Abstract
From chronic kidney disease (CKD) Stage 4 onwards, phosphate binders are needed in many patients to prevent the development of hyperphosphataemia, which can result in disturbed bone and mineral metabolism, cardiovascular disease and secondary hyperparathyroidism. In this review, we re-examine the use of magnesium-containing phosphate binders for patients with CKD, particularly as their use circumvents problems such as calcium loading, aluminum toxicity and the high costs associated with other agents of this class. The use of magnesium hydroxide in the 1980s has been superseded by magnesium carbonate, as the hydroxide salt was associated with poor gastrointestinal tolerability, whereas studies with magnesium carbonate show much better gastrointestinal profiles. The use of combined magnesium- and calcium-based phosphate binder regimens allows a reduction in the calcium load, and magnesium and calcium regimen comparisons show that magnesium may be as effective a phosphate binder as calcium. A large well-designed trial has recently shown that a drug combining calcium acetate and magnesium carbonate was non-inferior in terms of lowering serum phosphate to sevelamer-HCl and had an equally good tolerability profile. Because of the high cost of sevelamer and lanthanum carbonate, the use of magnesium carbonate could be advantageous and drug acquisition cost savings would compensate for the cost of introducing routine magnesium monitoring, if this is thought to be necessary and not performed anyway. Moreover, given the potential cost savings, it may be time to re-investigate magnesium-containing phosphate binders for CKD patients with further well-designed clinical research using vascular end points.
Keywords: chronic kidney disease, cost-effectiveness, magnesium, phosphate binder
Introduction
Declining renal function is associated with increasing serum phosphate levels, and while dietary restriction of phosphorus can help to control serum phosphate levels initially, most patients will have hyperphosphataemia from chronic kidney disease (CKD) Stages 3 or 4 onwards [1]. Phosphate binders are therefore required, primarily to prevent and to treat secondary hyperparathyroidism that results from hyperphosphataemia, but also because of increasing concern that phosphate, and possibly calcium also, may have a central role in vascular calcification in patients with CKD [2]. The most recent guidelines [Kidney Disease: Improving Global Outcomes (KDIGO)] suggest that individual values of serum calcium and phosphate, evaluated together, should be used to guide clinical practice rather than the calcium × phosphate product which probably adds nothing to clinical management of CKD patients [3]. Target ranges for serum phosphate and calcium are shown in Table 1 [3, 4], but it is important to note that so far, no randomized controlled trial has tested the benefits or otherwise of adhering to these targets—a situation that cannot be allowed to continue as the costs of treatment escalate. For this reason, the KDIGO guidelines suggest that serum phosphate should simply be lowered towards normal, pointing out that no prospective study has yet examined specific target ranges in relation to outcomes.
Table 1.
| Analyte (disease stage) | K/DOQI (2003) | KDIGO (2009) |
| Serum phosphorus (CKD 3–5) | 0.87–1.49 mmol/L (2.7–4.6 mg/dL) | 0.81–1.45 mmol/L (2.5–4.5 mg/dL) |
| Serum phosphorus (CKD 5D)a | 1.13–1.78 mmol/L (3.5–5.5 mg/dL) | 0.81–1.45 mmol/L (2.5–4.5 mg/dL) |
| Serum calcium | 2.10–2.37 mmol/L (8.4–9.5 mg/dL) | 2.10–2.62 mmol/L (8.4–10.5 mg/dL) |
| Dialysate calcium | 1.25 mmol/L (2.5 mEq/L) | 1.25 or 1.50 mmol/L (2.5 or 3.0 mEq/L) |
CKD stage 5D, CKD stage 5 and patient is on dialysis (HD or PD).
Oral phosphate binders have been used for many years to control phosphate levels in patients with CKD, but as yet we have not found the ideal binder(s) in terms of improved clinical outcomes, efficacy, patient adherence, safety and cost. Traditional phosphate binders containing aluminum or calcium have well-known drawbacks. While aluminum-containing agents are highly efficient phosphate binders, they are now prescribed less often in developed countries because of proven toxicity with long-term use but remain widely used in developing countries. Calcium-based salts are inexpensive, effective and very widely used, but there exists some concern about observational statistical associations between calcium load, hypercalcaemia and vascular calcification [5]. Newer non-aluminum and non-calcium agents, such as sevelamer hydrochloride/carbonate and lanthanum carbonate have the dual advantage of avoiding aluminum toxicity and decreasing the calcium burden, but at high cost. Lanthanum carbonate’s potency also reduces the high pill burden conferred by many binders including sevelamer, although the introduction of sevelamer carbonate granules is a welcome additional step towards improving patient adherence.
In this review, we will re-examine the use of magnesium-containing phosphate binders in patients with CKD. They share the advantages of lanthanum and sevelamer in not containing aluminum but are relatively inexpensive in comparison (Table 2). However, many clinicians are not fully familiar with current understanding of magnesium metabolism and usage in CKD. Therefore, there are various perceptions and concerns that need to be re-examined before nephrologists are likely to start using magnesium-containing phosphate binders more widely. Most of these concerns centre around hypermagnesaemia, the incidence of gastrointestinal disorders and the necessity for monitoring of serum magnesium. In this review, we will examine how the use of magnesium-containing phosphate binders developed from early studies with magnesium hydroxide as an adjunct to aluminum hydroxide to the later use of magnesium carbonate alone and as an adjunct to calcium carbonate or calcium acetate to reduce patients’ calcium load.
