Crop production can be limited by soil waterlogging. Tolerance to waterlogging can vary between and within species. This study quantified tolerance to soil waterlogging in two divergent genotypes of pea (Pisum sativum), two of lentil (Lens culinaris) and a grasspea (Lathyrus sativus) control at germination and during vegetative growth. Soil waterlogging at 10 mm depth had no significant effect on shoot and root dry mass after 14 days. Significant genetic variation in both pea and lentil in tolerance to waterlogging after germination and subsequent recovery was evident. Screening of additional pea and lentil germplasm for waterlogging conditions is clearly warranted.
Keywords: Chlorophyll, intra-species variation, legume, porosity, waterlogging
Abstract
Waterlogging reduces the yield of food crops. Tolerance to waterlogging could vary between and within species. This study aimed to quantify tolerance to soil waterlogging in two divergent genotypes of pea (Pisum sativum), two of lentil (Lens culinaris) and a grasspea (Lathyrus sativus) control at germination and during vegetative growth. Following germination, seeds were grown for 14 days in soil waterlogged with the water table 10 mm below the surface, and then by draining the pots and allowing to recover for 21 days—to be compared with 35 days of continuous waterlogging. In both pea and lentil, the pair of genotypes contrasted widely with large-seeded pea genotype Kaspa and lentil genotype Nugget showing higher (2-fold) root porosity and less effect on shoot nitrogen content under waterlogging than the other genotypes (NPE and ATC). During recovery, the same two genotypes—Kaspa pea and Nugget lentil—also recovered better than their smaller-seeded species pairs. Soil waterlogging at 10 mm depth had no significant effect on shoot and root dry mass after 14 days. Root penetration into waterlogged soil was restricted to ∼100 mm depth and its distribution altered for pea and lentil genotypes but not for grasspea. Within the small sample studied, we demonstrated a significant genetic variation in both pea and lentil in tolerance to waterlogging after germination and subsequent recovery for the first time. Screening of additional pea and lentil germplasm for waterlogging conditions is clearly warranted.
Introduction
Soil waterlogging is a common abiotic stress that impacts on crop production (Jackson and Colmer 2005). Seeds of some species can withstand waterlogged conditions (i.e. prolonged soaking) more than others (Crawford 1977). The effects of hypoxia and/or anoxia during vegetative growth of dryland crops are well documented; shoot and root growth decrease and nutrient uptake becomes inhibited (pea, Cannell et al. 1979; lupin, Davies et al. 2000; wheat, Malik et al. 2002; barley, Pang et al. 2004; chickpea, Palta et al. 2010). Photosynthesis is also severely inhibited in waterlogging-sensitive plants resulting in reduced dry matter accumulation (wheat, Malik et al. 2001; barley, Pang et al. 2004). Inhibition of nitrogen nutrition reduces chlorophyll concentration of leaves resulting in premature leaf senescence (wheat, Malik et al. 2002; soya bean, Youn et al. 2008). Increased porosity through the development of aerenchyma tissue in root systems enhances the tolerance to waterlogging by facilitating oxygen movement via diffusion from shoots to roots (Colmer 2003). However, the response of a crop to waterlogging depends on the timing (developmental stage of the plant when stress was imposed), duration (number of days) and genotypic variation in waterlogging tolerance (Setter and Waters 2003).
Food legumes, a major dietary protein source (Erskine 2009), are grown on 77 million hectare worldwide [Food and Agriculture Organization (FAO) 2013] and often affected by waterlogging. Legume crops such as lupin (Lupinus angustifolius), chickpea (Cicer arietinum), lentil (Lens culinaris subsp. culinaris) and field pea (Pisum sativum) are susceptible to waterlogging at vegetative stages (Siddique et al. 1993; Siddique and Sykes 1997; Yu and Rengel 1999; Palta et al. 2010). In a detailed comparison of the differing responses of food legumes to waterlogging during vegetative growth, Solaiman et al. (2007) found faba bean (Vicia faba) as the most tolerant and field pea the least; with yellow lupin (Lupinus luteus), grasspea (Lathyrus sativus), narrow-leafed lupin, chickpea and lentil as intermediate (Solaiman et al. 2007). Within-species genetic variation in waterlogging tolerance is present in lupin (Davies et al. 2000), faba bean (Solaiman et al. 2007), soya bean (Henshaw et al. 2007), lotus (Real et al. 2008) and chickpea (Palta et al. 2010).
