Abstract
We investigated whether hearing advertisement calls over several nights, as happens in natural frog choruses, modified the responses of the peripheral auditory system in the green treefrog, Hyla cinerea. Using auditory evoked potentials (AEP), we found that exposure to 10 nights of a simulated male chorus lowered auditory thresholds in males and females, while exposure to random tones had no effect in males, but did result in lower thresholds in females. The threshold change was larger at the lower frequencies stimulating the amphibian papilla than at higher frequencies stimulating the basilar papilla. Suprathreshold responses to tonal stimuli were assessed for two peaks in the AEP recordings. For the peak P1 (assessed for 0.8–1.25 kHz), peak amplitude increased following chorus exposure. For peak P2 (assessed for 2–4 kHz), peak amplitude decreased at frequencies between 2.5 and 4.0 kHz, but remained unaltered at 2.0 kHz. Our results show for the first time, to our knowledge, that hearing dynamic social stimuli, like frog choruses, can alter the responses of the auditory periphery in a way that could enhance the detection of and response to conspecific acoustic communication signals.
Keywords: plasticity, Hyla cinerea, hearing, audition, lek, social signals
1. Introduction
Acoustic communication is an integral part of social behaviour in a wide variety of vertebrates [1]. Individuals are often exposed to a complex, variable mixture of conspecific calls over a long period of time during a breeding season, especially when males congregate in leks or other types of communal display, breeding or nesting assemblies [2]. Anuran amphibians (frogs and toads) are prime examples of this. Many anuran species form a breeding chorus during their reproductive season in which many males produce their advertisement calls nightly [3]. Such calls attract females to the chorus and serve as mate choice criteria while also regulating spacing and protecting call sites from other males [3–5].
Much of the research on the sensory effects of hearing repeated acoustic communication signals has focused on habituation to a regularly repeated or otherwise familiar and predictable signal [6–8]. The neural, perceptual and behavioural diminution of responses in such circumstances has important consequences for natural animal behaviour, from reducing costly behaviours, such as predator avoidance, to non-threatening stimuli [9,10], to maintaining established territorial boundaries with familiar neighbours without overt aggression [6,11]. The effects of participating in a social group and hearing a dynamic, unpredictable assembly of varying acoustic signals are less well understood. However, there is evidence that regular experience with acoustic social signals can enhance, rather than diminish, responses to new acoustic signals at midbrain or telencephalic levels of the central auditory system. Exposure to a particular song type for one week enhanced telencephalic responses to novel exemplars of that type in European starlings (Sturnus vulgaris) [12,13]. Similarly, we recently found that green treefrogs (Hyla cinerea) that heard a simulated chorus nightly for 10 days had significantly increased immediate early gene responses to novel conspecific advertisement calls in the auditory midbrain [14].
Although often considered a relatively stable component of the auditory system, there is evidence that the peripheral auditory (ear and auditory nerve) responses can change in threshold or other neural response measures across reproductive state or following experimental manipulation of hormonal levels [15–17]. In the current study, we ask whether social experience in the form of exposure to conspecific vocal signals over several days could modify the auditory periphery's response to novel calls or other acoustic stimuli. We examined this by measuring auditory evoked potentials (AEPs) in the green treefrog (H. cinerea), a species with a well-described auditory system [18–21]. The H. cinerea advertisement call is a short, pulsatile, broad band signal with spectral peaks around 900 and 3000 Hz [22–25]. As in other amphibians, treefrogs have two main auditory organs in the inner ear, the amphibian papilla (AP) and basilar papilla (BP). They are considered matched filters which are tuned to the low- and high-frequency peaks in the advertisement calls, respectively, and their response and filtering properties are important in guiding male and female behavioural responses to the advertisement call. Green treefrogs have a prolonged summer breeding season [3,26,27]. Males form choruses in which they produce advertisement calls for many hours each night during the summer breeding season. An individual in or near a breeding chorus may therefore be exposed to many consecutive nights of acoustic social stimulation in a complex acoustic environment where the number and identity of calling males varies, and individuals produce calls that differ spectrally and temporally, vary in number and repetition rate, and change in their degree of overlap with other calling males. We previously found that hearing conspecific calls for 10 consecutive nights resulted in enhanced immediately early gene expression to novel calls in the auditory midbrain [14]. Our results here show that social stimulation is sufficient to alter the electrophysiological response properties of the peripheral auditory system in a way that could enhance reproduction through increased detection of and response to conspecific advertisement calls. Although previous work in other vertebrates shows that peripheral auditory system responses can vary seasonally [15,16], our results show for the first time to our knowledge, that social experience can also have this effect.
2. Material and methods
(a). Housing
The animals used in this experiment were acquired through a commercial vendor (Charles D. Sullivan Co. Inc.). We tested a total of 23 (seven males; 16 females) green treefrogs in late May through to July, 2014. Prior to our experiments animals were housed in single-sex 10 gallon aquariums, with four individuals per aquarium. During the experiment, animals were housed singly in custom-built acoustically isolated chambers equipped with a speaker. Both the aquarium and the experimental chambers contained a water dish, rock and artificial vegetation. Animals were fed gut-loaded crickets twice weekly and maintained on a 14 L : 10 D light cycle.
(b). General experimental procedure
We used a repeated measures design to assess the effects of social stimulus exposure on the frequency sensitivity of the green treefrog auditory periphery. On day 1, we used AEPs to determine the baseline frequency sensitivity of the auditory periphery. Animals were then transferred to an acoustic chamber, where they remained in silence for the remainder of day 1. On day 2, we began 10 consecutive nights of social stimulus or control exposure, in which the animals were exposed to 6 h of either green treefrog chorus recordings (four males, seven females) or random tone recordings (three males, nine females) presented during the dark-phase of the light cycle, as would occur in a natural chorus. The loudest portion of the recording was set to 80 dB in each chamber using a Larson Davis LxT sound level meter (C weighting, slow integration). The recordings we used were 30 min in duration, and variable in the number of animals vocalizing at a single time and included natural breakpoints that would be found in a natural chorus. The random tones were matched in duration, timing and overall amplitude envelope (figure 1). Additionally, the tones had a frequency distribution that was within the hearing range of the frogs and had an overall power spectrum that was very similar to that of the chorus stimulus. On day 12, the animals remained in the chambers but we discontinued the playbacks to minimize habituation effects. On day 13, we repeated our AEP measurements on the animals.
