Abstract
Magnesium (Mg2+) is the fourth most abundant cation in the body. Thus, magnesium homeostasis needs to be tightly regulated, and this is facilitated by intestinal absorption and renal excretion. Magnesium absorption is dependent on two concomitant pathways found in both in the intestine and the kidneys: passive paracellular transport via claudins facilitates bulk magnesium absorption, whereas active transcellular pathways mediate the fine-tuning of magnesium absorption. The identification of genes responsible for diseases associated with hypomagnesaemia resulted in the discovery of several magnesiotropic proteins. Claudins 16 and 19 form the tight junction pore necessary for mass magnesium transport. However, most of the causes of genetic hypomagnesaemia can be tracked down to transcellular magnesium transport in the distal convoluted tubule. Within the distal convoluted tubule, magnesium reabsorption is a tightly regulated process that determines the final urine magnesium concentration. Therefore, insufficient magnesium transport in the distal convoluted tubule owing to mutated magnesiotropic proteins inevitably leads to magnesium loss, which cannot be compensated for in downstream tubule segments. Better understanding of the molecular mechanism regulating magnesium reabsorption will give new opportunities for better therapies, perhaps including therapies for patients with chronic renal failure.
Keywords: human genetic disease, hypomagnesaemia, magnesium homeostasis, TRPM6
Introduction
As magnesium (Mg2+) is a cofactor of many enzymes, it is involved in all major cellular processes such as energy metabolism, DNA transcription and protein synthesis. Physiologically, Mg2+ plays an essential role in bone formation, neuromuscular stability and muscle contraction. Therefore, the tight regulation of plasma Mg2+ levels is of vital importance. Hypomagnesaemia occurs because of decreased gastrointestinal absorption or increased renal Mg2+ excretion and is associated with a wide spectrum of diseases, including Type 2 diabetes, hypertension, osteoporosis, tetany, seizures and depression [1–4]. This issue has been discussed in greater detail in the review by Jahnen-Dechent and Ketteler [5] and Geiger and Wanner [6] in this supplement. Certain drug therapies (e.g. diuretics, aminoglycosides, cetuximab therapy and immunosuppressive agents) can result in acquired renal Mg2+ wasting and associated low serum Mg2+ levels [7]. Hypomagnesaemia can be treated with oral Mg2+ supplements, though at high doses these might cause diarrhoea. Oral Mg2+ supplements are also often given with potassium (K+) supplements because Mg2+ deficiency is frequently associated with hypokalaemia [8]. In severely hypomagnesaemic patients, intravenous supplementation can be essential to restore the patient's Mg2+ levels without discomforting the patient. During the last decade, human hereditary disorders have given great insight into the molecular pathways involved in the regulation of Mg2+ homeostasis (Table 1). In this review, we will focus on the regulation of magnesium homeostasis and we will describe the genetic causes of hypomagnesaemia and the underlying molecular mechanisms.
Table 1.
Human genetic magnesium transport disordersa
| Disease | OMIM | Renal segment | Gene | Protein | Protein full name | Serum Mg2+ | Urine Mg2+ | Other symptoms |
| Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis | 248250 | TAL | Claudin-16 and Claudin-19 | Claudin-16 and claudin-19 | Claudin-16 and claudin-19 | ↓ | ↑ | Nephrocalcinosis and visual impairment |
| Bartter's syndrome | 241200 | TAL | SLC12A1, BSND, CLCNKB, KCNJ1 | NKCC, Barttin, ClC-Kb, ROMK | Na+-K+-2Cl− cotransporter, Barttin, ClC-Kb Cl channel, ROMK K channel | ↓ | Hypokalaemic alkalosis, elevated renin and aldosterone | |
| Hypomagnesaemia with secondary hypocalcemia | 602014 | DCT | TRPM6 | TRPM6 | Transient receptor potential melastatin member 6 | ↓ | ↑ | Epileptic seizures, muscle spasms and mental retardation |
| Isolated autosomal-recessive hypomagnesaemia | 611718 | DCT | EGF | EGF | Epiderminal growth factor | ↓ | ↑ | Epileptic seizures and mental retardation |
| Autosomal-dominant hypomagnesaemia | 176260 | DCT | KCNA1 | Kv1.1 | Voltage-gated K channel 1.1 | ↓ | Muscle cramps, tetany and tremor | |
| Gitelman syndrome | 263800 | DCT | NCC | NCC | Na–Cl cotransporter | ↓ | ↑ | Muscle weakness, tetany and fatigue |
| Isolated dominant hypomagnesaemia | 154020 | DCT | FXYD2 | Na+/K+-ATPase | Na+/K+-ATPase | ↓ | ↑ | Convulsions |
| Maturity-onset diabetes of the young | 137920 | DCT | HNF1B | HNF1B | Hepatocyte nuclear factor 1 | ↓ | ↑ | Neonatal diabetes and renal malformation |
| SeSAME syndrome | 612780 | DCT | KCNJ10 | Kir4.1 | Kir4.1 K channel | ↓ | Sensorineural deafness, seizures and mental retardation |
Overview of genetic disease associated with hypomagnesaemia, the genes held responsible. The table shows which proteins are mutated and the renal segment in which they are expressed. Effects on serum and urine Mg2+ concentrations and other symptoms of the disease are shown in the last columns. TAL, thick ascending limb of Henle’s loop; DCT, distal convoluted tubule; OMIM, online Mendelian inheritance in man.
Regulation of magnesium homeostasis
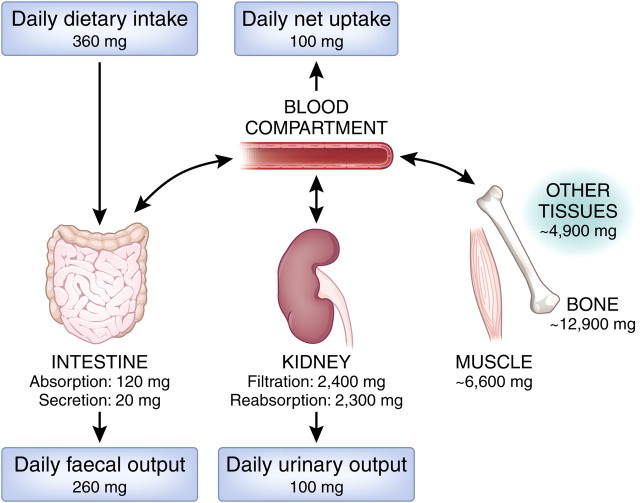
Many studies have shown intestinal Mg2+ absorption is balanced against renal Mg2+ excretion [9–11]. In times of a temporary Mg2+ deficit, the body depends on the availability of Mg2+ in bone to maintain constant serum levels [12]. Therefore, Mg2+ homeostasis depends on three organs: the intestine, facilitating Mg2+ uptake; bone, the Mg2+ storage system of the body and the kidneys, which are responsible for Mg2+ excretion.
