Abstract
Patients with chronic kidney disease (CKD) have a high prevalence of vascular calcification, and cardiovascular disease is the leading cause of death in this population. However, the molecular mechanisms of vascular calcification, which are multifactorial, cell-mediated and dynamic, are not yet fully understood. We need to address ways to improve outcomes in CKD patients, both in terms of vascular calcification and cardiovascular morbidity and mortality—and to these ends, we investigate the role of magnesium. Magnesium’s role in the pathogenesis of vascular calcification has not been extensively studied. Nonetheless, several in vitro and animal studies point towards a protective role of magnesium through multiple molecular mechanisms. Magnesium is a natural calcium antagonist and both human and animal studies have shown that low circulating magnesium levels are associated with vascular calcification. Clinical evidence from observational studies of dialysis patients has shown that low-magnesium levels occur concurrently with mitral annular calcification, peripheral arterial calcification and increased carotid intima–media thickness. Few interventional studies have been performed. Two interventional studies suggest that there may be benefits such as retardation of arterial calcification and/or reductions in carotid intima–media thickness in response to magnesium supplementation in CKD patients, though both studies have limitations. Finally, observational studies have shown that low serum magnesium may be an independent risk factor for premature death in CKD patients, and patients with mildly elevated serum magnesium levels could have a survival advantage over those with lower magnesium levels.
Keywords: atherosclerosis, chronic kidney disease, magnesium, survival, vascular calcification
Introduction
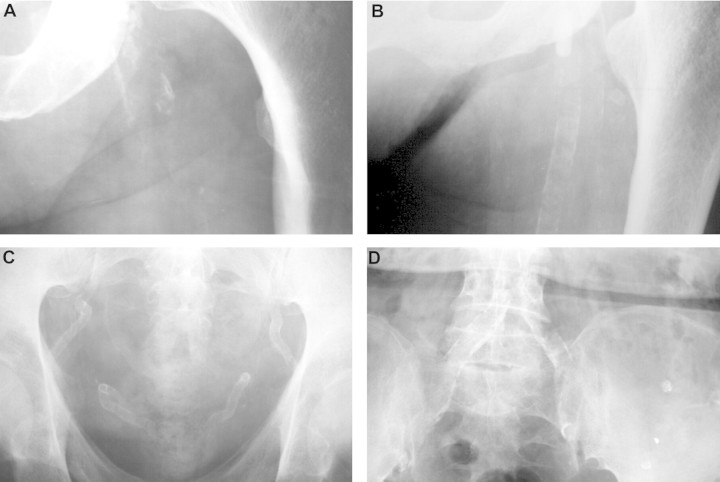
Cardiovascular disease is the leading cause of death in both chronic kidney disease (CKD) and peritoneal dialysis/haemodialysis patients. In fact, the risk of dying because of cardiovascular disease in adults with CKD is about an order of magnitude higher than for the general population, even after adjusting for age and diabetic status [1]. It is also notable that patients with CKD undergoing dialysis have 2- to 5-fold more coronary artery calcification (CAC) than age-matched individuals with angiographically proven coronary artery disease [2]. The high incidence of cardiovascular mortality appears, at least partially, attributable to increased medial calcification of the large arteries, including the aorta, which in turn can result in increased arterial wall stiffness and pulse pressure and decreased myocardial perfusion during diastole [3, 4]. However, intimal calcification associated with atherosclerosis is even more frequent than medial calcification, especially in patients with CKD Stages 3–5 before dialysis therapy is started [5]. Both intimal and medial calcification are probably major direct or indirect contributors to cardiovascular disease and excess cardiovascular mortality of CKD patients [6, 7]. Figure 1 shows typical x-ray aspects of intimal calcification, medial calcification and mixed intimal and medial calcifications in pelvic and femoral arteries. Vascular disease prevention is therefore important, with the aim to reduce the incidence of cardiovascular morbidity and mortality. While at least some traditional coronary risk factors (e.g. increased age, dyslipidaemia, diabetes and smoking) play a role in haemodialysis patients, several non-traditional factors associated with CKD are also likely to be involved [8, 9]. These include anaemia, uraemic toxins, oxidative stress, protein glycation and carbamylation and the disorder of mineral and bone metabolism (CKD–MBD) [4, 8, 10, 11].
Fig. 1.
Intima and media calcification in CKD patients. Arterial calcifications can be classified as intima calcification, present as discrete plaques with irregular and patchy distribution (A) or as media calcification, present as uniform linear railroad track-type (angiogram-like; B and C). The presence of both, intima and media calcification is reflected as discordances (D). Shown are soft tissue posteroanterior fine-detail native (unenhanced) radiographs of the pelvis and the thigh taken in CKD patients in the recumbent position. A and B, femoral artery; C and D, pelvic artery. With permission from Oxford University Press, London et al. [6].
Although being part of CKD–MBD, magnesium’s role in CKD–MBD has been underestimated and generally neglected. Here, we review the role of magnesium in vascular calcification with particular focus on CKD and look towards potential interventions to improve outcomes for this group of patients.
Magnesium and the pathogenesis of vascular calcification
Vascular calcification: in vitro evidence and potential pathogenic mechanisms
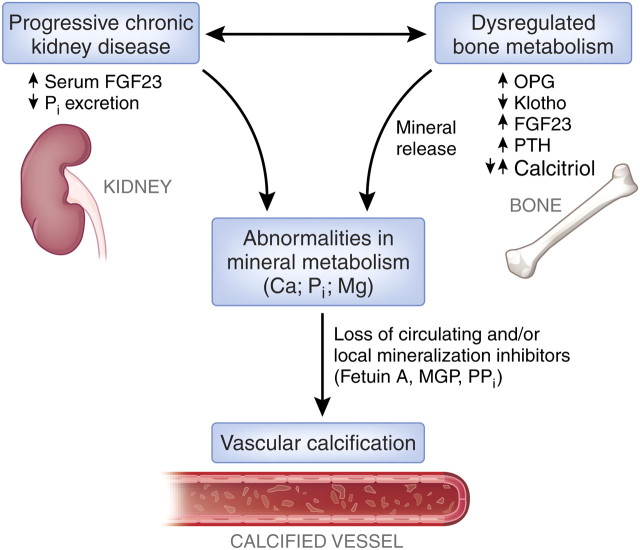
The process of vascular calcification may start early during the course of CKD, prior to the start of dialysis, and worsens progressively, often in an accelerated fashion compared with the general population [12, 13]. Disturbances in mineral and bone metabolism appear to play a major role in the pathogenesis and rapid progression of vascular calcification [11, 14–16] (see Figure 2 for details). However, it is notable that compared with calcium and phosphate, the role of magnesium in this pathologic process has been the subject of few studies.