Figure 1.
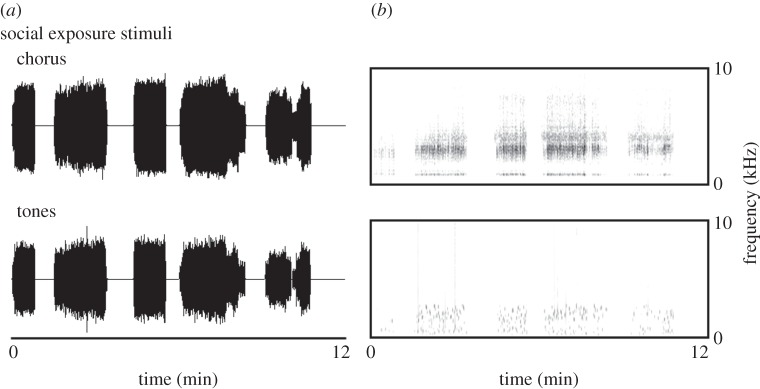
Examples of stimuli used for the nightly sound exposure during a 10 day period. (a) The waveforms of the stimuli and (b) the spectrograms of the stimuli. The tones had the same repetition rate, duration and amplitude envelope as the frog chorus.
(c). Auditory evoked potential experiments
All experiments were conducted in an IAC audiology booth using a Tucker Davis Technologies System 3 (Tucker Davis Technologies, Alachua, FL, USA). Stimuli were generated in SigGenRZ and consisted of 5 ms tone bursts that ranged in frequency from 0.4 to 5 kHz in third octave steps and ranged in intensity from 25 to 90 dB in 5 dB steps. AEPs were recorded from frogs that had been immobilized with an average (±s.d.) of 6.4 ± 1.3 µg g−1 tubocurarine hydrochloride pentahydrate (Sigma Aldrich). Responses were conducted from the subject to the System 3 with needle electrodes that were placed at apex of the subject's head and in either auditory meatus directly below the tympanum. Additional experimental detail can be found in the electronic supplementary material.
AEPs were analysed offline in Praat v. 5.3.55 [28]. First, we determined the auditory thresholds using the visual detection method. In this method, a trained observer determines the lowest stimulus intensity at which a response can be observed at a given frequency, using the gross morphology of the AEP waveform. The threshold is then estimated as lying halfway between this stimulus intensity and the next lowest intensity (in our case, 2.5 dB below the stimulus intensity of the last detectable response). This method consistently produces evoked potential thresholds that provide the greatest agreement with thresholds estimated with single unit or behavioural estimates [17,21,29].
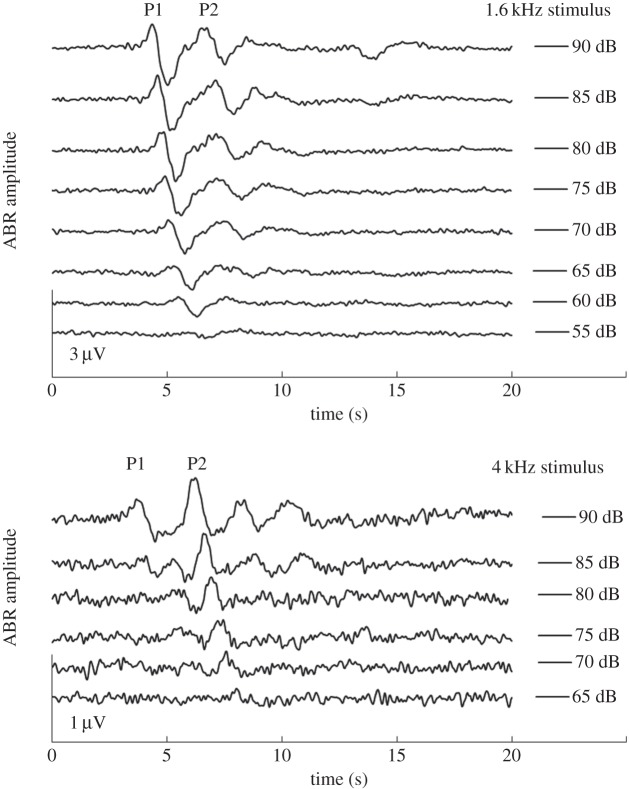
We also measured the amplitude of the AEP. Specifically, we measured the auditory brainstem response, a type of AEP which is generated by the auditory periphery, occurs in response to the onset of sound and is seen within 10 ms of the arrival of the stimulus at the ear. The green treefrog response consists of two peaks, P1 and P2 [21]. We found that P1 was only consistently measurable from frequencies of 0.8 to 1.6 kHz (figure 2). At low frequencies (0.4–0.63 kHz) and higher frequencies (2–5 kHz), we could reliably identify P2 but could not reliably identify P1 (figure 2). It has recently been suggested that these differences in the appearance of the evoked potential may be related to the three populations of afferent fibres [21,29]. Two populations of fibres, one at low and one at middle frequencies originate from the amphibian papilla, while a third population tuned to higher frequencies originates from the basilar papilla [18,19,21]. Therefore, we chose to analyse amplitude for P1 when the stimulus frequency was between 0.8 and 1.25 kHz, which are also the lower frequencies found in green treefrog calls [22–24]. For frequencies between 2 and 4 kHz, a range which includes the higher frequencies found in green treefrog calls, we choose to analyse P2. To determine amplitude, we measured the voltage difference between the positive peak and the subsequent negative peak of either P1 or P2.
