Abstract
The kidney has a vital role in magnesium homeostasis and, although the renal handling of magnesium is highly adaptable, this ability deteriorates when renal function declines significantly. In moderate chronic kidney disease (CKD), increases in the fractional excretion of magnesium largely compensate for the loss of glomerular filtration rate to maintain normal serum magnesium levels. However, in more advanced CKD (as creatinine clearance falls <30 mL/min), this compensatory mechanism becomes inadequate such that overt hypermagnesaemia develops frequently in patients with creatinine clearances <10 mL/min. Dietary calcium and magnesium may affect the intestinal uptake of each other, though results are conflicting, and likewise the role of vitamin D on intestinal magnesium absorption is somewhat uncertain. In patients undergoing dialysis, the effect of various magnesium and calcium dialysate concentrations has been investigated in haemodialysis (HD) and peritoneal dialysis (PD). Results generally show that dialysate magnesium, at 0.75 mmol/L, is likely to cause mild hypermagnesaemia, results for a magnesium dialysate concentration of 0.5 mmol/L were less consistent, whereas serum magnesium levels were mostly normal to hypomagnesaemic when 0.2 and 0.25 mmol/L were used. While dialysate magnesium concentration is a major determinant of HD or PD patients’ magnesium balance, other factors such as nutrition and medications (e.g. laxatives or antacids) also play an important role. Also examined in this review is the role of magnesium on parathyroid hormone (PTH) levels in dialysis patients. Although various studies have shown that patients with higher serum magnesium tend to have lower PTH levels, many of these suffer from methodological limitations. Finally, we examine the complex and often conflicting results concerning the interplay between magnesium and bone in uraemic patients. Although the exact role of magnesium in bone metabolism is unclear, it may have both positive and negative effects, and it is uncertain what the optimal magnesium levels are in uraemic patients.
Keywords: bone, CKD, haemodialysis, dialysate magnesium, diuretics, magnesium, magnesium supplements, peritoneal dialysis
Introduction
The regulation and elimination of magnesium in patients with renal disease is somewhat understudied. Despite this incomplete understanding, we know that serum magnesium levels increase when the glomerular filtration rate (GFR) falls below ∼20–30 mL/min, yet we do not know what happens to serum magnesium concentration in patients with more modest falls in GFR [e.g. chronic kidney disease (CKD) Stages 1–3; GFR > 30 mL/min] or what proportion of these patients are likely to be hypermagnesaemic [1]. In addition, we also need to consider the relationship between serum magnesium levels and total body magnesium content, as magnesium is predominantly an intracellular cation [2]. Oral medications containing magnesium (e.g. certain laxatives and antacids) may cause hypermagnesaemia, particularly in patients with renal dysfunction [3–6], and conversely, diuretic use can lower magnesium levels.
Haemodialysis (HD) and peritoneal dialysis (PD) provide different scenarios from CKD Stages 3 and 4, and we shall examine the extent to which serum magnesium concentrations depend on the dialysate magnesium concentration and magnesium supplements. Finally, we shall discuss the effect of magnesium on the parathyroid gland, the putative inverse relationship between serum magnesium levels and parathyroid hormone (PTH) levels in dialysis patients and its effect on bone.
Serum magnesium in CKD
In healthy people, intestinal magnesium absorption and renal excretion are regulated so as to maintain magnesium balance (see de Baaij et al. [7] in this supplement). The fractional absorption of magnesium (which occurs mainly in the small intestine) adapts to dietary intake. Under normal conditions, ∼30–50% of ingested magnesium is absorbed. However, the fractional absorption of magnesium rises to 80% if intake is low and falls to ∼25% when magnesium intake is high [8, 9]. Experiments conducted in eight volunteers given magnesium supplements with meals showed that as dietary magnesium increased from 1.5 mmol (3 mEq) to 40 mmol (80 mEq), the fraction of dietary magnesium absorbed fell progressively from 65 to 11% [10].
The kidney has a vital role in magnesium homeostasis: regulation of magnesium excretion is determined by filtration and reabsorption. In individuals with normal renal function, ∼74–100 mmol (1800–2400 mg) of magnesium are filtered everyday [2, 3] (see also Baaji et al. [7] in this supplement). About 70–80% of plasma magnesium is ultrafilterable, and ∼95% of the filtered magnesium load is subject to tubular reabsorption with 5% excreted in urine [9]. The renal handling of magnesium depends to a great extent on the plasma magnesium concentration: in hypermagnesaemia, the fractional excretion of magnesium is high, while during hypomagnesaemia, it is low [9].
Because the renal excretion of magnesium is so powerfully adaptable, impairment of renal function has long been recognized as a frequent prerequisite for the development of hypermagnesaemia. However, in moderate CKD, the increase in the fractional excretion of magnesium compensates for the loss of renal function, such that serum levels are maintained within in the normal range. Interestingly, there seem to be differences in diabetics and non-diabetics. When patients with and without diabetes (creatinine clearance ranging from 115 to >30 mL/min/1.73 m2) and not treated with diuretics were investigated, a significant inverse correlation was found between creatinine clearance and serum magnesium in non-diabetics, but not in diabetics (Figure 1). Serum total and ionized magnesium levels, still in the normal range in both groups, were significantly lower in diabetics (0.773 ± 0.07 and 0.489 ± 0.05 mmol/L, respectively) than in the non-diabetic group (0.834 ± 0.07 and 0.534 ± 0.05 mmol/L, respectively) (unpaired t-test: P < 0.001) [11].
Fig. 1.
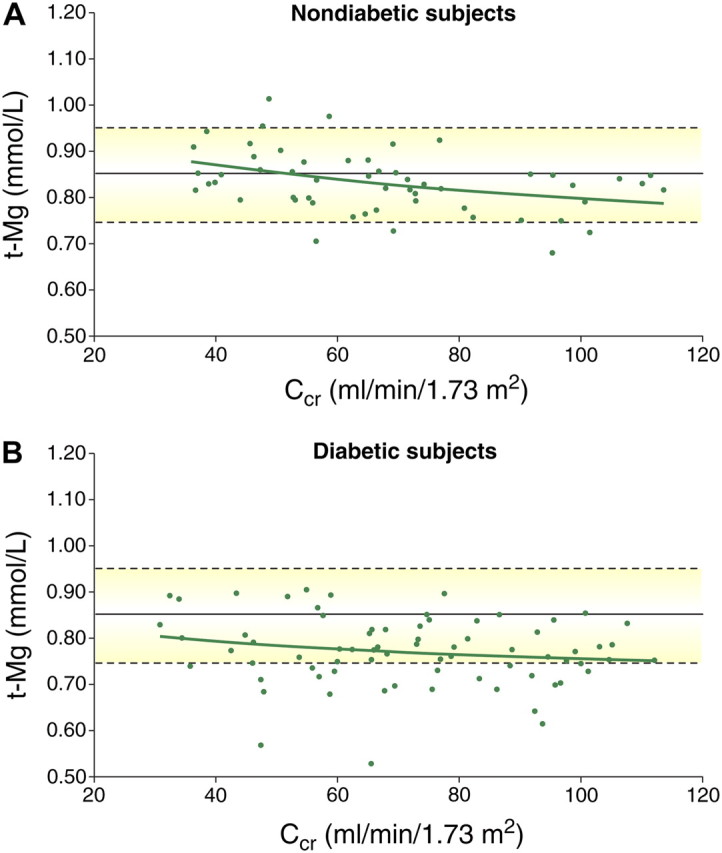
Distribution of serum total magnesium (t-Mg) values as a function of creatinine clearance (CCr) in non-diabetic (A) and diabetic (B) patients (adapted from [11]). (Solid green line shows the course of t-Mg as predicted by regression analysis, with the reference level mean and upper/lower limits shown by solid and dotted lines, respectively.) In non-diabetic patients, serum t-Mg increased significantly when CCr decreased from 115 to 30 ml/min/1.73 m2, (r = −0.38, P < 0.001), whereas this was not the case in the diabetic group (r = −0.18, P > 0.05). Diabetes Care by American Diabetes Association. Copyright 2004. Reproduced with permission of American Diabetes Association in the format Journal via Copyright Clearance Center.
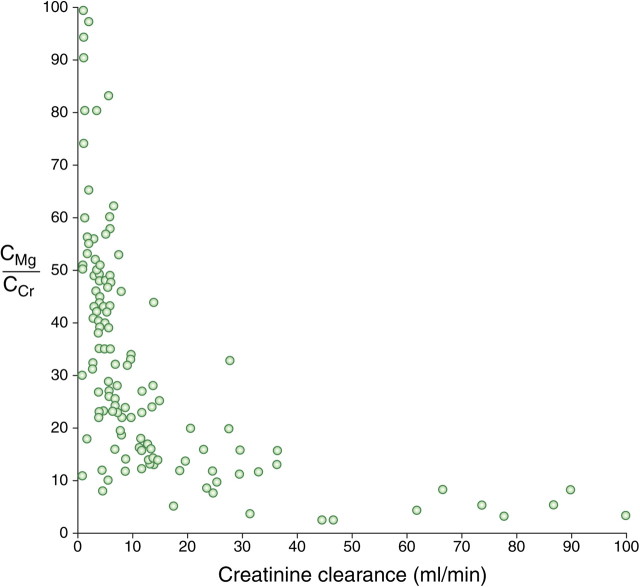
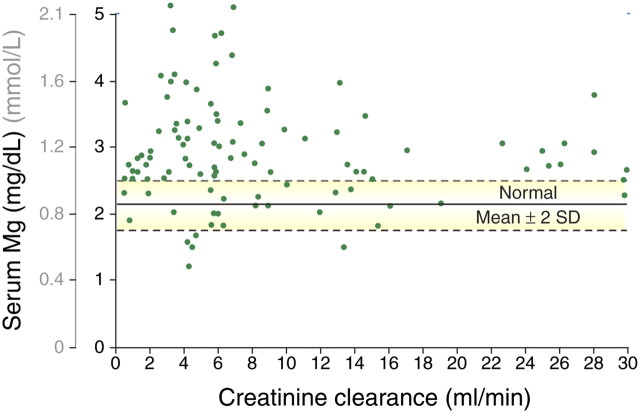
As renal function further deteriorates to CKD Stages 4 and 5, the quantitative excretion of magnesium tends to decrease [3] and cannot be compensated any longer by an increased fractional excretion of magnesium. This first becomes apparent as creatinine clearance falls <30 mL/min and particularly <10–15 mL/min (Figure 2) [12, 13]. Thus, overt hypermagnesaemia develops frequently in patients with creatinine clearances <10 mL/min [12] (Figure 3). As such, renal failure patients might be more vulnerable to changes in magnesium intake via the diet or via medication (e.g. antacids or phosphate binders) and/or the use of diuretics [9, 14, 15]. Furthermore, intestinal absorption of magnesium can also be influenced by calcium and vice versa (for review, see Hardwick et al. [16]). High intestinal calcium concentrations have been reported to reduce the absorption of magnesium [17, 18] but these findings could not be confirmed by others [19, 20]. In respect of the effect of magnesium on calcium uptake, results are conflicting, with some studies describing a decrease in calcium absorption with increased magnesium [18, 19], an effect that was not observed by others [21]. In addition, vitamin D may influence the intestinal absorption of magnesium, though data are conflicting. High doses of 1,25-dihydroxy vitamin D increase the absorption of magnesium, but magnesium is also absorbed independently of vitamin D and the intestinal vitamin D receptor [16].
