. Biol. 19, R935–R937. ( 10.1016/j.cub.2009.08.015) [DOI] [PubMed] [Google Scholar]
- 23.Graham P, Cheng K. 2009. Which portion of the natural panorama is used for view-based navigation in the Australian desert ant? J. Comp. Physiol. A 195, 681–689. ( 10.1007/s00359-009-0443-6) [DOI] [PubMed] [Google Scholar]
- 24.Reid SF, Narendra A, Hemmi JM, Zeil J. 2011. Polarised skylight and the landmark panorama provide night-active bull ants with compass information during route following. J. Exp. Biol. 214, 363–370. ( 10.1242/jeb.049338) [DOI] [PubMed] [Google Scholar]
- 25.Narendra A, Gourmaud S, Zeil J. 2013. Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc. R. Soc. B 280, 20130683 ( 10.1098/rspb.2013.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehner R. 2001. Polarization vision–a uniform sensory capacity? J. Exp. Biol. 204, 2589–2596. [DOI] [PubMed] [Google Scholar]
- 27.Bühlmann C, Cheng K, Wehner R. 2011. Vector-based and landmark-guided navigation in desert ants inhabiting landmark-free and landmark-rich environments. J. Exp. Biol. 214, 2845–2853. ( 10.1242/jeb.054601) [DOI] [PubMed] [Google Scholar]
- 28.Narendra A, Reid SF, Raderschall CA. 2013. Navigational efficiency of nocturnal Myrmecia ants suffers at low light levels. PLoS ONE 8, e58801 ( 10.1371/journal.pone.0058801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone T, Mangan M, Ardin P, Webb B. 2014. Sky segmentation with ultraviolet images can be used for navigation. Robot. Sci. Syst. 10, 47. [Google Scholar]
- 30.Möller R. 2002. Insects could exploit UV–green contrast for landmark navigation. J. Theor. Biol. 214, 619–631. ( 10.1006/jtbi.2001.2484) [DOI] [PubMed] [Google Scholar]
- 31.Stürzl W, Grixa I, Mair E, Narendra A, Zeil J. 2015. Three-dimensional models of natural environments and the mapping of navigational information. J. Comp. Physiol. A. ( 10.1007/s00359-015-1002-y) [DOI] [PubMed] [Google Scholar]
- 32.Jayatilaka P, Raderschall CA, Narendra A, Zeil J. 2014. Individual foraging patterns of the jack jumper ant Myrmecia croslandi (Hymenoptera: Formicidae). Myrmecol. News 19, 75–83. [Google Scholar]
- 33.Jayatilaka P, Narendra A, Reid SF, Cooper P, Zeil J. 2011. Different effects of temperature on foraging activity schedules in sympatric Myrmecia ants. J. Exp. Biol. 214, 2730–2738. ( 10.1242/jeb.053710) [DOI] [PubMed] [Google Scholar]
- 34.Greiner B, Narendra A, Reid SF, Dacke M, Ribi WA, Zeil J. 2007. Eye structure correlates with distinct foraging-bout timing in primitive ants. Curr. Biol. 17, R879–R880. ( 10.1016/j.cub.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 35.Jacobs GH, Neitz J, Krogh K. 1996. Electroretinogram flicker photometry and its applications. J. Opt. Soc. Am. A 13, 641–648. ( 10.1364/JOSAA.13.000641) [DOI] [PubMed] [Google Scholar]
- 36.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0; URL http://www.R-project.org/. [Google Scholar]
- 37.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. ( 10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 38.Warrant EJ, Nilsson DE. 1998. Absorption of white light in photoreceptors. Vis. Res. 38, 195–207. ( 10.1016/S0042-6989(97)00151-X) [DOI] [PubMed] [Google Scholar]
- 39.Mote MI, Wehner R. 1980. Functional characteristics of photoreceptors in the compound eye and ocellus of the desert ant, Cataglyphis bicolor. J. Comp. Physiol. A 137, 63–71. ( 10.1007/BF00656918) [DOI] [Google Scholar]