Waterlogged soil restricts food legume establishment in rice-based cropping systems in the Eastern Gangetic Plains of Bangladesh, Eastern India and Nepal (Ali et al. 2009). In such systems, pea, lentil, chickpea, grasspea and soya bean (Glycine max) are sown on residual soil moisture after paddy rice (Awadhwal et al. 2001). Relay sowing, the practice of broadcasting seed into a standing rice crop prior to harvest, predominates in Nepal for lentil and in Bangladesh for grasspea, and relay sown pea is also found (A. I. Malik, pers. obs.). Malik et al. (2015) recently found that the substitution of relay sown lentil for fallow was a useful option to intensify cropping in the Eastern Gangetic Plain. At the time of relay sowing, excess soil moisture and puddles are common features in puddled soil and such crops face transient soil waterlogging from an early developmental stage. Crawford (1977), Jackson (1979) and Sarlistyaningsih et al. (1995) demonstrated variation in germination of pea, bean and lupin seeds under different durations of waterlogging or anoxic conditions. Poor germination in waterlogged soil is a major impeding factor for crop establishment (Ramakrishna et al. 2000). Setter and Waters (2003) emphasized the need for future research on waterlogging tolerance during germination. Knowledge on waterlogging tolerance from germination to crop establishment in lentil and pea is scant. This study aimed to assess waterlogging tolerance in contrasting pairs (seed size and origins—Table 1) of pea and lentil genotypes—compared with a grasspea, considered waterlogging tolerant (Purseglove 1968), control—in the period after germination to vegetative growth and during subsequent recovery.
Table 1.
Names, country of origin and 100 seed weight of five legume genotypes.
| Crop | Name | Origin | 100 seed weight (g) |
|---|---|---|---|
| Pea | Kaspa | Australia | 27.3 ± 1.4 |
| NPE 1191.515 | Pakistan | 7.2 ± 0.3 | |
| Lentil | Nugget | Australia | 4.1 ± 0.5 |
| ATC 70856 | Bangladesh | 1.8 ± 0.6 | |
| Grasspea | Ceora | Australia | 11.4 ± 1.1 |
Methods
Experimental design
The experimental design was factorial with treatments of genotypes (5) (Table 1) × waterlogging (3) in a completely randomized block design with four replications. A pot was the experimental unit. Genotype pairs (pea and lentil) were selected for their contrasting seed size and origins (Table 1).
Genotypes were sown in pots either free-drained (control) or waterlogged to 10 mm below the soil surface at the start of the experiment. This water table depth best represents relay sowing conditions in the field (Ali 2011). Fourteen days after sowing (DAS), waterlogged pots were either continued as waterlogged or allowed to drain. This gave the three treatments: (i) drained control, (ii) waterlogged (i.e. continuously waterlogged for 35 days) and (iii) recovery (14 day of waterlogging followed by 21 days recovery).
After 14 DAS, 4 waterlogged and 4 control pots were harvested (H1); and 4 pots were allowed to drain from each genotype. The pots were randomly re-positioned every 7 days to minimize glasshouse positional effects.
The experiment was conducted during the winter (July–August) of 2012 in the glasshouse of the University of Western Australia Plant Growth Facility. For the period of the experiment, minimum and maximum temperatures during the day were 16.9 and 23.9 °C, respectively; and photoperiod 10.5 h, light intensity 1000 µmol m−2 s−1.
Plant culture
Seeds of two pea, two lentil and a grass pea genotype(s) (Table 1) were surface sterilized with 1 % commercial bleach (active ingredients NaOCl 40 mg L−1) for 1 min, washed 3–5 times with deionized water and placed on moist filter paper (Whatman no. 1) in 90 mm Petri dishes in a dark cabinet at room temperature overnight to imbibe. Seeds of grasspea were imbibed 3 days before the other genotypes (grasspea took longer to imbibe in a preliminary trial).
Four imbibed seeds were sown in each pot for lentil and grasspea and two seeds for pea genotypes. Seeding rate in the pots was decided according to reported field rates [i.e. 100–120 seeds m−2 for lentil and 45–55 seeds m−2 for pea (White et al. 2005)]. Seeds were placed on the surface of free-draining plastic pots (height 140 mm, diameter 120 mm) and each pot was placed in a closed-bottom plastic pot (height 180 mm, diameter 200 mm). Waterlogged treatment was imposed by filling the closed-bottom pots with DI-H2O up to 10 mm below the soil surface of free-draining pots. Water table was maintained by adding water every day. Control pots were weighed to 80 % of the field capacity at the start and retained at that weight by adding water every second day. Pots contained gravel at the bottom and 1.2 kg of mixed washed river sand and potting mix (1 : 1) (pH = 6.3 and EC (1 : 5 weight/volume in water extract) = 0.197 dS m−1). The potting mix contained 5 : 2 : 3 composted pine bark, coco peat and brown river sand. Rhizobium was not added to substrate. While filling the pots, platinum (Pt) electrodes were inserted in the substrate in 15 pots at a depth of 100 mm for redox measurement with three treatments for each genotype. Potting mix and river sand contained 0.004 and 0.018, 0.062 and 0.04, K+ 0.0005 and 0.0009 μmol kg−1 substrate, respectively.