Intestinal magnesium uptake
In healthy people, Mg2+ blood plasma concentrations range between 0.65 and 1.05 mmol/L. In order to maintain these levels, a daily Mg2+ intake of 320 mg for men and 420 mg for women is recommended by the US Food and Nutrition Board. Approximately 30–50% of dietary Mg2+ is absorbed by the intestine (Figure 1). However, when Mg2+intake is low, the absorption percentage can rise to ∼80% [13]. Mg2+absorption takes place mainly in the distal small intestine and in the colon [14]. Thus, shortening of the rat ileum, for example, results in a substantial decrease of Mg2+ absorption [15].
Fig. 1.
Magnesium homeostasis. Panels represent the daily amount of Mg2+ intake and excretion. A daily net intake of ∼100 mg in the intestine results in a balanced 100 mg excretion in the kidney. In times of Mg2+ shortage, other tissues such as bone and muscle provide Mg2+ to restore blood Mg2+ levels. See also “Magnesium basics” in this supplement [5]. The conversion factor from milligrams to millimole is 0.04113.
Absorption pathways.
Two Mg2+-absorbing pathways have been identified in the mammalian intestine (Figure 2). Paracellular transport involves the absorption of Mg2+ through the small spaces between the epithelial cells and is a passive mechanism. Secondly, the transcellular pathway involves the active transport of Mg2+ to the blood through the interior of the epithelial cell. This second type of Mg2+ transport is subject to tight regulation since the ions have to pass through two cell membranes.
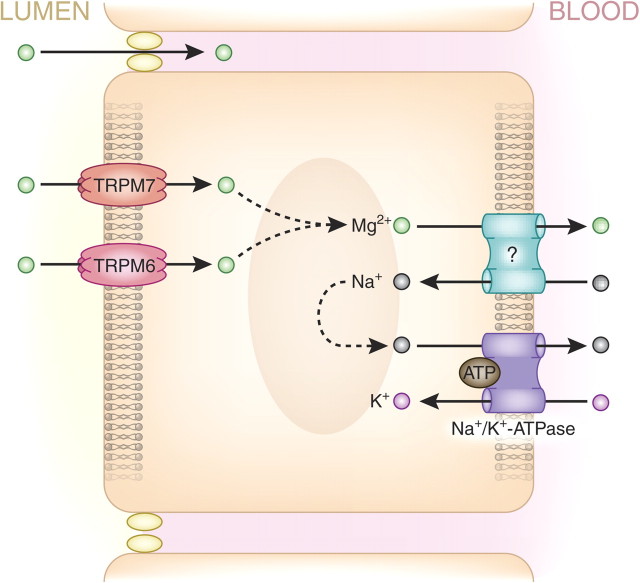
Fig. 2.
A schematic overview of magnesium absorption pathways in the intestine, showing proteins associated with Mg2+ transport in enterocytes. In the intestinal epithelia, paracellular Mg2+ transport occurs via unidentified claudins, occuring concurrently with transcellular Mg2+ transport via transient receptor potential channel melastatin member 6 (TRPM6) and TRPM7 to facilitate Mg2+ absorption.
Paracellular Mg2+ absorption is responsible for 80–90% of intestinal Mg2+ uptake. The driving force behind this passive Mg2+ transport is supplied by the high luminal Mg2+ concentration, which ranges between 1.0 and 5.0 mmol/L, and the lumen-positive transepithelial voltage of ∼+5 mV [2]. Paracellular Mg2+ absorption relies on tight junction permeability, which is still poorly understood. The ileum and distal parts of the jejunum are known to be the most permeable for ions because of the relatively low expression of ‘tightening’ claudins 1, 3, 4, 5 and 8 [16]. As such, paracellular Mg2+ transport seems mainly restricted to these areas that lack the ‘tightening’ claudins. Claudins 16 and 19, known to be involved in Mg2+ permeability [17], are not expressed in the intestine [16]. The exact mechanism facilitating paracellular Mg2+ absorption, therefore, remains unknown.
Transient receptor potential channel melastatin member 6 (TRPM6) and TRPM7 Mg2+ channels mediate transcellular absorption. Whereas TRPM7 is ubiquitously expressed; intestinal TRPM6 expression is mainly detected in the distal small intestine and colon in murine tissue at least, though this result needs to be confirmed in humans [18]. Both TRPM6 and TRPM7 expression is restricted to the luminal membrane of the enterocytes (Figure 2). The basolateral Mg2+ extrusion mechanism is unknown, but several publications have suggested that basolateral Mg2+ transport is coupled to the Na+ gradient, sodium concentrations being lower in the cytoplasm than the blood owing to the action of basolateral Na+/K+-ATPase [19]. This hypothesis, however, remains to be confirmed by the identification of the basolateral Mg2+ transporter.
Regulatory factors.
Intestinal Mg2+ absorption is regulated by a variety of factors. Mg2+ absorption is altered by dietary Mg2+ intake, as demonstrated by Mg2+ uptake studies [13]. This effect can be attributed, at least partly, to changes in TRPM6 expression in the colon [18]. It also, probably, depends on alterations in paracellular Mg2+ transport rates owing to changes in the electrochemical gradient. Furthermore, it has been shown that 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] can stimulate intestinal Mg2+ absorption [20]. Indeed, patients with chronic renal disease often associated with hypomagnesaemia have low 1,25(OH)2D3 levels [21, 22]. However, TRPM6 expression in the kidneys is not regulated by 1,25(OH)2D3 [18]. TRPM6 expression in colon in response to 1,25(OH)2D3 remains to be determined. Interestingly, claudins 2 and 12, which are involved in paracellular Ca2+ transport, are regulated by 1,25(OH)2D3 [23]. As such, one could hypothesize that these claudins are also involved in paracellular Mg2+ absorption. As early as in 1943, Mg2+ absorption was reported to be regulated by protein intake [24]. Fifty years later, this finding was developed when it was demonstrated that it was not Mg2+ absorption but rather intestinal Mg2+ excretion that is altered by high protein intake [25]. Finally, experiments in mice showed that low and high dietary Mg2+ affects Ca2+ balance via the kidney (i.e. increased reabsorption and elimination, respectively) [18]. The mechanisms responsible for these phenomena are unknown, but the authors suggest that a regulatory role for the calcium-sensing receptor (CaSR) could explain the interaction between Mg2+ and Ca2+.
Magnesium storage
While Mg2+ can be stored in muscle fibres, where it plays an important role in the regulation of muscle contraction by antagonizing the action of Ca2+ [26], bone tissue is the largest Mg2+ store in the human body (Figure 1), where it also contributes to the density and strength of the skeleton. Depletion of Mg2+ is, therefore, a risk factor for osteoporosis [27]. A model of Mg2+-induced bone loss has been proposed in which low blood plasma Mg2+ concentrations lead to activation of bone resorption by osteoclasts and decreased osteoblast bone formation [27]. Moreover, bone surface Mg2+ concentrations (of ∼30%) are closely related to serum Mg2+ concentrations, indicating a continuous exchange of Mg2+ between bone and blood [12].