Fig. 2.
Mechanisms of vascular calcification in CKD patients. Disturbances of mineral and bone metabolism are common in patients with CKD. The progressive loss of kidney function is accompanied—among other changes—by elevated serum FGF23 levels, a decrease in inorganic phosphate excretion and a dysregulation of bone metabolism. These anomalies are intimately interrelated. Indicators of this disturbed state are pathological changes of various biomarkers such as OPG, Klotho, FGF23, PTH and calcitriol. Whether their altered serum levels are the cause or the consequence of the skeletal abnormalities requires further study. The resulting derangements in mineral metabolism, as reflected by altered serum and vascular tissue levels of Ca, Pi and Mg are accompanied by additional metabolic changes and inflammation. This leads to loss of circulating and/or local mineralization inhibitors such as fetuin A, PPi and MGP, further supporting the development of vascular calcification. Ca, calcium; FGF23, fibroblast growth factor 23; Mg, magnesium; MGP, matrix Gla protein; OPG, osteoprotegerin; Pi , inorganic phosphate; PPi, inorganic pyrophosphate; PTH, parathyroid hormone. (modified after Schoppet, Shroff et al. [17]).
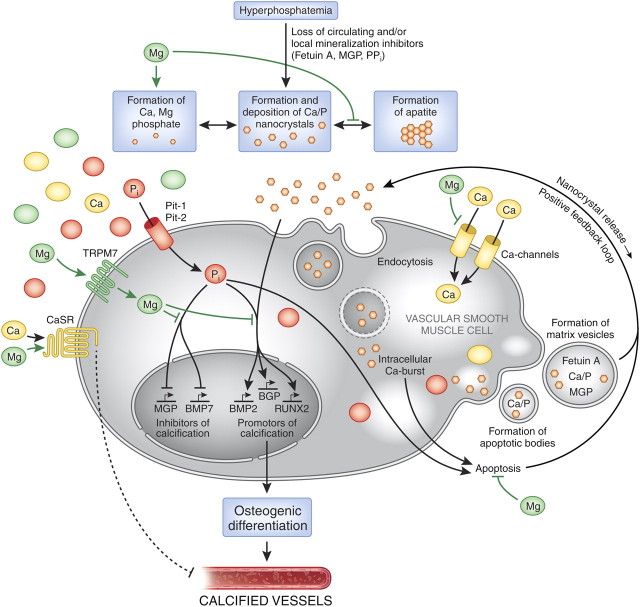
The pathogenesis of vascular calcification is not well understood, but it is likely to be multifactorial [9]. It appears to be a cell-mediated, dynamic and actively regulated process that closely resembles the formation of normal bone tissue [4, 17–20]. Several non-mutually exclusive theories or mechanisms have been advanced to explain the onset and progression of vascular calcification, during which a central role is played by the vascular smooth muscle cells (VSMCs) that compose the medial layer of the vessel wall. Figure 3 gives a comprehensive overview of the mechanisms and factors that act upon VSMCs, influencing their conversion into osteoblast-like cells—a phenotype that is commonly found in calcified vessels. Initially, a soluble amorphous calcium–phosphate complex is deposited in presence of excessive calcium phosphate mineral. It is unlikely to cause harm if stabilized effectively by inhibitory proteins, such as fetuin A, carboxylated matrix Gla protein (MGP) and osteopontin, and by the inorganic inhibitory compound pyrophosphate [17, 21–24]. According to three recent reports, however, the starting point could be the formation of nanocrystals that could directly stimulate calcification and vascular cell differentiation [25, 26, 27]. Subsequently, when there is an imbalance between calcification inhibitors and promoters, amorphous calcium phosphate and/or nanocrystals may be transformed into the stable hydroxyapatite crystal. Alterations in calcium and phosphate balance, as observed in patients with CKD, clearly promote vascular calcification and may be considered as non-traditional risk factors for cardiovascular disease in these patients [9, 28] (Figure 2).
Fig. 3.
The putative protective roles of magnesium in the course of vascular calcification. Abnormalities in mineral metabolism, particularly hyperphosphataemia as well as the loss of circulating and/or local mineralization inhibitors such as fetuin A, MGP or PPi, initially lead to the formation and deposition of Ca/P nanocrystals [25, 26]. These nanocrystals are taken up by VSMCs, most likely via endocytosis [29]. The lysosomal degradation of the endocytosed crystals results in an intracellular release of calcium and phosphate. Inorganic phosphate additionally accumulates in the cell via uptake through the sodium-dependent phosphate transporter Pit-1 (and Pit-2) [30, 31]. In an attempt to compensate for excess Ca/P, VSMCs form matrix vesicles loaded with Ca/P products as well as the mineralization inhibitors fetuin A and MGP [32]. The intracellular Ca-burst induced by endocytosed nanocrystals [33] as well as the phosphate uptake [34] trigger VSMC apoptosis, resulting in the formation of Ca/P containing apoptotic bodies [35]. Both apoptotic bodies and matrix vesicles are ultimately causing a positive feedback loop through nanocrystal release into the surrounding milieu, thus amplifying the calcification process. Furthermore, Ca/P nanocrystals as well as Pi induce the expression of genes that promote the calcification/mineralization process such as RUNX2, BMP2 and BGP, while at the same time repressing the expression of MGP or BMP7, factors that are known to inhibit the progression of calcification. This causes a transdifferentiation of VSMCs to osteoblast-like cells, ultimately resulting in vessel calcification. Magnesium interferes with this process of vascular calcification on different levels: firstly, Mg inhibits the transformation from amorphous Ca/P to any apatite (carbonatohydroxyapatite) [36, 37] and forms Mg-substituted tricalcium (whitlockite) under certain conditions, which is more soluble than apatite [37], resulting in smaller, more soluble deposits [37, 38]. Secondly, magnesium functions as a Ca-channel antagonist [39] and thus inhibits the entry of Ca into the cells. Thirdly, within the cell, via TRPM7, Mg restores the balance between the expression of calcification promotors and inhibitors by neutralizing the inhibition of MGP and BMP7 induced by phosphate [40]. Furthermore, it regresses the phosphate- and Ca/P nanocrystal-induced enhanced expression of RUNX2 and BMP2 [41] preventing the VSMCs from osteoblastic conversion and calcification. In addition, magnesium acts on the CaSR [42]; activation of this receptor by calcimimetics has been shown to inhibit VSMC calcification [43]. The underlying molecular mechanisms have not been identified so far. BGP, bone GLA protein, osteocalcin; BMP, bone morphogenetic protein; Ca, calcium; CaSR, calcium-sensing receptor; Mg, magnesium; MGP, matrix Gla protein; Pi, inorganic phosphate; Pit, inorganic phosphate transporter; PPi, inorganic pyrophosphate; RUNX2, runt-related transcription factor 2, cbfa1, core-binding factor subunit alpha-1; TRPM, transient receptor potential melastatin; VSMC, vascular smooth muscle cell.