Fig. 2.
The relationship between the fractional excretion of magnesium (CMg) and endogenous creatinine clearance (CCr) in patients with chronic renal disease. Each point represents a result from a single patient (With permission from Coburn et al. [12]. Copyright (1969), American Medical Association. All rights reserved.)
Fig. 3.
The relationship between serum magnesium concentration and creatinine clearance in patients with CKD. Each point represents a result from a single patient. Normal range serum magnesium: 0.65–1.05 mmol/L (1.58–2.55 mg/dL). (With permission from Coburn et al. [12] Copyright (1969), American Medical Association. All rights reserved.)
Magnesium homeostasis depends on the adaptability of magnesium absorption in the intestine and magnesium reabsorption in the kidneys.
Individuals with creatinine clearance down to ∼30 mL/min can generally compensate for the loss of renal function by increases in the fractional excretion of magnesium such that serum levels are maintained within the normal range.
Below this level of renal function, compensatory mechanisms fail and patients with creatinine clearance of <10 mL/min are likely to be hypermagnesaemic.
Magnesium in dialysis patients
In the normal population, total and ionized serum magnesium concentrations usually lie between 0.65 and 1.05 mmol/L and 0.45 and 0.74 mmol/L, respectively. In HD and PD patients, both total and ionized magnesium concentrations are often slightly elevated above the normal range and have been shown to be dependent on residual renal function [1], on pharmacological or dietary intake and on dialytic elimination (reviewed in Heaton and Parsons [21]; see also Hutchison and Wilkie [22] and Jahnen-Dechent and Ketteler [23] in this supplement). Mild hypermagnesaemia has been described when using a magnesium dialysate concentration of 0.75 mmol/L in both PD and HD patients, while the results when a lower dialysate concentration (0.5 and/or 0.25 mmol/L) was used were not as consistent (for detailed information, see Table 1, and also later in this article).
Table 1.
Total serum Mg levels and dialytic balance in PD and HD patientsa
| Study | n | Dialysate Mg concentration (mmol/L) | Total serum Mg (mmol/L) | Comments | |
| PD | Blumenkrantz et al. [39] | 8 | 0.75 | 1.27 ± 0.04 | Mild hypermagnesaemia reported. |
| Hutchison et al. [42] | 11 | 0.75 (b) | 1.24 ± 0.06 | Mild hypermagnesaemia at baseline. Mean serum magnesium dropped from 1.24 to 0.89 mmol/L (P < 0.001) and remained at the mean level of 0.94 mmol/L for the next 6 months. | |
| 11 | 0.25 (s) | 0.94 ± 0.04b | |||
| Katopodis et al. [37] | 34 | 0.75 | 1.15 ± 0.11 | Mild hypermagnesaemia in the 0.75 mmol/L group. Significant lower serum Mg in patients with low versus high Mg concentration in the dialysate (P < 0.01). Hypermagnesaemia was significantly more frequent in the 0.75 mmol/L group. None of the patients developed hypomagnesaemia. | |
| 34 | 0.50 | 0.94 ± 0.14 | |||
| Saha et al. [1] | 26 | (b) | 1.11 ± 0.22 | Mild hypermagnesaemia in the 0.75 group. Ionized serum Mg concentration was higher in patients with residual renal function than in patients without (0.77 ± 0.1 mmol/L versus 0.69 ± 0.11 mmol/L). | |
| 13 | 0.75 | 1.22 ± 0.20c | |||
| 10 | 0.50 | 1.02 ± 0.15c | |||
| 3 | 0.25 | 0.98 ± 0.35c | |||
| Eisenman et al. [54] | 5 | 0.75 | 0.85 ± 0.13 | Increasing the dialysate magnesium concentration raised serum Mg levels significantly in the patients. | |
| 0.25 | 0.71 ± 0.20 | ||||
| Ejaz et al. [45] | 33 | 0.5–0.6 (b) | 0.85 ± 0.02 | 64% of the patients developed hypermagnesaemia after a mean duration of CAPD treatment with 0.25 mmol/L Mg. 13 of these 21 patients retrieved oral Mg supplementation. | |
| 21 | 0.25 | 0.55 ± 0.01 | |||
| 12 | 0.25 | 0.8 ± 0.035 | |||
| Hutchison et al. [40] | 16 | 0.75 | Dialytic balance: 0.75 mmol/L, 1.36% Glu: −0.01 ± 0.08 mmol/exchange; 0.75 mmol/L, 3.86% Glu: −0.32 ± 0.11 mmol/exchange; 0.25 mmol/L, 1.36% Glu: −0.58 ± 0.13 mmol/exchange; 0.25 mmol/L, 3.86% Glu: −1.07 ± 0.11 mmol/exchange. | ||
| 0.25 | |||||
| Eddington et al. [41] | 12 | 0.75 | Dialytic balance: 0.75 mmol/L, 1.36% Glu: 0.49 ± 0.36 mmol/exchange; 0.25 mmol/L, 1.36% Glu: −1.65 ± 0.7 mmol/exchange; 0.25 mmol/L, icodextrin: −1.29 ± 0.34 mmol/exchange. Mean daily transperitoneal Mg loss was −0.8 mmol/24 h when using 0.75 mmol/L Mg plus icodextrin compared to a mean loss of −3.26 mmol/24 h with 0.25 mmol/L Mg plus icodextrin. | ||
| 12 | 0.25 | ||||
| Tattersall et al. [44] | 43 | 0.75 (b) | 1.07 ± 0.17 | There was a significant fall in serum Mg levels. | |
| 0.25 (s) | 0.87 ± 0.19d | ||||
| HD | Gonella et al. [47] | 13 | 0.75 | 1.17 ± 0.05 | Mild hypermagnesaemia in the 0.75 mmol/L group. |
| 12 | 0.25 | 0.78 ± 0.02 | |||
| Nilsson et al. [48] | 22 | (b) | 1.13 | Mild hypermagnesaemia in the 0.75 mmol/L group. Significant lower serum Mg in patients with low versus high Mg concentration in the dialysate (P < 0.01). No changes in muscle or lymphocyte magnesium. | |
| 0.75 | 1.13 ± 0.13d | ||||
| 0.20 | 0.94 ± 0.24d | ||||
| Navarro et al. [49] | 110 | 0.49 | 2.8 ± 0.4(b) | Mild hypermagnesaemia (serum Mg levels above 2.47 mg/dL) in 73% of patients. | |
| Saha et al. [29] | 47 | 0.50 | 1.01 ± 0.19 | Normal magnesium serum levels found. | |
| 0.25 | 0.94 ± 0.18 | ||||
| 47 | Controls | 0.82 ± 0.08 | |||
| Kelber et al. [50] | 8 | 0.75 | Pre: 1.32 ± 0.12 | Patients were treated with oral MgCO3 as the phosphate binder. Dialytic balance: 0.75 mmol/L: 23.03 ± 20.57 mmol/session; 0.25 mmol/L: 125.86 ± 28.38 mmol/session; 0.00 mmol/L: 199.89 ± 18.10 mmol/session. | |
| Post: 1.22 ± 0.04 | |||||
| 0.25 | Pre: 1.4 ± 0.12 | ||||
| Post: 0.86 ± 0.08 | |||||
| 0.00 | Pre: 1.36 ± 0.08 | ||||
| Post: 0.66 ± 0.08 |
b, baseline; s, study; Glu, glucose; pre, pre-dialytic; post, post-dialytic.
At 6 months.
At 13 months.
At 4 months.
Several studies have investigated ionized serum magnesium in dialysis patients in comparison with healthy controls to assess the utility of ionized and total magnesium assay in detecting magnesium overload. A lower ionized fraction could be due to a higher fraction of complexed magnesium (phosphates, citrate, sulphates) in dialysis patients than in healthy individuals [24], whereas reduced albumin levels, often present in dialysis patients, could lead to a higher fraction of ionized magnesium, thus opposing this effect [25, 26]. Some studies found reductions in ionized magnesium [24, 27, 28], whereas others did not [29, 30] (for detailed values, see Table 2). Thus, the ionized magnesium fraction in dialysis patients seems to be variable, at ∼60–70% of total magnesium. Complexed magnesium, evaluated in only one paper, accounts for ∼16% of total magnesium in PD patients [24].
Table 2.
