Abstract
It is now clear in many species that male and female genital evolution has been shaped by sexual selection. However, it has historically been difficult to confirm correlations between morphology and fitness, as genital traits are complex and manipulation tends to impair function significantly. In this study, we investigate the functional morphology of the elongate male intromittent organ (or processus) of the seed bug Lygaeus simulans, in two ways. We first use micro-computed tomography (micro-CT) and flash-freezing to reconstruct in high resolution the interaction between the male intromittent organ and the female internal reproductive anatomy during mating. We successfully trace the path of the male processus inside the female reproductive tract. We then confirm that male processus length influences sperm transfer by experimental ablation and show that males with shortened processi have significantly reduced post-copulatory reproductive success. Importantly, male insemination function is not affected by this manipulation per se. We thus present rare, direct experimental evidence that an internal genital trait functions to increase reproductive success and show that, with appropriate staining, micro-CT is an excellent tool for investigating the functional morphology of insect genitalia during copulation.
Keywords: genital evolution, genital ablation, micro-computed tomography, post-copulatory, cryptic female choice, functional morphology
1. Introduction
Male and female genitalia show extraordinary diversity across the animal kingdom, and there are numerous examples of highly divergent genital morphology among closely related species [1–4]. It is now widely accepted that both the elaboration and rapid evolution of genital traits is probably driven by sexual selection, with selection favouring the evolution of genital morphology (usually in males) that increases fertilization success relative to that of their rivals (whereas the ‘lock and key’ hypothesis for genital evolution is not well supported [2,4]). However, the specific mechanisms of sexual selection involved in genital evolution remain unclear for most species [3–6]. Evidence for the role of sexual selection in genital evolution comes primarily from studies correlating intraspecific variation in morphology with reproductive success (see [7] for examples of male genitalia in insects; female genitalia have been much less studied [8]). In males, the size and shape of both internal and external genitalia have been shown to influence post-copulatory traits such as sperm transfer and paternity [7].
An alternative approach is to experimentally manipulate male genitalia and record how reproductive success is influenced by such manipulation [7]. This has the advantage of establishing that the targeted trait actually functions to influence reproductive success (although of course other functions cannot be ruled out). Studies in which genital structures are removed or reduced in some way are known as genital ablation studies. Such studies have become much more sophisticated in recent years. For example, Hotzy et al. [9] used micro-laser surgery to ablate male genital spines in the seed beetle Callosobruchus maculatus. This manipulation, along with artificial selection lines, showed that males with longer spines gained more fertilizations in a competitive context and that this was possibly due to a larger proportion of the seminal fluid passing into the haemolymph of the female [9]. The traits targeted by such ablation studies tend to be tough sclerotized structures such as spines [9,10], teeth [11] and claspers [12] that are amenable to manipulation. Manipulation of the structures directly associated with sperm transfer is not likely to be possible in most species, as such structures tend to be highly complex so that manipulation impairs function [13] and vascularized so that manipulation leads to injury and the loss of blood/haemolymph (although see [14] for an experimental reduction of male gonopodium length in a fish, for which genital function was not tested).
Moreover, this approach has recently come under criticism, with Simmons [7] noting that complete removal or serious disruption of a trait may not tell us much about the selection pressures acting on it due to the inevitable detrimental effect on normal trait function. However, if genital traits can be manipulated while keeping normal reproductive functions intact, the major drawbacks of this potentially powerful approach are resolved. Such a manipulation has been performed in the tortoise beetle Chelymorpha alternans [15,16]. Male tortoise beetles possess an extremely long, thread-like flagellum that enters the female spermathecal duct, and experimental reduction of the flagellum leads to an increased incidence of sperm droplet formation after mating, a behaviour which may represent sperm rejection by the female [15,16]. We suggest that this is a potentially powerful approach to studying the functional morphology of genitalia that has not been fully explored.
In order to understand the function of male genital traits, it would be useful to be able to visualize the interactions between male and female genitalia while in copula. However, such interactions can be delicate, especially in insects, so that even the most careful dissections of copulating pairs may alter the normal positions of male and female genitalia. An alternative is to use non-destructive imaging techniques such as micro-computed tomography, or ‘micro-CT’. Micro-CT has been widely used to describe the morphology of fossil organisms [17,18] and, in recent years, has become increasingly prominent in anatomical studies of extant species [19], particularly in combination with contrast-enhancing agents [20]. The technique allows taxonomists to carry out non-destructive ‘virtual dissections’ of taxonomically important characters, such as genitalia [21]. Thus far, few studies have used micro-CT to study the functional morphology of genitalia (although see [22,23]).
Males of the seed bug Lygaeus simulans L (Heteroptera: Lygaeidae) possess an intromittent organ with a very long, thread-like posterior structure known as the processus gonopori (hereafter referred to as the processus) [24] (electronic supplementary material, figure S1), which is around two-thirds of a male's body length [25]. Such an extremely long male intromittent organ is common in the Heteroptera [26–28], and is also found in several other insect groups including the Coleoptera [15,16,29,30], Dermaptera [31,32] and Zoraptera [22]. A previous correlational study in L. simulans found stabilizing post-copulatory selection on processus length: males with an average processus length were most likely to inseminate a female [33]. The male processus is a long, thin, sclerotized tube through which the ejaculate is transferred via fluid pressure at the base, with no obvious musculature or vascularization. It therefore may be amenable to experimental manipulation without further damage to the male or complete loss of function.