Calcium phosphate deposition, mainly in the form of carbonate and hydroxyapatite, respectively [(Ca,Na)10(PO4,CO3)6(OH)2, Ca10(PO4)6CO3 and Ca10(PO4)6(OH)2], which also are the mineral compounds of bone, is the hallmark of vascular calcification and can occur in the blood vessels, myocardium and cardiac valves [36, 27, 28, 44]. (This issue has been discussed in greater detail in the review by Jahnen-Dechent and Ketteler [45] in this supplement.) A recent in vitro investigation of the role of calcium phosphate deposition in VSMC calcification suggests that calcium phosphate deposition is initially a passive phenomenon, which then triggers the aforementioned osteogenic changes, resulting in the formation of more organized apatite crystal ultrastructures [26].
An analysis of the crystalline composition of soft tissue calcification in uraemic patients identified non-visceral and arterial calcification to be hydroxyapatite, while heart, lung and skeletal muscle calcification was identified as an amorphous or microcrystalline compound composed of calcium, magnesium and phosphorus [46]. More recently, synchrotron X-ray-μ-fluorescence and diffraction data were used to examine vascular calcification. The aortic vessel wall mineral deposits in calcitriol- and non-calcitriol-treated rodent models of uraemia-induced vascular calcification [44] were composed of amorphous calcium phosphate precipitate, apatite and—in animals treated with calcitriol—also whitlockite (magnesium-substituted tricalcium phosphate or calcium magnesium orthophosphate: [(Ca,Mg)3(PO4)2]). In contrast to this animal work, a very recent detailed investigation of tissue samples taken from iliac arteries of uraemic patients revealed the colocalization of hydroxyapatite with whitlockite in three of six patients, indicating that this type of calcium phosphate crystals is not only found in soft tissue calcifications but also in the vascular space [27]. The presence of magnesium in calcification is not unexpected as it inhibits the formation of apatite and stabilizes amorphous calcium phosphate [36, 22, 47]. In addition, other inhibitors of calcifications, namely calcium binding or calcification-inhibitory proteins such as fetuin A, osteopontin and MGP, were found in close association with microcalcifications [27].
Several in vitro studies have shown that magnesium can have an inhibitory effect on hydroxyapatite formation and precipitation, as well as on the calcification process. Posner’s group showed in the 1970s and 1980s that magnesium stabilized amorphous calcium phosphate and inhibited the formation of calcium-acidic phospholipid–phosphate complexes in metastable calcium phosphate solutions [48, 49]. Of interest, Bennett et al. [50] found that magnesium was also able to inhibit calcium pyrophosphate dihydrate crystal formation in vitro. More recently, the effect of magnesium was examined on in vitro VSMC transformation into osteoblast-like cells and calcification [40]. The addition of 2.0–3.0 mM magnesium to a high-phosphate medium prevented osteogenic differentiation and calcification, in part via the restoration of the activity of the cation channel known as transient receptor potential melastatin 7 (TRPM7). Magnesium also increased the expression of anti-calcification proteins, including osteopontin and MGP [40]. Furthermore, it was shown that magnesium can stimulate the calcium-sensing receptor (CaSR), which is expressed on VSMCs [51, 52]. Stimulation of the CaSR by calcimimetics reduced mineral deposition in VSMCs and delayed the progression of both aortic calcification and atherosclerosis in uraemic apoE(−/− ) mice [43]. The exact underlying mechanisms have not been resolved so far but this suggests that one of the mechanisms of how magnesium influences VSMC calcification might be via the CaSR. Thus, magnesium could protect against vascular calcification via multiple molecular mechanisms.
Potential mechanisms of the inhibitory effects of magnesium in the calcification process are shown in Figure 3.
Evidence from animal experiments
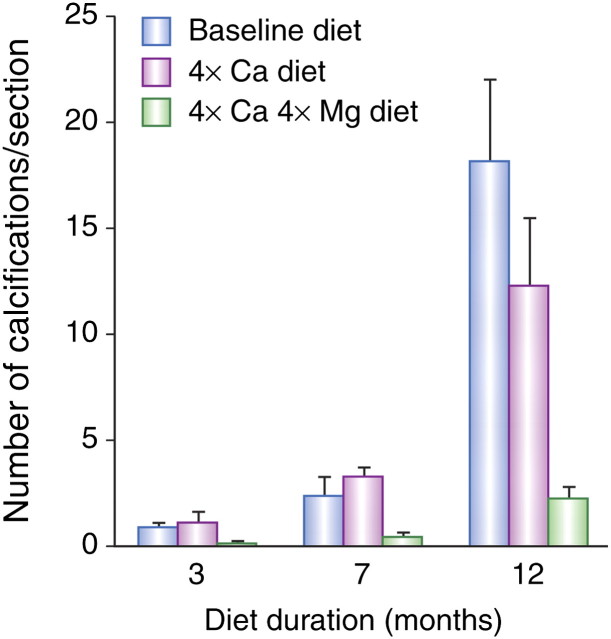
Low serum levels of magnesium are associated with vascular calcification, both in humans and in a number of experimental animal studies [53–56]. Several of the animal studies have demonstrated that changes in dietary magnesium levels can cause or prevent vascular calcification [53–55]. A rat model consisting of aortic transplantation associated with medial calcification of the grafted vessel was used to show that dietary supplementation with a combination of magnesium, alkali citrate and bases was capable of preventing aorta transplant-induced calcification [54]. The effect of dietary magnesium and calcium has also been examined in the Abcc6−/− mouse, a pseudoxanthoma elasticum (PXE) mouse model which mimics the clinical features of PXE (a genetic disorder characterized by calcification of connective tissue in skin, Bruch’s membrane of the eye and blood vessel walls) [53]. Disease severity was measured by quantifying calcification after up to 12 months dietary treatment. An increase in dietary intake of calcium and magnesium resulted in significantly fewer calcifications of kidney blood vessels than mice given an unsupplemented diet or fed a calcium-enriched diet alone (both P < 0.05) [53] (Figure 4). In the same mouse model, it was shown that treatment with the phosphate binder magnesium carbonate prevented the onset as well as the progression of calcification, whereas treatment with lanthanum carbonate had no effect [57].