Figure 2.
Example traces of AEPs to a low-frequency (1.6 kHz) and high-frequency (4 kHz) stimulus at several stimulus intensities. The amplitude of P1 was measured from the vertex of the first positive peak (marked P1) to the subsequent negative trough. P2 amplitude was measured from the vertex of the second positive peak to the subsequent negative trough.
(d). Statistical analyses
We analysed our data with repeated measures mixed models in SAS v. 9.3, with the identity of each frog as the subject. We had three models, one for the dependent variable auditory thresholds and one model each for the amplitude of P1 and P2. Independent variables in the auditory threshold model included the within-subject factors of frequency and time (before and after exposure), the between subject factor stimulus type (chorus or tones) and their interactions. Independent variables in the AEP amplitude models included the within-subject independent variables frequency, intensity and time (before or after exposure), and the between subject factor stimulus type (chorus or tones) and their interactions. We removed non-significant higher order interactions from the model according to p-value and the resulting Akaike information criteria value for the new model. We also included sex as a between subject factor in each model, to account for any variation that might be due to the sex of our subjects.
We modelled covariance with an autoregressive structure (AR(1)) and used the Kenward–Rogers algorithm to calculate degrees of freedom. Significant main effects and interaction terms were explored post-hoc with tests of simple effects or pairwise comparisons. Statistics were Bonferoni corrected for multiple comparisons as appropriate. AEP amplitude data were log transformed to achieve normality and homogeneity of variance. We therefore report back-transformed marginal means (±s.e.) throughout.
3. Results
(a). Auditory thresholds
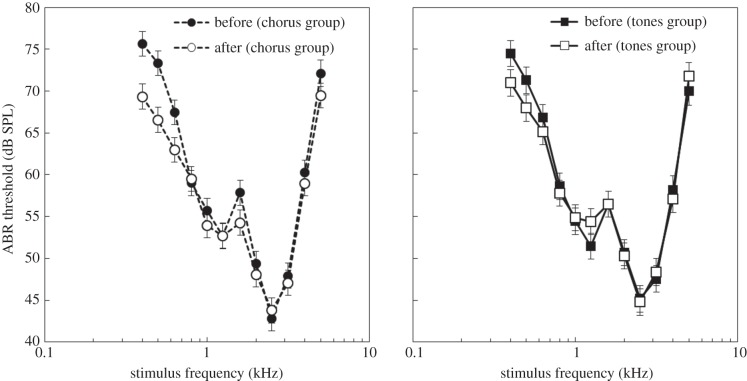
We found significant main effects of frequency (F11,323 = 99.3, p < 0.001) and time (before versus after: F1,216 = 10.4, p = 0.0015), but not sex (F1,132 = 0.1, p = 0.75) or stimulus (chorus versus tones: F1,131 = 0.09, p = 0.76) on auditory thresholds. As predicted, we found that auditory thresholds were significantly influenced by the stimulus × time interaction (figure 3; F1,215 = 4.8, p = 0.03). We also found a significant effect of the stimulus × sex (F1,132 = 4.9, p = 0.03), time × sex (F1,214 = 6.2, p = 0.01) and frequency × time (figure 3; F11,356 = 2.6, p = 0.003) interaction on auditory thresholds. Finally, we found a significant effect of the three-way interaction stimulus × time × sex (F1,209 = 4.1, p = 0.045) on auditory thresholds.
Figure 3.
Auditory thresholds of animals in the (a) chorus group and (b) tone groups before and after 10 days of nightly stimulus exposure. Thresholds (±s.e.) are plotted as a function of frequency.
When the sexes were combined we found that individuals in the chorus group (figure 3; t212 = 4.4, p < 0.0001), but not the tone group (t217 = 0.52, p = 0.91) had a significant decrease in their auditory thresholds. The average thresholds of the chorus group and tones group did not differ prior to exposure (t326 = 1.02, p = 0.74). The three-way interaction stimulus × time × sex was driven by differences between male and female animals in their response to chorus and tone exposure. Female thresholds decreased in both the chorus (F1,209 = 15.8, p < 0.0001), and the tone playbacks conditions (F1,213 = 19.3, p < 0.0001), while male thresholds were reduced in response to chorus exposure (F1,211 = 6.2, p = 0.014), but not tone exposure (F1,216 = 1.5, p = 0.23).
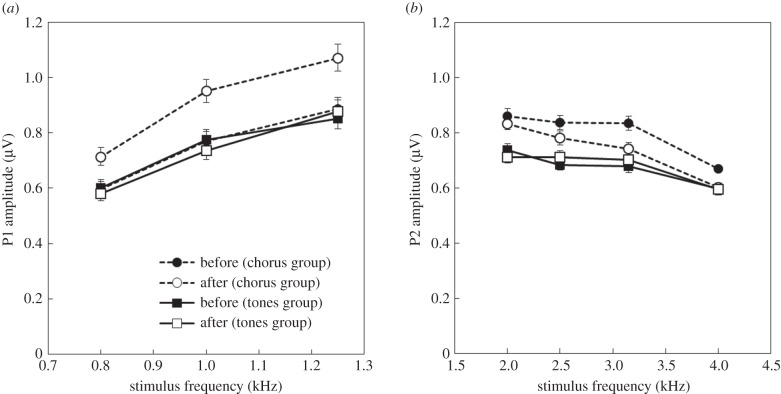
(b). P1 amplitude (0.8–1.25 kHz)
We found significant main effects of frequency (F2,252 = 52.8, p < 0.001), intensity (F5,923 = 95.6, p < 0.001), stimulus (F1,204 = 12.14, p < 0.001) and time (F1,1394 = 49.5, p < 0.001), but not sex (F1,255 = 0.73, p = 0.39) on the amplitude of P1. As predicted there was a significant stimulus × time interaction (figure 4a; F1,1375 = 66.5, p < 0.001); however, there were no other significant interactions (F < 1.24, p > 0.3). Prior to 10 days of nightly sound exposure, there was no difference between the chorus and tones group (t264 = 0.47, p = 0.6377); but a significant difference emerged after 10 days of nightly sound exposure (t260 = 6.07, p < 0.001). This appears to be due to plasticity in the chorus group, as their AEP amplitude increased significantly after 10 days (t1399 = 10.6, p < 0.001), while there was no change in the group exposed to tones (t1369 = 0.78, p = 0.87).