In this study, we investigate the functional morphology of the male processus in L. simulans in two ways. First, we present micro-CT scans of flash-frozen copulating pairs and show that this technique can be used to non-destructively visualize the interactions between male and female genitalia. We then confirm that male processus length influences sperm transfer directly by experimental reduction of processus length by differing amounts over three experiments. We consider four measures of reproductive success: male mating, copulation duration, insemination success and fertilization success (see §2e). We show first that the processus can be manipulated while maintaining its sperm transfer function, and second that male post-copulatory reproductive success decreases as a greater proportion of the processus is removed.
2. Material and methods
(a). Insect husbandry
All individuals were maintained at 29°C, with a 22 L : 2 D cycle to prevent reproductive diapause. Prior to experiments, individuals were moved from large stock populations into small plastic deli tubs (108 × 82 × 55 mm) as nymphs. These tubs were checked every day for newly eclosed adults, which were then separated into single-sex tubs, with 8 to 10 individuals per tub. All tubs were provisioned with de-husked organic sunflower seeds (Helianthus annuus) ad libitum, plastic tubes containing distilled water stopped with cotton wool, and a piece of dry cotton wool as shelter. Water was replaced every 7 days, and prior to mating trials. All mating trials were performed when males and females were sexually mature (7–14 days post adult eclosion).
(b). Micro-computed tomography
A single male and female were allowed to copulate for 2 h, and then flash-frozen in liquid nitrogen. This gives time for the processus to reach the entrance to the spermatheca (this typically takes around 1 h), but is shorter than the average copulation duration of 200–250 min [33,34]. Samples were fixed by placing in Alcoholic Bouin's solution for 4 h. The fixative was then washed out using 70% ethanol, and then the pairs were stained with 1% iodine in 100% ethanol (I2E) for 4 days prior to scanning. This served to enhance the X-ray attenuation contrast of non-mineralized tissues, which are otherwise difficult to distinguish using micro-CT [20]. Prior to transportation to the scanning facility, mated pairs were washed several times in 70% ethanol to remove excess I2E, and then all ethanol was pipetted out (ethanol residue on the sides of the tubes was sufficient to prevent the samples from drying out).
Micro-CT was performed on a Nikon (formerly Metris X-Tek) XT H 225 cabinet scanner at the Natural History Museum, London. Samples were scanned dry, in an Eppendorf tube mounted on florist's foam. Scans were performed using a current/voltage of 105 kV/190 µA and 3142 projections. This generated datasets of slice images with voxel sizes ranging from about 5 to 7 µm. Digital visualization was undertaken using the freely available SPIERS software suite [35]. For each scan, a global linear threshold was applied to the dataset, creating binary images in which all pixels brighter than a user-defined grey level were turned ‘on’ (white). The ‘on’ pixels identified as belonging to the bugs were then manually assigned to distinct regions of interest, which corresponded to important anatomical characters (e.g. processus, aedaegus, claspers, spermatheca and bursa). Finally, these regions of interest were rendered as separate isosurfaces, producing interactive three-dimensional virtual reconstructions in which the different anatomical structures could be independently manipulated (see the electronic supplementary material). High-quality images and animations were produced in the open-source program Blender (www.blender.org).
Two mating pairs were scanned in total, but reconstructions for only one of the pairs are presented here, as the results for the other pair are very similar. A scan was also performed of a single male with aedeagus everted from the genital capsule following mating. Additional figures and videos are presented in the electronic supplementary material. The raw slices obtained from the scans, plus spiersview (VAXML) files and 3D PDFs showing scan reconstructions, have been deposited in Dryad (doi:10.5061/dryad.4tp56).
(c). Processus cutting
In order to manipulate male processus length, virgin males and females were first placed together in a mating arena and observed until copulation occurred. After approximately 5 min, copulation was interrupted using a fine paintbrush, which caused the male to disengage from the female with his intromittent organ everted from the genital capsule. The male was then sedated by placing in a freezer at –18°C for 4 min, and then the processus was cut using a pair of micro-scissors. The removed portion of the processus was kept for measurement. A sham treatment was also performed in which males were placed in the freezer and the processus manipulated but not cut. Males were given at least 1 day to recover before being introduced to new, naive females: the females used for this pre-trial stage were not re-used. Prior to the experiment, the lumen of the processus was confirmed as remaining open after cutting by taking images using a dissecting microscope and a scanning electron microscope (figure 2). During the experiment, each processus was checked by eye following cutting to ensure the cut was performed cleanly.
Figure 2.
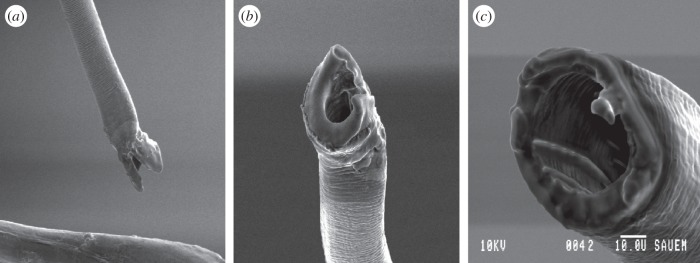
Scanning electron microscope images showing (a,b) the normal tip of the processus and (c) the intact lumen after experimental manipulation.
(d). Experimental design
Three manipulation experiments were performed. In the first experiment, the processus was shortened by an average of 2 mm in 39 males, which is 29% of the total processus length. This is far outside the natural phenotypic range of the processus [33]. A further 39 males were subjected to the same procedure but without cutting (sham treatment). Males were then given the opportunity for a single mating with a virgin female.