Fig. 4.
Effect of diet on the number of calcifications in blood vessels in the kidney cortex of Abcc6−/− mice. Histograms represent the average number of calcifications per kidney section as a function of diet and diet duration. Diets supplemented with calcium plus magnesium (‘4 × Ca, 4 × Mg’ diet) slowed down calcification significantly [compared with baseline unsupplemented diet or diet supplemented with calcium alone (4 × Ca diet)] after 3, 7 and 12 months (Kruskal–Wallis test, P < 0.05 for all comparisons)] [53]. With kind permission from Springer Science + Business Media, Gorgels et al. [53] (Figure 2).
The effect of phosphate and magnesium intake has been investigated in a mouse model (DBA/2) associated with dystrophic cardiac calcification [55]. DBA/2 mice with either a low-magnesium or a high-phosphate intake developed marked cardiac calcifications; moreover, a combination of low-magnesium and high-phosphate intake caused severe calcification of cardiac and renal tissues. However, when increasing dietary magnesium content and reducing dietary phosphate, cardiac calcification could be partially prevented.
Magnesium is also considered to be ‘a natural calcium antagonist’ as one of its major functions in biological systems is to modulate the neuromuscular activity of calcium ions [39, 58]. Thus, contractility of all types of muscle is dependent upon the actions and interactions of these two divalent cations. The magnesium ion can block calcium movement across VSMC membranes and lower peripheral and cerebral vascular resistance [39]. More generally speaking, magnesium deficiency appears to enhance the activity of calcium in the body, while an excess of magnesium may block it, and as such magnesium may help to control cardiovascular function [58].
Mechanisms of vascular calcification are poorly understood but are likely to be multifactorial, cell-mediated and dynamic processes.
Low serum magnesium levels are associated with vascular calcification in human and in animal studies. Animal studies show that dietary magnesium can prevent, or help mitigate, vascular calcification.
Magnesium has potential to protect against vascular calcification via multiple molecular mechanisms.
Clinical evidence for the role of magnesium in calcification, atherosclerosis and survival
Vascular calcification, atherogenesis and magnesium: observational studies
The influence of serum magnesium levels on vascular calcification has been suspected for a considerable time, as shown by a number of observational studies in patients with CKD (Table 1). In an early observational study, 44 end-stage renal disease (ESRD) patients receiving peritoneal dialysis therapy were followed up for a mean duration of 27 months [59]. Half of the patients (n = 22) developed peripheral arterial calcifications (detected in the hands, ankles or feet), while the remainder either did not develop any calcifications or had calcifications that regressed. The arterial calcification group had significantly lower mean serum magnesium levels (±SD) than the group without calcifications [1.11 ± 0.21 mmol/L (2.69 ± 0.52 mg/dL) and 1.24 ± 0.21 mmol/L (3.02 ± 0.51 mg/dL), respectively; P < 0.001]. Moreover, there were no significant between-group differences in parameters such as serum concentrations of calcium, phosphorus, calcium × phosphorus product, total alkaline phosphatases or intact parathyroid hormone (iPTH). Although the difference between serum magnesium levels in the calcification and no-calcification/calcification regression groups was striking, the study was limited by the semi-quantitative nature of vascular calcification assessment. Nevertheless, these results suggested that there may be a role for modest hypermagnesaemia as a preventative strategy for arterial calcification in patients with ESRD.
Table 1.
Observational and interventional studies investigating the influence of serum magnesium levels on vascular calcificationa
| Authors (year) | Patients | Study design | Parameter | Assessment technique | P-valueb |
| Observational studies | |||||
| Ishimura et al. (2007) [56] | 390 (non-diabetic haemodialysis) | Prospective single blind follow-up over 4 months | Calcification of the hand arteries | Radiographic findings of the hands | 0.036 |
| Tzanakis et al. (2004) [62] | 93 (haemodialysis) and 182 age- and sex-matched healthy controls | Cross-sectional analysis | Carotid intima–media thickness | B-mode ultrasound | 0.001 |
| Tzanakis et al. (1997) [60] | 56 (haemodialysis) | Retrospective analysis of 8 years | Mitral annular calcification | Doppler echocardiography | 0.008 |
| Meema et al. (1987) [59] | 44 (CAPD) | Prospective follow-up | Progression/regression of arterial calcification | Radiographic surveys | 0.001 |
| Interventional studies | |||||
| Spiegel et al. (2009) [71] | 7 (haemodialysis) | Prospective interventional follow-up over 18 months (Mg carbonate) | CAC | Electron beam tomography | 0.0737c |
| Turgut et al. (2008) [75] | 47 (haemodialysis) | Prospective interventional follow-up over 2 months (Mg citrate) | Intima–media thickness of the carotid artery | Ultrasound | 0.014d |
Results indicate that higher serum magnesium correlates with reduced vascular calcification and reduced intima–media thickness.
P-values indicate the significance level related to lower and higher serum magnesium levels or
progression vs baseline and
intervention versus no intervention, respectively. CAPD, continuous ambulatory peritoneal dialysis; CAC, coronary artery calcification.
Another observational study has been conducted in 390 patients undergoing maintenance haemodialysis that excluded patients with diabetes, and which used hand radiography to detect visible calcification of hand arteries in an examiner blinded manner [56]. Phalangeal vessel calcification was detected in 52 patients (13%). Mean serum magnesium levels (±SD) measured over a 4-month period were significantly lower in patients with vascular calcification [1.11 ± 0.12 mmol/L (2.69 ± 0.28 mg/dL)] than in those without [1.14 ± 0.14 mmol/L (2.78 ± 0.33 mg/dL); P < 0.05]. In addition, multivariate analysis showed that serum magnesium concentration was a significant independent factor associated with vascular calcification [odds ratio 0.28; 95% confidence interval (CI) 0.09–0.92 per 0.41 mmol/L (1 mg/dL) increase in serum magnesium levels; P = 0.036] after adjustment for age, sex, duration of haemodialysis and serum calcium, phosphate and iPTH concentrations. However, as in the study by Meema et al., the authors only used a semi-quantitative assessment of the small hand arteries by X-ray examination [56, 59].