Renal magnesium elimination
Approximately 2400 mg of Mg2+ is filtered daily by the glomeruli. Along the nephron, 90–95% of Mg2+ is retrieved; the remaining 100 mg leaves the body via the urine (Figure 1). The specific roles of the various parts of the nephron are considered in the following sections.
Proximal tubule.
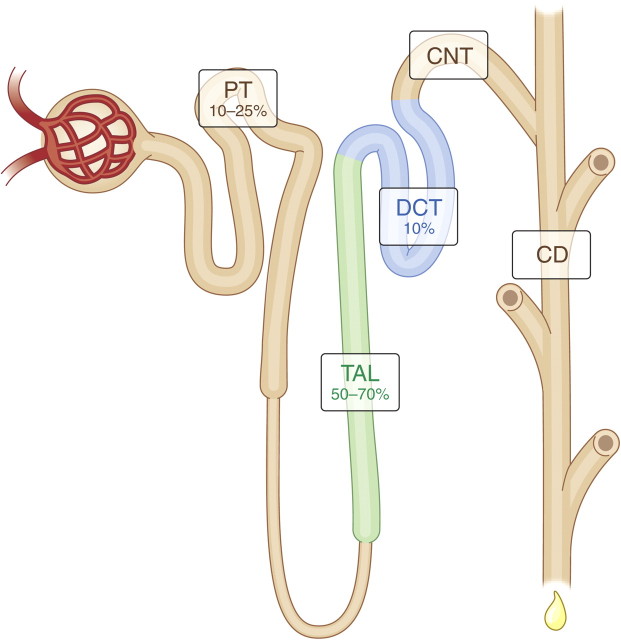
Surprisingly, little Mg2+ is reabsorbed in the proximal tubule in comparison with other electrolytes such as Na+, K+ and Cl− (Figure 3). Mg2+ concentrations increase as water is reabsorbed, but once a high concentration gradient is obtained then Mg2+ reabsorption occurs via passive paracellular transport, leading to the reabsorption of 10–25% of Mg2+ [28].
Fig. 3.
Magnesium reabsorption along the nephron. The glomerulus filters the blood and facilitates thereby the entrance of Mg2+ into the tubular system that subsequently mediates the reabsorption of 90–95% of Mg2+. Approximately 10–25% of Mg2+ is reabsorbed in the proximal tubule (PT). Bulk transport (50–70%) of Mg2+ is achieved along the thick ascending limb (TAL) of the loop of Henle. The final Mg2+ concentration in urine is determined in the distal convoluted tubule (DCT) where only 10% of Mg2+ is reabsorbed [28]. CNT, connecting tubule; CD, collecting duct.
Thick ascending limb.
The majority of filtered Mg2+ is reabsorbed in the loop of Henle, mostly in the thick ascending limb which accounts for up to 70% of total Mg2+ reabsorption (Figure 3). In fact, Mg2+ is the only bulk-transported ion in the thick ascending limb of the loop of Henle. Mg2+ reabsorption in the proximal tubule and thick ascending limb is, in fact, the opposite to the reabsorption of Na+ and K+, which occurs mainly in the proximal tubule rather than in the thick ascending limb.
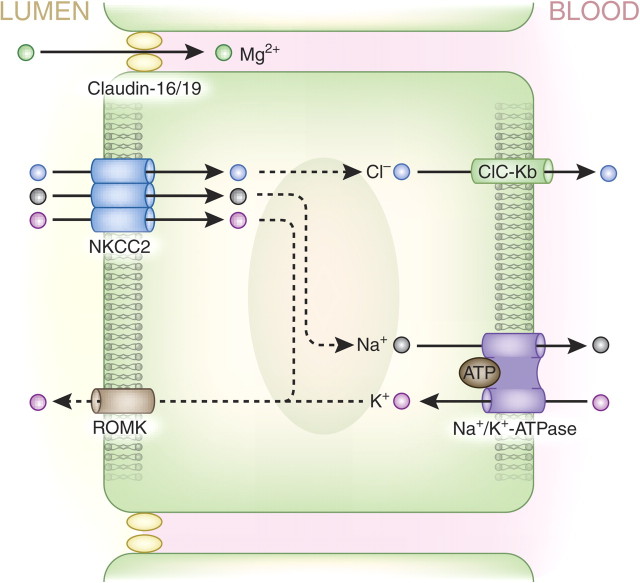
The transepithelial voltage gradient is the driving force behind the passive paracellular transport of Mg2+ in the thick ascending limb (voltages in the tubular lumen are positive relative to blood). Furthermore, it has been shown recently that claudins 16 and 19 form a cation-selective tight junction, facilitating the paracellular transport of Mg2+ in the thick ascending limb [17, 29]. The role of these claudins in bulk Mg2+ transport can still be questioned, however, since their Mg2+ transport capacity is fairly low when they are reconstituted in proximal tubule cells [30]. Nevertheless, the current theory proposes that NaCl enters thick ascending loop cells via the apical furosemide-sensitive Na+–K+–2Cl− cotransporter (NKCC2) (Figure 4). K+ is recycled into the luminal space via the renal outer medullary K+ (ROMK) channel, whereas Na+ and Cl− are extruded from the cell basolaterally via the Na+/K+-ATPase and the kidney-specific Cl− channel, CLC-Kb, respectively. This process establishes the aforementioned lumen-positive potential that drives paracellular Mg2+ transport. It is interesting to note here that inhibition of NKCC2 activity by the use of the diuretic furosemide diminishes this positive charge, can lead to excessive Mg2+ excretion and thus can result in hypomagnesaemia [31, 32].
Fig. 4.
Schematic overview of Mg2+ transport pathways in the thick ascending limb of the loop of Henle. The majority of Mg2+ is transported in this part of the nephron. Mg2+ absorption takes place in a paracellular fashion via claudins-16 and -19 of the tight junction complex. The driving force behind Mg2+ transport in the thick ascending limb is the transepithelial voltage gradient.
Distal convoluted tubule.