The following nutrients were applied once 15 DAS which allow plants to establish on seed reserve prior to fertilization. Nutrients (μmol kg−1 substrate): KNO3 0.15, Ca(NO3)2 · 4H2O 0.375, KH2PO4 0.191, MgSO4 · 7H2O 0.025, KCl 0.0030, H3BO3 0.0012, MnSO4 · H2O 0.0002, ZnSO4 · 7H2O 0.0005, CuSO4 · 5H2O 0.00008, Na2MoO4 · 2H2O 0.00008 and NiSO4 · 6H2O 0.00025.
Measurements
Redox
Redox potential was measured daily in 15 pots (i.e. 6 waterlogged, 6 recovery and 3 control) with Pt electrodes and a silver/silver chloride reference electrode attached to a millivolt-meter. The reading was corrected as described by Patrick et al. (1996).
Growth
Harvested plants were divided into shoots and roots. All plant parts were dried for 72 h at 59 °C. Plant biomass at H1 and final harvest was measured by weighing the dry mass of the shoots and roots. The root lengths of main axis and the longest lateral roots were measured with a ruler at harvest.
Porosity
Porosity (% gas space per volume) of main and lateral roots was determined at in the final harvest following the principle described by Raskin (1983) and as modified by Thomson et al. (1990). Briefly, roots were lined up and cut into 50 mm segments on a tray containing water to avoid root drying. Roots were weighed submerged and then vacuum infiltration was carried out by subjecting the submerged tissue to low pressure with a vacuum pump three times to ensure the exit of all air from the roots. The fresh mass of the roots in the air was recorded and corrected using the equation of Thomson et al. (1990).
Nitrogen, chlorophyll concentration and chlorophyll fluorescence
Oven-dried pulverized samples were analysed for total nitrogen (N) in shoots using an auto analyser (Elementer, Model: Vario Macro, Hanau, Germany) against ethylenediaminetetraacetic acid and rice flour as standards. Total N was calculated on shoot dry mass basis. Relative changes in chlorophyll concentration were assessed thrice during the experiment at 21, 27 and 34 DAS on the youngest fully-expanded leaves of all plants in each pot with a hand-held chlorophyll meter (Minolta SPAD 502, Osaka, Japan).
Chlorophyll fluorescence was measured at 34 DAS on the youngest fully-expanded leaves of 1 plant in each pot after keeping the leaves in the dark for 30 min prior to measurement by using plant efficiency analyser (PEA) (Hansatech Instrument Ltd, UK). Measurements were taken at 50 % light intensity and the exposure time was set at 5 s. To obtain optimum light saturation point, a series of measurements taken in a preliminary experiment to ensure photosynthetic apparatus response to light and the accuracy of measurement for fluorescence. The F0 (minimal fluorescence), Fm (maximal fluorescence), Fv (variable fluorescence) and Fv/Fm were recorded.
Statistical analysis
Two-way analyses of variance (ANOVAs) were performed using GenStat 14th edition (VSN International) to determine the effects of plant genotype (2 pea, 2 lentil and 1 grass pea), waterlogging (well drained, waterlogged for 14 days, waterlogged for 35 days) and their interaction on the following response variables: main root length, main root porosity, shoot chlorophyll and nitrogen concentration, shoot and root mass. If main effects or interactions were significant, we then proceeded with multiple comparison tests to compare differences among means using Tukey's test. Separate analyses were performed on data collected by Day 14 and on data collected after 35 days of treatment.
Results
Redox potential
Redox potential was 365 ± 23 mV in the drained control pots and 200 ± 4 mV in the waterlogged pots at the start of the experiment. After 14 days the redox potential had declined to 173 ± 40 mV and it was 70 ± 10 mV by 35 days in waterlogged pots at the end of the experiment. After draining waterlogged pots on 14 DAS, the redox potential increased to 248 ± 55 mV after 2 days and to 403 ± 21 mV after 7 days of recovery. The redox potential of control pots remained close to 400 mV throughout the experimental period (data not shown).
Root growth
Roots of plants grown in drained soil reached the base of pots after 14 days of treatment for all genotypes. Overall root length was significantly (P < 0.001, Table 2) shorter in plants grown in waterlogged soil for 14 days than in the drained control in all pea and lentil genotypes (Fig. 1A). However, root length in grasspea was unaffected by waterlogging (Fig. 1A).
Table 2.