Table 2.
| Drug | Dose | Pack size | Mean MSP | DDD | Mean cost per DDD |
| Sevelamer hydrochloride | 800 mg | 180 | €145.59 D,Fr,UK,It,E | 6.4 g | €6.47 D,Fr,UK,It,E |
| Lanthanum carbonate | 750 mg | 90 | €225.23 D,Fr,UK,It,E | 2.25 g | €7.51 D,Fr,UK,It,E |
| Calcium acetate/magnesium carbonate | 435 mg | 180 | €25.91 D,UK,It,E | 2.6 g | €0.86 D,UK,It,E |
| Calcium acetate | 667 mg | 200 | €15.57 UK,It,E | 6.0 g | €0.60 D,UK,It,E |
| 950 mg | 200 | €9.17 D | |||
| Calcium carbonate | 500–1750 mg | 30–200 | €2.64–€16.65 D,Fr,UK,It,E | 10.0 g | €1.15 D,Fr,UK,It,E |
DDD, defined daily dose; MSP, manufacturer selling price.
D, Germany; Fr, France; UK, United Kingdom; It, Italy; E, Spain.
Magnesium-containing phosphate binders have certain intrinsic advantages: they do not contain aluminum, and so their use avoids aluminum toxicity and allows calcium intake to be reduced or eliminated; they have been in use for many years and magnesium-containing phosphate binders are relatively inexpensive compared with sevelamer- or lanthanum-based binders.
Studies of magnesium as a phosphate binder in dialysis patients
Early studies: magnesium as a substitute for aluminum hydroxide.
The first studies to use magnesium compounds as a phosphate binder in dialysis patients date from the early 1980s as an attempt to reduce aluminum hydroxide use (Table 3). This was understandable as some patients found aluminum hydroxide to be unpalatable (so leading to poor compliance), associated with constipation and occasionally nausea and vomiting. More significantly, aluminum is partially absorbed in the gut, accumulates in the body and leads to aluminum toxicity [6, 7, 8]. The very early clinical studies tended to use magnesium hydroxide, and these trials were complicated by diarrhoeal symptoms or mild hyperkalaemia associated with this salt of magnesium [6, 9]. Although these studies also suffered from low numbers of patients, they nevertheless showed that magnesium-containing phosphate binders could reduce the use of aluminum [6, 9] and that parathyroid hormone (PTH) levels were also reduced when they were used alone or in combination with aluminum [9].
Table 3.
Summary of clinical trials involving magnesium-containing phosphate binders in patients undergoing dialysisa
| Year | Author | Journal | Product | Modality | Patients (N) | Design/duration | Dialysate | Result |
| 1982 | Guillot et al. [6] | Nephron | Mg(OH)2 and Al(OH)3—separately and in combination | HD | 9 | Four open study phases: no phosphate binders (period I: 2 weeks), Mg(OH)2 alone (II: 2–5 weeks), Al(OH)3 plus Mg(OH)2 (III: 4–10 weeks), Al(OH)3 alone (IV: 4 weeks) | Mg, 0.5–0.75 mmol/L (1.0–1.5 mEq/L) and Ca, 1.5–1.6 mmol/L (3.0–3.25 mEq/L) | Best control of serum P levels when Al(OH)3 and Mg(OH)2 were used together. |
| 1987 | Oe et al. [9] | Clin Nephrol | Mg(OH)2 and Al(OH)3—separately and in combination | HD | 18 | Open, sequential: Al(OH)3 alone (period I: 6–9 months), Mg(OH)2 alone (II: 2–6.5 months) then Al(OH)3 plus Mg(OH)2 (III: 4–13 months) | Period I: Mg, 0.75 mmol/L; Periods II and III: Mg, 0.00 mmol/L | Allowed reduced aluminum usage. PTH levels fell on Mg(OH)2 treatment (both when used as monotherapy or in conjunction with Al(OH)3). |
| 1986 | O’Donovan et al. [7] | Lancet | MgCO3 versus Al(OH)3 | HD | 50 | Two-year open-label study: 28 pts (chronic hospital-based haemodialysis) given MgCO3, 22 pts (home-based dialysis) given Al(OH)3 | MgCO3 group: Mg < 0.2 mmol/L and Ca, 1.65 mmol/L; Al(OH)3 group: Mg, 0.85 mmol/L and Ca, 1.65 mmol/L | MgCO3 suitable for long-term control of serum phosphate levels when used alone, but difficult to compare groups owing to different dialysis regimens. |
| 1988 | Moriniere et al. [8] | Nephrol Dial Transplant | Mg(OH)2 versus Al(OH)3 | HD | 20 | Sequential open-label study: 20 pts for 6 months; 12 pts for 20 months. | Control period (with Al(OH)3): Mg, 0.75 mmol/L; during Mg(OH)2 period: Mg, 0.375 mmol/L | Replaced Al(OH)3 with Mg(OH)2· Diarrhoea common. |
| Bone histomorphometry performed | Reduced requirement for supplemental calcium during Mg(OH)2 treatment. During Mg(OH)2 treatment, serum PTH levels declined non-significantly; stable serum concentrations of P, Ca, Mg and alkaline phosphatase. | |||||||