Total and ionized serum magnesium concentrations in dialysis patients
| Saha et al. [29]; serum ionized versus total Mg and Ca in randomly selected HD patients | |||||||||
| Group |
Dialysate (mmol/L) |
n
|
Pre-dialysis |
Post-dialysis |
|||||
| Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Comments |
|||
| HD | 0.50 | 41 | 1.01 ± 0.19 | 0.69 ± 0.11 | 68.6 ± 2.9 | 0.89 ± 0.21 | 0.60 ± 0.14 | 68.1 ± 7.7 | Normal magnesium serum levels found. Similar ionized Mg fraction in HD versus control. |
| 0.25 | 6 | 0.94 ± 0.18 | 0.63 ± 0.11 | 0.67 ± 0.22 | 0.46 ± 0.13 | ||||
| Control | 47 | 0.82 ± 0.08 | 0.56 ± 0.06 | 68.7 ± 5.3 | |||||
| Truttmann et al. [27]; influence of HD treatment on the relationship between ionized and total Mg | |||||||||
| Group |
Dialysate (mmol/L) |
n
|
Pre-dialysis |
Post-dialysis |
|||||
| Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Free fraction (%) |
Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Free fraction (%) |
Comments |
|||
| HD | 0.75 | 46 | 1.19 (1.05–1.33) | 0.71 (0.67–0.78) | 61 (57–64) | 1.10 (1.02–1.16) | 0.65 (0.63–0.69) | 60 (56–62) | Tendency toward a reduced free Mg fraction (pre- and post-dialyses) in HD versus controls. Reduction of total and ionized serum Mg concentration during the HD session. |
| Control | 25 | 0.82 (0.80–0.92) | 0.57 (0.54–0.59) | 68 (65–70) | |||||
| Dewitte et al. [30]; clinical relevance of ionized fraction of serum Mg in HD patients | |||||||||
| Group |
Dialysate (mmol/L) |
n
|
Pre-dialysis |
Post-dialysis |
|||||
| Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Comments |
|||
| HD | 0.50 | 49 | 0.97 ± 0.12 | 0.62 ± 0.07 | 64.2 ± 1.9 | 0.84 ± 0.06 | 0.55 ± 0.03 | 66.2 ± 1.9 | No difference between ionized fraction of total serum Mg between HD patients and healthy controls. |
| Control | 30 | 0.86 ± 0.08 | 0.56 ± 0.04 | 64.9 ± 1.8 | |||||
| Markell et al. [28]; correlation between ionized and total Mg levels in both HD and CAPD | |||||||||
| Group | Dialysate (mmol/L) |
n
|
Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Comments |
|||
| HD | 0.375 | 26 | 0.99 ± 0.04 | 0.55 ± 0.02 | 55.6 ± 0.93 | Lower ionized serum Mg in HD and CAPD patients versus age matched controls. | |||
| CAPD | 0.25 | 10 | 0.85 ± 0.04 | 0.50 ± 0.02 | 59.2 ± 1.05 | ||||
| Control | 66 | 0.84 ± 0.008 | 0.60 ± 0.004 | 72 ± 0.61 | |||||
| Saha et al. [1]; 13 months (mean) observational study assessing ionized and total serum Mg levels with three different dialysate Mg concentrations | |||||||||
| Group |
Dialysate (mmol/L) |
n
|
Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Comments |
|||
| CAPD | 0.75 | 13 | 1.22 ± 0.20a | 0.87 ± 0.10 | 66.1 ± 0.07 | Mild hypermagnesaemia in the 0.75 group. Lower fraction of ionized Mg in CAPD patients than in controls, P < 0.06. Ionized serum Mg concentration higher in patients with residual renal function than in patients without (0.77 ± 0.1 versus 0.69 ± 0.11 mmol/L). | |||
| 0.50 | 10 | 1.02 ± 0.15a | 0.70 ± 0.07 | ||||||
| 0.25 | 3 | 0.98 ± 0.35a | 0.67 ± 0.19 | ||||||
| Control | 26 | 1.11 ± 0.22 | 0.73 ± 0.11 | 69.4 ± 0.05 | |||||
| Huijgen et al. [24]; validation and comparison of measurement methods for ionized Mg fraction | |||||||||
| Group |
Dialysate (mmol/L) |
n
|
Total Mg (mmol/L) |
Ionized Mg (mmol/L) |
Ionized Mg fraction (%) |
Protein bound Mg fraction (%) |
Complexed Mg fraction (%) |
Comments |
|
| PD | 0.75 | 29 | 1.24 ± 0.18 | 0.76 ± 0.08 | 62 ± 4 | 22 ± 5 | 16 ± 5 | Lower ionized fraction of total serum Mg in dialysis patients versus controls (P < 0.05). Complexed Mg accounts for ∼16% of total Mg in PD patients. | |
| Control | 81 | 0.88 ± 0.06 | 0.56 ± 0.05 | 65 ± 4 | 27 ± 4 | 8 ± 3 | |||
At 13 months.
In searching for a better marker for magnesium overload, several studies have investigated not only serum magnesium concentrations but also the total and ionized magnesium concentration in body tissues or blood cells (i.e. erythrocytes and mononuclear blood cells) in patients undergoing HD or PD, with a variety of results. Because young erythrocytes have a higher magnesium concentration than older cells [31], it might be expected that patients on dialysis would have a higher red blood cell magnesium concentration. In fact, the average magnesium concentration of erythrocytes was found to be consistently higher in several studies when patients on dialysis were compared with healthy volunteers [32, 33, 34] or to non-dialysed patients with impaired renal function [35]. However, patients with higher haematocrit levels (i.e. >30%; [32]) or undergoing erythropoietin treatment [34] had little difference in their erythrocyte magnesium concentration compared to healthy controls and moreover there was a significant inverse correlation between the magnesium concentration of erythrocytes and haematocrit in patients on chronic dialysis [32]. In one study, there was a correlation between total and ionized serum magnesium concentrations and total intracellular erythrocyte magnesium concentrations [33]. In contrast, total magnesium levels in mononuclear cells were not related to serum magnesium levels and were not discriminative for detection of magnesium overload [33].
Based on the above, neither ionized serum, ionized mononuclear nor red blood cell measurements are helpful in detecting magnesium overload. In contrast, the measurement of magnesium content in hair, which was found to be significantly higher in patients on dialysis (n = 31) than in non-dialysed patients with impaired renal function (n = 15): 104.0 μg/g hair versus 21.0 μg/g hair, respectively (P < 0.001) [35], could give an indication of overload but cannot be used as a routine marker.
Levels of total and ionized magnesium are often slightly elevated above the normal range (0.65–1.05 and 0.45–0.74 mmol/L, respectively) in PD and HD patients.
Ionized serum magnesium and many other potential markers have been examined to see if they would be better than total serum magnesium for detecting magnesium overload.
Ionized serum, ionized mononuclear or red blood cell measurements are not helpful in identifying magnesium overload.
Serum magnesium levels and dialysate magnesium
Diffusible magnesium
Magnesium concentration of the dialysate is one of the major determinants of HD or PD patients’ magnesium balance. Magnesium crosses the dialysis and peritoneal membranes readily, and the amount eliminated depends on ultrafiltration and on the diffusible magnesium concentration gradient between serum and dialysis fluid in both HD and PD patients [9, 36, 37]. Ionized magnesium ranges between 60 and 70% of the total serum value depending on protein concentration and percentage complexed magnesium (see above). Taking also into account the Gibbs–Donnan effect (i.e. the impairment of free diffusion of cations and thus higher concentrations of cations in the compartment containing non-diffusible anionic proteins compared to the one not containing any protein), which makes multiplication of the dialysate magnesium concentration by 0.962 necessary [38], leads to the conclusion that in most cases, only a dialysate magnesium concentration of ∼0.5 mmol/L (× 0.962 = 0.46 mmol/L) or lower (Figure 4) will result in a diffusive elimination of magnesium.
Fig. 4.
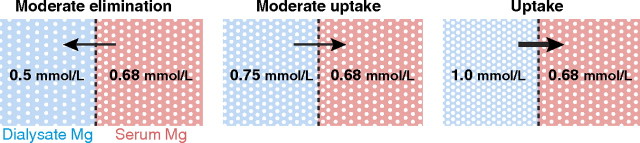
Dialytic magnesium removal or uptake is dependent on the dialysate–serum magnesium gradient (here based on a total serum magnesium concentration of 1.05 and an ionized (i.e. diffusible) serum magnesium of 65%).
Peritoneal dialysis
For many years, PD solutions with a magnesium concentration of 0.75 mmol/L and calcium concentration of 1.75 mmol/L were used as ‘standard’ dialysate for the majority of patients although there has been a trend to lower dialysate calcium over the past decade. Patients using these solutions are slightly hypermagnesaemic [37, 39, 40] (see Table 1). This is because the transfer of magnesium is, if diffusive, mostly into the patient and thus positive [39, 41].
As an example, in a study using such ‘standard’ dialysate, mild hypermagnesaemia was observed (n = 8) (Table 1) [39]. The dialytic balance [2.0 ± 0.3 mmol/day (46 ± 8 mg/day)] and the overall metabolic balance of magnesium were slightly positive in patients on a 1.0 and 1.4 g/kg body weight protein diet (+1.9 ± 0.4 mmol/day ≈ 46 ± 10 mg/day and 2.7 ± 1.1 mmol/day ≈ 66 ± 28 mg/day), respectively [39]. In contrast, when patients are treated with a low magnesium dialysate (0.25 mmol/L), serum magnesium levels normalize after some time, as dialytic magnesium balance is constantly negative, independent of glucose concentration, use of icodextrin (i.e. ultrafiltration) or treatment modality (continuous ambulatory PD or automated PD) [40–42] (Table 1).
As already discussed, serum magnesium is a poor indicator of the overall magnesium content of different tissues, and normomagnesaemia does not necessarily exclude magnesium depletion [43]. Moreover, there may well be a slow continuous loss of body magnesium when using low magnesium dialysate (0.25 mmol/L) over extended treatment periods: 4, 8 and 12 months after introducing the solution, persistent hypomagnesaemia occurred in 21, 64 and 37% of patients, respectively [42–46]. In many of these patients, oral supplementation was needed to normalize serum magnesium levels [45, 46], and in one study, oral magnesium supplements were insufficient and so 15% of patients were given magnesium sulphate in their PD fluid [46].
Other PD solutions contain 0.5 mmol/L magnesium, and patients treated with these solutions tend to have normal serum magnesium levels [1, 37] with no development of hypomagnesaemia even after a mean treatment period of ∼28 months [37].