- 40.Reid SF. 2010. Life in the dark: vision and navigation in a nocturnal bull ant. PhD thesis, The Australian National University, Canberra, Australia. [Google Scholar]
- 41.Schmeling F, Wakakuwa M, Tegtmeier J, Kinoshita M, Bockhorst T, Arikawa K, Homberg U. 2014. Opsin expression, physiological characterization and identification of photoreceptor cells in the dorsal rim area and main retina of the desert locust, Schistocerca gregaria. J. Exp. Biol. 217, 3557–3568. ( 10.1242/jeb.108514) [DOI] [PubMed] [Google Scholar]
- 42.Shaw SR. 1969. Interreceptor coupling in ommatidia of drone honeybee and locust compound eyes. Vis. Res. 9, 999–1029. ( 10.1016/0042-6989(69)90044-3) [DOI] [PubMed] [Google Scholar]
- 43.De Souza JM, Ventura DF. 1989. Comparative study of temporal summation and response form in hymenopteran photoreceptors. J. Comp. Physiol. A 165, 237–245. ( 10.1007/BF00619198) [DOI] [PubMed] [Google Scholar]
- 44.Laughlin SB, Weckström M. 1993. Fast and slow photoreceptors—a comparative study of the functional diversity of coding and conductances in the Diptera. J. Comp. Physiol. A 172, 593–609. ( 10.1007/BF00213682) [DOI] [Google Scholar]
- 45.Heimonen K, Salmela I, Kontiokari P, Weckström M. 2006. Large functional variability in cockroach photoreceptors: optimization to low light levels. J. Neurosci. 26, 13 454–13 462. ( 10.1523/JNEUROSCI.3767-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warrant EJ, Dacke M. 2011. Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 56, 239–254. ( 10.1146/annurev-ento-120709-144852) [DOI] [PubMed] [Google Scholar]
- 47.Frederiksen R, Wcislo WT, Warrant EJ. 2008. Visual reliability and information rate in the retina of a nocturnal bee. Curr. Biol. 18, 349–353. ( 10.1016/j.cub.2008.01.057) [DOI] [PubMed] [Google Scholar]
- 48.Warrant EJ. 2008. Seeing in the dark: vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 211, 1737–1746. ( 10.1242/jeb.015396) [DOI] [PubMed] [Google Scholar]
- 49.Ward PS, Brady SG. 2003. Phylogeny and biogeography of the ant subfamily Myrmeciinae (Hymenoptera: Formicidae). Invertebr. Syst. 17, 361–386. ( 10.1071/IS02046) [DOI] [Google Scholar]
- 50.Jackowska M, Bao R, Liu Z, McDonald EC, Cook TA, Friedrich M. 2007. Genomic and gene regulatory signatures of cryptozoic adaptation: loss of blue sensitive photoreceptors through expansion of long wavelength-opsin expression in the red flour beetle Tribolium castaneum. Front. Zool. 4, 24 ( 10.1186/1742-9994-4-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeil J, Narendra A, Stürzl W. 2014. Looking and homing: how displaced ants decide where to go. Phil. Trans. R. Soc. B 369, 20130034 ( 10.1098/rstb.2013.0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangan M, Webb B. 2012. Spontaneous formation of multiple routes in individual desert ants (Cataglyphis velox). Behav. Ecol. 23, 944–954. ( 10.1093/beheco/ars051) [DOI] [Google Scholar]
- 53.Cheng K, Collett TS, Wehner R. 1986. Honeybees learn the colours of landmarks. J. Comp. Physiol. A 159, 69–73. ( 10.1007/BF00612497) [DOI] [Google Scholar]
- 54.Chittka L. 1996. Does bee color vision predate the evolution of flower color? Naturwissenschaften 83, 136–138. ( 10.1007/BF01142181) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.