Degrees of freedom (df), F values and probabilities of two-way ANOVA. 1Values in parentheses indicate number of missing plots.
| Character | Source of variation | Genotype (G) | Treatment (T) | G × T | Residual |
|---|---|---|---|---|---|
| Waterlogging 14 days | |||||
| Shoot mass | df | 4 | 1 | 4 | 25 (5)1 |
| F value | 9.11 | 0.05 | 1.24 | ||
| Probability | <0.001 | 0.825 | 0.318 | ||
| Root mass | F value | 9.31 | 2.82 | 0.86 | |
| Probability | <0.001 | 0.105 | 0.502 | ||
| Main root length | F value | 1.31 | 29.28 | 3.12 | |
| Probability | 0.295 | <0.001 | 0.033 | ||
| Shoot nitrogen | df | 4 | 1 | 4 | 22 (8)1 |
| F value | 18.31 | 21.60 | 0.49 | ||
| Probability | <0.001 | 0.002 | 0.746 | ||
| Waterlogging 35 days | |||||
| Shoot mass | df | 4 | 2 | 8 | 42 (3)1 |
| F value | 88.01 | 0.56 | 0.30 | ||
| Probability | <0.001 | 0.574 | 0.961 | ||
| Root mass | F value | 21.85 | 1.13 | 0.26 | |
| Probability | <0.001 | 0.331 | 0.976 | ||
| Main root length | F value | 4.30 | 19.54 | 1.09 | |
| Probability | 0.005 | <0.001 | 0.388 | ||
| Shoot nitrogen | F value | 2.55 | 30.93 | 3.17 | |
| Probability | 0.053 | <0.001 | 0.007 | ||
| Chlorophyll concentration | F value | 31.63 | 341.93 | 29.01 | |
| Probability | <0.001 | <0.001 | <0.001 | ||
| Chlorophyll fluorescence | F value | 2.09 | 4.59 | 1.60 | |
| Probability | 0.100 | 0.016 | 0.153 | ||
| Root porosity | df | 4 | 2 | 8 | 30 (15)1 |
| F value | 8.59 | 6.07 | 0.99 | ||
| Probability | <0.001 | 0.006 | 0.463 | ||
Figure 1.
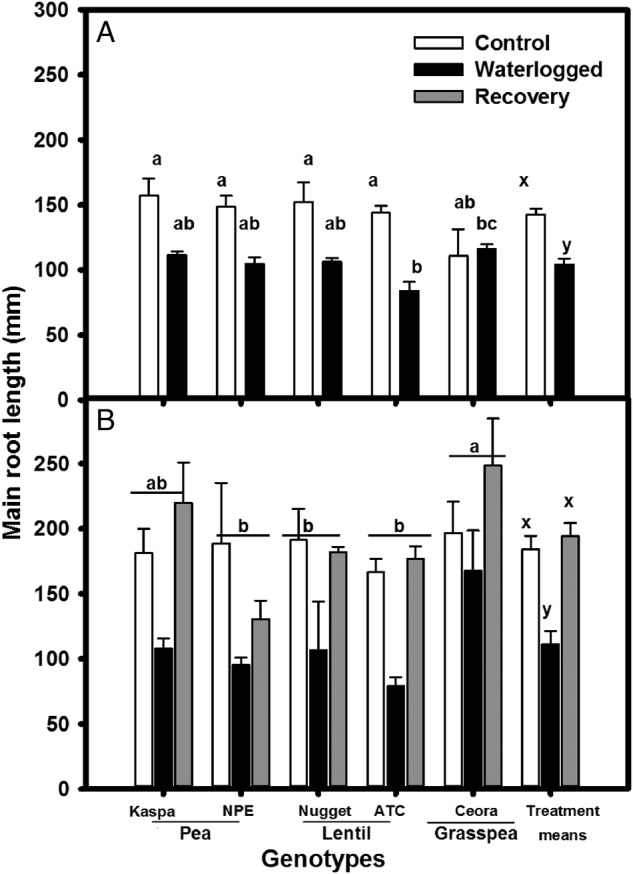
Main root length (mm) of legume genotypes (A) after 14 days and (B) after 35 days of waterlogging. Treatments were drained control (white bars), continuously waterlogged (black bars), 14 days waterlogged and subsequent 21 days of recovery (grey bars). Values are the means of four replicates standard errors. Overall treatment (+SE) means are shown grouped on the right. Means associated with different letters are significantly different (P < 0.05) by Tukey's test.
Again with the exception of grasspea, the extended waterlogging treatment stopped main root extension in all genotypes when the duration of waterlogging was prolonged from 14 to 35 days (Fig. 1A and B). On those pots, where the waterlogging stress was ceased after 14 days, main roots continued to elongate and reach the length of roots on continuously drained pots in all genotype except pea—NPE (Fig. 1B).
As with root length, root dry mass differed significantly (P < 0.001, Table 2) among legume genotypes when grown in drained soil; however, there was no effect of waterlogging on root dry mass (Table 3).
Table 3.