Haemodialysis
In the past, dialysis fluid concentration usually contained 0.75 mmol/L magnesium, with mild hypermagnesaemia observed quite frequently [27, 47, 48]. Both mild hypermagnesaemia and normomagnesaemia were found when patients were dialysed against a dialysis fluid magnesium concentration of 0.5 mmol/L [29, 49].
There are several studies investigating serum magnesium changes and magnesium flux during HD in greater detail. During HD treatment with 0.75 mmol/L magnesium, a significant reduction of serum magnesium concentration was observed in one study, even though the dialysate concentration was higher than the actual ionized serum magnesium concentrations, possibly reflecting the importance of the Gibbs–Donnan effect [27], whereas the decrease in serum concentration was not significant in another study using a magnesium dialysate concentration of 0.74 mmol/L (≈1.8 mg/dL) [50]. In this latter study, a large variability in magnesium elimination was found, both negative or positive, which was significantly influenced by initial serum concentrations. Magnesium elimination and a fall in serum magnesium concentration was seen in every patient when lower concentrations, such as 0.0 and 0.25 mmol/L, were used [48, 50] (see Table 1). However, magnesium-free dialysate was poorly tolerated as most of the patients participating in the clinical stage of this study experienced leg cramps, though these resolved quickly after dialysate was switched back to 0.75 mmol/L (1.8 mg/dL) [50]. Perhaps surprisingly, but in line with other investigations [33], in the investigation by Nilsson et al. [48], muscle and lymphocyte magnesium levels did not change significantly from pre-study levels after a period of 4 months of using a 0.2 mmol/L magnesium dialysate concentration.
The fact that neither using 0.75 mmol/L nor 0.5 mmol/L as a dialysis fluid leads to consistent serum magnesium results is emphasized by an epidemiological study in 27 544 patients in which the baseline prescribed dialysate magnesium concentration exhibited only a weak correlation with patients’ serum magnesium (r = 0.22, P < 0.0001), suggesting that factors other than dialysate magnesium, such as nutrition and magnesium supplements (e.g. antacids), may also play an important role in determining serum magnesium levels in end-stage renal disease (ESRD) patients [51].
The haemodynamic effect of various dialysate magnesium concentrations is potentially of great importance. A study focussing on haemodynamic changes, in which dialysate calcium and magnesium concentrations were changed sequentially in a group of eight HD patients, revealed that the combined use of a dialysate containing calcium, 1.25 mmol/L, and magnesium, 0.25 mmol/L, led to a significant drop in mean arterial pressure induced by a drop in cardiac index that was not compensated by an increase in total peripheral resistance [52]. However, the combination of a dialysate containing calcium, 1.25 mmol/L, and magnesium, 0.75 mmol/L, could help to prevent blood pressure deterioration and a combination with magnesium, 0.5 mmol/L, showed intermediate results [52]. In contrast, another study has investigated cardiovascular and haemodynamic effects of high and low magnesium dialysate concentrations (1.0 and 0.5 mmol/L) in HD patients (n = 20), using serial echocardiography [53]. No positive influence of an increased magnesium dialysate concentration was found on intradialytic blood pressure stability or cardiovascular performance [53].
Various magnesium and calcium dialysate concentrations have been investigated in patients undergoing HD or PD.
Results indicate that magnesium, 0.75 mmol/L, is likely to cause mild hypermagnesaemia.
Results for lower magnesium dialysate concentrations (e.g. 0.5 mmol/L) were less consistent.
Serum magnesium levels were mostly normal to hypomagnesaemic when 0.2 and 0.25 mmol/L were used.
Factors other than dialysate magnesium concentration, such as nutrition and medications (e.g. laxatives or antacids), are also likely to play an important role in determining serum magnesium concentrations in dialysis patients.
The intradialytic cardiovascular and haemodynamic benefits of varying magnesium concentration in patients’ dialysate are unclear.
Effect of magnesium on PTH levels
Secondary hyperparathyroidism is an almost inevitable complication of chronic renal insufficiency and can occur in patients with mild, moderate or severe renal dysfunction [55]. Serum calcium, as well as calcitriol, fibroblast growth factor-23 (FGF-23) and serum phosphate are known to have key roles in regulating the synthesis and secretion of PTH [56–58]. Calcium is the dominant activator of the calcium-sensing receptor (CaSR), but other divalent and trivalent cations are also potent Type I activators of this receptor. Serum magnesium levels may, therefore, have an important regulatory role in PTH secretion [59, 60].
The regulation of intact PTH (iPTH) by extracellular calcium has been studied extensively, but the mechanism whereby serum magnesium affects PTH has still not been fully elucidated. In vitro [61] and in vivo animal and human studies [59, 61, 62] have demonstrated that high magnesium concentrations modulate PTH secretion in a similar manner to calcium. High calcium concentrations activate the extracellular CaSR which eventually results in decreased PTH secretion [56, 63]. The CaSR is a G-protein-coupled receptor that leads to an increase in intracellular calcium, stimulation of phospholipase A and inhibition of cAMP accumulation via Gαi and Gαq proteins. It is known that magnesium is able to activate the CaSR, though with a potency 2- to 3-fold less than calcium in terms of activating the effector molecule phospholipase C. In addition, binding sites for calcium and magnesium seem to be different [61, 64, 65]. Moreover, an in vitro study showed that addition of extracellular magnesium to single isolated bovine parathyroid cells affects intracellular calcium concentrations via two independent mechanisms that modify PTH secretion [66]. So in addition to the CaSR, other mechanisms possibly involving further receptors may exist and contribute to PTH regulation by magnesium. A caveat concerns the concentration of extracellular magnesium used: only at concentrations >1.2 mM magnesium were effects on intracellular calcium seen and the secretory response was evaluated using extremely high magnesium concentrations of 4.5 and 9.0 mM [66].
On the other hand, a phenotype of blunted PTH secretion is seen in patients with severe hypomagnesaemia, even if calcium concentrations are low [67–69]. In vitro studies investigating the biphasic regulation of PTH secretion from parathyroid cells by magnesium showed that at levels <0.5 mM magnesium, PTH secretion is suppressed by an intracellular disinhibition of Gαi/q signalling, leading to a constitutive activation of the CaSR [70, 71]. These findings provide an explanation for the observed decrease in PTH secretion at any given calcium concentration under magnesium deficiency in vitro and in patients with severe hypomagnesaemia [68, 70, 72]. In these situations, calcium-dependent regulation can be restored by elevating magnesium concentrations [70, 72].
Two studies in patients undergoing PD have investigated the influence of different calcium and magnesium concentrations in dialysis solutions on serum magnesium and serum PTH levels [73, 74] (Table 3). The study by Wei et al. investigated patients (n = 46) retrospectively, whom the authors described as having been treated with either a ‘standard’ dialysis solution containing calcium (1.62 mmol/L) and magnesium (0.75 mmol/L) or a ‘low’ calcium/magnesium dialysate (calcium, 1.25 mmol/L, and magnesium, 0.25 mmol/L). Laboratory data from a 6-month period were analysed and the results showed that the only significant between-group differences were higher serum magnesium in the standard dialysate group and higher serum PTH levels in the low dialysate group, with a significant inverse correlation between serum magnesium and PTH levels (r = −0.357; P < 0.05). In contrast, the study by Sanchez et al. was conducted over a 12-month period and patients undergoing PD (n = 44) were randomized to either a standard or a low-calcium/magnesium dialysate (dialysate concentrations: ‘standard’ calcium: 1.75 mmol/L; magnesium: 0.75 mmol/L and ‘low’ calcium: 1.25 mmol/L; magnesium: 0.25 mmol/L). Patients underwent bone biopsies at baseline and after 12 months [73]. The results showed an increase in serum PTH levels and a higher intake of calcium salts in the low-calcium/magnesium dialysate group (P < 0.01). While serum calcium, phosphate levels or bone histological outcome did not differ between the two groups, serum magnesium levels were lower in the low-calcium/magnesium dialysate group than in the high-calcium/magnesium group (month 6: P < 0.009; month 12: P < 0.005).
Table 3.
Interventional and observational studies investigating the relationship between PTH and magnesium levels in dialysis patientsa
| Author | Patients (n), (modality) | Dialysate cation concentration [mmol/L] | Design | r-value | P-value | Notes | |
| Interventional studies | |||||||
| Wei et al. 2006 [74] | 46 (CAPD, n = 13; APD, n = 33) | [Ca 1.62/Mg 0.75], [Ca 1.25/Mg 0.25] | Prospective 6-month study (standard Ca/Mg versus low Ca/Mg dialysate) | −0.36 | <0.05 | The only significant between-group differences were higher serum PTH and lower serum Mg levels in the low Ca/Mg group with an inverse correlation between serum PTH and serum Mg. | |
| Sanchez et al. 2004 [73] | 44 (CAPD) | [Ca 1.75/Mg 0.75], [Ca 1.25/Mg 0.25] | Prospective, randomized, 12-month study (standard Ca/Mg versus low Ca/Mg dialysate) | Not determined | NA | Higher serum Mg in the standard dialysate group (P = 0.005) and higher serum PTH levels in the low dialysate group (P = 0.0004). | |
| Observational studies | |||||||
| Cho et al. 2002 [78] | 56 (CAPD) | [Ca 1.75/Mg 0.25] | Retrospective analysis over at least 6-month period | −0.174 | 0.200 | Results did not show a significant correlation between Mg and iPTH. | |
| Navarro et al. 1999 [77] | 110 (HD) | [Ca 1.5/Mg 0.50] | Prospective 6-month analysis | −0.58 | <0.001 | Serum magnesium concentrations were independently and inversely associated with PTH levels in HD patients. | |
| Navarro et al. 1999 [49] | 51 (CAPD) | [Ca 1.75/Mg 0.75] | Prospective 6-month analysis | −0.57 | <0.001 | The significant inverse relationship between serum Mg and iPTH levels was independent of factors regulating parathyroid gland function (calcium, phosphorus and calcitriol levels). | |
| Saha et al. 1997 [1] | 26 (CAPD) | [Ca 1.75/Mg 0.75], [Ca 1.75/Mg 0.50], [Ca 1.25/Mg 0.25], [Ca 1.00/Mg 0.50] | Cross-sectional study | −0.42 | <0.05 | An inverse relationship was found between serum ionized Mg and iPTH levels. | |
APD, automated PD; Ca, calcium; CAPD, continuous ambulatory PD; (i)PTH, (intact) PTH; Mg, magnesium; NA, not applicable.