A second experiment was performed in which proportionally less of the processus was removed experimentally. The processus of 13 males was shortened by an average of 1 mm (14% of total length), while 12 males were left untreated. In order to confirm that sperm transfer was possible after experimental manipulation, each male was housed with a single virgin female for two weeks, thus allowing the opportunity for multiple matings. This gave each male several opportunities to successfully inseminate the female. Pairs were checked two to three times a day for copulation.
Finally, a third experiment was performed in which treated males had their processi reduced by a smaller amount, this time within the natural phenotypic range. A third treatment was also added in which only the very tip of the processus was removed, for two reasons. First, this controls for any effect of ablation itself, as males receive the cutting procedure but with a negligible reduction in processus length. Second, the processus ends in a cup-like structure with a V-shaped cleft, which may be important for normal sperm transfer (figure 2). Males were thus given one of three treatments: (i) reduction by 0.4 mm (5.7% of total length, n = 56), (ii) reduction by 0.1 mm (n = 54) or (iii) no reduction (sham treatment, n = 55). Males were then given the opportunity for a single mating with a virgin female as before.
(e). Measures of reproductive success
For experiments 1 and 3, no-choice mating trials were performed in which virgin males were introduced to a virgin female in small plastic Petri dishes (55 mm diameter). Dishes were observed continuously for 2 h, and then checked every 10 min for a further 8 h. If a copulation ended during the trial, the pair were separated so as to restrict the female to a single mating. This was done for any copulation that lasted 15 min: pairs that copulated for less than this time were not separated as sperm transfer is not possible (sperm transfer has been shown to take at least 30 min [34]). Copulations that did not end during the trial were separated manually using a fine paintbrush (this does not damage the male or female). We recorded the proportion of males that mated for all treatments. Copulation duration was recorded of all mated pairs, as this is shown to significantly influence insemination success [36]. For experiment 2, each male was housed with a single virgin female in a tub with food and water ad libitum for two weeks. For this treatment, the proportion of times a pair was seen in copula was used as a proxy for male mating frequency.
All males were euthanized once mating trials were finished. Mated females were kept in isolated tubs with food and water for two weeks to oviposit. After two weeks, mated females and all offspring were frozen, and the number of offspring produced was recorded. Hereafter, we refer to whether a female produced offspring or not as ‘insemination success', and the number of offspring produced by a female as ‘fertilization success’.
(f). Processus measurements
After the experiments were performed, male processi were dissected and placed onto a microscope slide using Sellotape double-sided sticky tape for measurement [37]. Images were taken with an Olympus SZX10 stereo microscope (Olympus Corp.) and an attached ColorView IIIu camera (Soft Imaging System, Olympus Corp.). Measurements were made from these images using the programCell^D v. 2.8 (Soft Imaging System, Olympus Corp.). Processus length was measured from the middle of the ‘turning point’, the curved region just before the fleshy aedeagus ends to the tip (point A to point B in electronic supplementary material, figure S1), following Tadler [33]. Both the removed portion of the processus as well as the intact portion was measured.
(g). Statistical analysis
Analyses were performed separately for the four measures of male reproductive success. All models (with the exception of those concerning copulation duration for experiment 3; see below) were first run including treatment, male body length and their interaction as response variables. In all cases, the interaction was not significant and so was removed from the model. Male body lengths were not measured for experiment 2, so those models include only experimental treatment as a response variable.
Determinants of male mating were tested in two ways. For experiments 1 and 3, logistic regression was used, with male mating as a binary response variable (whether a male mated or not). For experiment 2, general linear models were used, with the proportion of times a male was seen mating (square-root transformed) as the response variable. Determinants of copulation duration were tested in two ways. For experiment 1, a general linear model was used, including both experimental treatment and male body length as response variables. However, the residuals for experiment 3 were not normally distributed, and so the effects of treatment and male body length were tested separately, using non-parametric tests. The effect of experimental treatment was tested using a Kruskall–Wallis test, and the effect of male body length using Spearman's rank correlation. Determinants of insemination success were tested using logistic regression with insemination as a binary response variable (whether a mating resulted in offspring or not). Finally, determinants of fertilization success were tested using general linear models, with offspring number as the response variable. For experiment 3, additional pairwise comparisons were performed between the three experimental treatments using Tukey tests, using the multcomp package in R [38].
Additionally, for experiment 3, logistic regression was used to estimate the relationship between male processus length and insemination success (as a binomial response) separately for each of the three experimental treatments. Processus length was included as both a linear and quadratic term. This relationship was then plotted for males with 0.4 mm of the processus removed using a non-parametric curve [39]. The curve was estimated using a general additive model, with insemination success as a binomial response (whether the mating resulted in offspring or not) and processus length as the predictor variable (using the R package mcgv), and visualized using a cubic spline [39]. All statistical analyses were performed in R v. 3.1.0 [40]. All data for the three experiments have been deposited in Dryad (doi:10.5061/dryad.4tp56).
3. Results
(a). Micro-computed tomography
Three-dimensional virtual reconstructions of an L. simulans copulating pair, obtained via micro-CT scanning, can be seen in figure 1. Iodine staining served to greatly enhance the contrast of non-mineralized tissues—which are otherwise difficult to resolve with micro-CT because they show limited X-ray contrast [20]—allowing visualization of the entire male intromittent organ, including the processus and fleshy base of the aedeagus, within the female tract. The sclerotized nature of the processus meant that it was clearly differentiated from the surrounding tissues in micro-CT images (figure 1), so that its path could be traced both inside the female, and also posteriorly within the base of the aedeagus (figure 1a). The female internal reproductive morphology was also reconstructed in detail; specifically, the bursa (which appears as a large cavity) and the spermatheca, which is sclerotized (figure 1b,c). The positions of the male aedeagus and processus within the female bursa have not previously been reported, and physical dissection invariably causes distortion of the natural shape of the bursa, which is very fragile; consequently, this virtual approach was an ideal way of imaging these structures in situ. It appears that the processus is coiled inside the bursa for slightly more than half of its length and performs one and a half turns once in the spermathecal duct (figure 1b,c) [34]. Furthermore, the high resolution of the scans (down to about 5–7 µm) meant that very fine-scale anatomical features could be detected, such as the tight corkscrew region at the entrance to the spermatheca (point D in figure 1b) [41].