Root mass of five legume genotypes after 14 and 35 days of treatment in control, fully-drained soil; waterlogged, water table 10 mm below soil surface; recovery, after 14 days of waterlogging pots were allowed to drain. Means associated with different letters are significantly different (P < 0.05) by Tukey's test. Values are the means of four replicates.
| Crop | Name | Root mass (mg plant−1) 14 days |
Root mass (mg plant−1) 35 days |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Waterlogged | Mean | Control | Waterlogged | Recovery | Mean | ||
| Pea | Kaspa | 114 ± 22 | 91 ± 20 | 102.5 ac | 359 ± 12 | 391 ± 30 | 348 ± 30 | 332.5 a |
| NPE | 42 ± 10 | 40 ± 9 | 40.9 ab | 432 ± 14 | 384 ± 110 | 433 ± 90 | 416.3 a | |
| Lentil | Nugget | 55 ± 10 | 38 ± 8 | 46.6 ab | 177 ± 23 | 133 ± 11 | 169 ± 32 | 159.6 bc |
| ATC | 20 ± 7 | 27 ± 8 | 23.3 c | 97 ± 21 | 46 ± 1 | 84 ± 3 | 75.6 c | |
| Grasspea | Ceora | 90 ± 20 | 48 ± 4 | 69.6 ab | 151 ± 110 | 192 ± 20 | 244 ± 24 | 195.8 b |
Porosity of main and lateral roots
In drained soil, main root porosity was highest (i.e. 4.6 %) for lentil—Nugget; and this value was ∼2-fold higher than any other legume genotype in the study. Thirty-five days of waterlogging significantly (P > 0.001, Table 2) increased main root porosity in all legume genotypes (Fig. 2). Porosity increased for Kaspa, Nugget and grasspea by 2-fold, NPE by 1.1-fold and ATC by 1.4-fold in plants grown in waterlogged soil compared with the drained control.
Figure 2.
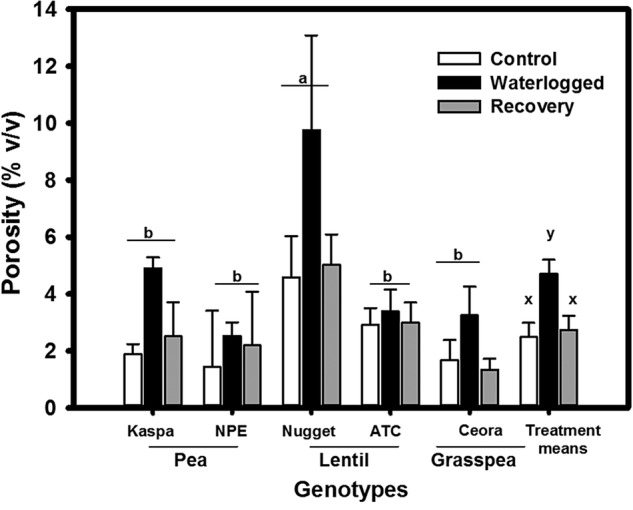
Effect of waterlogging treatment on root systems of legume genotypes main root porosity (% gas per unit volume after 35 days of growth. Treatments were drained control (white bars), continuously waterlogged (black bars), 14 days waterlogged and subsequent 21 days of recovery (grey bars). Values are the means of four replicates standard errors. Overall treatment (+SE) means are shown grouped on the right. Means associated with different letters are significantly different (P < 0.05) by Tukey's test.
Main root (i.e. tap root) porosity of plants that were allowed to drain after 14 days of waterlogging returned towards the control value in all genotypes (Fig. 2A) due to resumed growth after drainage of the pots. Lateral root porosity demonstrated a similar pattern to main roots when grown in drained and in waterlogged soil as well as at recovery (data not shown). Waterlogging increased (both main and lateral) porosity over the control due to the formation of aerenchyma (visual observation under microscope—data not shown).
Shoot growth and chlorophyll concentration
Shoot dry mass differed significantly among legume genotypes grown in drained soil. Waterlogging had no effect on shoot dry mass compared with the drained control (Table 4). However, waterlogging reduced leaf chlorophyll concentration significantly in all genotypes when compared with the drained control (P < 0.001, Table 2) (Fig. 3A). At the end of the waterlogging treatment period (35 DAS), there was a significant interaction (P < 0.001, Table 2) of treatment × within-species variation, indicating that chlorophyll concentration in small-seeded pea (NPE) and lentil (ATC) were markedly reduced compared with the larger-seeded pea Kaspa and lentil Nugget. After only 7 days of recovery, chlorophyll concentration started to recover (data not shown) and recovered to the control value by the end of the experiment (Fig. 3A).
Table 4.