Of course, more than one variable has been changed (both calcium and magnesium concentrations) in both studies, and so both cations may have affected PTH levels, with calcium possibly playing a predominant role. However, in both studies serum calcium and phosphate levels were similar between groups, and serum PTH levels in the low-calcium/magnesium dialysate groups were significantly higher. Both groups of authors suggested that high serum magnesium levels may suppress the synthesis and/or secretion of serum PTH, and low serum magnesium stimulates PTH synthesis and/or secretion independently of serum calcium and phosphate concentrations [73, 74]. However, even though serum calcium levels were not different, another interpretation may be also possible: in both studies, lower dialysate magnesium and lower calcium dialysate concentrations were used, and thus, a transient decrease of serum calcium could have occurred, in which case PTH probably maintained serum calcium levels.
A double crossover study has evaluated the impact of changes in dialysate magnesium concentration with constant calcium dialysate concentrations (1.3 mmol/L) in HD patients (n = 26) [75]. They were exposed to increasing (0.75 to 1.25 mmol/L) and then decreasing (to 0.25 mmol/L) concentrations of magnesium in the dialysate, and vice versa, with dialysate calcium kept constant at 1.3 mmol/L [75]. An increase in dialysate magnesium concentration was associated with a reduction in PTH levels and a decrease of dialysate magnesium with an increase in serum PTH levels. It is noteworthy that the strongest effects were observed when changing the dialysate concentration from very low (0.25 mmol/L) to high (1.25 mmol/L) concentrations and vice versa. PTH changes were much less pronounced when changing magnesium from ‘normal’ (0.75 mmol/L) to low or high [75]. Other studies have also noted that the use of low magnesium dialysate (0.25 and 0.20 mmol/L) in HD patients resulted in significant increases in serum PTH levels [48, 76]. All but one of the patients who entered the study with normal plasma PTH showed an increase of the circulating hormone after 18 months on a low magnesium dialysis. In contrast, patients who had raised PTH levels initially responded variably, some showing an increase and others a decrease of the hormone concentrations. These studies show that magnesium affects PTH secretion; however, PTH assays used in these early studies measured carboxyterminal PTH fragments and therefore it is difficult to judge from these results to what extent PTH is really influenced by magnesium.
Two 6-month observational studies have also investigated the potential relationship between serum magnesium and PTH levels, both in patients undergoing HD (n = 110) and PD (n = 51) [49, 77]. Serum magnesium concentrations were independently and inversely associated with PTH levels in HD patients [49]. In fact, both magnesium and calcium levels showed an inverse correlation with PTH levels (r = −0.48; P < 0.001 and r = −0.21; P < 0.05, respectively). After adjusting for calcium and phosphate (partial correlation analysis), magnesium and PTH remained inversely correlated (r = −0.58; P < 0.001). A stepwise multiple regression analysis also showed that PTH levels were predicted by magnesium (P < 0.001), alkaline phosphatase (P < 0.01) and phosphate levels (P < 0.05; multiple r = 0.57; P < 0.001), but not calcium concentration. Furthermore, patients with relative hypoparathyroidism (PTH < 120 pg/mL; n = 52) also had greater serum magnesium concentrations than the other patients (P < 0.001). Similar results were found in PD patients—a significant inverse relationship between serum magnesium and PTH levels—an association that was statistically independent of important factors regulating parathyroid gland function (i.e. calcium, phosphorus and calcitriol levels) [77]. (Note that in the two studies by Navarro et al. [49, 77], PTH levels were in most patients <400 pg/mL.)
However, a further observational study conducted in 56 PD patients receiving 0.25 mmol/L magnesium dialysate for at least 6 months failed to show a correlation between serum magnesium and serum iPTH levels (though iPTH levels correlated inversely with ionized calcium levels: r = −0.515; P < 0.001) [78]. Nevertheless, in 49 of these patients whose serum iPTH levels were <300 pg/mL, serum iPTH levels correlated inversely with these patients’ serum magnesium levels (r = −0.295; P = 0.039) [78]. It would be of interest to know if in patients with advanced hyperparathyroidism treated with calcimimetics (which acts on the parathyroid calcium receptor) differences in serum magnesium will still be correlated inversely with serum PTH.
It should be noted, however, that at best the studies investigating the potential effect of magnesium on PTH constitute indirect evidence. Nevertheless, there may be an assumption of increased risk of adynamic bone disease from the association of low PTH levels and elevated serum magnesium concentrations. On the other hand, iPTH assays may not be particularly good methods to determine bone turnover in patients with ESRD [79]. It is important to note, however, that severe magnesium depletion leads to inhibition of PTH secretion which can be restored by elevating magnesium concentrations [72]. Clearly, this is an area that would benefit from further research to elucidate the mechanisms whereby magnesium may influence PTH levels, particularly under conditions prevalent in patients with renal dysfunction.
In general, research has shown that patients with higher serum magnesium levels have lower PTH levels.
However, many variables other than serum magnesium levels influence PTH levels.
Many of the studies evaluating the influence between PTH and magnesium are not controlled well enough or suffer from other methodological drawbacks to draw firm conclusions.
Magnesium and bone in uraemic patients
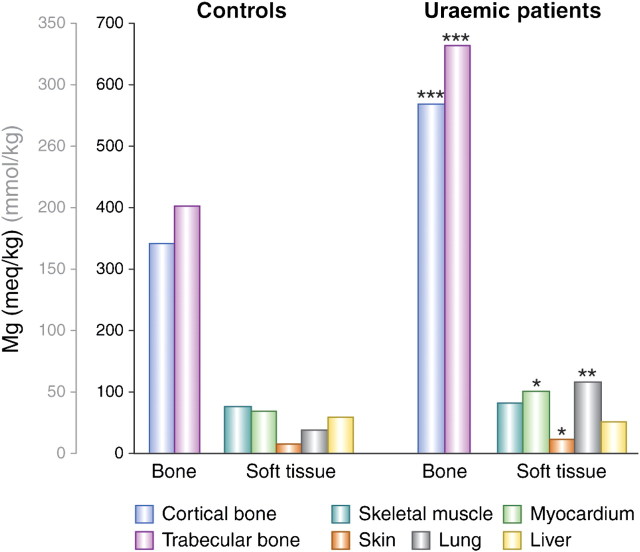
About 55% of the body’s magnesium content is found in bone, but this only represents a tiny proportion of the total bone ash (∼0.5% or 14 g). Calcium and phosphate as hydroxyapatite account for ∼2700 g or 90% [80] (Figure 5). Various studies have looked at magnesium concentrations in the bone and other tissues in patients with CKD and in those without kidney disease. The common thread throughout these studies is the high degree of variability in results. For example, in a detailed investigation, Pellegrino and Biltz [80] found no difference in bone magnesium content, whether in trabecular or cortical bone, between uraemic patients (n = 22) and controls (n = 32), but found reductions in calcium and carbonate content. In a later investigation by the same group, decreases in calcium and mineral carbonate were confirmed, but simultaneous increases in phosphate and magnesium concentrations were also found [81]. The changes in bone mineral composition were ascribed to a concomitant loss of fixed base and calcium carbonate as well as a replacement of calcium carbonate by phosphate [81]. This may reflect the effect of acidosis. Likewise, another group found elevated magnesium concentrations in trabecular and cortical bones, at the same time as lowered calcium and increased phosphorus concentration [82]. In contrast, other investigators found increases in both calcium and magnesium content [83], and yet others found an increase in magnesium in both cortical and trabecular bones by 50–66% (wet and dry weight, respectively) and no difference in calcium concentrations [84]. In the same investigation, a significantly higher magnesium concentration was found in myocardium, lung and skin tissue of uraemic patients compared with controls (Figure 6). The uraemic patients had been treated for a longer period of time with HD and presented increased serum magnesium levels, together with a relatively high dialysis fluid magnesium concentration.
Fig. 5.
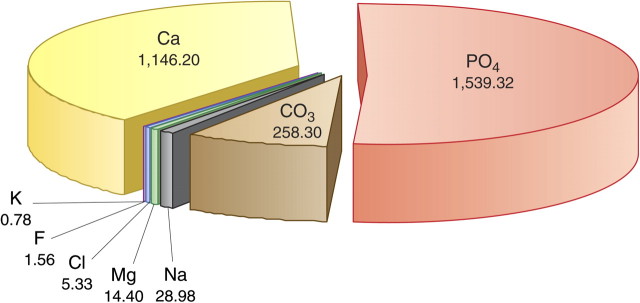
Bone composition in normal individuals (in grams). Mean values from 109 human bone specimens using Trotter’s estimate for total bone mass: 4459.9 g. Reproduced with permission from Pellegrino and Biltz [80].
Fig. 6.
Bone and tissue magnesium content in uraemic patients compared with controls [84]. Tissues were obtained from 33 patients with ESRD and 11 non-uraemic patients after death. Twenty-five of the renal patients had been dialysed for a period of ∼23 months with a dialysis fluid containing 1.3 mEq/L (0.65 mmol/L) magnesium. Plasma magnesium concentrations were 2.57 ± 0.41 mEq/L (1.29 ± 0.21 mmol/L). Most of the controls had died in road traffic accidents. Their plasma magnesium concentrations were 1.57 ± 0.08 mEq/L (0.79 ± 0.04) mmol/L. The soft tissue samples were dried and defatted. The result is given as milliequivalent per kilogram fat-free dry solids. For myocardium, skin and lung, mean magnesium concentrations were significantly higher in uraemic patients; there was no difference for skeletal muscle or liver. The bone was defatted and then dried. Results are given as milliequivalent per kilogram ashed weight. Although the mineral content of uraemic trabecular bone was decreased by 8% and of cortical bone by 5%, there was still a significant increase in bone magnesium content on a dry weight basis in uraemic patients (*P < 0.01; **P < 0.0005; ***P < 0.0001 versus controls) (2 mEq/L = 1 mmol/L). Reproduced with permission from Contiguglia et al. [84].