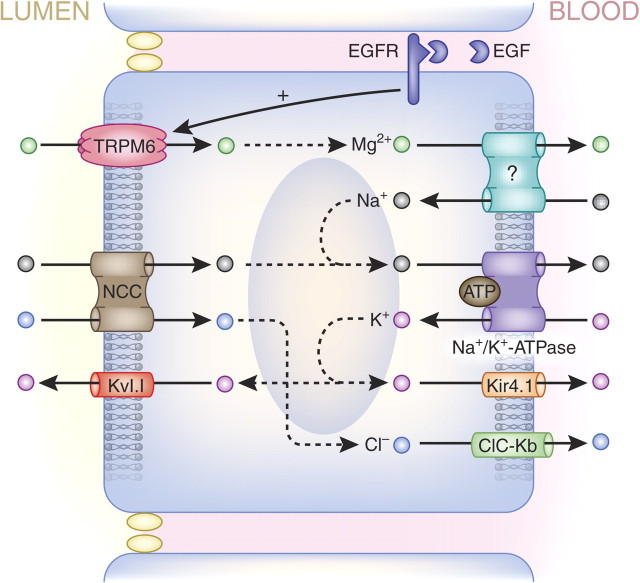
The ‘fine-tuning’ of Mg2+ reabsorption takes place along the distal convoluted tubule, where ∼10% of filtered Mg2+ is reabsorbed (Figure 3). Here, Mg2+ reabsorption is an active transcellular process that is tightly regulated by several recently discovered factors, each playing an important role in Mg2+ homeostasis [33]. The TRPM6 Mg2+ channel allows Mg2+to enter the cell [34], and while the basolateral Mg2+ extrusion mechanism remains to be identified, it may be dependent on the inward Na+ gradient that is mediated by the Na+/K+-ATPase (Figure 5). Notably, thiazide diuretics mimic the effects of Gitelman's syndrome by enhancing Na+ excretion via inhibition of the Na+–Cl− cotransporter (NCC) (Figure 5). In addition, these drugs are known to affect the Mg2+ balance, inducing hypomagnesaemia, which may be explained by the down-regulation of TRPM6 expression in response to chronic thiazide treatment [35]. Hypomagnesaemia is frequently linked with hypokalaemia owing to disturbances in renal K+ secretion in the connecting tubule and collecting duct (Figure 3). Low intracellular Mg2+ levels release the Mg2+-dependent inhibition of ROMK channels, resulting in increased renal K+ secretion, often leading to hypokalaemia [8].
Fig. 5.
Ion transport pathways in the distal convoluted tubule. The final possibility of Mg2+ reabsorption is the tightly regulated transcellular transport in the distal convoluted tubule. In this schematic overview, all the proteins whose mutations cause hypomagnesaemia are shown. Mg2+ enters the cell via the TRPM6 Mg2+ channel that is regulated by EGF. The Kv1.1 K+ channel maintains transmembrane voltage that is the driving force for Mg2+ transport. The key molecule at the basolateral membrane is the Na+/K+-ATPase, whose expression is regulated by transcription factor HNF1B (not shown). The Na+/K+-ATPase activity is stimulated by its γ–subunit. Kir4.1 is responsible for recycling of K+ at the basolateral site of the cell. The basolateral Mg2+ transporter remains to be identified. Gitelman's-associated proteins NCC and ClC-Kb are responsible for Na+ and Cl− transport in the distal convoluted tubule.
Regulatory factors.
Epidermal growth factor (EGF) regulates TRPM6 activity and plasma membrane availability. Interestingly, basolaterally expressed pro-EGF is almost exclusively found in the distal convoluted tubule. Pro-EGF is cleaved to yield EGF, activating the EGF receptor (EGFR), which in turn triggers an intracellular cascade that regulates TRPM6 activity [36].
Oestrogen is known to stimulate TRPM6 expression [18]. Thus, oestrogen substitution therapy is used to normalize hypermagnesuria, which occurs frequently in post-menopausal women [37]. Interestingly, TRPM6 expression appears to be regulated by plasma Mg2+ levels and oestrogens, but not by 1,25(OH)2D3 or parathyroid hormone (PTH) action [18].
Mg2+ homeostasis depends on three organs: the intestine, facilitating Mg2+ uptake; bone, the main Mg2+ storage system of the body and the kidneys, which are responsible for Mg2+ excretion.
In the intestine, about 80–90% of Mg2+ is absorbed passively through paracellular transport. The remaining Mg2+ is absorbed via active Mg2+ transporters, which account for the fine-tuning of Mg2+ regulation.
Bone tissue constitutes the largest Mg2+ store in the human body, though it is also stored in muscle where it acts to antagonize Ca2+ during muscle contraction.
Mg2+ is mainly excreted in the kidney. About 90–95% of daily filtrated Mg2+ is reabsorbed in the kidney. Again, the interplay between passive mechanisms and adjustment via active transporters determine the final Mg2+ concentration.
Magnesium transporters and genetic disease
In the last decade, gene-linkage studies in families with hereditary forms of hypomagnesaemia have helped identify several proteins involved in renal Mg2+ reabsorption (Table 1). Causative mutations for several genetic disorders have been described and have provided insight in the molecular regulation of Mg2+ transport. In this review, we will present an overview of the implicated diseases and proteins.
Impaired Mg2+ reabsorption in the thick ascending limb
Claudins 16 and 19.
Claudins are small transmembrane proteins and are regarded as the most important components of the tight junction barrier, which is thought to have a key role in passive paracellular reabsorption (responsible for most Mg2+ reabsorption, which occurs in the thick ascending limb). Mutations in the claudin-16 gene (formerly known as paracellin-1 gene) have been shown to be responsible for the rare inherited disorder known as familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC) [38]. Claudin-19 was then identified in Swiss and Spanish families without claudin-16 mutations, but who suffered from the same symptoms of nephrocalcinosis, progressive renal failure and visual impairment [39]. Claudin-16 and claudin-19 colocalize in the thick ascending limb to form a cation-specific complex [29]. This complex is involved in paracellular Mg2+ reabsorption in the thick ascending limb but is also important in voltage-dependent paracellular Na+ transport (reviewed by Hou and Goodenough [40]). Knockout models showed that mice deficient in claudin-16 and claudin-19 also developed FHHNC [17]. Claudin-16 and claudin-19 mutations reduce the cation specificity of the paracellular pathway, diminishing the transepithelial voltage potential. Ultimately, this reduces the driving force for bulk Mg2+ reabsorption in the thick ascending limb and results in renal Mg2+ wasting characteristic of patients with FHHNC [17].
Bartter's syndrome-associated genes.
Bartter's syndrome is a group of autosomal-recessive disorders characterized by reduced salt absorption in the thick ascending limb, the target segment of the furosemide diurectics. This salt wasting often coincides with hypokalaemic metabolic alkalosis, elevated renin and aldosterone levels and low blood pressure [7]. Mutations in five different genes have been shown to induce the Bartter phenotype [41, 42]. Firstly, loss-of-function mutations in NKCC2 are responsible for reduced salt reabsorption. The second gene involved encodes the apical K+ channel ROMK that recycles K+ into the luminal space. Other gene mutations are those encoding the basolateral Cl− channel (CLC-Kb) responsible for basolateral Cl− extrusion; Barttin, a protein that regulates CLC-Kb activity and finally, gain-of-function mutations in the CaSR, which can cause Bartter's syndrome via inhibitory action on NKCC2 activity. Bartter's syndrome is often linked to mild hypomagnesaemia owing to the dissipation of the lumen-positive transepithelial voltage that is the driving force for paracellular Mg2+ transport [43]. Compensatory mechanisms in the distal convoluted tubule may, however, at least partly compensate for the impairment of bulk Mg2+ reabsorption.
Impaired Mg2+ reabsorption in the distal convoluted tubule
Transient receptor potential channel melastatin member 6 (TRPM6).