Figure 4.
(a) Amplitude (±s.e.) of peak one (P1) of the AEP as a function of stimulus, time, and frequency. The AEP amplitudes are average across intensity. (b) Amplitude (±s.e.) of peak two (P2) of the AEP as a function of stimulus, time and frequency. The difference in P2 amplitude between the two groups before exposure is probably due to the relative numbers of males and females in each group, as females had a higher average P2 amplitude.
(c). P2 amplitude (2–4 kHz)
We found significant main effects of sex (F1,300 = 80.7, p < 0.001), frequency (F3,364 = 28.1, p < 0.001), intensity (F5,1151 = 41.4, p < 0.001), stimulus (F1,300 = 33.1, p < 0.001) and time (F1,1842 = 16.4, p < 0.001) on the P2 amplitude. We also found significant effects of the interaction terms stimulus × time (F1,1818 = 24.9, p < 0.001) and frequency × stimulus × time (figure 4; F3,1837 = 3.1, p = 0.026) on the P2 amplitude.
Prior to exposure the two groups differed in average P2 amplitude, with the chorus exposure group having a higher overall amplitude than the tones group (t418 = 7.24, p < 0.001). The groups remained different after 10 days of exposure (t417 = 3.35, p = 0.005). There was plasticity in the chorus group (t1803 = 6.52, p < 0.001), but not in the tones group (t1803 = 0.6, p = 0.82). Counter to our expectations, this plasticity in the chorus group led to decreases in the P2 amplitude after 10 days of nightly sound exposure. The plasticity in the chorus group was frequency-specific. We found plasticity in the chorus group at 2.5 (F1,1865 = 7.36, p = 0.006), 3.15 (F1,1867 = 21.12, p < 0.001) and 4 kHz (F1,1909 = 17.34, p < 0.001), but not at 2 kHz (F1,1893 = 1.74, p = 0.18), while the tones group did not differ at any frequency (F < 2.55, p > 0.11).
Females had significantly greater P2 amplitudes than males (mean ± s.e. males = 0.65 ± 0.012 µV; females = 0.80 ± 0.01 µV). This is likely the reasons that the two groups differed in AEP amplitude prior to sound exposure, as females were represented in the chorus group to a greater extent than they were in the tones group.
4. Discussion
We found that nightly exposure to a dynamic conspecific stimulus resulted in sensitization of the auditory periphery in the green treefrog. Hearing a chorus playback for 10 nights led to lower peripheral auditory thresholds and enhanced P1 suprathreshold responses (measured in response to low frequencies), while exposure to 10 nights of random tones did not affect these responses. Surprisingly, we found that exposure to the chorus led to decreased P2 suprathrehold responses (measured in response to higher frequencies); while again, exposures to tones did not induce plasticity in the P2 response. The magnitude of change in P1 amplitude was much greater than the magnitude of the change in P2 amplitude, suggesting greater plasticity in P1. Additionally, in males, auditory thresholds were lower after exposure to the chorus exposure, but not the tones exposure. However, in females, auditory thresholds were lower after exposure to both the tones and the chorus exposure. Although socially induced plasticity has been shown in other areas of the auditory system, we believe this to be the first report of a social stimulus inducing plasticity in peripheral auditory responses.
(a). Implications for acoustic communication
Changes in peripheral auditory properties are especially important in shaping an individual's response to social signals. Peripheral sense organs are gatekeepers, determining to a large extent what information is passed on to the central nervous system, and at what level relative to other stimuli, and thus greatly impact the responses of neurons throughout the central auditory system and beyond. A change in threshold can potentially increase the salience of a perceived signal or the distance over which it can be detected. Detection should be primarily a function of the threshold level of the auditory system, with detection ability increasing as threshold is lowered. However, the salience of a stimulus may be more influenced by the perceived amplitude of the component parts of the stimulus. For example, Gerhardt [23,24] found that the relative amplitude of high- and low-frequency peaks in the male advertisement call affected female treefrog responses to the call. Thus, differentially up- and downregulating the suprathreshold response of the ear to different parts of the advertisement calls could result in different populations of neurons downstream of the periphery being activated in response to call reception, thereby modulating the salience of a conspecific stimulus by changing the gating of these signals to downstream processing areas. Depending on the exact contour of the resultant frequency-dependent response, it could also change the signal-to-noise ratio of the stimuli of interest. Our results show that both phenomena occur when treefrogs listen to an assembly of species typical advertisement calls over several nights, similar to their experience in a natural mating chorus. They become more sensitive to advertisement calls, and their suprathreshold responses are changed to accentuate the lower frequencies of the call.