| 1993 | Parsons et al. [13] | Nephron | MgCO3/CaCO3 versus CaCO3 versus Al(OH)3 | CAPD | 50 | One-year, open-label, parallel-group study: MgCO3 + CaCO3 (Group I: n = 32); CaCO3 alone (II: n = 10), Al(OH)3 alone (III: n = 8) | All patients given MgCO3 + CaCO3 were given Mg-free dialysate (Ca, 1.65 mmol/L) | Serum P levels were controlled equally well in the MgCO3 + CaCO3 group as in the other groups, without evidence of increased Mg levels. No significant between-group difference in PTH. |
| 1996 | Delmez et al. [10] | Kidney Int | MgCO3/CaCO3 versus CaCO3 | HD | 29 | Two-year, randomized, controlled, crossover trial: MgCO3 + CaCO3 (Phase I); CaCO3 alone (Phase II) | MgCO3 + CaCO3 group: Mg, 0.25 mmol/L (0.6 mg/dL), Ca, 1.25 mmol/L (5 mg/dL) CaCO3 alone: Mg, 0.74 mmol/L (1.8 mg/dL), Ca, 1.25 mmol/L (5 mg/dL) | Serum levels of Ca, P and Mg stable in both phases. Use of MgCO3 enabled higher doses of i.v. calcitriol without hypercalcaemia (0.8 μg/treatment with CaCO3 monotherapy, to 1.5 μg/treatment with MgCO3 + CaCO3; P < 0.02) and reduced Ca intake (from 2.9 to 1.2 g/day, respectively; P < 0.0001). |
| 2004 | Deuber [14] | Nieren- und Hockdruck-krankheiten | CaAc/MgCO3 versus CaCO3 | HD | 50 | Three-year, randomized parallel-group trial: MgCO3 + CaAc (Group I); CaCO3 alone (Group II) | For both groups: Mg, 0.5 mmol/L and Ca, 1.5 mmol/L | Serum levels of Ca and P and plasma iPTH levels were lower in the MgCO3 + CaAc group (all P < 0.05 versus CaCO3 group). |
| 2007 | Spiegel et al. [15] | J Ren Nutr | MgCO3/CaCO3 versus CaAc | HD | 30 | Twelve-week, randomized open-label pilot study: MgCO3 + CaCO3 (Group I; n = 20); CaAc alone (Group II; n = 10) | For both groups: Mg, 0.375 mmol/L (0.75 meq/L) and Ca, 1.25 mmol/L (2.5 meq/L) | Both regimens were generally well tolerated and MgCO3/CaCO3 was at least as effective in control of serum P as CaAc alone, but required less elemental Ca ingestion. |
| 2008 | Tzanakis et al. [16] | Int Urol Nephrol | MgCO3 versus CaCO3 | HD | 46 | Six-month, randomized open-label study: MgCO3 (Group I; n = 25) CaCO3 (Group II; n = 21) | MgCO3 group: Mg, 0.3 mmol/L and Ca, 1.50 mmol/L; CaCO3 group: Mg, 0.48 mmol/L and Ca, 1.50 mmol/L. | The Mg regimen showed equally effective control of serum P and Mg, but better control of serum Ca, than the Ca regimen. Good tolerability profile for Mg regimen: 2 of 25 (8%) withdrew because of diarrhoea or high Mg levels. |
| 2009 | Spiegel et al. [17] | Hemodial Int | MgCO3/CaCO3 | HD | 7 | Eighteen-month open-label pilot study to monitor CAC and V-BMD | Composition of dialysate not mentioned | There was no significant progression of the CAC score and no significant change in V-BMD, and thus Mg may have a favourable effect on these parameters (though the size of the study precludes any firm conclusions). |
| 2009 | McIntyre et al. [19] | Clin J Am Soc Nephrol | Fe–Mg hydroxycarbonate | HD | 63 | Five-week, randomized, placebo-controlled, double-blind parallel-group study: placebo (Group I; n = 21); Fe–Mg hyroxycarbonate, 1 g tds (Group II; n = 21); Fe–Mg hyroxycarbonate, 2 g tds (Group III; n = 21) | Composition of dialysate not mentioned | Lower dose had an acceptable tolerability profile, but only about half of this group had acceptable serum phosphorous control (<1.78 mmol/L). Higher dose group had acceptable phosphate control, with 81% achieving levels < 1.78 mmol/L, but tolerability profile was poor (13 of 21 [61.9%] discontinued owing to adverse events). Serum Mg levels were significantly elevated in both Fe–Mg groups versus placebo. |
| 2010 | de Francisco et al. [20] | Nephrol Dial Transplant | CaAc/MgCO3 versus sevelamer-HCl | HD/HDF | 255 | Twenty-four-week, randomized, controlled, parallel-group investigator-blinded multicentre study: CaAc/MgCO3 (Group I; n = 126); sevelamer-HCl (Group II; n = 129) | For both groups: Mg, 0.5 mmol/L and Ca, 1.5 or 1.25 mmol/L (dependent on prior prescription) | CaAc/MgCO3 was non-inferior to sevelamer, with both treatments significantly lowering serum P by 25 weeks of therapy. Both treatments were equally well tolerated, with minimal increases in serum Ca and Mg levels in the CaAc/MgCO3 group. |
Al(OH)3, aluminium hydroxide; Ca, calcium; CaAc, calcium acetate; CAC, coronary artery calcification; CaCO3, calcium carbonate; CAPD, continuous ambulatory peritoneal dialysis; HD, hemodialysis; HDF, hemodiafiltration; i.v., intravenous; Mg, Magnesium; Mg(OH)2, magnesium hydroxide; MgCO3, magnesium carbonate; P, phosphate; pts, patients; tds, three-times daily; V-BMD, vertebral bone mineral density.