These highly variable results should not be surprising given the many factors that affect serum magnesium levels in CKD patients undergoing dialysis. Magnesium levels may be increased by intake of over-the-counter drugs containing magnesium (e.g. antacids, laxatives) and high magnesium dialysate concentrations. A negative magnesium balance may be caused by excessive use of diuretics (in patients with mild-to-moderate CKD) or reduced gastrointestinal uptake (due to acidosis and poor nutrition and/or absorption) [85, 86]. In fact, it is not unusual for the magnesium balance to be normal or even decreased in uraemic patients owing to decreased dietary intake of magnesium combined with the impaired intestinal magnesium absorption characteristic of CKD [36].
The role of magnesium in bone mineralization and in the pathogenesis of renal bone disease has been a matter of some debate in recent years and a hypothesis has been advanced in the past to the effect that magnesium is directly involved in the development of osteomalacia and/or renal osteodystrophy [9, 85, 87–89]. This is on the one hand because of magnesium’s known effect on the CaSR and thus on PTH levels, (mentioned earlier in this review) and on the other hand because of its ability to prevent mineralization and/or calcification.
Only two small clinical studies involving therapeutic interventions with magnesium (both in <10 renal failure patients) have used bone biopsies to investigate the issue of whether changes in serum magnesium concentrations have affected bone. In one study, patients switched from aluminum hydroxide to magnesium hydroxide as a phosphate binder while reducing magnesium dialysate concentration from 0.75 mmol/L to 0.375 mmol/L (serum magnesium levels increased from 0.96 ± 0.2 mmol/l to 1.54 ± 0.2 mmol/L) [90]. After 8 and 20 months of treatment, bone histomorphometry showed no change in mineralization or osteoid formation. In the second study, patients switched from a dialysate magnesium concentration of 0.5 mmol/L (1.0 mEq/L) to 0.25 mmol/L (0.5 mEq/L) and their predialysis serum magnesium levels decreased from 1.24 ± 0.15 mmol/L (2.47 ± 0.3 mEq/L) to 1.03 ± 0.6 mmol/L (2.05 ± 0.11 mEq/L; P < 0.02) [89]. In this study, bone biopsies demonstrated a decrease in osteoid volume and surface. One reason for these contradictory results could be the possible confounding by the use of aluminum. A larger, more recent cross-sectional observational study which focused on trace metal alterations in renal patients and possible associations with bone diseases [91] did not find a higher absolute bone magnesium content in these patients, though an increased magnesium-to-calcium ratio was found compared with controls. In particular, no association was observed between bone magnesium content and adynamic bone disease.
At present, the role of magnesium in influencing bone metabolism remains unclear. There are many other factors such as vitamin D status, PTH level, calcium and phosphate concentration, and metabolic acidosis, which contribute to bone metabolism and function [92]. Recently investigated molecules such as FGF-23 or Klotho [93] factors such as vitamin K [94] and matrix Gla protein for which magnesium serves as a cofactor and/or modulator [95] may also play important roles in regulating bone turnover and mineralization. In addition, it seems that magnesium is essential for the activity of pyrophosphatases [96, 97], and magnesium pyrophosphate itself is a substrate for pyrophosphatases [98]. Thus, the presence of magnesium in bone is necessary as magnesium deficiency is likely to be a problem in osteoporosis as it is crucial for regulation of osteoblast and osteoclast function and activity in bone remodelling [99–101]. Moreover, epidemiological studies have linked insufficient magnesium in the diet to low bone mass and osteoporosis, as have genetic causes of hypomagnesaemia (which result in low bone mass), and magnesium deficiency in animal models (linked to osteopenia and skeletal fragility) [100–102].
Too little is known about the interaction of the different factors to be able to describe the exact role of magnesium in uraemic bone metabolism.
How much magnesium is necessary, and how much is too much, is unknown.
Acknowledgments
The authors thank Richard Clark and Martina Sintzel for providing writing and editorial assistance on behalf of Fresenius Medical Care Deutschland GmbH. Fresenius also made an unrestricted educational grant to meet the cost of preparing this article. These declarations are in line with the European Medical Writers’ Association guidelines.
Conflict of interest statement. J.C. has received speakers’ and/or consultancy honoraria from Amgen, Abbott, Fresenius, Cytochroma, Vifor and Shire and research support from Abbott and Amgen. M.R. has received speakers’ or consultancy honoraria from and participated in experimental work with Amgen, Abbott, Fresenius and Shire. P.M. has received speakers’ or consultancy honoraria from and participated in clinical trials with Abbott, Amgen, Genzyme and Fresenius.
References
- 1.Saha HH, Harmoinen AP, Pasternack AI. Measurement of serum ionized magnesium in CAPD patients. Perit Dial Int. 1997;17:347–352. [PubMed] [Google Scholar]
- 2.Massry SG, Seelig MS. Hypomagnesemia and hypermagnesemia. Clin Nephrol. 1977;7:147–153. [PubMed] [Google Scholar]
- 3.Mordes JP, Wacker WE. Excess magnesium. Pharmacol Rev. 1977;29:273–300. [PubMed] [Google Scholar]
- 4.Lembcke B, Fuchs C. Magnesium load induced by ingestion of magnesium-containing antacids. Contrib Nephrol. 1984;38:185–194. doi: 10.1159/000408085. [DOI] [PubMed] [Google Scholar]
- 5.Castelbaum AR, Donofrio PD, Walker FO, Troost BT. Laxative abuse causing hypermagnesemia, quadriparesis, and neuromuscular junction defect. Neurology. 1989;39:746–747. doi: 10.1212/wnl.39.5.746-a. [DOI] [PubMed] [Google Scholar]
- 6.Krendel DA. Hypermagnesemia and neuromuscular transmission. Semin Neurol. 1990;10:42–45. doi: 10.1055/s-2008-1041252. [DOI] [PubMed] [Google Scholar]
- 7.de Baaij JHF, Joost GJ, et al. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J. 2012;5(Suppl 1):i15–i24. doi: 10.1093/ndtplus/sfr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham L, Caesar J, Burgen A. Gastrointestinal absorption and excretion of Mg 28 in man. Metabolism. 1960;9:646–659. [PubMed] [Google Scholar]
- 9.Navarro-Gonzalez JF, Mora-Fernandez C, Garcia-Perez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22:37–44. doi: 10.1111/j.1525-139X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 10.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88:396–402. doi: 10.1172/JCI115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewitte K, Dhondt A, Giri M, et al. Differences in serum ionized and total magnesium values during chronic renal failure between nondiabetic and diabetic patients: a cross-sectional study. Diabetes Care. 2004;27:2503–2505. doi: 10.2337/diacare.27.10.2503. [DOI] [PubMed] [Google Scholar]
- 12.Coburn JW, Popovtzer MM, Massry SG, Kleeman CR. The physicochemical state and renal handling of divalent ions in chronic renal failure. Arch Intern Med. 1969;124:302–311. [PubMed] [Google Scholar]
- 13.Massry SG. Magnesium homeostasis in patients with renal failure. Contrib Nephrol. 1984;38:175–184. doi: 10.1159/000408084. [DOI] [PubMed] [Google Scholar]
- 14.Schelling JR. Fatal hypermagnesemia. Clin Nephrol. 2000;53:61–65. [PubMed] [Google Scholar]
- 15.Zaman F, Abreo K. Severe hypermagnesemia as a result of laxative use in renal insufficiency. South Med J. 2003;96:102–103. doi: 10.1097/01.SMJ.0000049844.49028.1D. [DOI] [PubMed] [Google Scholar]
- 16.Hardwick LL, Jones MR, Brautbar N, Lee DB. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991;121:13–23. doi: 10.1093/jn/121.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Norman DA, Fordtran JS, Brinkley LJ, et al. Jejunal and ileal adaptation to alterations in dietary calcium: changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J Clin Invest. 1981;67:1599–1603. doi: 10.1172/JCI110194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix Z, Alcock N, Archibald R. Competition between Calcium, Strontium, and Magnesium for Absorption in the Isolated Rat Intestine. Clin Chem. 1963;12:734–744. [PubMed] [Google Scholar]
- 19.O'Donnell JM, Smith MW. Uptake of calcium and magnesium by rat duodenal mucosa analysed by means of competing metals. J Physiol. 1973;229:733–749. doi: 10.1113/jphysiol.1973.sp010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leichsenring J, Norris L, Lamison S. Magnesium metabolism in college women: observations on the effect of calcium and phosphorus intake levels. J Nutr. 1951;45:477–485. doi: 10.1093/jn/45.4.477. [DOI] [PubMed] [Google Scholar]
- 21.Heaton F, Parsons F. The metabolic effect of high magnesium intake. Clin Sci. 1961;21:273–284. [PubMed] [Google Scholar]