Mitral valve calcification is common in patients undergoing haemodialysis, and magnesium may exert a protective role against this type of cardiovascular calcification as well [60, 61]. This question has been investigated in a cross-sectional observational study of chronic haemodialysis patients (n = 56) in which 23 patients (41%) had mitral annular calcification [60]. There were no significant differences between patients with or without mitral annular calcification with regard to serum phosphate, calcium, calcium × phosphate product or iPTH, but magnesium levels were significantly lower in patients with calcification (P < 0.05). Further statistical analysis showed that patients with serum magnesium levels <1.23 mmol/L (3.0 mg/dL) were twice as likely to develop mitral valve calcification as those with magnesium levels >1.23 mmol/L (3.0 mg/dL) (χ2 = 6.98; P = 0.008). Moreover, multiple logistical regression showed that serum magnesium levels could predict the occurrence of mitral annular calcification with 86% accuracy when controlling for patients’ age and biochemical factors other than magnesium levels [60].
More recently, Tzanakis et al. [62] in a cross-sectional study reported a negative association of both serum and intracellular magnesium levels with carotid intima–media thickness in patients undergoing haemodialysis, using multivariate analysis. The authors compared 93 stable chronic haemodialysis patients with 182 age- and sex-matched healthy control subjects with normal renal function. Intima–media thickness of both common carotids was assessed by ultrasonography: it was found to be significantly larger in the haemodialysis patients than the healthy controls (P < 0.001). Thus, for a 0.5 mmol/L (1.0 mEq/L) change in serum magnesium concentrations, a 0.35-mm change in carotid intima–media thickness was observed (P = 0.01).
Results from an observational study conducted within the general population in Japan (n = 728) point to a similar direction. Lower serum magnesium levels were significantly and independently associated with greater mean intima–media thickness (P = 0.004) and the risk of at least two carotid plaques (P = 0.03) [63] (see also Geiger and Wanner [64] in this supplement). Magnesium deficiency has also been reported to be related to the progression of atherosclerosis in several studies, including the observational Atherosclerosis Risk in Communities (ARIC) Study in middle-aged adults [65, 66].
Overall, these observational data suggest that magnesium may play an important protective role in the development and/or acceleration of arterial atherosclerosis in both patients with chronic kidney failure and in the general population since carotid intima–media thickness as measured by ultrasonography is thought to be a surrogate marker for increased risk of myocardial infarction and stroke [67].
Thus, it appears that magnesium deficiency, caused either by poor diet or impaired magnesium metabolism, may be the missing link between various cardiovascular risk factors and atherosclerosis [68]. This issue is discussed further in the review by Geiger and Wanner [64] in this supplement.
Evidence based on observational studies:
ESRD patients with peripheral arterial calcification had lower mean serum magnesium levels than those without calcifications or whose calcifications had regressed.
Mitral annular calcification in chronic haemodialysis patients was strongly associated with low serum magnesium levels.
The above effects were independent of several other commonly involved factors such as serum phosphate, calcium, calcium × phosphate product or parathyroid hormone (PTH) levels.
There was a strong association between lower serum magnesium levels and increased carotid intima–media thickness in patients undergoing long-term haemodialysis treatment.
Similar associations were also observed in the general population.
Vascular calcification, atherogenesis and magnesium: intervention studies
Given the potential involvement of low-magnesium levels in vascular calcification, as shown by various observational studies, some interventional studies have investigated the use of magnesium, though as yet there is no hard evidence in patients with ESRD (Table 1). A case study has described the resolution of soft tissue calcification after treatment with a dialysate containing a high concentration of magnesium [69]. Interestingly, clinical symptoms of soft tissue calcification such as joint swelling or pain reappeared when magnesium concentration in dialysate was subsequently reduced and again improved when the high dialysate magnesium level was re-instituted.
A pilot study conducted in 30 stable haemodialysis patients suggested that oral magnesium carbonate was generally well tolerated and effective in controlling serum phosphorus while reducing elemental calcium ingestion [70]. Following-on from this study, magnesium carbonate was given as a long-term phosphate binder to seven haemodialysis patients, all of whom had baseline CAC scores >30 and were treated with a dialysate containing 1.25 mmol/L (2.5 mEq/L) calcium and 0.375 mmol/L (0.75 mEq/L) magnesium, three times weekly [71]. This open-label, prospective pilot study evaluated changes in CAC scores from baseline and at 6, 12 and 18 months using electron beam computed tomography. There was no significant CAC score progression throughout the study (median per cent change in CAC score from baseline was 8, 4 and 8% at 6, 12 and 18 months, respectively). Furthermore, a paired test for directional change of CAC score was not statistically significant from baseline to 18 months (P = 0.0737). However, this study did not have a control group. For comparison, in other studies that included comparator groups, CAC in patients with CKD showed progression rates from baseline to month 12 in the range of 5–33% for sevelamer and 25–75% for calcium-containing phosphate binders. [72–74].
A larger study randomized 47 chronic haemodialysis patients to two groups: a magnesium group in which patients were given oral magnesium citrate at a dosage of 610 mg every other day in addition to daily oral calcium acetate and a control group in which patients received only calcium acetate as a phosphate binder [75]. The study lasted 2 months. Mean serum calcium and phosphorus concentrations did not change in either group. Serum magnesium concentration also did not change in the control group but increased in the magnesium group by the end of the study. Magnesium supplementation was generally well tolerated: none of the patients presented with signs of magnesium toxicity such as arrhythmia or neuromuscular manifestations, none developed severe hypermagnesaemia and only one discontinued treatment because of diarrhoea. Carotid intima–media thickness was measured by ultrasound. At baseline, both groups had similar carotid intima–media thickness values. After 2 months, mean carotid intima–media thickness was reduced significantly in the magnesium group (0.70 versus 0.97 mm for left carotid artery, P = 0.001; 0.78 versus 0.95 mm for right carotid artery, P = 0.002) but not in the control group. In addition, there was a significant inverse association between the absolute change in serum magnesium concentrations and in right (but not left) carotid intima–media thickness after 2 months of magnesium treatment (R = −0.443; P = 0.014). Serum PTH levels were also reduced in the magnesium group, but not in the control group. This led the authors to suggest that the beneficial effect of magnesium supplementation on carotid intima–media thickness might, among other factors, also be linked to a better control of hyperparathyroidism. However, baseline serum PTH was two times higher in the magnesium group than the control group. The authors concluded that magnesium supplementation might be useful in reducing the progression of atherosclerosis in chronic dialysis patients [75].