Shoot mass of five legume genotypes after 14 and 35 days of growth in control, fully-drained soil; waterlogged, water table 10 mm below soil surface; recovery, after 14 days of waterlogging pots were allowed to drain. Means associated with different letters are significantly different (P < 0.05) by Tukey's test. Values are the means of four replicates.
| Crop | Name | Shoot mass (mg plant−1) 14 days |
Shoot mass (mg plant−1) 35 days |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Waterlogged | Mean | Control | Waterlogged | Recovery | Mean | ||
| Pea | Kaspa | 84 ± 21 | 59 ± 20 | 71.3 a | 1680 ± 140 | 1406 ± 140 | 1475 ± 250 | 1496 a |
| NPE | 42 ± 4 | 60 ± 20 | 50.8 ab | 1157 ± 110 | 1014 ± 110 | 1060 ± 200 | 1077 b | |
| Lentil | Nugget | 21 ± 4 | 23 ± 2 | 21.7 b | 293 ± 40 | 232 ± 52 | 274 ± 23 | 266 c |
| ATC | 25 ± 10 | 32 ± 11 | 28.6 b | 194 ± 31 | 155 ± 24 | 202 ± 33 | 184 c | |
| Grasspea | Ceora | 20 ± 3 | 26 ± 2 | 21.9 b | 341 ± 110 | 427 ± 32 | 429 ± 53 | 399 c |
Figure 3.
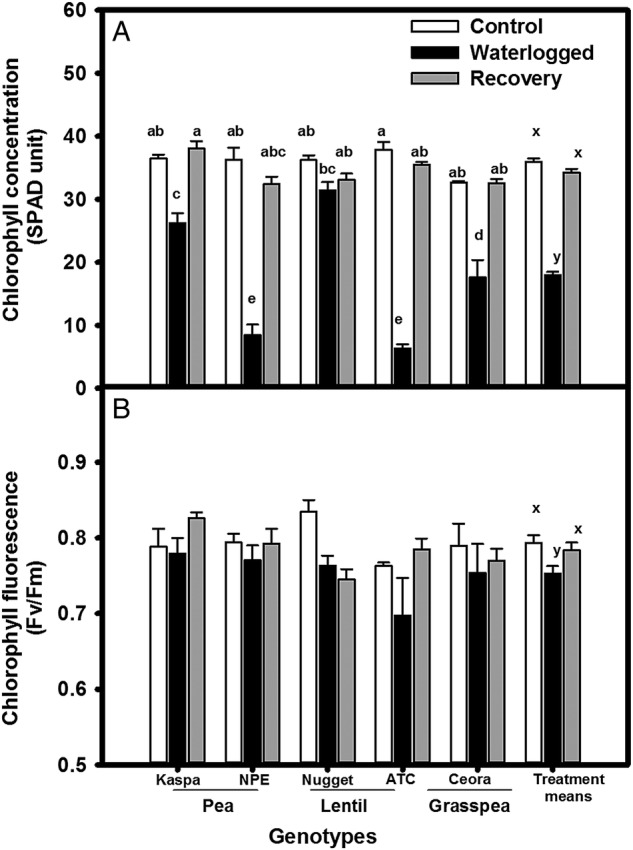
Effect of waterlogging treatment on youngest fully-expanded leaves. (A) Chlorophyll concentration (SPAD unit) and (B) chlorophyll fluorescence (Fv/Fm) of legume genotypes after 35 days of growth. Treatments were drained control (white bars), continuously waterlogged (black bars), 14 days waterlogged and subsequent 21 days of recovery (grey bars). Values are the means of four replicates standard errors. Overall treatment (+SE) means are shown grouped on the right. Means associated with different letters are significantly different (P < 0.05) by Tukey's test.
Chlorophyll fluorescence (maximum quantum efficiency Fv/Fm) on the youngest fully developed leaves was reduced by waterlogging in all legume genotypes compared with drained control (P < 0.05, Table 2) (Fig. 3B). The reduction was more pronounced in lentil than in pea. The chlorophyll fluorescence of the youngest fully-expanded leaf of plants previously waterlogged recovered to the control value for all legumes except for lentil Nugget (Fig. 3B).
Nitrogen concentration
Total shoot nitrogen concentration decreased over time in all plants grown in drained conditions (Fig. 4A and B). Fourteen days of waterlogging significantly (P < 0.01, Table 2) reduced nitrogen concentration on average (Fig. 4A), which decreased further when the treatment was prolonged to 35 days (Fig. 4B). The reduction of total shoot nitrogen varied after 35 days of treatment and the interaction of treatments with both between-species and within-species reached significance. This reduction was greatest in the small-seeded pea NPE and lentil ATC with 35 days of waterlogging. With 21 days of recovery following waterlogging, the nitrogen level in both pea genotypes and grasspea had recovered to the level of plants grown continuously in drained soil (Fig. 4B). However, the lentil genotypes did not recover similarly.
Figure 4.