(b). The anuran auditory periphery
Our results show that response changes following exposure to conspecific vocal signals occur in both of the two primary inner ear auditory end organs found in amphibians, the AP and BP [30–33]. The sensitivity range of the larger AP is relatively conserved across species. Its hair cells are tuned collectively to a range of low- and mid-frequencies with individual AP eighth nerve fibres showing characteristic frequencies ranging from about 100 to 1200 Hz. The P1 peak in our recordings most probably reflects AP responses [21]. Tuning of the smaller BP varies greatly across anuran species ranging from a best frequency of 1500 Hz in large frogs to a best frequency of over 6000 Hz in small frogs. The H. cinerea BP is centred on average around 3000 Hz [18,25]. The P2 peak in our recordings probably arose from BP responses [21]. When sex differences have been found in anuran auditory tunings, they have been found in the BP rather than the AP [34–37]. We found one here as well in that the P2 peak amplitude was significantly greater in females than in males.
The AP and BP are thought to differ in the mechanism of hair cell tuning. AP tuning is based on a travelling wave in the tectorial membrane sharpened through electrical tuning in the hair cells [31,33,38,39]. AP tuning may be more malleable, in general, owing to the physiological component of its tuning. For example, AP thresholds, but not BP thresholds, are susceptible to degradation by anoxia and antibiotics, and manifest tuning shifts with temperature changes [31,40]. Our results in fact indicate greater plasticity at frequencies associated with the AP (P1 response) than at frequencies associated with the BP (P2 response). Lower thresholds were more prominent in the AP frequency range as indicated by a frequency × time interaction based on a greater difference (lower after chorus exposure) in threshold values below 2 kHz, and a concomitant increase in P1 amplitude to suprathreshold stimuli between 0.8 and 1.25 kHz. It is important to note, however, that changes also occurred at BP frequencies, as shown by the chorus-induced changes in P2 amplitude at higher frequencies.
(c). Mechanisms of auditory plasticity
Modulation of the electrical tuning properties of the hair cells is an attractive mechanism for the changes we observed, particularly for changes in P1 amplitude and low-frequency thresholds which are generated by the amphibian papilla. Electrical tuning of hair cells contributes (in varying degrees) to the spectral sensitivity of hair cells across most vertebrate taxa [41–44] including anurans [31,33,38]. These electrical tuning properties are derived, in part, from the differential expression of ion channel splice variants, particularly voltage-gated calcium and calcium-sensitive big potassium (BK) ion channels in the hair cells. The kinetic properties of different splice variants produce frequency-specific resonances in different hair cells [45,46]. Recent work in fishes suggests that seasonal changes in auditory sensitivity may be due in part to changes in BK channel expression [47,48]. In midshipman fish, these seasonal changes correlate with seasonal changes in sensitivity to frequencies found in mating vocalizations [48]. In mammals, oestrogen responsive elements are involved in the regulation of Slo transcription suggesting that differences in circulating hormone levels may lead to differential expression of BK channels [49,50]. This suggests that seasonally or socially induced changes in auditory sensitivity could be mediated by hormonally regulated expression of BK channels. It is less clear what mechanisms could be responsible for changes in P2 amplitude, as the basilar papilla is thought to have tuning that is primarily mechanically derived.
(d). Hormones and auditory plasticity
Changes in auditory thresholds, response strengths or other aspects of signal processing have been documented in many species as a function of reproductive state, season or experimentally manipulated steroid levels [16,51–62]. These have most often been assessed by measuring activity in the central nervous system. Studies in several species show, however, that gonadal steroids (or reproductive state) can also change peripheral responses to conspecific vocal signals [15,16,63–65]. Call stimulation has a significant effect on the level of circulating gonadal steroids of both male and female frogs, while stimulation by a non-salient tone stimulus has no effect [66–70]. Therefore, one model for the effects we observed is that hearing conspecific calls elevates circulating gonadal steroid levels, which then feedback on to both the central and peripheral auditory systems to lower thresholds and increase response amplitude to that same class of stimuli.
Although this is a simple and attractive hypothesis consistent with previous findings, it is important to note that a combination of events occurs during chronic stimulation by conspecific calls. While gonadal steroids are elevated so may be other hormones and brain neuromodulators sensitive to salient signals [71,72]. Moreover, both peripheral and central auditory neurons are repeatedly depolarized during the call stimulation; that in itself may induce neural plasticity leading to changes in excitation by call stimuli (for example of experience dependent changes in brainstem auditory processing, see [73–75]).
An optimal stimulus (i.e. one with a frequency profile that most closely matches the best frequencies of the periphery) may result in a greater experience dependent change in peripheral processing than a stimulus with a less optimal frequency profile. In the case of our stimuli, the more variable frequency profile of the tone stimuli may be less optimal than the more restricted frequency profile of the chorus stimulus. Therefore, the chorus stimulus could have a larger influence on peripheral sensitivity than the tone stimulus because of its optimal match to the frequency characteristics of the ear, rather than the behavioural salience of the stimulus. In fact, we did find that the tone stimulus was capable of altering thresholds in females (not in males), although it did not influence suprathreshold responses in either sex. Carefully controlled experiments are needed to dissect the mechanisms underlying the auditory changes caused by chronic call stimulation.
5. Conclusion
In summary, we found that experience with a social communication signal can modulate the peripheral auditory sensitivity of the green treefrog. We believe this is the first report of social stimulus exposure influencing the sensitivity of the peripheral auditory system in adult animals. The modulation of peripheral sensitivity could alter the central processing of social stimuli and thus change stimulus detectability and salience. Investigating the relationship between peripheral sensitivity and mate choice and/or aggressive behaviours would help elucidate the influence of peripheral tuning modulation on stimulus salience. The mechanisms underlying this phenomenon are currently unknown, however previous work in a variety of taxa suggest that gonadal steroid hormone regulation of electrical tuning is a promising area for investigation.
Supplementary Material
Supplementary Material
Ethics statement
All experiments conformed to the Animal Behaviour Society Guidelines for the Use of Animals in Research and were approved by the Georgia State University Institutional Animal Care and Use Committee (protocol A12036).
Funding statement
This work was supported by the National Science Foundation (IBN 0751573 to W.W.) and funding from Vassar College and the Georgia State University Neuroscience Institute.