The last study to use magnesium hydroxide as a phosphate binder was published in the late 1980s [8]. Although haemodialysis patients participating in this study had the gastrointestinal symptoms typical of other magnesium hydroxide studies, it was notable for three main reasons. Firstly, it was a long-term study, in which aluminum hydroxide was replaced by magnesium hydroxide (given in addition to high doses of oral calcium) for 6 months in 20 patients and for 20 months in 12 patients. Secondly, in addition to the control of serum phosphate, calcium, magnesium and alkaline phosphatase, switching from aluminum to magnesium hydroxide lowered PTH (C terminal) levels (from 260 ± 214 pg/mL to 185 ± 182 pg/mL) and intake of elemental calcium [from 4.4 (110 mmol/day) to 3.0 g/day (76 mmol/day)], non-significantly. Thirdly, bone mineralization (mineral apposition rate) remained constant throughout the study at approximately normal levels (between 0.63 and 0.65 μm/day) as shown by bone histomorphometry.
A final study that compared aluminum- and magnesium-containing phosphate binders used magnesium carbonate rather than magnesium hydroxide [7]. Although this was a 2-year study and involved a reasonable number of patients, it was difficult to compare the two phosphate-binding regimens: magnesium carbonate was used in hospital-based chronic maintenance haemodialysis patients (n = 28), while aluminum hydroxide was used in haemodialysis patients at home (n = 24). This study demonstrated that magnesium carbonate was suitable for long-term use and was an effective phosphate binder when used with magnesium-free dialysate. Some reduction in PTH was noted, although no mention is made of the tolerability profiles of the two regimens in this short paper. However, it was suggested that magnesium carbonate has a better tolerability profile than magnesium hydroxide, particularly with regard to gastrointestinal side effects, but there have been no direct comparisons of these regimens [10].
Early studies comparing the use of aluminum hydroxide and magnesium hydroxide or magnesium carbonate showed that magnesium could be used as an effective phosphate binder, but it is widely acknowledged that magnesium hydroxide caused gastrointestinal side effects, whereas the carbonate salt is much better tolerated.
Later studies: magnesium- and calcium-containing phosphate binders.
Calcium carbonate and calcium acetate started to be used as phosphate binders in the 1980s as an alternative to aluminum [11, 12]. Patients undergoing continuous ambulatory peritoneal dialysis participated in a 1-year, open-label parallel-group study to compare a combination of magnesium carbonate plus calcium carbonate (n = 32) in equal proportions, with calcium carbonate alone (n = 10) or aluminum hydroxide alone (n = 8) [13]. A magnesium-free peritoneal dialysate was used in the group given magnesium as a phosphate binder. Serum phosphate levels were controlled equally well with combined magnesium and calcium carbonate treatment as with calcium carbonate or aluminum hydroxide used alone. Mean (±SD) phosphate levels after 1 year were 1.36 (±0.41) mmol/L for combined treatment, 1.38 (±0.27) mmol/L (calcium carbonate monotherapy) and 1.46 (±0.5) mmol/L (aluminum hydroxide monotherapy). There was, moreover, no significant difference (P > 0.05) between groups in serum magnesium concentration or PTH levels.
The use of combined magnesium carbonate/calcium carbonate phosphate-binder treatment was compared with calcium carbonate monotherapy in a 2-year, randomized, controlled crossover trial in 29 haemodialysis patients [10]. The dialysate magnesium concentration was 0.25 mmol/L (0.6 mg/dL) in the magnesium carbonate plus calcium carbonate group and 0.74 mmol/L (1.8 mg/dL) in the calcium carbonate monotherapy group. Calcium carbonate and magnesium carbonate doses were adjusted in both groups to achieve target serum calcium concentrations of 2.37–2.62 mmol/L (9.5–10.5 mg/dL) and serum phosphate concentrations of <1.94 mmol/L (<6.0 mg/dL). Serum levels of calcium, phosphate and magnesium were similar in the combined and the monotherapy phases. However, the control of phosphate levels in the combined magnesium and calcium phase occurred despite the ingestion of significantly less calcium binder. Mean elemental calcium used (±SD) was 2.9 (±0.4) g/day during calcium carbonate monotherapy and 1.2 (±0.2) g/day during magnesium carbonate plus calcium carbonate treatment; P < 0.0001. Moreover, the use of magnesium carbonate allowed higher doses of intravenous calcitriol to be used without hypercalcaemia (0.8 μg/treatment with calcium carbonate monotherapy to 1.5 μg/treatment with magnesium carbonate plus calcium carbonate; P < 0.02). During this study, the mean (±SD) elemental magnesium intake was 465 ± 52 mg/day (range 214–858 mg/day), and despite this high intake of magnesium, this was generally well tolerated: no patients reported any gastrointestinal symptoms including loose stools, diarrhoea or bloating. Serum magnesium levels were slightly elevated but not different from the run-in levels.
Treatment with combined magnesium carbonate/calcium carbonate phosphate binders was generally well tolerated: no patient reported gastrointestinal symptoms.
Another study compared a combined phosphate-binder regimen of magnesium carbonate plus a calcium salt to monotherapy calcium carbonate [14]. In this 3-year parallel-group study, 50 haemodialysis patients were randomized to either combination therapy with calcium acetate [pills containing 435 mg calcium acetate (110 mg elemental calcium) plus 235 mg magnesium carbonate (60 mg elemental magnesium)] or calcium carbonate monotherapy. The dialysate was the same for both groups with calcium of 1.5 mmol/L and magnesium of 0.5 mmol/L. Dose adjustment of both phosphate-binder groups was based on serum calcium and phosphate concentrations and plasma intact parathyroid hormone (iPTH) levels. Combination treatment with magnesium carbonate and calcium acetate resulted in significantly lower serum phosphate and calcium concentrations compared with calcium carbonate monotherapy (both P < 0.05) (Figure 1), and a significant decrease in plasma iPTH levels (P < 0.05). Indeed, iPTH levels rose somewhat in the calcium monotherapy group. Combination therapy resulted in a significant increase in serum magnesium concentration (P < 0.05), although this remained within normal values (0.65–1.06 mmol/L). Safety and tolerability of the regimens were not mentioned in this publication [14].