Figure 1.
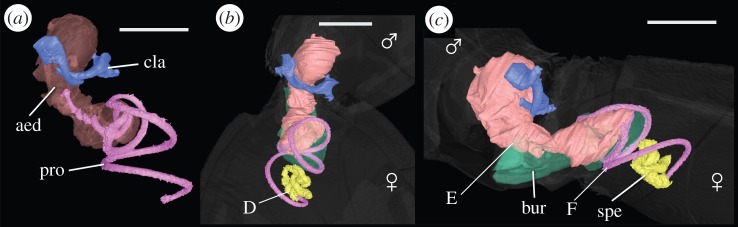
Reconstructions of reproductive anatomy of L. simulans obtained from micro-CT scans, showing male and female in copula. (a) The male genitalia in isolation and (b,c) the interaction between the male and female genitalia (with the body transparent) in dorsal and lateral view, respectively. The fleshy base of the aedeagus can be seen in orange/brown (aed), and the coiled processus in purple (pro). The paired male claspers are shown in blue (cla). The female bursa is shown in green (bur), and the spermatheca in yellow (spe). The corkscrew region at the entrance to the spermatheca is shown at point D. The aedeagus enters the female at point E. The approximate point where the processus enters the female spermathecal duct is shown at point F. Scale bar, 1 mm.
Scans also confirm that the male processus is able to reach the spermatheca after copulation for 2 h, and can thus be inferred to extend all the way along the spermathecal duct (as previous studies have reported [34]). However, the spermathecal duct could not be distinguished from the male processus; this may be because the spermathecal duct is a very fine structure, and hence is difficult to resolve with micro-CT, even after the use of contrast-enhancing agents to increase differential attenuation [20]. The starting position of the spermathecal duct can be inferred from the point where the processus appears to break through the wall of the bursa (point F in figure 1c). Furthermore, the resolution of the CT scans was insufficient to reveal the fine-scale structure of the processus tip, which is better resolved using scanning electron microscope (SEM) imaging (figure 2).
(b). Experimental reduction in processus length
The average processus length for each treatment across all experiments can be seen in table 1. Across all three experiments, experimental treatment did not appear to alter male mating behaviour.
Table 1.
Table showing mean processus lengths for all three manipulation experiments, split by experimental treatment.
| experiment | treatment | n | amount removed (mm) | s.d. | length after cutting (mm) | s.d. | proportion of total removed |
|---|---|---|---|---|---|---|---|
| 1 | sham | 39 | 0.00 | — | 6.90 | 0.22 | — |
| manipulated | 39 | 2.00 | 0.24 | 4.84 | 0.30 | 0.29 | |
| 2 | sham | 12 | 0.00 | — | 6.92 | 0.26 | — |
| manipulated | 13 | 1.02 | 0.39 | 5.80 | 0.48 | 0.16 | |
| 3 | sham | 55 | 0.00 | — | 6.80 | 0.16 | — |
| tip removed | 54 | 0.10 | 0.03 | 6.74 | 0.20 | 0.01 | |
| manipulated | 56 | 0.39 | 0.13 | 6.48 | 0.20 | 0.05 |
(i). Experiment 1
The proportion of males that mated did not differ between the two experimental treatments (logistic regression; 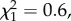 p = 0.44). However, larger males were more likely to mate (
p = 0.44). However, larger males were more likely to mate (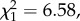 p = 0.01). Copulation duration was significantly shorter for males with a shortened processus compared with sham males (GLM; F1,56 = 7.04, p = 0.01; figure 3a). Larger males also copulated for longer (F1,56 = 4.23, p = 0.044). Males with a shortened processus also had significantly reduced insemination success (
p = 0.01). Copulation duration was significantly shorter for males with a shortened processus compared with sham males (GLM; F1,56 = 7.04, p = 0.01; figure 3a). Larger males also copulated for longer (F1,56 = 4.23, p = 0.044). Males with a shortened processus also had significantly reduced insemination success (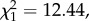 p < 0.001; figure 3b): only 2 out of 28 matings by manipulated males led to offspring, compared with 15 out of 31 matings for sham males. Insemination success was not influenced by male body length (
p < 0.001; figure 3b): only 2 out of 28 matings by manipulated males led to offspring, compared with 15 out of 31 matings for sham males. Insemination success was not influenced by male body length (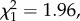 p = 0.16). For those matings that produced offspring, there was no significant difference in the number of offspring between reduced and sham males (F1,14 = 3.22, p = 0.09; figure 3c), which is likely to be due to the small number of successful inseminations by manipulated males. Additionally, larger males produced more offspring following fertile matings (F1,14 = 6.03, p = 0.027).
p = 0.16). For those matings that produced offspring, there was no significant difference in the number of offspring between reduced and sham males (F1,14 = 3.22, p = 0.09; figure 3c), which is likely to be due to the small number of successful inseminations by manipulated males. Additionally, larger males produced more offspring following fertile matings (F1,14 = 6.03, p = 0.027).
Figure 3.