Author contributions
M.D.G. and W.W. jointly conceived of the project and contributed equally to the writing of the manuscript; M.D.G. designed the AEP experiments, and collected and analysed the AEP data; W.W. designed the social stimulus exposure paradigm. All authors gave final approval for publication.
References
- 1.Bradbury JW, Vehrenkamp SL. 2011. Principles of animal communication, 3rd edn New York, NY: Sinaur Associates Inc. [Google Scholar]
- 2.Höglund J, Alatalo RV. 1995. Leks. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Wells KD. 1977. The social behaviour of anuran amphibians. Anim. Behav. 25, 666–693. ( 10.1016/0003-3472(77)90118-X) [DOI] [Google Scholar]
- 4.Brenowitz EA, Rose GJ, Adler T. 2001. The neuroethology of acoustic communicatin in Pacific treefrogs. In Anuran communication (ed. Ryan MJ.), pp. 145–155. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 5.Ryan MJ. 2009. Communication in frogs and toads. In Encyclopedia of neuroscience, vol. 2 (ed. Squire LR.), pp. 1159–1166. Oxford, UK: Academic Press. [Google Scholar]
- 6.Bee MA, Gerhardt HC. 2001. Habituation as a mechanism of reduced aggression between neighboring territorial male bullfrogs (Rana catesbeiana). J. Comp. Psychol. 115, 68–82. ( 10.1037/0735-7036.115.1.68) [DOI] [PubMed] [Google Scholar]
- 7.Dong S, Clayton DF. 2009. Habituation in song birds. Neurobiol. Learn. Mem. 92, 183–188. ( 10.1016/j.nlm.2008.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mello C, Nottebohm F, Clayton D. 1995. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J. Neurosci. 15, 6919–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Prieto I, Martín J, Fernández-Juricic E. 2011. Individual variation in behavioural plasticity: direct and indirect effects of boldness, exploration and sociability on habituation to predators in lizards. Proc. R. Soc. B 278, 266–273. ( 10.1098/rspb.2010.1194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raderschall CA, Magrath RD, Hemmi JM. 2011. Habituation under natural conditions: model predators are distinguished by approach direction. J. Exp. Biol. 214, 4209–4216. ( 10.1242/jeb.061614) [DOI] [PubMed] [Google Scholar]
- 11.Peeke HVS. 1984. Habituation and the maintenance of territorial boundaries. In Habituation, sensitization and behaviour (eds Peeke HVS, Petrinovich L.), pp. 393–421. London, UK: Academic Press. [Google Scholar]
- 12.Sockman KW, Gentner TQ, Ball GF. 2005. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J. Neurobiol. 62, 72–81. ( 10.1002/neu.20068) [DOI] [PubMed] [Google Scholar]
- 13.Sockman KW, Gentner TQ, Ball GF. 2002. Recent experience modulates forebrain gene–expression in response to mate–choice cues in European starlings. Proc. R. Soc. Lond. B 269, 2479–2485. ( 10.1098/rspb.2002.2180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gall MD, Wilczynski W. 2014. Prior experience with conspecific signals enhances auditory midbrain responsiveness to conspecific vocalizations. J. Exp. Biol. 217, 1977–1982. ( 10.1242/jeb.096883) [DOI] [PubMed] [Google Scholar]
- 15.Sisneros JA, Bass AH. 2003. Seasonal plasticity of peripheral auditory frequency sensitivity. J. Neurosci. 23, 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Cui J, Tang Y. 2012. Plasticity of peripheral auditory frequency sensitivity in Emei music frog. PLoS ONE 7, e45792 ( 10.1371/journal.pone.0045792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gall MD, Salameh TS, Lucas JR. 2013. Songbird frequency selectivity and temporal resolution vary with sex and season. Proc. R. Soc. B 280, 20122296 ( 10.1098/rspb.2012.2296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehret G, Capranica RR. 1980. Masking patterns and filter characteristics of auditory nerve fibers in the green treefrog (Hyla cinerea). J. Comp. Physiol. A 141, 1–12. ( 10.1007/BF00611872) [DOI] [Google Scholar]
- 19.Moss CF, Simmons AM. 1986. Frequency selectivity of hearing in the green treefrog Hyla cinerea. J. Comp. Physiol. A 159, 257–266. ( 10.1007/BF00612308) [DOI] [PubMed] [Google Scholar]
- 20.Klump GM, Benedix JH, Jr, Gerhardt HC, Narins PM. 2004. AM representation in green treefrog auditory nerve fibers: neuroethological implications for pattern recognition and sound localization. J. Comp. Physiol. A 190, 1011–1021. ( 10.1007/s00359-004-0558-8) [DOI] [PubMed] [Google Scholar]
- 21.Buerkle NP, Schrode KM, Bee MA. 2014. Assessing stimulus and subject influences on auditory evoked potentials and their relation to peripheral physiology in green treefrogs (Hyla cinerea). Comp. Biochem. Physiol. A 178, 68–81. ( 10.1016/j.cbpa.2014.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardt HC. 1981. Mating call recognition in the green treefrog (Hyla cinerea): importance of two frequency bands as a function of sound pressure level. J. Comp. Physiol. A 144, 9–16. ( 10.1007/BF00612792) [DOI] [Google Scholar]
- 23.Gerhardt HC. 1974. The significance of some spectral features in mating call recognition in the green treefrog (Hyla cinerea). J. Exp. Biol. 61, 229–241. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt HC. 1976. Significance of two frequency bands in long distance vocal communication in the green treefrog. Nature 261, 692–694. ( 10.1038/261692a0) [DOI] [Google Scholar]
- 25.Gerhardt HC, Schwartz JJ. 2001. Auditory tuning and frequency preferences in anurans. In Anuran communication (ed. Ryan MJ.), pp. 73–85. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 26.Garton JS, Brandon RA. 1975. Reproductive ecology of the green treefrog, Hyla cinerea, in southern Illinois (Anura: Hylidae). Herpetologica 31, 150–161. [Google Scholar]
- 27.Elliott L, Gerhardt HC, Davidson C. 2009. The frogs and toads of North America: a comprehensive guide to their identification, behavior and calls. Boston, MA: Mariner Books. [Google Scholar]
- 28.Boersma P, Weenink D. Praat: doing phonetics by computer, version 5.3.51 ed2013. See http://www.fon.hum.uva.nl/praat/.