Two groups identified TRPM6, simultaneously and independently, as the causative gene in the rare genetic disorder of hypomagnesaemia with secondary hypocalcaemia (HSH) [44, 45]. HSH-affected individuals have abnormally low serum Mg2+ levels (<0.40 mmol/L) that indirectly lead to hypocalcaemia, probably because of concomitant parathyroid failure. Patients suffer from neurological symptoms such as seizures and muscle spasms, and eventually, the disorder may be fatal or cause neurological damage if not treated with high-dose Mg2+.
TRPM6 belongs to the family of transient receptor potential channels that facilitate electrolyte transport. TRPM6 consists of six membrane-spanning domains with a pore-forming region and intracellular C- and N-terminal. In the nephron, TRPM6 expression is limited to the apical membrane of distal convoluted tubule cells, whereas intestinal TRPM6 expression is highest in the colon and caecum [18]. Patch-clamp analysis showed that TRPM6 is a Mg2+- and Ca2+-permeable cation channel, which preferentially transports Mg2+ [34]. TRPM6 proteins form homotetrameric functional complexes as well as heterotetrameric complexes with TRPM7, the closest homologue of TRPM6 [46]. Recently, there has been controversy about the necessity of TRPM7 in TRPM6 functioning, as TRPM7-dependent as well as TRPM7-independent TRPM6 activity has been reported [47, 48]. The TRPM6 protein contains a C-terminal α-kinase domain that seems important in regulatory functions. Interactions between the α-kinase domain and regulatory factors such as receptor for activated C-kinase 1 (RACK1) and repressor of oestrogen receptor activity (REA) have been shown to modulate TRPM6 activity [49]. TRPM6 knockout mice are lethal, but TRPM6 heterozygous mice survive and have significantly lower plasma Mg2+ levels owing to reduced intestinal and renal expression of TRPM6 [50]. Several TRPM6 loss-of-function mutations have been demonstrated to decrease TRPM6 activity [51]. TRPM6 mutations often affect tetramerization or trafficking to the plasma membrane, but recently, a missense mutation in the pore-forming domain has been reported which leads to a functionally inactive channel [52].
Epidermal growth factor.
The first reports of the involvement of EGF in the regulation of Mg2+ reabsorption dates from 2005, when symptomatic hypomagnesaemia was observed in a patient with colorectal cancer who was treated with cetuximab, a monoclonal antibody directed against the EGFR [53]. Two years later, EGF was linked to isolated renal hypomagnesaemia (IRH), a rare disorder of low serum Mg2+ concentrations because of renal Mg2+ wasting. Whole-genome linkage analysis and subsequent candidate gene analysis in a family with IRH (Mg2+ serum levels ∼0.5 mmol/L) resulted in the identification of mutations in the EGF gene [54]. In addition to hypomagnesaemia, the IRH phenotype consists of moderate mental retardation and epileptic seizures. The EGF gene encodes a membrane-bound precursor molecule, pro-EGF, which is proteolytically cleaved to release the active form of the EGF hormone. Pro-EGF is expressed both on luminal and basolateral membranes of distal convoluted tubule cells, but EGFR itself is found only on the basolateral membrane.
Hypomagnesaemia was also found as a side effect in a prospective study in colorectal cancer patients given cetuximab therapy, and the authors suggested the involvement of EGF in TRPM6 regulation [55]. Follow-up studies revealed that EGF increases activity and membrane trafficking of TRPM6, and thus, it is the first hormone directly regulating renal Mg2+ reabsorption [56]. The expression of EGF is restricted to the TRPM6-expressing distal convoluted tubule cells. In the kidney, EGF is, therefore, a dedicated hormone regulating the Mg2+ reabsorption in a para- or autocrine fashion. Moreover, Ikari et al. [36, 57] reported that EGF regulates TRPM6 transcription via a MEK/ERK/AP-1 pathway, and activation of TRPM6 expression by EGF was confirmed recently in vivo in a study with EGFR-inhibitor erlotinib [58]. The mutation that led to the discovery of EGF as magnesiotropic hormone causes impaired basolateral expression of pro-EGF as it disrupts the basolateral-sorting motif. As a consequence, TRPM6 activity and membrane expression are reduced, and thus less Mg2+ is reabsorbed in the distal convoluted tubule.
Thiazide-sensitive Na+–Cl− cotransporter (NCC).
Gitelman's syndrome is an inherited disorder of impaired salt transport in the distal convoluted tubule and is associated with hypomagnesaemia, hypocalciuria and secondary aldosteronism [7]. Patients generally suffer from tetany, fatigue, chondrocalcinosis and muscle weakness. Gitelman's syndrome is caused by mutations in the thiazide-sensitive Na+–Cl− cotransporter (NCC) gene, resulting in loss of function of the protein. NCC is responsible for apical Na+ and Cl− entry into distal convoluted tubule cells and is sensitive to thiazide blocking [59, 60]. This characteristic of NCC was used in a study investigating the effect of NCC blocking on Ca2+ and Mg2+ reabsorption. The authors showed that thiazide-treated mice have abnormally low expression levels of TRPM6 in the distal convoluted tubule, causing hypomagnesaemia [35]. However, the interesting cross-talk mechanism between NCC activity and TRPM6 expression remains to be resolved.
Indirect reduction of Mg2+ transport in the distal convoluted tubule: transmembrane voltage
Kv1.1 K+ channel (KCNA1).
Single nucleotide polymorphism-based linkage analysis in a family with autosomal-dominant hypomagnesaemia resulted in the identification of mutations in the KCNA1 gene, encoding the Kv1.1 K+ channel. Affected individuals have severe hypomagnesaemia (<0.40 mmol/L) and suffer from muscle cramps, tetanic episodes, tremor and muscle weakness. Kv1.1 is a voltage-gated K+ channel and consists of six transmembrane domains, one of which (S4) acts as the voltage sensor and two (S5/S6) that form the pore region. Immunohistochemistry showed that kidney Kv1.1 localization is restricted to the luminal membrane of distal convoluted tubule cells (Figure 5). In these cells, Kv1.1 is thought to be involved in maintenance of the optimal membrane voltage, which is the driving force behind Mg2+ reabsorption via TRPM6 [61]. Interestingly, the Kv1.1 K+ current is blocked by intracellular Mg2+, thereby preventing intracellular Mg2+ overload [62]. The N255H mutation, leading to the discovery of the involvement of the KCNA1 gene in Mg2+ transport, locates in the third membrane-spanning domain and results in a non-functional channel. The asparagine at Position 255 is required for normal channel gating and voltage dependence [63]. It would be interesting to further characterize Mg2+ wasting in Kv1.1 null mice that currently are mainly studied to explain the epileptic seizures they suffer from [64].
Na+/K+-ATPase γ-subunit (FXYD2).