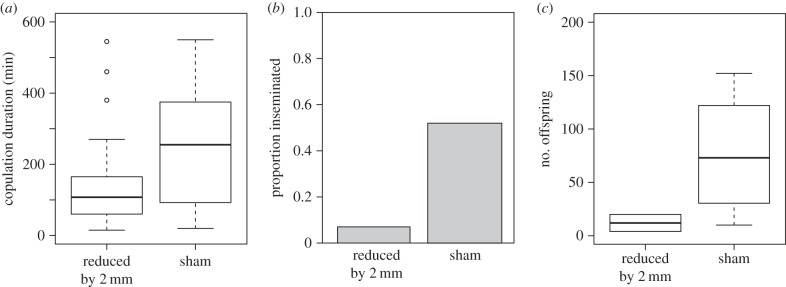
The influence of experimental reduction in processus length on male reproductive success in experiment 1. The male processus was either shortened by 2 mm (n = 39) or manipulated but not cut (sham, n = 39). Following a single mating, three measures of reproductive success were recorded: (a) copulation duration, (b) insemination success (whether a mating resulted in offspring or not) and (c) fertilization success (the number of offspring produced).
(ii). Experiment 2
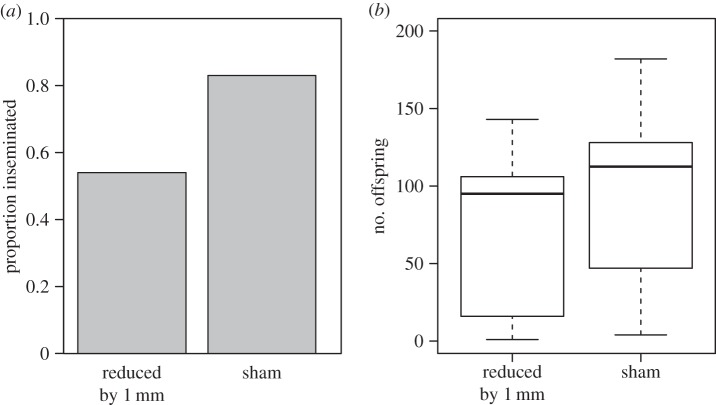
There was no significant difference in male mating frequency (proportion of observations seen in copula) between the two treatments (F1,23 = 0.95, p = 0.34). Reduction of processus length by 1 mm led to no difference in male insemination success (including all males, even those that were not seen mating) compared with sham males (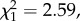 p = 0.11; figure 4a). However, the sample size for this experiment is small, and there is a non-significant trend towards a reduction in the insemination success of manipulated males. Nevertheless, this confirms that males can successfully transfer sperm after experimental manipulation, at least when the processus has been shortened by around 1 mm. There was also no significant difference in the fertilization success of manipulated males compared with sham males (F1,15 = 1.14, p = 0.3; figure 4b).
p = 0.11; figure 4a). However, the sample size for this experiment is small, and there is a non-significant trend towards a reduction in the insemination success of manipulated males. Nevertheless, this confirms that males can successfully transfer sperm after experimental manipulation, at least when the processus has been shortened by around 1 mm. There was also no significant difference in the fertilization success of manipulated males compared with sham males (F1,15 = 1.14, p = 0.3; figure 4b).
Figure 4.
The influence of experimental reduction in processus length on male reproductive success in experiment 2. The male processus was either shortened by 1 mm (n = 13) or manipulated but not cut (sham, n = 12). Males and females were kept together for two weeks, after which we recorded (a) insemination success (whether a pair produced offspring or not) and (b) fertilization success (the number of offspring produced).
(iii). Experiment 3
The proportion of males that mated was not significantly influenced by experimental treatment (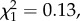 p = 0.94) or male body length (
p = 0.94) or male body length (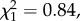 p = 0.36). Copulation duration was also not significantly influenced by experimental treatment (Kruskal–Wallis test, H2 = 0.54, p = 0.76; figure 5a). However, larger males copulated for longer (Spearman's rank correlation, rs = 0.18, d.f. = 1, p = 0.026). Insemination success was not significantly influenced by experimental treatment (
p = 0.36). Copulation duration was also not significantly influenced by experimental treatment (Kruskal–Wallis test, H2 = 0.54, p = 0.76; figure 5a). However, larger males copulated for longer (Spearman's rank correlation, rs = 0.18, d.f. = 1, p = 0.026). Insemination success was not significantly influenced by experimental treatment (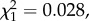 p = 0.99; figure 5b), though matings with larger males were more likely to result in insemination (
p = 0.99; figure 5b), though matings with larger males were more likely to result in insemination (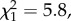 p = 0.016). Among the males that produced offspring, there is a positive relationship between processus length and insemination success for males that had 0.4 mm of processus removed (
p = 0.016). Among the males that produced offspring, there is a positive relationship between processus length and insemination success for males that had 0.4 mm of processus removed (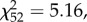 p = 0.023; figure 6), but no relationship for sham males (
p = 0.023; figure 6), but no relationship for sham males (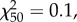 p = 0.75) or those that had just the tip removed (
p = 0.75) or those that had just the tip removed (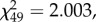 P = 0.16).
P = 0.16).
Figure 5.
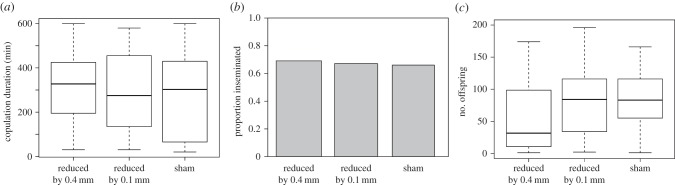
The influence of experimental reduction in processus length on male reproductive success in experiment 3. The male processus was shortened by 0.4 mm (n = 56) or 0.1 mm (n = 54), or manipulated but not cut (sham, n = 55). Following a single mating, three measures of reproductive success were recorded: (a) copulation duration, (b) insemination success (whether a mating resulted in offspring or not) and (c) fertilization success (the number of offspring produced).