- 29.Schrode K, Buerkle N, Brittan-Powell E, Bee M. 2014. Auditory brainstem responses in Cope's gray treefrog (Hyla chrysoscelis): effects of frequency, level, sex and size. J. Comp. Physiol. A 200, 221–238. ( 10.1007/s00359-014-0880-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilczynski W, Capranica RR. 1984. The auditory system of anuran amphibians. Progr. Neurobiol. 22, 1–38. ( 10.1016/0301-0082(84)90016-9) [DOI] [PubMed] [Google Scholar]
- 31.Zakon H, Wilczynski W. 1988. The physiology of the anuran eighth nerve. In The Evolution of the amphibian auditory system (eds Fritzsch B, Walkowiak W, Ryan R, Wilczynski W, Heatherington T.), pp. 125–155. New York, NY: Wiley. [Google Scholar]
- 32.Smotherman MS, Narins PM. 2000. Hair cells, hearing and hopping: a field guide to hair cell physiology in the frog. J. Exp. Biol. 203, 2237–2246. [DOI] [PubMed] [Google Scholar]
- 33.Simmons DD, Meenderink SWF, Vassilakis PN. 2007. Anatomy, physiology, and function of the auditory end-organs in the frog inner ear. In Hearing and sound communication in amphibians (eds Narins PM, Feng AS, Fay RR, Popper AN.), pp. 184–220. New York, NY: Springer. [Google Scholar]
- 34.Narins PM, Capranica RR. 1976. Sexual difference in the auditory system of the treefrog Eleutherodactylus coqui. Science 192, 378–380. ( 10.1126/science.1257772) [DOI] [PubMed] [Google Scholar]
- 35.Wilczynski W, Keddy-Hector AC, Ryan MJ. 1992. Call patterns and basilar papilla tuning in cricket frogs. I. Differences among populations and between sexes. Brain Behav. Evol. 39, 229–237. ( 10.1159/000114120) [DOI] [PubMed] [Google Scholar]
- 36.McClelland BE, Wilczynski W, Rand AS. 1997. Sexual dimorphism and species differences in the neurophysiology and morphology of the acoustic communication system of two neotropical hylids. J. Comp. Physiol. A 180, 451–462. ( 10.1007/s003590050062) [DOI] [PubMed] [Google Scholar]
- 37.Liu W-R, Shen J-X, Zhang Y-J, Xu Z-M, Qi Z, Xue M-Q. 2014. Auditory sexual difference in the large odorous frog Odorrana graminea. J. Comp. Physiol. A 200, 311–316. ( 10.1007/s00359-014-0885-3) [DOI] [PubMed] [Google Scholar]
- 38.Lewis ER, Lombard RE. 1988. The amphibian inner ear. In The evolution of the amphibian auditory system (eds Fritzsch B, Walkowiak W, Ryan MJ, Wilczynski W, Heatherington T.), pp. 93–123. New York, NY: Wiley. [Google Scholar]
- 39.Salvi RJ. 2008. Overview: regeneration and repair. In Hair cell, regeneration, repair, and protection (eds Salvi RJ, Popper AN, Fay RR.), pp. 1–38. New York, NY: Springer. [Google Scholar]
- 40.Narins PM. 2001. Ectothermy's last stand: hearing in the heat and cold. In Anuran communication (ed. Ryan MJ.), pp. 61–70. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 41.Ashmore JF. 1983. Frequency tuning in a frog vestibular organ. Nature 304, 536–538. ( 10.1038/304536a0) [DOI] [PubMed] [Google Scholar]
- 42.Fuchs P, Nagai T, Evans M. 1988. Electrical tuning in hair cells isolated from the chick cochlea. J. Neurosci. 8, 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugihara I, Furukawa T. 1989. Morphological and functional aspects of two different types of hair cells in the goldfish sacculus. J. Neurophysiol. 62, 1330–1343. [DOI] [PubMed] [Google Scholar]
- 44.Steinacker A, Romero A. 1992. Voltage-gated potassium current and resonance in the toadfish saccular hair cell. Brain Res. 574, 229–236. ( 10.1016/0006-8993(92)90821-P) [DOI] [PubMed] [Google Scholar]
- 45.Fettiplace R, Fuchs PA. 1999. Mechanisms of hair cell tuning. Annu. Rev. Physiol. 61, 809–834. ( 10.1146/annurev.physiol.61.1.809) [DOI] [PubMed] [Google Scholar]
- 46.Ramanathan K, Michael TH, Jiang G-J, Hiel H, Fuchs PA. 1999. A molecular mechanism for electrical tuning of cochlear hair cells. Science 283, 215–217. ( 10.1126/science.283.5399.215) [DOI] [PubMed] [Google Scholar]
- 47.Rohmann KN, Tripp JA, Genova RM, Bass AH. 2014. Manipulation of BK channel expression is sufficient to alter auditory hair cell thresholds in larval zebrafish. J. Exp. Biol. 217, 2531–2539. ( 10.1242/jeb.103093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohmann Kevin N, Fergus Daniel J, Bass Andrew H. 2013. Plasticity in ion channel expression underlies variation in hearing during reproductive cycles. Curr. Biol. 23, 678–683. ( 10.1016/j.cub.2013.03.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. 2005. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 579, 4856–4860. ( 10.1016/j.febslet.2005.07.069) [DOI] [PubMed] [Google Scholar]
- 50.Kundu P, Alioua A, Stefani E, Toro L. 2007. Regulation of mouse Slo gene expression: multiple promoters, transcription start sites, and genomic action of estrogen. J. Biol. Chem. 282, 27 478–27 492. ( 10.1074/jbc.M704777200) [DOI] [PubMed] [Google Scholar]