Fig. 1.
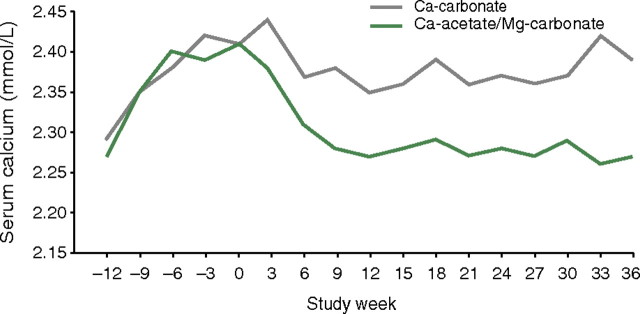
Significant decrease in serum calcium concentration in patients given a phosphate binder consisting of calcium acetate plus magnesium carbonate (versus calcium carbonate alone) [14]. (Reprinted from Deuber [14], with permission from Dustri-Verlag, Dr. Karl Feistle GmbH & Co.).
Combination treatment with magnesium carbonate and calcium acetate resulted in significantly lower serum phosphate and calcium concentrations compared with calcium carbonate monotherapy (both P < 0.05).
A pilot study has compared combined magnesium carbonate plus calcium carbonate with calcium acetate monotherapy [15]. Patients undergoing haemodialysis were randomized in a 2:1 ratio to receive either calcium carbonate plus magnesium carbonate (n = 20; 86 mg elemental magnesium, 100 mg elemental calcium) or calcium acetate alone (n = 10) at their previous dose, or equivalent, of elemental calcium, for 12 weeks. Phosphate-binder doses were titrated to a target serum phosphate concentration of under 5.5 mg/dL (1.78 mmol/L), and the dialysate was the same for both groups, including calcium at 1.25 mmol/L (2.5 meq/L) and magnesium at 0.375 mmol/L (0.75 meq/L). Combination therapy with magnesium carbonate and calcium carbonate offered control of serum phosphate that was at least as good as that with calcium acetate alone. The proportion of patients with serum phosphate <5.5 mg/dL (the K/DOQI guideline threshold) was 70.6 and 62.5%, respectively, in the efficacy phase (last 8 weeks) of the study (P > 0.05). Moreover, this equivalent control was obtained with a significant decrease in mean (±SD) elemental calcium consumption in the magnesium/calcium carbonate group (908 ± 24 mg/day versus 1743 ± 37 mg/day, respectively; P < 0.0001). Serum calcium concentrations were also significantly higher in the calcium acetate monotherapy group than in those taking magnesium carbonate plus calcium carbonate (Figure 2; P < 0.003). Serum magnesium levels were higher in patients receiving magnesium carbonate than in the calcium acetate group (P < 0.03), but no patients experienced symptoms related to hypermagnesaemia [the highest serum magnesium reading was 1.95 mmol/L (3.9 meq/L)]. The pill burden per meal was significantly, but not dramatically, lower in patients receiving magnesium carbonate plus calcium acetate versus those receiving calcium acetate alone (3.0 ± 0.1 versus 3.4 ± 0.1 pill per meal, P < 0.0001). In this study, safety and tolerability were investigated, and there were no significant between-group differences, with both regimens being generally well tolerated with a similar incidence of gastrointestinal symptoms.
Fig. 2.
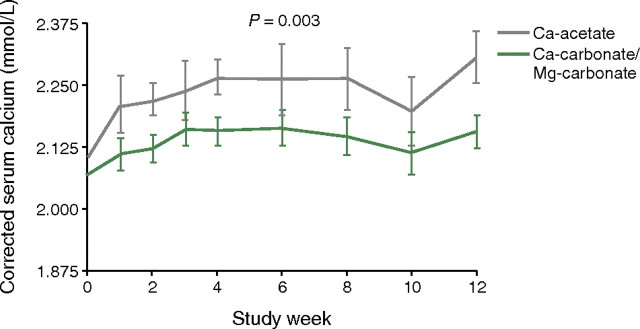
Lower serum calcium levels in patients given a magnesium-containing phosphate binder (magnesium carbonate plus calcium carbonate) than in those taking calcium acetate alone (P = 0.003) Reprinted from Spiegel et al. [15], Copyright 2007, with permission from Elsevier.
Combination therapy with magnesium carbonate and calcium carbonate offered control of serum phosphate that was at least as good as that with calcium acetate alone, but with significantly decreased elemental calcium consumption.
A 6-month, randomized open-label study involving 46 haemodialysis patients investigated whether magnesium carbonate was as effective as calcium carbonate (both agents were the sole phosphate binder used in each group) [16]. This study used lower magnesium concentrations (0.3 mmol/L) in the dialysate for the magnesium group compared with 0.48 mmol/L in the calcium group to reduce the risk of hypermagnesaemia, whereas dialysate calcium was 1.50 mmol/L for both groups. There were no significant differences in mean values for Months 1–6 between the magnesium and calcium groups for the control of serum phosphate [1.77 mmol/L (5.47 mg/dL) versus 1.71 mmol/L (5.29 mg/dL), respectively; P = NS] or magnesium [1.06 mmol/L (2.57 mg/dL) versus 0.99 mmol/L (2.41 mg/dL), respectively; P > 0.05], but serum calcium concentration was lower in the magnesium than in the calcium group [2.28 mmol/L (9.13 mg/dL) versus 2.4 mmol/L (9.60 mg/dL), respectively; P < 0.001]. Levels of iPTH were not significantly different between the magnesium and calcium groups after 6 months (251 versus 212 pg/mL, respectively; P > 0.05). At Month 6, the percentages of patients with serum levels of phosphate, Ca × P product and iPTH that fell within the K/DOQI guidelines were similar in both groups, whereas more patients in the magnesium group (17/23; 73.91%) than in the calcium group (5/20, 25%) had serum calcium levels that fell within these guidelines (P < 0.01). Furthermore, magnesium carbonate was generally well tolerated with only 2 of 25 patients (8%) withdrawn owing to adverse events (one because of diarrhoea, the other because of recurrent hypermagnesaemia).