- 22.Hutchison A, Wilkie M. Use of magnesium as a drug in chronic kidney disease. Clin Kidney J. 2012;5(Suppl 1):i62–i70. doi: 10.1093/ndtplus/sfr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huijgen HJ, van Ingen HE, Kok WT, Sanders GT. Magnesium fractions in serum of healthy individuals and CAPD patients, measured by an ion-selective electrode and ultrafiltration. Clin Biochem. 1996;29:261–266. doi: 10.1016/0009-9120(96)84729-b. [DOI] [PubMed] [Google Scholar]
- 25.Saha H, Harmoinen A, Karvonen AL, Mustonen J, Pasternack A. Serum ionized versus total magnesium in patients with intestinal or liver disease. Clin Chem Lab Med. 1998;36:715–718. doi: 10.1515/CCLM.1998.126. [DOI] [PubMed] [Google Scholar]
- 26.Kulpmann WR, Rossler J, Brunkhorst R, Schuler A. Ionised and total magnesium serum concentrations in renal and hepatic diseases. Eur J Clin Chem Clin Biochem. 1996;34:257–264. doi: 10.1515/cclm.1996.34.3.257. [DOI] [PubMed] [Google Scholar]
- 27.Truttmann AC, Faraone R, Von Vigier RO, Nuoffer JM, Pfister R, Bianchetti MG. Maintenance hemodialysis and circulating ionized magnesium. Nephron. 2002;92:616–621. doi: 10.1159/000064109. [DOI] [PubMed] [Google Scholar]
- 28.Markell MS, Altura BT, Sarn Y, et al. Deficiency of serum ionized magnesium in patients receiving hemodialysis or peritoneal dialysis. ASAIO J. 1993;39:M801–M804. [PubMed] [Google Scholar]
- 29.Saha H, Harmoinen A, Pietila K, Morsky P, Pasternack A. Measurement of serum ionized versus total levels of magnesium and calcium in hemodialysis patients. Clin Nephrol. 1996;46:326–331. [PubMed] [Google Scholar]
- 30.Dewitte K, Dhondt A, Lameire N, Stockl D, Thienpont LM. The ionized fraction of serum total magnesium in hemodialysis patients: is it really lower than in healthy subjects? Clin Nephrol. 2002;58:205–210. doi: 10.5414/cnp58205. [DOI] [PubMed] [Google Scholar]
- 31.Walser M. Magnesium metabolism. Ergeb Physiol. 1967;59:185–296. doi: 10.1007/BF02269144. [DOI] [PubMed] [Google Scholar]
- 32.Gonella M, Betti G, Buzzigoli G, Bartolini V. The relationship between hematocrit and erythrocyte magnesium concentration in patients on regular hemodialysis. Nephron. 1979;24:134–137. doi: 10.1159/000181701. [DOI] [PubMed] [Google Scholar]
- 33.Huijgen HJ, Sanders R, van Olden RW, Klous MG, Gaffar FR, Sanders GT. Intracellular and extracellular blood magnesium fractions in hemodialysis patients; is the ionized fraction a measure of magnesium excess? Clin Chem. 1998;44:639–648. [PubMed] [Google Scholar]
- 34.Pietrzak I, Bladek K, Bulikowski W. Comparison of magnesium and zinc levels in blood in end stage renal disease patients treated by hemodialysis or peritoneal dialysis. Magnes Res. 2002;15:229–236. [PubMed] [Google Scholar]
- 35.Dlugaszek M, Szopa M, Rzeszotarski J, Karbowiak P. Magnesium, calcium and trace elements distribution in serum, erythrocytes, and hair of patients with chronic renal failure. Magnes Res. 2008;21:109–117. [PubMed] [Google Scholar]
- 36.Mountokalakis TD. Magnesium metabolism in chronic renal failure. Magnes Res. 1990;3:121–127. [PubMed] [Google Scholar]
- 37.Katopodis KP, Koliousi EL, Andrikos EK, Pappas MV, Elisaf MS, Siamopoulos KC. Magnesium homeostasis in patients undergoing continuous ambulatory peritoneal dialysis: role of the dialysate magnesium concentration. Artif Organs. 2003;27:853–857. doi: 10.1046/j.1525-1594.2003.07193.x. [DOI] [PubMed] [Google Scholar]
- 38.Rippe B, Venturoli D. Optimum electrolyte composition of a dialysis solution. Perit Dial Int. 2008;28(Suppl 3):S131–S136. [PubMed] [Google Scholar]
- 39.Blumenkrantz MJ, Kopple JD, Moran JK, Coburn JW. Metabolic balance studies and dietary protein requirements in patients undergoing continuous ambulatory peritoneal dialysis. Kidney Int. 1982;21:849–861. doi: 10.1038/ki.1982.109. [DOI] [PubMed] [Google Scholar]
- 40.Hutchison AJ, Merchant M, Boulton HF, Hinchcliffe R, Gokal R. Calcium and magnesium mass transfer in peritoneal dialysis patients using 1.25 mmol/L calcium, 0.25 mmol/L magnesium dialysis fluid. Perit Dial Int. 1993;13:219–223. [PubMed] [Google Scholar]
- 41.Eddington H, Hurst H, Ramli MT, Speake M, Hutchison AJ. Calcium and magnesium flux in automated peritoneal dialysis. Perit Dial Int. 2009;29:536–541. [PubMed] [Google Scholar]
- 42.Hutchison AJ, Were AJ, Boulton HF, Mawer EB, Laing I, Gokal R. Hypercalcaemia, hypermagnesaemia, hyperphosphataemia and hyperaluminaemia in CAPD: improvement in serum biochemistry by reduction in dialysate calcium and magnesium concentrations. Nephron. 1996;72:52–58. doi: 10.1159/000188806. [DOI] [PubMed] [Google Scholar]
- 43.Ryan MF. The role of magnesium in clinical biochemistry: an overview. Ann Clin Biochem. 1991;28(Pt 1):19–26. doi: 10.1177/000456329102800103. [DOI] [PubMed] [Google Scholar]
- 44.Tattersall JE, Dick C, Doyle S, Greenwood RN, Farrington K. Alkalosis and hypomagnesaemia: unwanted effects of a low-calcium CAPD solution. Nephrol Dial Transplant. 1995;10:258–262. [PubMed] [Google Scholar]
- 45.Ejaz AA, McShane AP, Gandhi VC, Leehey DJ, Ing TS. Hypomagnesemia in continuous ambulatory peritoneal dialysis patients dialyzed with a low-magnesium peritoneal dialysis solution. Perit Dial Int. 1995;15:61–64. [PubMed] [Google Scholar]
- 46.Amirmokri P, Morgan P, Bastani B. Intra-peritoneal administration of potassium and magnesium: a practical method to supplement these electrolytes in peritoneal dialysis patients. Ren Fail. 2007;29:603–605. doi: 10.1080/08860220701392215. [DOI] [PubMed] [Google Scholar]
- 47.Gonella M, Buzzigoli G, Bencivelli W, Bartolini V, Betti G. The determination of whole blood magnesium concentration in uremics on chronic dialysis. Nephron. 1981;28:88–89. doi: 10.1159/000182120. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson P, Johansson SG, Danielson BG. Magnesium studies in hemodialysis patients before and after treatment with low dialysate magnesium. Nephron. 1984;37:25–29. doi: 10.1159/000183202. [DOI] [PubMed] [Google Scholar]
- 49.Navarro JF, Mora C, Jimenez A, Torres A, Macia M, Garcia J. Relationship between serum magnesium and parathyroid hormone levels in hemodialysis patients. Am J Kidney Dis. 1999;34:43–48. doi: 10.1016/s0272-6386(99)70106-x. [DOI] [PubMed] [Google Scholar]
- 50.Kelber J, Slatopolsky E, Delmez JA. Acute effects of different concentrations of dialysate magnesium during high-efficiency dialysis. Am J Kidney Dis. 1994;24:453–460. doi: 10.1016/s0272-6386(12)80902-4. [DOI] [PubMed] [Google Scholar]
- 51.Passlick-Deetjen J, Wang W. Magnesium and mortality risk in hemodialysis patients. 2010. Munich, Germany, European Renal Association (ERA) and European Dialysis and Transplant Association (EDTA), XLVII Congress, 25-28 June, Presentation. [DOI] [PubMed] [Google Scholar]
- 52.Kyriazis J, Kalogeropoulou K, Bilirakis L, et al. Dialysate magnesium level and blood pressure. Kidney Int. 2004;66:1221–1231. doi: 10.1111/j.1523-1755.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 53.Jefferies HJ, McIntyre CW. Use of High Magnesium Dialysate Does Not Abrogate Intradialytic Haemodynamic Instability or Haemodialysis-Induced Myocardial Stunning. J Am Soc Nephrol. 2010;21(Suppl):223A. TH-PO490. [Google Scholar]
- 54.Eisenman K, Holley JL. A higher magnesium dialysate concentration treats hypomagnesemia. Perit Dial Int. 2005;25:604–605. [PubMed] [Google Scholar]
- 55.Portale AA, Morris RC., Jr Pathogenesis of secondary hyperparathyroidism in chronic renal insufficiency. Miner Electrolyte Metab. 1991;17:211–220. [PubMed] [Google Scholar]
- 56.Kumar R, Thompson JR. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol. 2011;22:216–224. doi: 10.1681/ASN.2010020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakai K, Komaba H, Fukagawa M. New insights into the role of fibroblast growth factor 23 in chronic kidney disease. J Nephrol. 2010;23:619–625. [PubMed] [Google Scholar]
- 58.Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatr Nephrol. 2010;25:2241–2245. doi: 10.1007/s00467-010-1565-3. [DOI] [PubMed] [Google Scholar]
- 59.Massry SG, Coburn JW, Kleeman CR. Evidence for suppression of parathyroid gland activity by hypermagnesemia. J Clin Invest. 1970;49:1619–1629. doi: 10.1172/JCI106379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferment O, Garnier PE, Touitou Y. Comparison of the feedback effect of magnesium and calcium on parathyroid hormone secretion in man. J Endocrinol. 1987;113:117–122. doi: 10.1677/joe.0.1130117. [DOI] [PubMed] [Google Scholar]
- 61.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 62.Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med. 1984;310:1221–1225. doi: 10.1056/NEJM198405103101904. [DOI] [PubMed] [Google Scholar]
- 63.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 64.Chang W, Pratt S, Chen TH, Nemeth E, Huang Z, Shoback D. Coupling of calcium receptors to inositol phosphate and cyclic AMP generation in mammalian cells and Xenopus laevis oocytes and immunodetection of receptor protein by region-specific antipeptide antisera. J Bone Miner Res. 1998;13:570–580. doi: 10.1359/jbmr.1998.13.4.570. [DOI] [PubMed] [Google Scholar]