Figure 6.
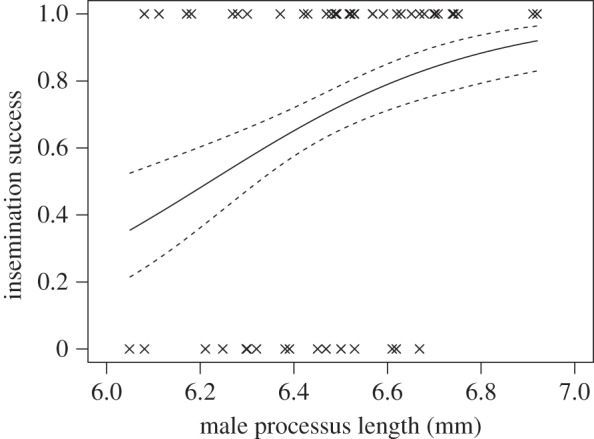
Relationship between male processus length and likelihood of insemination in experiment 3, for mated males with 0.4 mm removed (n = 52). Dashed lines indicate 1 s.e. above and below the predicted curve.
Fertilization success was not influenced by male body length (F1,98 = 1.89, p = 0.17), but was significantly influenced by the experimental treatment (F2,98 = 4.59, p = 0.012; figure 5c). Post hoc tests show that removal of the tip did not influence the number of offspring produced compared with sham males (t65 = 0.35, p = 0.94; figure 5c); however, females mated to males with a processus shortened by 0.4 mm had significantly fewer offspring compared with both sham males (t68 = 2.4, p = 0.046) and those with just the tip removed (t68 = 2.76, p = 0.019).
4. Discussion
We use two approaches to investigate the functional morphology of the male processus in L. simulans. We first use micro-CT to produce high-resolution virtual dissections of male and female reproductive anatomy in copula. Our results show that it is possible to distinguish between soft (non-sclerotized) structures even of small invertebrates; for example, from the scans we were able to resolve structures less than 10 µm long. This method may be especially useful when coupling with flash-freezing to investigate the positioning of genitalia at different stages of copulation, and also to determine the normal shape of internal structures (such as the female bursa). This has traditionally been investigated using serial sections; however, micro-CT has the advantage of not requiring the destruction of samples. Our results confirm that this technique is an excellent tool for the non-destructive visualization of internal reproductive morphology, including the interaction between male and female genitalia in copula.
Experimental reduction in processus length confirms that males with shorter processi have reduced insemination and fertilization success in a non-competitive context. Furthermore, the effect that manipulation has on male reproductive success depends on which proxy measure of success we use: if we remove 0.5% of the total processus length (which is within the natural phenotypic range), we cannot detect a significant reduction in insemination success, but we can detect a reduction in the number of eggs fertilized (experiment 3). By contrast, reduction of the processus by 29% (which is far outside the natural phenotypic range) leads to a significant reduction in copulation duration, insemination success and the number of offspring produced (experiment 3).
Across all three manipulation experiments, the manipulation of processus length had no effect on the proportion of males seen mating, or male mating frequency. By removing only the tip of the processus in experiment 3, we also show that the experimental ablation itself does not influence post-copulatory reproductive success. This result, and the fact that processus morphology is the same over the region manipulated here, suggests that the reduction in reproductive success seen in experiments 1 and 2 is not due to injury caused by cutting, but rather a direct result of the reduction in processus length. Additionally, in experiment 2, we show that insemination success when the processus is reduced by around 15% (which is still outside the natural phenotypic range) is comparable with that from a non-manipulated processus, when males were allowed to mate multiple times. However, it is not clear if males mated significantly more often following this manipulation.
Interestingly, the relationship between processus length and insemination success is positive and linear following reduction by 0.4 mm (figure 6), in contrast to the stabilizing selection found in previous studies [33]. This demonstrates how directional selection may act strongly following perturbation to return processus length to its optimum. We note that we were unable to detect stabilizing selection on processus length for the sham males in experiment 3; however, this is likely to be because the sample size was insufficient to be able to detect the much weaker quadratic selection gradient.
Studies on the functional morphology of genitalia are lacking in general [23], and an experimental approach such as this is rarely taken, probably due to the perceived difficulties of manipulating traits while maintaining function. However, we demonstrate that this approach may be fruitful in some cases, though probably only when targeting sclerotized structures that do not cause damage to subjects. Despite this, the exact mechanisms through which processus length increases sperm transfer success remain unclear. The simplest possibility is that successful insemination could only occur if sperm are released in the distal region of the spermathecal duct, after passing the valve at the entrance to the spermatheca, through which sperm seem unable to pass [34,40]. However, it should be noted that the female spermathecal duct is approximately 1.9 mm long [41], which is considerably shorter than even the shortest processus length [33], and it can be seen from figure 1 that a large proportion of the processus remains in the female bursa during sperm transfer. This suggests that mechanical considerations are more likely. For example, processi that are much shorter or longer than average may be harder to manoeuvre into the entrance to the spermathecal duct if the number of coils the processus makes within the female bursa is important for positioning of the tip [34].