Patients with dominant renal hypomagnesaemia associated with hypocalciuria suffer from renal Mg2+ wasting and convulsions. Linkage studies followed by candidate screening led to the discovery of the FXYD domain containing the ion transport regulator 2 (FXYD2) gene coding for the γ-subunit of the Na+/K+-ATPase as the causative gene [65]. The basolateral Na+/K+-ATPase allows the active transport of Na+ and K+ transport in the opposite direction (Figure 5). The γ-subunit regulates the kinetics of Na+/K+-ATPase-mediated exchange of Na+ and K+. Immunohistochemistry confirmed colocalization of the γa- and γb-subunits at the basolateral membrane of the distal convoluted tubule [66]. Moreover, Na+/K+-ATPase activity is highest in this part of the nephron [67]. The currently leading hypothesis states that reduced Na+ transport lowers the membrane potential across the luminal membrane that acts as the inward electrical driving force for Mg2+. As a consequence, Mg2+ reabsorption is reduced. The glycine to arginine mutation at amino acid Position 41 (G41R) found in a Dutch family causes misrouting of the FXYD2 complex to the basolateral membrane [65]. G41R-mutated subunits oligomerize with wild-type FXYD2, thereby retaining the complex in the cell [68]. A recent study of the G41R mutation proposed a role for FXYD2 as an inward rectifying channel. These findings resulted in the suggestion that FXYD2 mediates basolateral Mg2+ extrusion [69]. Future studies, however, need to address the exact role of the γ-subunit of the Na+/K+-ATPase in the distal convoluted tubule.
Hepatocyte nuclear factor 1 homeobox B (HNF1B).
Mutations in the HNF1B transcription factor are associated with many human disorders such as neonatal diabetes and renal malformation. Recently, HNF1B mutations were linked to hypomagnesaemia in a 13-year-old Pakistani boy and a cohort of patients with HFN1B mutations and renal malformations. Forty-four per cent of the HNF1B mutation carriers have hypomagnesaemia, hypermagnesuria and hypocalciuria [70]. Luciferase reporter assays demonstrated that HNF1B stimulates transcriptional expression of the FXYD2a gene [66]. Mutations in HFN1B prevented transcriptional activation of the γa-subunit of the Na+/K+-ATPase, underlining the importance of the Na+/K+-ATPase in renal Mg2+ reabsorption.
Kir4.1 K+ channel (KCNJ10).
Quantitative trait loci mapping studies of seizure-sensitive mice led to the nomination of KCNJ10 as the responsible gene [71]. Recently, two groups confirmed that hypomagnesaemia (∼0.6 mmol/L) associated with seizures, sensorineural deafness, ataxia, mental retardation and electrolyte imbalance (SeSAME syndrome, also referred to as EAST) is provoked by mutations in the KCJN10 gene, which encodes the Kir4.1 K+ channel [72, 73]. Kir4.1 is expressed in glial cells, epithelium of the inner ear and the basolateral side of kidney distal convoluted tubule cells, where it is involved in K+ recycling necessary for optimal Na+/K+-ATPase activity [74] (Figure 5). By this mechanism, it is indirectly involved in the regulation of the intracellular voltage that is required for Mg2+ transport, explaining the hypomagnesaemia observed in patients with Kir4.1 mutations.
Kir4.1 and the CaSR have recently been shown to physically interact in human embryonic kidney cells and in kidney homogenates, and the CaSR appears to regulate Kir4.1 activity by decreasing Kir4.1 membrane availability via a Gα- and caveolin-dependent pathway [75]. A Kir4.1 knockout mouse model is available, but no data on kidney Mg2+ transport from this model has been published. The Kir4.1−/− mice have severe neurological problems and die prematurely (in the first few weeks after birth) [76, 77]. Several Kir4.1 mutations identified in SeSAME syndrome patients have been studied, and although all mutations modified the channel function, the mechanisms underlying these disturbances are different. Some mutations resulted in a shift in pH sensitivity causing changes in pore gating, while others impaired correct protein folding and decreased surface expression [78].
Studies of genetic human diseases associated with hypomagnesaemia have extended our understanding of Mg2+ reabsorption in the nephron.
Claudins are thought to have a key role in passive paracellular reabsorption in the thick ascending limb.
Active transcellular Mg2+ reabsorption occurs in the distal convoluted tubule, where TRPM6 has been identified as the luminal Mg2+ channel. The process is regulated by a variety of factors, including EGF.
Gitelman's-associated proteins NCC and ClC-Kb are responsible for Na+ and Cl− transport in the distal convoluted tubule. The disease is associated with hypomagnesaemia caused by a low TRPM6 expression.
Kv1.1 K+ channel maintains the apical transmembrane voltage, thought to be the driving force behind Mg2+ reabsorption via TRPM6 in the distal convoluted tubule.
The key molecule at the distal convoluted tubule basolateral membrane is the Na+/K+-ATPase, whose expression is regulated by transcription factor HNF1B.
Kir4.1 is responsible for recycling of K+ at the basolateral site of the cell and so is indirectly involved in intracellular voltage regulation needed for Mg2+ transport.
Conclusions and future perspectives
Mg2+ plays a vital physiological role in the body, and therefore control of plasma Mg2+ level is of major importance. Mg2+ homeostasis depends on its uptake in the intestine, storage in bone tissue and its excretion by the kidneys. When Mg2+ intake is low, its absorption can rise from 30 to 50% of dietary Mg2+ to ∼80%. Within the nephron, filtered Mg2+ is mainly reabsorbed in the loop of Henle, particularly in the thick ascending limb. However, the ‘fine-tuning’ of Mg2+ reabsorption occurs along the distal convoluted tubule, where ∼10% of filtered Mg2+ is reabsorbed in a tightly regulated and active transcellular process.
During the last decade, studies of human diseases have helped to extend the understanding of Mg2+ reabsorption in the nephron. In the thick ascending limb, claudins 16 and 19 form the pore permitting paracellular Mg2+ reabsorption, a process which depends on the transepithelial voltage which is maintained by transcellular salt transport. Active transcellular Mg2+ reabsorption takes place in the distal convoluted tubule, where TRPM6 has been identified as the luminal Mg2+ channel, and it is regulated by a variety of factors (of which EGF is the best studied). Pro-EGF is cleaved from the distal convoluted tubule cell, releasing EGF, where it can activate the EGFR at the basolateral membrane. TRPM6 can form homotetrameric complexes and heterotetrameric complexes with TRPM7; however, the necessity of TRPM7 for TRPM6 functioning is controversial. TRPM6 activity is dependent on the transmembrane voltage that is kept intact by the luminal K+ channel Kv1.1, but the exact role of Kv1.1 has to be further examined.
The basolateral Mg2+ extrusion mechanism is less well understood but is known to be linked to the basolateral Na+/K+-ATPase. Na+/K+-ATPase activity is regulated by the HNF1B transcriptional factor, which stimulates the expression of FXYD2. Also, the Kir4.1 K+ channel is involved in the Na+/K+-ATPase activity since it facilitates the availability of K+ ions. The involvement of the Na+/K+-ATPase in transcellular Mg2+ transport is poorly understood. Many genes of transporters and regulatory proteins have been identified, but several questions remain unanswered. A review summarizing these newly identified magnesiotropic proteins has been recently published [79]. Most importantly, the basolateral Mg2+ extrusion mechanism still has to be identified. Recently, patients with hypomagnesaemia have been screened for several candidate genes, but without result. New generation DNA sequencing techniques may contribute to the final identification of the missing transporter.