- 65.Ruat M, Snowman AM, Hester LD, Snyder SH. Cloned and expressed rat Ca2+-sensing receptor. J Biol Chem. 1996;271:5972–5975. doi: 10.1074/jbc.271.11.5972. [DOI] [PubMed] [Google Scholar]
- 66.Miki H, Maercklein PB, Fitzpatrick LA. Effect of magnesium on parathyroid cells: evidence for two sensing receptors or two intracellular pathways? Am J Physiol. 1997;272:E1–E6. doi: 10.1152/ajpendo.1997.272.1.E1. [DOI] [PubMed] [Google Scholar]
- 67.Anast CS, Mohs JM, Kaplan SL, Burns TW. Evidence for parathyroid failure in magnesium deficiency. Science. 1972;177:606–608. doi: 10.1126/science.177.4049.606. [DOI] [PubMed] [Google Scholar]
- 68.Duran MJ, Borst GC, III, Osburne RC, Eil C. Concurrent renal hypomagnesemia and hypoparathyroidism with normal parathormone responsiveness. Am J Med. 1984;76:151–154. doi: 10.1016/0002-9343(84)90764-2. [DOI] [PubMed] [Google Scholar]
- 69.Mennes P, Rosenbaum R, Martin K, Slatopolsky E. Hypomagnesemia and impaired parathyroid hormone secretion in chronic renal disease. Ann Intern Med. 1978;88:206–209. doi: 10.7326/0003-4819-88-2-206. [DOI] [PubMed] [Google Scholar]
- 70.Quitterer U, Hoffmann M, Freichel M, Lohse MJ. Paradoxical block of parathormone secretion is mediated by increased activity of G alpha subunits. J Biol Chem. 2001;276:6763–6769. doi: 10.1074/jbc.M007727200. [DOI] [PubMed] [Google Scholar]
- 71.Vetter T, Lohse MJ. Magnesium and the parathyroid. Curr Opin Nephrol Hypertens. 2002;11:403–410. doi: 10.1097/00041552-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Mahaffee DD, Cooper CW, Ramp WK, Ontjes DA. Magnesium promotes both parathyroid hormone secretion and adenosine 3′,5′-monophosphate production in rat parathyroid tissues and reverses the inhibitory effects of calcium on adenylate cyclase. Endocrinology. 1982;110:487–495. doi: 10.1210/endo-110-2-487. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez C, Lopez-Barea F, Sanchez-Cabezudo J, et al. Low vs standard calcium dialysate in peritoneal dialysis: differences in treatment, biochemistry and bone histomorphometry. A randomized multicentre study. Nephrol Dial Transplant. 2004;19:1587–1593. doi: 10.1093/ndt/gfh214. [DOI] [PubMed] [Google Scholar]
- 74.Wei M, Esbaei K, Bargman JM, Oreopoulos DG. Inverse correlation between serum magnesium and parathyroid hormone in peritoneal dialysis patients: a contributing factor to adynamic bone disease? Int Urol Nephrol. 2006;38:317–322. doi: 10.1007/s11255-006-0082-6. [DOI] [PubMed] [Google Scholar]
- 75.Pletka P, Bernstein DS, Hampers CL, Merrill JP, Sherwood LM. Relationship between magnesium and secondary hyperparathyroidism during long-term hemodialysis. Metabolism. 1974;23:619–630. doi: 10.1016/s0026-0495(74)80021-1. [DOI] [PubMed] [Google Scholar]
- 76.Parsons V, Papapoulos SE, Weston MJ, Tomlinson S, O'Riordan JL. The long-term effect of lowering dialysate magnesium on circulating parathyroid hormone in patients on regular haemodialysis therapy. Acta Endocrinol (Copenh) 1980;93:455–460. doi: 10.1530/acta.0.0930455. [DOI] [PubMed] [Google Scholar]
- 77.Navarro JF, Mora C, Macia M, Garcia J. Serum magnesium concentration is an independent predictor of parathyroid hormone levels in peritoneal dialysis patients. Perit Dial Int. 1999;19:455–461. [PubMed] [Google Scholar]
- 78.Cho MS, Lee KS, Lee YK, et al. Relationship between the serum parathyroid hormone and magnesium levels in continuous ambulatory peritoneal dialysis (CAPD) patients using low-magnesium peritoneal dialysate. Korean J Intern Med. 2002;17:114–121. doi: 10.3904/kjim.2002.17.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herberth J, Monier-Faugere MC, Mawad HW, et al. The five most commonly used intact parathyroid hormone assays are useful for screening but not for diagnosing bone turnover abnormalities in CKD-5 patients. Clin Nephrol. 2009;72:5–14. doi: 10.5414/cnp72005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pellegrino ED, Biltz RM. The composition of human bone in uremia. Observations on the reservoir functions of bone and demonstration of a labile fraction of bone carbonate. Medicine (Baltimore) 1965;44:397–418. doi: 10.1097/00005792-196509000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Pellegrino ED, Biltz RM, Letteri JM. Inter-relationships of carbonate, phosphate, monohydrogen phosphate, calcium, magnesium and sodium in uraemic bone: comparison of dialysed and non-dialysed patients. Clin Sci Mol Med. 1977;53:307–316. doi: 10.1042/cs0530307. [DOI] [PubMed] [Google Scholar]
- 82.Alfrey AC, Miller NL. Bone magnesium pools in uremia. J Clin Invest. 1973;52:3019–3027. doi: 10.1172/JCI107500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berlyne GM, Ben-Ari J, Szwarcberg J, Kaneti J, Danovitch GM, Kaye M. Increase in bone magnesium content in renal failure in man. Nephron. 1972;9:90–93. doi: 10.1159/000180137. [DOI] [PubMed] [Google Scholar]
- 84.Contiguglia SR, Alfrey AC, Miller N, Butkus D. Total-body magnesium excess in chronic renal failure. Lancet. 1972;1:1300–1302. doi: 10.1016/s0140-6736(72)91032-x. [DOI] [PubMed] [Google Scholar]
- 85.Drueke T. Does magnesium excess play a role in renal osteodystrophy? Contrib Nephrol. 1984;38:195–204. [PubMed] [Google Scholar]
- 86.Kanbay M, Goldsmith D, Uyar ME, Turgut F, Covic A. Magnesium in chronic kidney disease: challenges and opportunities. Blood Purif. 2010;29:280–292. doi: 10.1159/000276665. [DOI] [PubMed] [Google Scholar]
- 87.Brautbar N, Gruber HE. Magnesium and bone disease. Nephron. 1986;44:1–7. doi: 10.1159/000183902. [DOI] [PubMed] [Google Scholar]
- 88.Brunner FP, Thiel G. Re: the use of magnesium-containing phosphate binders in patients with end-stage renal disease on maintenance haemodialysis. Nephron. 1982;32:266. doi: 10.1159/000182860. [DOI] [PubMed] [Google Scholar]
- 89.Gonella M, Ballanti P, Della RC, et al. Improved bone morphology by normalizing serum magnesium in chronically hemodialyzed patients. Miner Electrolyte Metab. 1988;14:240–245. [PubMed] [Google Scholar]
- 90.Moriniere P, Vinatier I, Westeel PF, et al. Magnesium hydroxide as a complementary aluminium-free phosphate binder to moderate doses of oral calcium in uraemic patients on chronic haemodialysis: lack of deleterious effect on bone mineralisation. Nephrol Dial Transplant. 1988;3:651–656. doi: 10.1093/oxfordjournals.ndt.a091722. [DOI] [PubMed] [Google Scholar]
- 91.D'Haese PC, Couttenye MM, Lamberts LV, et al. Aluminum, iron, lead, cadmium, copper, zinc, chromium, magnesium, strontium, and calcium content in bone of end-stage renal failure patients. Clin Chem. 1999;45:1548–1556. [PubMed] [Google Scholar]
- 92.Vaporean ML, Van stone JC. Dialysate magnesium. 6(1)(Seminars in dialysis), 46–51. 1993. [Google Scholar]
- 93.Suzuki H, Amizuka N, Oda K, Noda M, Ohshima H, Maeda T. Histological and elemental analyses of impaired bone mineralization in klotho-deficient mice. J Anat. 2008;212:275–285. doi: 10.1111/j.1469-7580.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amizuka N, Li M, Maeda T. [The interplay of magnesium and vitamin K2 on bone mineralization] Clin Calcium. 2005;15:57–61. [PubMed] [Google Scholar]
- 95.Nakatani S, Mano H, Ryanghyok IM, Shimizu J, Wada M. Excess magnesium inhibits excess calcium-induced matrix-mineralization and production of matrix gla protein (MGP) by ATDC5 cells. Biochem Biophys Res Commun. 2006;348:1157–1162. doi: 10.1016/j.bbrc.2006.07.180. [DOI] [PubMed] [Google Scholar]
- 96.Kesselring K, Siebert G. [Properties of a soluble inorganic pyrophosphatase from rat liver cell nuclei] Hoppe Seylers Z Physiol Chem. 1967;348:585–598. [PubMed] [Google Scholar]
- 97.Baykov AA, Shestakov AS. Two pathways of pyrophosphate hydrolysis and synthesis by yeast inorganic pyrophosphatase. Eur J Biochem. 1992;206:463–470. doi: 10.1111/j.1432-1033.1992.tb16947.x. [DOI] [PubMed] [Google Scholar]
- 98.Zyryanov AB, Shestakov AS, Lahti R, Baykov AA. Mechanism by which metal cofactors control substrate specificity in pyrophosphatase. Biochem J. 2002;367:901–906. doi: 10.1042/BJ20020880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roy ME, Nishimoto SK. Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment: calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone. 2002;31:296–302. doi: 10.1016/s8756-3282(02)00821-9. [DOI] [PubMed] [Google Scholar]
- 100.Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am J Physiol Cell Physiol. 2009;297:C360–C368. doi: 10.1152/ajpcell.00614.2008. [DOI] [PubMed] [Google Scholar]
- 101.Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28:131–141. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 102.Rude RK, Kirchen ME, Gruber HE, Meyer MH, Luck JS, Crawford DL. Magnesium deficiency-induced osteoporosis in the rat: uncoupling of bone formation and bone resorption. Magnes Res. 1999;12:257–267. [PubMed] [Google Scholar]