Alternatively, we cannot rule out mechanisms of cryptic female choice that might prevent successful insemination by the male. For example, the valve at the entrance to the spermatheca may give some degree of control to the female over the amount of sperm stored [34]. This might be likely in a species such as L. simulans, where males can overcome female resistance to mating and seem able to extend copulation duration as a form of mate-guarding [25], and may also explain the observed high frequency of insemination failures [33,36]. However, active choice would require that the female is able to assess the size of the male processus during copula (independent of other male traits), which has not yet been shown.
In conclusion, we confirm that male processus length significantly influences insemination and fertilization success in L. simulans, by experimentally reducing processus length while keeping the sperm transfer ability intact. Further, we show that the greater the reduction in processus length, the greater the reduction in male reproductive success. We suggest that recent criticisms regarding genital ablation can be overcome if traits can be manipulated in such a way as to maintain reproductive function. This is probably not plausible for the majority of taxa, and for this reason L. simulans may prove to be a useful model system for the study of male genital evolution and sexual selection.
Supplementary Material
Acknowledgements
We thank Dan Sykes and Rebecca Summerfield at the London Natural History Museum for assistance with micro-CT and advice on staining, and Irvine Davidson for taking SEM images. Two anonymous referees generously provided many useful comments that greatly improved the manuscript.
Funding statement
This work was funded by the Natural Environment Research Council DTG PHD studentships to L.R.D., E.R.B.-S. and E.V.G., and an 1851 Royal Commission Research Fellowship to I.A.R.
Authors' contributions
L.R.D. conceived of the study, designed the study, performed all experiments and statistical analysis, and drafted the manuscript. I.A.R. arranged for and supervised the micro-CT scans, produced all scan reconstructions and helped draft the manuscript. E.R.B.-S. and E.V.G. helped in the preparation of animals for micro-CT scanning. D.M.S. conceived of the study, supervised the study and helped draft the manuscript. All authors gave final approval for publication.
Conflict of interests
We declare that we have no competing interests.
References
- 1.Eberhard W. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Eberhard WG. 1985. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Arnqvist G. 1997. The evolution of animal genitalia: distinguishing between hypotheses by single species studies. Biol. J. Linn. Soc. 60, 365–379. ( 10.1111/j.1095-8312.1997.tb01501.x) [DOI] [Google Scholar]
- 4.Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93. ( 10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- 5.Arnqvist G. 1998. Comparative evidence for the evolution of genitalia by sexual selection. Nature 393, 784–786. ( 10.1038/31689) [DOI] [Google Scholar]
- 6.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Simmons LW. 2014. Sexual selection and genital evolution. Austral Entomol. 53, 1–17. ( 10.1111/aen.12053) [DOI] [Google Scholar]
- 8.Ah-King M, Barron AB, Herberstein ME. 2014. Genital evolution: why are females still understudied? PLoS Biol. 12, e1001851 ( 10.1371/journal.pbio.1001851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotzy C, Polak M, Rönn JL, Arnqvist G. 2012. Phenotypic engineering unveils the function of genital morphology. Curr. Biol. 22, 2258–2261. ( 10.1016/j.cub.2012.10.009) [DOI] [PubMed] [Google Scholar]
- 10.Grieshop K, Polak M. 2012. The precopulatory function of male genital spines in Drosophila ananassae [Doleschall] (Diptera: Drosophilidae) revealed by laser surgery. Evolution 66, 2637–2645. ( 10.1111/j.1558-5646.2012.01638.x) [DOI] [PubMed] [Google Scholar]
- 11.Briceño R, Eberhard W. 2009. Experimental modifications imply a stimulatory function for male tsetse fly genitalia, supporting cryptic female choice theory. J. Evol. Biol. 22, 1516–1525. ( 10.1111/j.1420-9101.2009.01761.x) [DOI] [PubMed] [Google Scholar]
- 12.Moreno-García M, Cordero C. 2008. On the function of male genital claspers in Stenomacra marginella (Heteroptera: Largidae). J. Ethol. 26, 255–260. ( 10.1007/s10164-007-0058-8) [DOI] [Google Scholar]
- 13.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Kahn AT, Mautz B, Jennions MD. 2009. Females prefer to associate with males with longer intromittent organs in mosquitofish. Biol. Lett. 6, 55–58. ( 10.1098/rsbl.2009.0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez V. 1995. Relation of flagellum length to reproductive success in male Chelymorpha alternans Boheman (Coleoptera: Chrysomelidae: Cassidinae). Coleopterists’ Bull. 49, 201–205. [Google Scholar]
- 16.Rodriguez V, Windsor D, Eberhard W. 2004. Tortoise beetle genitalia and demonstrations of a sexually selected advantage for flagellum length in Chelymorpha alternans (Chrysomelidae, Cassidini, Stolaini). In New developments in the biology of Chrysomelidae (eds Jolivet P, Santiago-Blay JA, Schmitt M.), pp. 739–748. The Hague, The Netherlands: SPB Academic Publishing. [Google Scholar]