Suggestive evidence from interventional studies:
Long-term administration of oral magnesium supplements to CKD patients on intermittent haemodialysis therapy might retard arterial calcification (based on a pilot study).
Oral magnesium supplementation over a 2-month period led to a significant reduction in carotid intima–media thickness.
Magnesium and survival of haemodialysis patients: observational studies
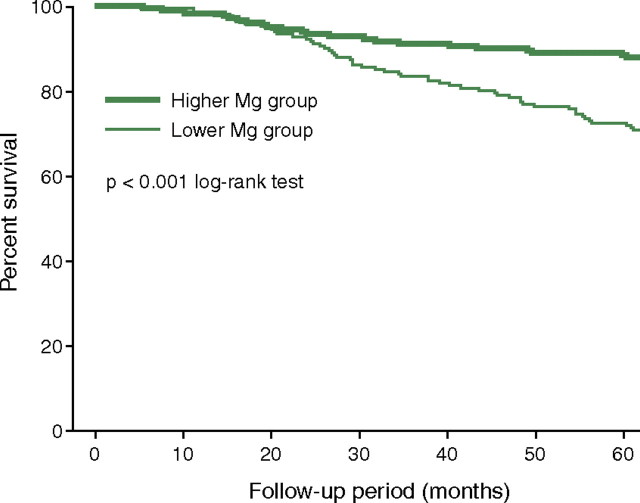
The potential relationship between serum magnesium levels and survival has been investigated in 515 ESRD patients undergoing intermittent haemodialysis treatment [76]. During a mean (±SD) follow-up of 51 (±17) months, 103 patients died (41 from cardiovascular causes). When analysing the results according to the patients’ baseline serum magnesium levels, mortality rates were significantly higher in the group with lower baseline magnesium levels [<1.14 mmol/L (2.77 mg/dL), n = 261] than in the group with higher baseline magnesium levels [≥1.14 mmol/L (2.77 mg/dL); n = 254] (P < 0.001) (Figure 5). It is important to note, at this point, that serum magnesium concentration at baseline correlated strongly with concentrations 1 year later (r = 0.835, P = 0.0001). Multivariate Cox proportional hazard analysis showed that serum magnesium levels were a significant and independent predictor of overall mortality {hazard ratio [per 0.41 mmol/L (1 mg/dL) increase in magnesium], 0.485 [95% CI, 0.241–0.975], P = 0.0424} after adjustment for confounding factors such as patients’ age, sex, duration of haemodialysis and presence of diabetes. Lower serum magnesium concentration was also a significant and independent predictor of death owing to non-cardiovascular causes [hazard ratio 0.318 (95% CI, 0.132–0.769), P = 0.011], but not for death from cardiovascular causes [hazard ratio 0.983 (95% CI, 0.313–3.086), P = 0.976]. The authors concluded that it might be worthwhile considering a higher dietary intake of magnesium and/or an adjustment of dialysate magnesium concentrations.
Fig. 5.
Kaplan–Meier analysis of all-cause mortality rates during a 51-month follow-up of 515 chronic haemodialysis patients. The relative risk of mortality was significantly greater in the group with lower baseline serum magnesium levels (<1.14 mmol/L, n = 261) than in that whith the higher baseline serum magnesium levels (≥1.14 mmol/L; n = 254) [76]. All-cause mortality, P < 0.001 (log-rank test); after adjustment by Cox multivariate analysis, P < 0.05. Reprinted from Ishimura et al. [76], with permission.
Another observational, retrospective and as yet preliminary study showed an association between increased serum magnesium concentrations and reduced relative risk of mortality in a large haemodialysis patient cohort [77, 78]. The analysis was done in a subgroup of all chronic haemodialysis patients treated at Fresenius Medical Care North America facilities, n = 110 271 (total sample) who had at least one serum magnesium result between 1 October and 31 December 2007 (baseline) and who survived until 1 January 2008, n = 27 544 (subsample). The subgroup was considered to be representative of the entire patient cohort. Mortality was followed until the end of 2008 and Cox models were constructed. A quarter of the patients (27 544 of 110 271) had serum magnesium levels recorded at baseline. The mean (±SD) concentration was 0.93 ± 0.16 mmol/L (2.26 ± 0.38 mg/dL). Compared with magnesium levels of 0.80–0.95 mmol/L (1.94–2.31 mg/dL) (mid-normal levels, used as a reference), the unadjusted hazard ratio for mortality in the observation period decreased significantly with increasing magnesium concentrations [beginning at 0.95–1.05 mmol/L (2.31–2.55 mg/dL); P < 0.0001], to a hazard ratio of 0.68 [magnesium concentration >1.15 mmol/L (>2.80 mg/dL); P < 0.0001]. Results for Cox models were similar when adjusting for case mix or for five quality indicators at baseline (serum albumin and phosphorus, haemoglobin, eKt/V and vascular access). More recently, similar results were found in a European database [79]. However, it must be pointed out that both these studies have not yet been published in a peer review journal.
What is clearly needed are prospective randomized trials examining the question whether increased serum or cytoplasmic magnesium levels or a magnesium intake in amounts such as those provided by magnesium containing phosphate binders is beneficial or not, in terms of hard outcomes in patients with CKD.
Magnesium and survival in haemodialysis patients: (partly based on data presented in abstract form only)
Patients with slightly elevated serum magnesium concentrations may have a survival advantage.
Low serum magnesium concentrations may be independent predictors of death.
Conclusions
A growing body of evidence from in vitro investigations, animal models and both observational as well as interventional clinical studies point to the possibility that low magnesium levels are associated with vascular calcification. Moreover, several observational studies suggest a relationship between increased serum magnesium concentrations and better survival rates for patients receiving long-term dialysis treatment. Preliminary results from an uncontrolled interventional trial suggest that long-term intervention with magnesium in dialysis patients may retard arterial calcification. However, many questions remain unanswered and hard evidence is as yet lacking. In order to conclusively show possible benefits and to demonstrate the absence of harm with the long-term intake of oral magnesium in patients with CKD, we still have to wait for the results of randomized controlled trials.