Mg2+ reabsorption in the distal convoluted tubule is tightly regulated by plasma Mg2+ levels [18], suggesting the existence of a Mg2+-sensing mechanism. The pathway that is involved in this mechanism, regulating for instance TRPM6 expression, may be discovered in the near future. The unidentified protein involved in Mg2+-sensing might be regulated by hormones since it has been shown that 1,25(OH)2D3 regulates intestinal Mg2+ absorption [20]. Next to 1,25(OH)2D3, several other factors, such as PTH or oestrogen, might be involved.
Genetic research in families with hypomagnesaemia has expanded our understanding of renal Mg2+ reabsorption. Understanding these processes can lead to new therapies for chronic hypomagnesaemia, and new state-of-the-art DNA screening techniques can help identify the missing magnesiotropic genes. Recent developments in renal Mg2+ research are excellent examples of bedside-to-bench research cooperation between clinicians and fundamental researchers and continuation of these collaborations may also allow new insights into the sequence of events that occurs when chronic renal failure develops.
Acknowledgments
Funding. This work was financially supported by grants from the Dutch Kidney Foundation (C08.2252), the Netherlands Organization for Scientific Research (ZonMw 40-00812-98-08026). J. Hoenderop is supported by a EURYI award.
Acknowledgements. We thank Martina Sintzel, Sjoerd Verkaart and Anke Lameris for critical reading of the manuscript. We also thank Richard Clark, Dunchurch, UK, for his comments on the final manuscript, on behalf of Fresenius GmbH.
Conflict of interest statement. This manuscript was sponsored by Fresenius Medical Care Deutschland GmbH. Dr J.H.F.B. and Dr J.G.J.H. declare no conflict of interest. Dr R.J.M.B. has received a consultancy honoraria from Fresenius.
References
- 1.Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41:357–362. doi: 10.1007/s11255-009-9548-7. [DOI] [PubMed] [Google Scholar]
- 2.Quamme GA. Recent developments in intestinal magnesium absorption. Curr Opin Gastroenterol. 2008;24:230–235. doi: 10.1097/MOG.0b013e3282f37b59. [DOI] [PubMed] [Google Scholar]
- 3.Pham PC, Pham PM, Pham SV, et al. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 4.Dimke H, Hoenderop JG, Bindels RJ. Hereditary tubular transport disorders: implications for renal handling of Ca2+ and Mg2+ . Clin Sci (Lond) 2010;118:1–18. doi: 10.1042/CS20090086. [DOI] [PubMed] [Google Scholar]
- 5.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiger H, Wanner C. Magnesium in disease. Clin Kidney J. 2012;5(Suppl 1):i25–i38. doi: 10.1093/ndtplus/sfr165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoers NV. Inherited forms of renal hypomagnesemia: an update. Pediatr Nephrol. 2009;24:697–705. doi: 10.1007/s00467-008-0968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007;18:2649–2652. doi: 10.1681/ASN.2007070792. [DOI] [PubMed] [Google Scholar]
- 9.Kesteloot H, Joossens JV. The relationship between dietary intake and urinary excretion of sodium, potassium, calcium and magnesium: Belgian Interuniversity Research on Nutrition and Health. J Hum Hypertens. 1990;4:527–533. [PubMed] [Google Scholar]
- 10.Kopple JD, Coburn JW. Metabolic studies of low protein diets in uremia. II. Calcium, phosphorus and magnesium. Medicine (Baltimore) 1973;52:597–607. doi: 10.1097/00005792-197311000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Spencer H, Lesniak M, Gatza CA, et al. Magnesium absorption and metabolism in patients with chronic renal failure and in patients with normal renal function. Gastroenterology. 1980;79:26–34. [PubMed] [Google Scholar]
- 12.Alfrey AC, Miller NL, Trow R. Effect of age and magnesium depletion on bone magnesium pools in rats. J Clin Invest. 1974;54:1074–1081. doi: 10.1172/JCI107851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham L, Caesar J, Burgen A. Gastrointestinal absorption and excretion of Mg28 in man. Metabolism. 1960;9:646–659. [PubMed] [Google Scholar]
- 14.Kayne LH, Lee DB. Intestinal magnesium absorption. Miner Electrolyte Metab. 1993;19:210–217. [PubMed] [Google Scholar]
- 15.Aliaga IL, Miller DL, Wilson HD, et al. Effects of resection on absorption and secretion of divalent cations by small intestine of rat. Am J Clin Nutr. 1990;52:867–871. doi: 10.1093/ajcn/52.5.867. [DOI] [PubMed] [Google Scholar]
- 16.Amasheh S, Fromm M, Gunzel D. Claudins of intestine and nephron - a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 2011;201:133–140. doi: 10.1111/j.1748-1716.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 17.Hou J, Renigunta A, Gomes AS, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groenestege WM, Thebault S, van der WJ, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2006;117:2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Schmulen AC, Lerman M, Pak CY, et al. Effect of 1,25-(OH)2D3 on jejunal absorption of magnesium in patients with chronic renal disease. Am J Physiol. 1980;238:G349–G352. doi: 10.1152/ajpgi.1980.238.4.G349. [DOI] [PubMed] [Google Scholar]
- 21.Brannan PG, Vergne-Marini P, Pak CY, et al. Magnesium absorption in the human small intestine. Results in normal subjects, patients with chronic renal disease, and patients with absorptive hypercalciuria. J Clin Invest. 1976;57:1412–1418. doi: 10.1172/JCI108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mawer EB, Taylor CM, Backhouse J, et al. Failure of formation of 1,25-dihydroxycholecalciferol in chronic renal insufficiency. Lancet. 1973;1:626–628. doi: 10.1016/s0140-6736(73)92197-1. [DOI] [PubMed] [Google Scholar]
- 23.Fujita H, Sugimoto K, Inatomi S, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCance RA, Widdowson EM, Lehmann H. The effect of protein intake on the absorption of calcium and magnesium. Biochem J. 1942;36:686–691. doi: 10.1042/bj0360686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbeek MJ, Van den Berg GJ, Lemmens AG, et al. High protein intake raises apparent but not true magnesium absorption in rats. J Nutr. 1993;123:1880–1887. doi: 10.1093/jn/123.11.1880. [DOI] [PubMed] [Google Scholar]
- 26.Potter JD, Robertson SP, Johnson JD. Magnesium and the regulation of muscle contraction. Fed Proc. 1981;40:2653–2656. [PubMed] [Google Scholar]
- 27.Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem. 2004;15:710–716. doi: 10.1016/j.jnutbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Le Grimellec C. Micropuncture study along the proximal convoluted tubule. Electrolyte reabsorption in first convolutions. Pflugers Arch. 1975;354:133–150. doi: 10.1007/BF00579944. [DOI] [PubMed] [Google Scholar]
- 29.Hou J, Renigunta A, Konrad M, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou J, Shan Q, Wang T, et al. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg A. Diuretic complications. Am J Med Sci. 2000;319:10–24. [PubMed] [Google Scholar]
- 32.Cohen N, Alon I, Almoznino-Sarafian D, et al. Metabolic and clinical effects of oral magnesium supplementation in furosemide-treated patients with severe congestive heart failure. Clin Cardiol. 2000;23:433–436. doi: 10.1002/clc.4960230611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaudemans B, Knoers NV, Hoenderop JG, et al. New molecular players facilitating Mg2+ reabsorption in the distal convoluted tubule. Kidney Int. 2010;77:17–22. doi: 10.1038/ki.2009.358. [DOI] [PubMed] [Google Scholar]