- 17.Cunningham JA, Rahman IA, Lautenschlager S, Rayfield EJ, Donoghue PC. 2014. A virtual world of paleontology. Trends Ecol. Evol. 29, 347–357. ( 10.1016/j.tree.2014.04.004) [DOI] [PubMed] [Google Scholar]
- 18.Sutton M, Rahman I, Garwood R. 2014. Techniques for virtual palaeontology. Chichester, UK: John Wiley and Sons. [Google Scholar]
- 19.Ziegler A, Ogurreck M, Steinke T, Beckmann F, Prohaska S, Ziegler A. 2010. Opportunities and challenges for digital morphology. Biol. Direct 5, 45 ( 10.1186/1745-6150-5-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metscher BD. 2009. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 9, 11 ( 10.1186/1472-6793-9-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonsen TJ, Kitching IJ. 2014. Virtual dissections through micro-CT scanning: a method for non-destructive genitalia ‘dissections’ of valuable Lepidoptera material. Syst. Entomol. 39, 606–618. ( 10.1111/syen.12067) [DOI] [Google Scholar]
- 22.Matsumura Y, et al. 2014. Two intromittent organs in Zorotypus caudelli (Insecta, Zoraptera): the paradoxical coexistence of an extremely long tube and a large spermatophore. Biol. J. Linn. Soc. 112, 40–54. ( 10.1111/bij.12260) [DOI] [Google Scholar]
- 23.Wojcieszek J, Austin P, Harvey M, Simmons L. 2012. Micro-CT scanning provides insight into the functional morphology of millipede genitalia. J. Zool. 287, 91–95. ( 10.1111/j.1469-7998.2011.00892.x) [DOI] [Google Scholar]
- 24.Ludwig W. 1926. Untersuchungen über den Copulationsapparat der Baumwanzen. Zoomorphology 5, 291–380. [Google Scholar]
- 25.Sillén-Tullberg B. 1981. Prolonged copulation: a male ‘postcopulatory’ strategy in a promiscuous species, Lygaeus equestris (Heteroptera: Lygaeidae). Behav. Ecol. Sociobiol. 9, 283–289. ( 10.1007/BF00299884) [DOI] [Google Scholar]
- 26.Bonhag PF, Wick JR. 1953. The functional anatomy of the male and female reproductive systems of the milkweed bug, Oncopeltus fasciatus (Dallas) (Heteroptera: Lygaeidae). J. Morphol. 93, 177–283. ( 10.1002/jmor.1050930202) [DOI] [Google Scholar]
- 27.Deckert J. 1990. Zum Bau von Parameren, Phallus und Pygophore der Lygaeinae und Bemerkungen zur Systematik der Unterfamilie (Heteroptera, Lygaeidae). Mitteilungen aus dem Museum für Naturkunde in Berlin. Zoologisches Museum und Institut für Spezielle Zoologie (Berlin) 66, 91–119. ( 10.1002/mmnz.19900660112) [DOI] [Google Scholar]
- 28.Rodriguez R. 1998. Possible female choice during copulation in Ozophora baranowskii (Heteroptera: Lygaeidae): female behavior, multiple copulations, and sperm transfer. J. Insect Behav. 11, 725–741. ( 10.1023/A:1022303010790) [DOI] [Google Scholar]
- 29.Gack C, Peschke K. 2005. ‘Shouldering'exaggerated genitalia: a unique behavioural adaptation for the retraction of the elongate intromittent organ by the male rove beetle (Aleochara tristis Gravenhorst). Biol. J. Linn. Soc. 84, 307–312. ( 10.1111/j.1095-8312.2005.00432.x) [DOI] [Google Scholar]
- 30.Matsumura Y, Akimoto S-I. 2009. Mating behavior and genital damage during copulation in the leaf beetle Lema coronata (Chrysomelidae: Criocerinae). Entomol. Sci. 12, 215–217. ( 10.1111/j.1479-8298.2009.00315.x) [DOI] [Google Scholar]
- 31.van Lieshout E, Elgar MA. 2011. Longer exaggerated male genitalia confer defensive sperm-competitive benefits in an earwig. Evol. Ecol. 25, 351–362. ( 10.1007/s10682-010-9422-1) [DOI] [Google Scholar]
- 32.Aspöck U, Aspöck H. 2008. Phylogenetic relevance of the genital sclerites of Neuropterida (Insecta: Holometabola). Syst. Entomol. 33, 97–127. ( 10.1111/j.1365-3113.2007.00396.x) [DOI] [Google Scholar]
- 33.Tadler A. 1999. Selection of a conspicuous male genitalic trait in the seedbug Lygaeus simulans. Proc. R. Soc. Lond. B 266, 1773–1777. ( 10.1098/rspb.1999.0845). [DOI] [Google Scholar]
- 34.Micholitsch T, Krugel P, Pass G. 2000. Insemination and fertilization in the seed bug Lygaeus simulans (Heteroptera: Lygaeidae). Eur. J. Entomol. 97, 13–18. ( 10.14411/eje.2000.003) [DOI] [Google Scholar]
- 35.Sutton MD, Garwood RJ, Siveter DJ, Siveter DJ. 2012. SPIERS and VAXML; A software toolkit for tomographic visualisation and a format for virtual specimen interchange. Palaeontol. Electron. 15, 1–14. [Google Scholar]
- 36.Dougherty LR, Shuker DM. 2014. Pre-copulatory sexual selection in the seed bug Lygaeus equestris: a comparison of choice and no-choice paradigms. Anim. Behav. 89, 207–214. ( 10.1016/j.anbehav.2014.01.005) [DOI] [Google Scholar]
- 37.Higgins SL, Hosken DJ, Wedell N. 2009. Phenotypic and genetic variation in male genitalia in the seedbug, Lygaeus equestris (Heteroptera). Biol. J. Linn. Soc. 98, 400–405. ( 10.1111/j.1095-8312.2009.01292.x) [DOI] [Google Scholar]
- 38.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 39.Schluter D. 1988. Estimating the form of natural selection on a quantitative trait. Evolution 42, 849–861. ( 10.2307/2408904) [DOI] [PubMed] [Google Scholar]
- 40.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 41.Gschwentner R, Tadler A. 2000. Functional anatomy of the spermatheca and its duct in the seed bug Lygaeus simulans (Heteroptera: Lygaeidae). Eur. J. Entomol. 97, 305–312. ( 10.14411/eje.2000.047) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.