Acknowledgments
The authors thank Dr Richard Clark and Dr Martina Sintzel for providing writing and editorial assistance on behalf of Fresenius Medical Care Deutschland GmbH. Fresenius also made an unrestricted educational grant to meet the cost of preparing this article. These declarations are in line with the European Medical Writers’ Association guidelines.
Conflict of interest statement. Z.A.M. has received speakers’ honoraria and research grants from Amgen, Genzyme, Fresenius Medical Care and Shire. T.B.D has received advisor/consultancy honoraria from Abbott, Amgen, Baxter, Fresenius, Genzyme, KAI Pharmaceuticals, KfH-Stiftung Präventivmedizin, Leo, Mitsubishi, Roche, Vifor and Theraclion; speaker honoraria from Abbott, Amgen, Chugai, Genzyme, Kirin, Roche, Takeda and grant/research support from Amgen, Baxter and Shire.
References
- 1.Foley RN, Parfrey PS. Cardiovascular disease and mortality in ESRD. J Nephrol. 1998;11:239–245. [PubMed] [Google Scholar]
- 2.Braun J, Oldendorf M, Moshage W, et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394–401. doi: 10.1016/s0272-6386(96)90363-7. [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Demuth K, Guerin AP, et al. Influence of biochemical alterations on arterial stiffness in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 1998;18:535–541. doi: 10.1161/01.atv.18.4.535. [DOI] [PubMed] [Google Scholar]
- 4.Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. doi: 10.1016/S1054-8807(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S, Ishibashi-Ueda H, Niizuma S, et al. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 7.Noordzij M, Cranenburg EM, Engelsman LF, et al. Progression of aortic calcification is associated with disorders of mineral metabolism and mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26:1662–1669. doi: 10.1093/ndt/gfq582. [DOI] [PubMed] [Google Scholar]
- 8.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 9.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 10.Ivanovski O, Szumilak D, Nguyen-Khoa T, et al. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 2005;67:2288–2294. doi: 10.1111/j.1523-1755.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 11.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 12.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 13.Temmar M, Liabeuf S, Renard C, et al. Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. J Hypertens. 2010;28:163–169. doi: 10.1097/HJH.0b013e328331b81e. [DOI] [PubMed] [Google Scholar]
- 14.Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Nikolov IG, Mozar A, Drueke TB, et al. Impact of disturbances of calcium and phosphate metabolism on vascular calcification and clinical outcomes in patients with chronic kidney disease. Blood Purif. 2009;27:350–359. doi: 10.1159/000209248. [DOI] [PubMed] [Google Scholar]
- 17.Schoppet M, Shroff RC, Hofbauer LC, et al. Exploring the biology of vascular calcification in chronic kidney disease: what's circulating? Kidney Int. 2008;73:384–390. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]
- 18.Shanahan CM. Mechanisms of vascular calcification in renal disease. Clin Nephrol. 2005;63:146–157. doi: 10.5414/cnp63146. [DOI] [PubMed] [Google Scholar]
- 19.Moe SM, Drueke T, Lameire N, et al. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2004;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Giachelli CM, Jono S, Shioi A, et al. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34–S37. doi: 10.1053/ajkd.2001.27394. [DOI] [PubMed] [Google Scholar]
- 21.Jahnen-Dechent W, Schafer C, Ketteler M, et al. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med. 2008;86:379–389. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill WC, Lomashvili KA. Recent progress in the treatment of vascular calcification. Kidney Int. 2010;78:1232–1239. doi: 10.1038/ki.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennenberg RJ, Schurgers LJ, Kroon AA, et al. Arterial calcifications. J Cell Mol Med. 2010;14:2203–2210. doi: 10.1111/j.1582-4934.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riser BL, Barreto FC, Rezg R, et al. Daily peritoneal administration of sodium pyrophosphate in a dialysis solution prevents the development of vascular calcification in a mouse model of uraemia. Nephrol Dial Transplant. 2011;26:3349–3357. doi: 10.1093/ndt/gfr039. [DOI] [PubMed] [Google Scholar]
- 25.Sage AP, Lu J, Tintut Y, et al. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villa-Bellosta R, Millan A, Sorribas V. Role of calcium-phosphate deposition in vascular smooth muscle cell calcification. Am J Physiol Cell Physiol. 2011;300:C210–C220. doi: 10.1152/ajpcell.00229.2010. [DOI] [PubMed] [Google Scholar]
- 27.Schlieper G, Aretz A, Verberckmoes SC, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Zeng XR, Wenger L, et al. Basic calcium phosphate crystals stimulate the endocytotic activity of cells—inhibition by anti-calcification agents. Biochem Biophys Res Commun. 2003;312:1053–1059. doi: 10.1016/j.bbrc.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 30.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 33.Ewence AE, Bootman M, Roderick HL, et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–e34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 34.Son BK, Kozaki K, Iijima K, et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–1031. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 35.Proudfoot D, Skepper JN, Hegyi L, et al. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 36.LeGeros RZ. Formation and transformation of calcium phosphates: relevance to vascular calcification. Z Kardiol. 2001;90:III/116–III/124. doi: 10.1007/s003920170032. [DOI] [PubMed] [Google Scholar]
- 37.Cheng PT, Grabher JJ, LeGeros RZ. Effects of magnesium on calcium phosphate formation. Magnesium. 1988;7:123–132. [PubMed] [Google Scholar]
- 38.Peters F, Epple M. Simulating arterial wall calcification in vitro: biomimetic crystallization of calcium phosphates under controlled conditions. Z Kardiol. 2001;90(Suppl 3):81–85. doi: 10.1007/pl00022850. [DOI] [PubMed] [Google Scholar]
- 39.Altura BM, Altura BT, Carella A, et al. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol. 1987;65:729–745. doi: 10.1139/y87-120. [DOI] [PubMed] [Google Scholar]
- 40.Montezano AC, Zimmerman D, Yusuf H, et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension. 2010;56:453–462. doi: 10.1161/HYPERTENSIONAHA.110.152058. [DOI] [PubMed] [Google Scholar]