- 34.Voets T, Nilius B, Hoefs S, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 35.Nijenhuis T, Vallon V, van der Kemp AW, et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikari A, Okude C, Sawada H, et al. TRPM6 expression and cell proliferation are up-regulated by phosphorylation of ERK1/2 in renal epithelial cells. Biochem Biophys Res Commun. 2008;369:1129–1133. doi: 10.1016/j.bbrc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 37.McNair P, Christiansen C, Transbol I. Effect of menopause and estrogen substitutional therapy on magnesium metabolism. Miner Electrolyte Metab. 1984;10:84–87. [PubMed] [Google Scholar]
- 38.Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 39.Konrad M, Schaller A, Seelow D, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou J, Goodenough DA. Claudin-16 and claudin-19 function in the thick ascending limb. Curr Opin Nephrol Hypertens. 2010;19:483–488. doi: 10.1097/MNH.0b013e32833b7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeck N, Schlingmann KP, Reinalter SC, et al. Salt handling in the distal nephron: lessons learned from inherited human disorders. Am J Physiol Regul Integr Comp Physiol. 2005;288:R782–R795. doi: 10.1152/ajpregu.00600.2004. [DOI] [PubMed] [Google Scholar]
- 42.Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens. 2003;12:527–532. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Ellison DH. Divalent cation transport by the distal nephron: insights from Bartter's and Gitelman's syndromes. Am J Physiol Renal Physiol. 2000;279:F616–F625. doi: 10.1152/ajprenal.2000.279.4.F616. [DOI] [PubMed] [Google Scholar]
- 44.Walder RY, Landau D, Meyer P, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 45.Schlingmann KP, Weber S, Peters M, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 46.Chubanov V, Waldegger S, Schnitzler M, et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitz C, Dorovkov MV, Zhao X, et al. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao G, Thebault S, van der WJ, et al. RACK1 inhibits TRPM6 activity via phosphorylation of the fused alpha-kinase domain. Curr Biol. 2008;18:168–176. doi: 10.1016/j.cub.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 50.Woudenberg-Vrenken TE, Sukinta A, van der Kemp AW, et al. Transient receptor potential melastatin 6 knockout mice are lethal whereas heterozygous deletion results in mild hypomagnesemia. Nephron Physiol. 2011;117:11–19. doi: 10.1159/000320580. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Du J, Jiang J, et al. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chubanov V, Schlingmann KP, Waring J, et al. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J Biol Chem. 2007;282:7656–7667. doi: 10.1074/jbc.M611117200. [DOI] [PubMed] [Google Scholar]
- 53.Schrag D, Chung KY, Flombaum C, et al. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005;97:1221–1224. doi: 10.1093/jnci/dji242. [DOI] [PubMed] [Google Scholar]
- 54.Groenestege WM, Hoenderop JG, van den HL, et al. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol. 2007;17:1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- 55.Tejpar S, Piessevaux H, Claes K, et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8:387–394. doi: 10.1016/S1470-2045(07)70108-0. [DOI] [PubMed] [Google Scholar]
- 56.Thebault S, Alexander RT, Tiel Groenestege WM, et al. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20:78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikari A, Sanada A, Okude C, et al. Up-regulation of TRPM6 transcriptional activity by AP-1 in renal epithelial cells. J Cell Physiol. 2010;222:481–487. doi: 10.1002/jcp.21988. [DOI] [PubMed] [Google Scholar]
- 58.Dimke H, van der WJ, Alexander TR, et al. Effects of the EGFR inhibitor erlotinib on magnesium handling. J Am Soc Nephrol. 2010;21:1309–1316. doi: 10.1681/ASN.2009111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gamba G, Saltzberg SN, Lombardi M, et al. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci USA. 1993;90:2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 61.Glaudemans B, van der WJ, Scola RH, et al. A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest. 2009;119:936–942. doi: 10.1172/JCI36948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez-Hernandez JM, Lorra C, Pardo LA, et al. Molecular basis for different pore properties of potassium channels from the rat brain Kv1 gene family. Pflugers Arch. 1997;434:661–668. doi: 10.1007/s004240050449. [DOI] [PubMed] [Google Scholar]
- 63.van der Wijst J, Glaudemans B, Venselaar H, et al. Functional analysis of the Kv1.1 N255D mutation associated with autosomal dominant hypomagnesemia. J Biol Chem. 2010;285:171–178. doi: 10.1074/jbc.M109.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smart SL, Lopantsev V, Zhang CL, et al. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 65.Meij IC, Koenderink JB, van BH, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na+/ K+-ATPase gamma-subunit. Nat Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 66.Ferre S, Veenstra GJ, Bouwmeester R, et al. HNF-1B specifically regulates the transcription of the gamma a-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun. 2011;404:284–290. doi: 10.1016/j.bbrc.2010.11.108. [DOI] [PubMed] [Google Scholar]
- 67.Katz AI, Doucet A, Morel F. Na+/K+-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979;237:F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- 68.Cairo ER, Friedrich T, Swarts HG, et al. Impaired routing of wild type FXYD2 after oligomerisation with FXYD2-G41R might explain the dominant nature of renal hypomagnesemia. Biochim Biophys Acta. 2008;1778:398–404. doi: 10.1016/j.bbamem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Sha Q, Pearson W, Burcea LC, et al. Human FXYD2 G41R mutation responsible for renal hypomagnesemia behaves as an inward-rectifying cation channel. Am J Physiol Renal Physiol. 2008;295:F91–F99. doi: 10.1152/ajprenal.00519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adalat S, Woolf AS, Johnstone KA, et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferraro TN, Golden GT, Smith GG, et al. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome. 2004;15:239–251. doi: 10.1007/s00335-003-2270-3. [DOI] [PubMed] [Google Scholar]
- 72.Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scholl UI, Choi M, Liu T, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10 . Proc Natl Acad Sci USA. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito M, Inanobe A, Horio Y, et al. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- 75.Cha SK, Huang C, Ding Y, et al. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem. 2011;286:1828–1835. doi: 10.1074/jbc.M110.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neusch C, Rozengurt N, Jacobs RE, et al. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marcus DC, Wu T, Wangemann P, et al. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- 78.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, et al. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–36048. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferre S, Hoenderop JG, Bindels RJ. Insight into renal Mg2+ transporters. Curr Opin Nephrol Hypertens. 2011;20:169–176. doi: 10.1097/MNH.0b013e3283435ee4. [DOI] [PubMed] [Google Scholar]