Treatment with magnesium carbonate in comparison to calcium carbonate resulted in a similar proportion of patients whose serum levels of phosphate, Ca × P product and iPTH fell within the K/DOQI guidelines, but more patients in the magnesium group than in the calcium group had serum calcium levels that fell within these guidelines (P < 0.01). Moreover, magnesium carbonate was generally well tolerated.
A very small, 18-month open-label pilot study of magnesium carbonate/calcium carbonate (elemental magnesium, 86 mg/elemental calcium, 100 mg) in haemodialysis patients (n = 7) is worth noting, as coronary artery calcification (CAC) and vertebral bone mineral density (V-BMD) were monitored [17]. Overall, there was no significant progression of the CAC score and no significant change in V-BMD, raising the possibility that magnesium may have a favourable effect on these parameters (though the size of the study precludes any firm conclusions). This study is discussed further in this supplement, in the review concerning magnesium, vascular calcification and outcomes in patients with CKD [18].
Another study that examined magnesium levels in dialysis patients in 2009 investigated the use of iron–magnesium hydroxycarbonate (Fermagate) as a novel oral phosphate binder [19]. Although this had a robust clinical study design (5-week, randomized, placebo-controlled, parallel-group three-arm trial testing two doses of phosphate binder) and a reasonable number of haemodialysis patients (n = 63), the results stand alone from the rest of the magnesium phosphate-binding literature. Iron–magnesium hydroxycarbonate has a more complex structure and different phosphate-binding characteristics than ‘plain’ magnesium-containing phosphate binders such as magnesium carbonate. It contains magnesium and ferric iron contained in an insoluble hydrotalcite structure with the iron and magnesium held in tight crystalline layers, with carbonate groups which are exchanged for phosphate, lying between the layers. In this short-term study, the proportion of patients achieving serum phosphate levels below the upper K/DOQI recommended value (1.78 mmol/L) in the placebo, 1 and 2 g iron–magnesium hydroxycarbonate groups was 30.0, 52.4 and 81.0%, respectively. Both treatment groups had significantly increased serum magnesium levels compared with the placebo arm, with the higher dose group suffering a greater number of adverse events such as diarrhoea, vomiting and dyspepsia. The most frequent reason for withdrawal during the treatment and follow-up periods was an adverse event, accounting for 20 (31.8%) patients (placebo, n = 6; 1 g arm, n = 1; 2 g arm, n = 13).
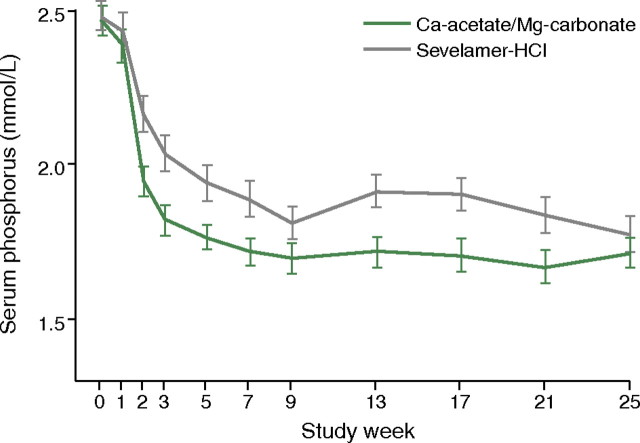
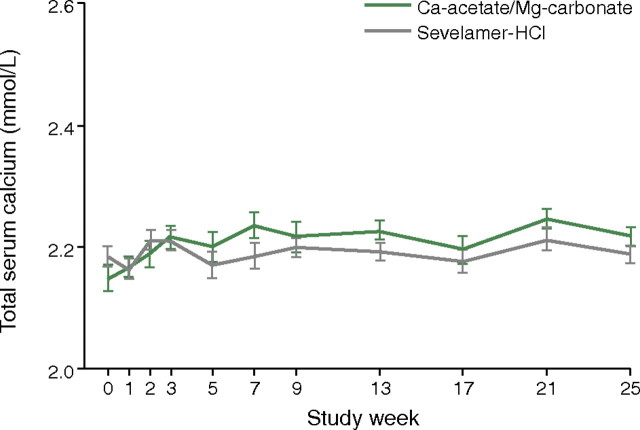
A large well-designed clinical trial published in 2010 compared the combination product calcium acetate and magnesium carbonate with sevelamer [20]. In this randomized, controlled, parallel-group, investigator-blinded multicentre study, 255 haemodialysis or haemodiafiltration patients were randomized to receive calcium acetate/magnesium carbonate (n = 126) or sevelamer (n = 129). The primary efficacy measure was to show non-inferiority of calcium acetate/magnesium carbonate to sevelamer in lowering serum phosphate levels to below K/DOQI targets after 24 weeks of treatment. This was fulfilled, and there was no significant difference between groups regarding the lowering of serum phosphate levels at the end of the study (P = 0.069) (Figure 3). However, the area under the curve for serum phosphate (P = 0.0042) and the number of visits above K/DOQI (≤1.78 mmol/L, P = 0.0198) and KDIGO targets (≤1.45 mmol/L, P = 0.0067) were significantly lower with calcium acetate/magnesium carbonate. Also, the time to reach serum phosphate levels of ≤1.78 and ≤1.45 mmol/L when compared for the calcium acetate/magnesium carbonate and sevelamer regimens was significantly shorter (i.e. 16 versus 30 days, P = 0.0018 and 57 versus 140 days, P = 0.0052), using an identical dose titration protocol. Average (±SD) daily study medication intake was also significantly lower in the calcium acetate/magnesium carbonate group by Week 25 than in the sevelamer group (7.3 ± 3.03 versus 8.1 ± 2.87 tablets/day, respectively; P = 0.0420). While ionized serum calcium levels were not significantly different between groups, total serum calcium increased slightly in the calcium acetate/magnesium carbonate group (Figure 4: treatment difference 0.0477 mmol/L; P = 0.0032) but this was not associated with a higher risk of hypercalcaemia. A small and asymptomatic increase in serum magnesium also occurred in this group (treatment difference 0.2597 mmol/L, P < 0.0001). The tolerability profiles were not affected as both groups had a similar number of adverse events and both regimens were equally well tolerated. There was, moreover, no significant difference between groups with regard to the Gastrointestinal Quality of Life Index. Overall, the authors concluded that calcium acetate/magnesium carbonate had a good tolerability profile and was at least as efficient in lowering serum phosphate as sevelamer and so may represent an effective treatment for hyperphosphataemia.