- 41.Kircelli F, Peter ME, Sevinc OE, et al. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr321. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 43.Ivanovski O, Nikolov IG, Joki N, et al. The calcimimetic R-568 retards uremia-enhanced vascular calcification and atherosclerosis in apolipoprotein E deficient (apoE-/-) mice. Atherosclerosis. 2009;205:55–62. doi: 10.1016/j.atherosclerosis.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 44.Verberckmoes SC, Persy V, Behets GJ, et al. Uremia-related vascular calcification: more than apatite deposition. Kidney Int. 2007;71:298–303. doi: 10.1038/sj.ki.5002028. [DOI] [PubMed] [Google Scholar]
- 45.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Contiguglia SR, Alfrey AC, Miller NL, et al. Nature of soft tissue calcification in uremia. Kidney Int. 1973;4:229–235. doi: 10.1038/ki.1973.104. [DOI] [PubMed] [Google Scholar]
- 47.LeGeros RZ, Contiguglia SR, Alfrey AC. Pathological calcifications associated with uremia: two types of calcium phosphate deposits. Calcif Tissue Res. 1973;13:173–185. doi: 10.1007/BF02015408. [DOI] [PubMed] [Google Scholar]
- 48.Boskey AL, Posner AS. Effect of magnesium on lipid-induced calcification: an in vitro model for bone mineralization. Calcif Tissue Int. 1980;32:139–143. doi: 10.1007/BF02408533. [DOI] [PubMed] [Google Scholar]
- 49.Termine JD, Peckauskas RA, Posner AS. Calcium phosphate formation in vitro. II. Effects of environment on amorphous-crystalline transformation. Arch Biochem Biophys. 1970;140:318–325. doi: 10.1016/0003-9861(70)90072-x. [DOI] [PubMed] [Google Scholar]
- 50.Bennett RM, Lehr JR, McCarty DJ. Factors affecting the solubility of calcium pyrophosphate dihydrate crystals. J Clin Invest. 1975;56:1571–1579. doi: 10.1172/JCI108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 52.Smajilovic S, Hansen JL, Christoffersen TE, et al. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- 53.Gorgels TG, Waarsing JH, de WA, et al. Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med. 2010;88:467–475. doi: 10.1007/s00109-010-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwille PO, Schmiedl A, Schwille R, et al. Media calcification, low erythrocyte magnesium, altered plasma magnesium, and calcium homeostasis following grafting of the thoracic aorta to the infrarenal aorta in the rat—differential preventive effects of long-term oral magnesium supplementation alone and in combination with alkali. Biomed Pharmacother. 2003;57:88–97. [PubMed] [Google Scholar]
- 55.van den Broek FA, Beynen AC. The influence of dietary phosphorus and magnesium concentrations on the calcium content of heart and kidneys of DBA/2 and NMRI mice. Lab Anim. 1998;32:483–491. doi: 10.1258/002367798780599758. [DOI] [PubMed] [Google Scholar]
- 56.Ishimura E, Okuno S, Kitatani K, et al. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. doi: 10.5414/cnp68222. [DOI] [PubMed] [Google Scholar]
- 57.Li Q, Larusso J, Grand-Pierre AE, et al. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6(-/-) mice - potential for treatment of pseudoxanthoma elasticum. Clin Transl Sci. 2009;2:398–404. doi: 10.1111/j.1752-8062.2009.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iseri LT, French JH. Magnesium: nature's physiologic calcium blocker. Am Heart J. 1984;108:188–193. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 59.Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int. 1987;32:388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 60.Tzanakis I, Pras A, Kounali D, et al. Mitral annular calcifications in haemodialysis patients: a possible protective role of magnesium. Nephrol Dial Transplant. 1997;12:2036–2037. [PubMed] [Google Scholar]
- 61.Mazzaferro S, Coen G, Bandini S, et al. Role of ageing, chronic renal failure and dialysis in the calcification of mitral annulus. Nephrol Dial Transplant. 1993;8:335–340. [PubMed] [Google Scholar]
- 62.Tzanakis I, Virvidakis K, Tsomi A, et al. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res. 2004;17:102–108. [PubMed] [Google Scholar]
- 63.Hashimoto T, Hara A, Ohkubo T, et al. Serum magnesium, ambulatory blood pressure, and carotid artery alteration: the Ohasama study. Am J Hypertens. 2010;23:1292–1298. doi: 10.1038/ajh.2010.168. [DOI] [PubMed] [Google Scholar]
- 64.Geiger H, Wanner C. Magnesium in disease. Clin Kidney J. 2012;5(Suppl 1):i25–i38. doi: 10.1093/ndtplus/sfr165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998;136:480–490. doi: 10.1016/s0002-8703(98)70224-8. [DOI] [PubMed] [Google Scholar]
- 66.Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol. 1995;48:927–940. doi: 10.1016/0895-4356(94)00200-a. [DOI] [PubMed] [Google Scholar]
- 67.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 68.Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–359. [PubMed] [Google Scholar]
- 69.Izawa H, Imura M, Kuroda M, et al. Proceedings: effect of magnesium on secondary hyperparathyroidism in chronic hemodialysis: a case with soft tissue calcification improved by high Mg dialysate. Calcif Tissue Res. 1974;15:162. [PubMed] [Google Scholar]
- 70.Spiegel DM, Farmer B, Smits G, et al. Magnesium carbonate is an effective phosphate binder for chronic hemodialysis patients: a pilot study. J Ren Nutr. 2007;17:416–422. doi: 10.1053/j.jrn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Spiegel DM, Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13:453–459. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 72.Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 73.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 74.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 75.Turgut F, Kanbay M, Metin MR, et al. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 76.Ishimura E, Okuno S, Yamakawa T, et al. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 77.Passlick-Deetjen J, Wang W, et al. Magnesium and mortality risk in hemodialysis patients. European Renal Association (ERA) and European Dialysis and Transplant Association (EDTA), XLVII Congress, 25–28 June, Munich, Germany. Oral presentation. [Google Scholar]
- 78.Lacson E, Wang W, Lazarus M, et al. Magnesium and mortality risk in hemodialysis patients. American Society of Nephrology, 42nd Annual Meeting & Scientific Exposition (Renal Week), 27 October–1 November, San Diego, CA, USA. 2009 Poster F-PO1488. [Google Scholar]
- 79.Marzell B, Arkossy O, et al. Association of serum magnesium with mortality—results from a large European database. ERA-EDTA Congress, Prague, 26 June 2011. Abstract SuO008. [Google Scholar]