- 51.Wilczynski W, Lynch KS. 2011. Female sexual arousal in amphibians. Horm. Behav. 59, 630–636. ( 10.1016/j.yhbeh.2010.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoder KM, Vicario DS. 2012. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav. Neurosci. 126, 17–28. ( 10.1037/a0026673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yovanof S, Feng AS. 1983. Effects of estradiol on auditory evoked responses from the frog's auditory midbrain. Neurosci. Lett. 36, 291–297. ( 10.1016/0304-3940(83)90015-0) [DOI] [PubMed] [Google Scholar]
- 54.Penna M, Capranica R, Somers J. 1992. Hormone-induced vocal behavior and midbrain auditory sensitivity in the green treefrog, Hyla cinerea. J. Comp. Physiol. A 170, 73–82. ( 10.1007/BF00190402) [DOI] [PubMed] [Google Scholar]
- 55.Lucas J, Freeberg T, Krishnan A, Long G. 2002. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J. Comp. Physiol. A 188, 981–992. ( 10.1007/s00359-002-0359-x) [DOI] [PubMed] [Google Scholar]
- 56.Lucas J, Freeberg T, Long G, Krishnan A. 2007. Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J. Comp. Physiol. A 193, 201–215. ( 10.1007/s00359-006-0180-z) [DOI] [PubMed] [Google Scholar]
- 57.Goense JBM, Feng AS. 2005. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. J. Neurobiol. 65, 22–36. ( 10.1002/neu.20172) [DOI] [PubMed] [Google Scholar]
- 58.Lynch KS, Wilczynski W. 2008. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav. Evol. 71, 143–150. ( 10.1159/000111460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arch VS, Narins PM. 2009. Sexual hearing: the influence of sex hormones on acoustic communication in frogs. Hearing Res. 252, 15–20. ( 10.1016/j.heares.2009.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miranda JA, Wilczynski W. 2009. Sex differences and androgen influences on midbrain auditory thresholds in the green treefrog, Hyla cinerea. Hearing Res. 252, 79–88. ( 10.1016/j.heares.2009.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miranda JA, Wilczynski W. 2009. Female reproductive state influences the auditory midbrain response. J. Comp. Physiol. A 195, 341–349. ( 10.1007/s00359-008-0410-7) [DOI] [PubMed] [Google Scholar]
- 62.Maney DL, Pinaud R. 2011. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrin. 32, 287–302. ( 10.1016/j.yfrne.2010.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson SJ, Dengerink HA. 1988. Changes in pure-tone thresholds and temporary threshold shifts as a function of menstrual-cycle and oral contraceptives. J. Speech Hear. Res. 31, 569–574. [PubMed] [Google Scholar]
- 64.Sisneros JA, Forlano PM, Deitcher DL, Bass AH. 2004. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science 305, 404–407. ( 10.1126/science.1097218) [DOI] [PubMed] [Google Scholar]
- 65.Rohmann KN, Bass AH. 2011. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. J. Exp. Biol. 214, 1931–1942. ( 10.1242/jeb.054114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burmeister S, Wilczynski W. 2000. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea). Horm. Behav. 38, 201–209. ( 10.1006/hbeh.2000.1605) [DOI] [PubMed] [Google Scholar]
- 67.Chu J, Wilczynski W. 2001. Social influences on androgen levels in the southern leopard frog, Rana sphenocephala. Gen. Comp. Endocrinol. 121, 66–73. ( 10.1006/gcen.2000.7563) [DOI] [PubMed] [Google Scholar]
- 68.Lynch KS, Wilczynski W. 2006. Social regulation of plasma estradiol concentration in a female anuran. Horm. Behav. 50, 101–106. ( 10.1016/j.yhbeh.2006.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Bryant EL, Wilczynski W. 2010. Changes in plasma testosterone levels and brain AVT cell number during the breeding season in the green treefrog. Brain Behav. Evol. 75, 271–281. ( 10.1159/000316084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilczynski W, Lynch KS, O'Bryant EL. 2005. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm. Behav. 48, 440–450. ( 10.1016/j.yhbeh.2005.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leary CJ, Harris S. 2013. Steroid hormone levels in calling males and males practicing alternative non-calling mating tactics in the green treefrog, Hyla cinerea. Horm. Behav. 63, 20–24. ( 10.1016/j.yhbeh.2012.11.006) [DOI] [PubMed] [Google Scholar]
- 72.Moncalvo VR, Burmeister S, Pfennig K. 2013. Social signals increase monoamine levels in the tegmentum of juvenile Mexican spadefoot toads (Spea multiplicata). J. Comp. Physiol. A 199, 681–691. ( 10.1007/s00359-013-0826-6) [DOI] [PubMed] [Google Scholar]
- 73.de Boer J, Thornton ARD. 2008. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J. Neurosci. 28, 4929–4937. ( 10.1523/JNEUROSCI.0902-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tzounopoulos T, Kraus N. 2009. Learning to encode timing: mechanisms of plasticity in the auditory brainstem. Neuron 62, 463–469. ( 10.1016/j.neuron.2009.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miranda JA, Shepard KN, McClintock SK, Liu RC. 2014. Adult plasticity in the subcortical auditory pathway of the maternal mouse. PLoS ONE 9, e101630 ( 10.1371/journal.pone.0101630) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.