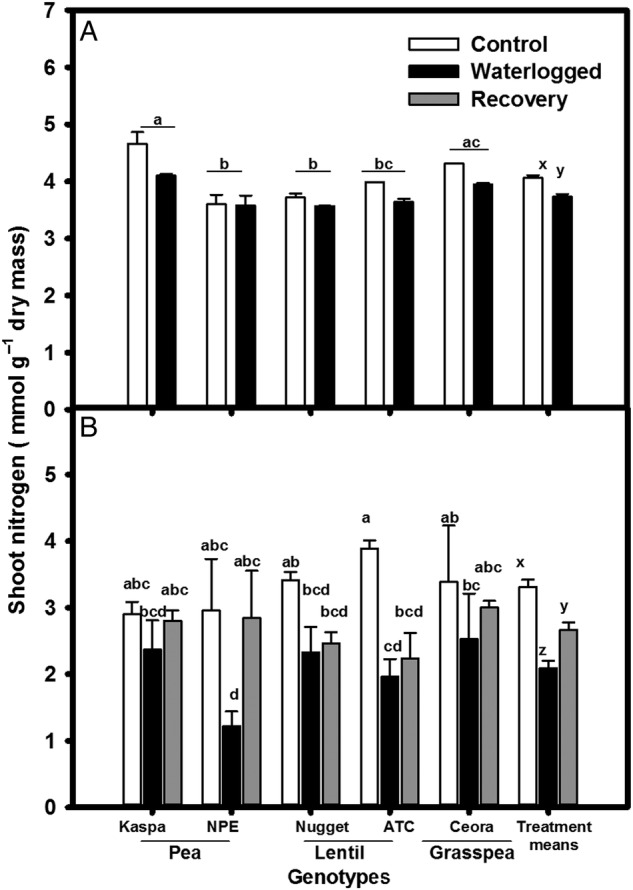
Effect of waterlogging treatment on shoot nitrogen (mmol g−1 dry mass) of legume genotypes (A) after 14 days and (B) after 35 days of growth. Treatments were drained control (white bars), continuously waterlogged (black bars), 14 days waterlogged and subsequent 21 days of recovery (grey bars). Values are the means of four replicates standard errors. Overall treatment (+SE) means are shown grouped on the right. Means associated with different letters are significantly different (P < 0.05) by Tukey's test.
Discussion
The results demonstrated a significant variation in waterlogging tolerance within legumes. In contrast to other studies, in which seedlings of food legumes were exposed to waterlogging (Davies et al. 2000; Solaiman et al. 2007; Palta et al. 2010; Bramley et al. 2011), the current study focussed on transient waterlogging tolerance: after aerobic imbibition where seeds were germinated on the surface of waterlogged soil, and their growth in waterlogged soil and recovery from waterlogging stress were assessed.
Variation in waterlogging tolerance among legume genotypes
Genetic variation in waterlogging tolerance within legume species has been demonstrated in lupin (Davies et al. 2000), faba bean (Solaiman et al. 2007), soya bean (Henshaw et al. 2007; Youn et al. 2008), lotus (Real et al. 2008), chickpea (Palta et al. 2010) and in the current study in both lentil and pea. The genotypes for this experiment were selected as contrasting pairs of pea and lentil with one small-seeded genotype each from South Asia, where there is a history of relay-sowing and germination under waterlogged conditions, and the other genotype—a larger-seeded Australian cultivar.
Following germination, important traits for waterlogging tolerance are the ability to increase root porosity during waterlogging, and for roots to recover during transient waterlogging; and to maintain shoot nitrogen and leaf chlorophyll (Malik et al. 2001). Considering these traits, the large-seeded genotypes from Australia (pea–Kaspa and lentil–Nugget) demonstrated greater waterlogging tolerance than their contrasting small-seeded pairs originating from South Asia (pea–NPE and lentil–ATC), presumably, due to greater carbohydrate pool and early vigour.
Enhanced root porosity contributes to better aeration in the root system in waterlogged soil (Colmer 2003). In both food (Solaiman et al. 2007) and pasture legumes (Gibberd et al. 1999) higher root porosity was observed in tolerant genotypes compared with sensitive genotypes. In the current experiment, tolerant legume cultivars showed enhanced root porosity by the formation of aerenchyma when grown in waterlogged substrate compared with sensitive genotypes. We confirmed the presence of aerenchyma by cross section of the main axis of the root (data not shown). Root porosity increased (up to 10 %) with growth in waterlogged conditions; however, constitutive root porosity was very low (∼2 %) and these values are much lower than those reported for waterlogging-tolerant legumes (Gibberd et al. 2001); all the species (and genotypes within species) responded to the treatment by increasing porosity with different degree of augmentation. Formation of adventitious roots is one trait that contributes to waterlogging tolerance; however, we did not observe adventitious root development in any of the legume genotypes; although, there is evidence for lentil to form adventitious roots when grown in waterlogged substrate (Erskine et al. 1994; Solaiman et al. 2007). In pea there are no reports of the development of adventitious roots (Jackson 1979). The discrepancy of the results from previous findings could be due to the experimental conditions. In the current study, the seeds were germinated aerobically on waterlogged soil in contrast to earlier experiments where 12- to 21-day-old seedlings were exposed to waterlogging.