Fig. 3.
Reductions in serum phosphate levels with treatment with either a magnesium-containing phosphate binder (calcium acetate plus magnesium carbonate) or sevelamer hydrochloride [20]. Reprinted from de Francisco et al. [20], by permission of Oxford University Press.
Fig. 4.
Total serum calcium levels in patients given a magnesium-containing phosphate binder (calcium acetate plus magnesium carbonate) or sevelamer hydrochloride [20]. Reprinted from de Francisco et al. [20], by permission of Oxford University Press.
When used as a phosphate binder, the combination of calcium acetate/magnesium carbonate had a good tolerability profile and was non-inferior to sevelamer-HCl.
Benefits and concerns associated with use of magnesium in CKD
Current usage of magnesium as a phosphate binder is not as high as might be expected, and the reasons behind this are difficult to discern. Partly it is due to the previous absence of major backing and promotion from a pharmaceutical company, and perhaps concerns remain about the gastrointestinal effects of the early hydroxide compounds as opposed to the more recent carbonate. As a result of both these factors, the CKD magnesium literature has been relatively sparse up to this time. However, further research is clearly required since magnesium usage has the potential to minimize vascular calcification in dialysis patients as first suggested by Meema in 1987 [21] (see the review in this supplement by Massy and Drüeke [18]).
Concern about the potential for adverse events resulting from hypermagnesaemia is genuine, but the few reports that exist in the literature mostly relate to magnesium levels of up to three times the upper limit of normal (which are not reached in any of the studies looking at magnesium as a phosphate binder) or the use of intravenous magnesium infusions such as in pre-eclampsia. The reduction in both HD and PD dialysate magnesium levels to 0.5–0.25 mmol/L in many countries greatly reduces the likelihood of severe hypermagnesaemia in dialysis patients, and it is often forgotten that prior to this reduction, many dialysis patients were routinely moderately hypermagnesaemic without apparent ill effects. However, a very practical concern around greater use of magnesium as a phosphate binder is that serum magnesium levels are not routinely measured in many dialysis clinics. Some may argue that routine monitoring is not necessary since the safety margin appears significant, but this is unlikely to satisfy the concerns of nephrologists unfamiliar with usage of magnesium-containing drugs. The cost of introducing magnesium monitoring would be offset by the lower cost of magnesium-containing phosphate binders over sevelamer or lanthanum, and most modern multi-channel biochemistry analysers can report magnesium if required without major cost implications (note that magnesium measurements are also covered by Jahnen-Dechent et al. [22] in this supplement). In the UK, the approximate cost of performing a serum magnesium test is £2–£3 (UK sterling); in Germany, it is mostly the same price as serum calcium (e.g. 1.40 €, for private insurance 2.33 €).
Discussion
Over the last 20 years, magnesium-based phosphate binders have been tried as an alternative or supplement, first to aluminum- and then to calcium-based phosphate binders. In the first instance, this was to avoid aluminum toxicity and secondly to avoid or minimize the risk of hypercalcaemia. The discussion of magnesium-containing phosphate binder based on clinical trials in the literature is, however, complicated for several reasons (Table 3). There are comparatively few published studies and of those that have been published most are poor quality with either small numbers of subjects or conducted over a short duration. In addition, the older studies tended to use magnesium hydroxide rather than magnesium carbonate, and it appears that magnesium hydroxide is associated with a high degree of gastrointestinal adverse events. Newer formulations using magnesium carbonate instead of magnesium hydroxide appeared to result in an improved tolerability profile [15, 16, 20]. The only side effects in these studies were mild diarrhoea in few patients [15, 16]. Although magnesium carbonate appears to be well tolerated, concerns about gastrointestinal side effects still persist. Without doubt, the best quality trial to date is also the most recent [20]. It is notable that there was no difference in phosphate lowering and in tolerability using the combination product magnesium carbonate/calcium acetate compared to sevelamer-HCl and calculated costs for the treatment in this trial were 80% lower for the combination product.
Given the high costs associated with sevelamer and lanthanum, the way ahead for magnesium-containing phosphate binders must include further clinical outcome trials as well as pharmaco-economic evaluations. The designs of such trials should be simple and of clinical utility, such as randomization of new dialysis patients to either calcium acetate or a non-calcium containing phosphate binder or magnesium carbonate (alone or in combination with calcium acetate) and should focus on vascular end points. Moreover, as health care costs become an ever more important factor, re-examination of the use of magnesium as a drug in CKD is timely.
Acknowledgments
The authors thank Dr Richard Clark and Dr Martina Sintzel, for providing writing and editorial assistance on behalf of Fresenius Medical Care Deutschland GmbH. Fresenius also made an unrestricted educational grant to meet the cost of preparing this article. These declarations are in line with the European Medical Writers’ Association guidelines.
Conflict of interest statement. M.W. has received speakers’ or consultancy honoraria from and participated in clinical trials with Abbott, Amgen, Fresenius and Ineos. A.J.H. has received speakers' or consultancy honoraria from and participated in clinical trials with Shire Pharmaceuticals, Ineos, Amgen Corp, Fresenius Inc. and Mitsubishi Pharmaceuticals.
References
- 1.Rees L, Shroff RC. Phosphate binders in CKD: chalking out the differences. Pediatr Nephrol. 2010;25:385–394. doi: 10.1007/s00467-009-1329-0. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 3.Moe SM. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;76:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation Inc. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 5.Hutchison AJ. Oral phosphate binders. Kidney Int. 2009;75:906–914. doi: 10.1038/ki.2009.60. [DOI] [PubMed] [Google Scholar]
- 6.Guillot AP, Hood VL, Runge CF, et al. The use of magnesium-containing phosphate binders in patients with end-stage renal disease on maintenance hemodialysis. Nephron. 1982;30:114–117. doi: 10.1159/000182446. [DOI] [PubMed] [Google Scholar]
- 7.O'Donovan R, Baldwin D, Hammer M, et al. Substitution of aluminium salts by magnesium salts in control of dialysis hyperphosphataemia. Lancet. 1986;1:880–882. doi: 10.1016/s0140-6736(86)90987-6. [DOI] [PubMed] [Google Scholar]
- 8.Moriniere P, Vinatier I, Westeel PF, et al. Magnesium hydroxide as a complementary aluminium-free phosphate binder to moderate doses of oral calcium in uraemic patients on chronic haemodialysis: lack of deleterious effect on bone mineralisation. Nephrol Dial Transplant. 1988;3:651–656. doi: 10.1093/oxfordjournals.ndt.a091722. [DOI] [PubMed] [Google Scholar]
- 9.Oe PL, Lips P, van der MJ, et al. Long-term use of magnesium hydroxide as a phosphate binder in patients on hemodialysis. Clin Nephrol. 1987;28:180–185. [PubMed] [Google Scholar]
- 10.Delmez JA, Kelber J, Norword KY, et al. Magnesium carbonate as a phosphorus binder: a prospective, controlled, crossover study. Kidney Int. 1996;49:163–167. doi: 10.1038/ki.1996.22. [DOI] [PubMed] [Google Scholar]
- 11.Malberti F, Surian M, Poggio F, et al. Efficacy and safety of long-term treatment with calcium carbonate as a phosphate binder. Am J Kidney Dis. 1988;12:487–491. doi: 10.1016/s0272-6386(88)80099-4. [DOI] [PubMed] [Google Scholar]
- 12.Mai ML, Emmett M, Sheikh MS, et al. Calcium acetate, an effective phosphorus binder in patients with renal failure. Kidney Int. 1989;36:690–695. doi: 10.1038/ki.1989.247. [DOI] [PubMed] [Google Scholar]
- 13.Parsons V, Baldwin D, Moniz C, et al. Successful control of hyperparathyroidism in patients on continuous ambulatory peritoneal dialysis using magnesium carbonate and calcium carbonate as phosphate binders. Nephron. 1993;63:379–383. doi: 10.1159/000187238. [DOI] [PubMed] [Google Scholar]
- 14.Deuber HJ. Long-term Efficacy and safety of an oral phosphate binder containing both calcium acetate and magnesium carbonate in hemodialysis patients. Nieren- und Hochdruckkrankheiten. 2004;33:403–408. [Google Scholar]
- 15.Spiegel DM, Farmer B, Smits G, et al. Magnesium carbonate is an effective phosphate binder for chronic hemodialysis patients: a pilot study. J Ren Nutr. 2007;17:416–422. doi: 10.1053/j.jrn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Tzanakis IP, Papadaki AN, Wei M, et al. Magnesium carbonate for phosphate control in patients on hemodialysis. A randomized controlled trial. Int Urol Nephrol. 2008;40:193–201. doi: 10.1007/s11255-007-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel DM, Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13:453–459. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 18.Massy ZA, Drüeke TB. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis, and survival. Clin Kidney J. 2012;5(Suppl 1):i52–i61. doi: 10.1093/ndtplus/sfr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre CW, Pai P, Warwick G, et al. Iron-magnesium hydroxycarbonate (fermagate): a novel non-calcium-containing phosphate binder for the treatment of hyperphosphatemia in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:401–409. doi: 10.2215/CJN.02630608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Francisco AL, Leidig M, Covic AC, et al. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol Dial Transplant. 2010;25:3707–3717. doi: 10.1093/ndt/gfq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int. 1987;32:388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 22.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]