Despite enhanced root porosity, waterlogging restricted root penetration into the substrate of pea and lentil (present study), wheat (Malik et al. 2001, 2002), soya bean (Henshaw et al. 2007), lupin (Davies et al. 2000; Real et al. 2008; Bramley et al. 2011) and chickpea (Palta et al. 2010). Restricted main root length was due to inadequate O2 diffusion to the root tip in waterlogged soil. According to the model of Armstrong (1979) with a given porosity (4–8 %, see Fig. 2) O2 could only diffuse down to 80–160 mm without any radial loss of oxygen along the root length. This demonstrates that the radial leakage of O2 along the root length and/or higher O2 uptake rate restricted roots to achieve desired root length. In contrast, grasspea main root length was not restricted by waterlogging (Fig. 1A). Presumably, grasspea formed a barrier to radial loss of O2. Solaiman et al. (2007) demonstrated the greater waterlogging tolerance of grasspea compared with lentil and pea. Further research is warranted to confirm the hypothesis.
Decreased shoot nitrogen in response to waterlogging has been well documented—wheat (Huang et al. 1994; Malik et al. 2001, 2002), soya bean (Riche 2004) and food legumes (Solaiman et al. 2007). Nitrogen reduction varied between legume genotypes under waterlogging due to effects on nitrogen fixation and the number of nodules (Cannell et al. 1979; Bacanamwo and Purcell 1999; Riche 2004; Bedard-Haughn 2009). The reduction of shoot nitrogen in waterlogged crops was due to a decrease in nutrient uptake (Malik et al. 2002; Solaiman et al. 2007; Palta et al. 2010). Waterlogging causes stelar anoxia to develop in the root (Aguilar et al. 2003), which reduced the loading of nutrients into the translocation stream, and restricted nitrogen supply to the shoot (Malik et al. 2001). In the current experiment, those genotypes able to enhance root porosity under waterlogging managed to maintain shoot N better—as in lotus (James and Sprent 1999; Striker et al. 2012, 2014) and soya bean (Shimamura et al. 2003; Thomas et al. 2005)—than those with low root porosity under waterlogging. Variation within species in chlorophyll retention in waterlogging conditions was reported in soya bean (Youn et al. 2008) and canola (Ashraf and Mehmood 1990), and tolerant genotypes with higher chlorophyll concentration identified (Talbot et al. 1987; Ashraf and Mehmood 1990; Pang et al. 2004; Youn et al. 2008). Once again, in this respect pea–Kaspa and lentil–Nugget showed better performance than other genotype pairs while the control grasspea also performed well.
Effect of waterlogging on growth
Waterlogging reduced shoot growth in wheat (Malik et al. 2002), lotus (Mendoza et al. 2005) and chickpea (Palta et al. 2010). Other studies mentioned no significant effect of waterlogging on shoot growth in lupin, pea, lentil, faba bean, chickpea and grasspea during the treatment period (i.e. 7 days), but the effect of waterlogging appeared on plants during the recovery period (Yu and Rengel 1999; Solaiman et al. 2007). We did not find an effect of waterlogging on shoot and root mass in our experiment. In previous studies, seedlings of 12–21 days were exposed to waterlogging; however, in the current experiment seeds were germinated on waterlogged soil and exposed to soil waterlogging at initial establishment; and as the water table in the pots was 10 mm below the soil surface, roots accessed aerobic conditions on the top layer of the substrate. Increase in shoot dry weight in wheat has been reported when exposed to a short period (e.g. 8 days) of waterlogging due to carbohydrate accumulation (Trought and Drew 1980). Moreover, roots responded to waterlogging by altering their distribution pattern and producing numerous lateral roots (visual observation). These roots grew close to the soil surface to obtain oxygen under waterlogging conditions (Voesenek et al. 1999), as was found in various subspecies of maize (Zea mays ssp. huehuetenangensis) (Mano et al. 2005a, b) and in Brassica napus (Cannell and Belford 1980).
Recovery from waterlogging
Root length recovers from waterlogging once allowed to grow in drained conditions. In wheat, stored carbohydrate was preferentially allocated to the re-growth of the root system during recovery (Malik et al. 2001, 2002). Root length recovered to the control value for tolerant legumes in the present experiment. This resulted in increasing net uptake of nitrogen transported to the shoot (Buwalda et al. 1988) and recovered leaf chlorophyll. Plants had to direct their energies into renewed pigment production, and re-greened chlorotic leaves at the onset of recovery (Smethurst et al. 2005). A previous study showed that pea and grasspea did not recover within 10 days after termination of waterlogging (Solaiman et al. 2007). Presumably, in our study, a longer recovery period led to the different result.
In the present study, relatively waterlogging-tolerant genotypes had an altered root distribution (i.e. near the soil surface) pattern while grown in waterlogged conditions as demonstrated by shallow root system-root length was short (∼100 mm) in waterlogged plants. But the overall root dry weight was similar for both drained and waterlogged treatments. However, there are disadvantages to the formation of the lateral roots—Armstrong et al. (1983) in pea and Malik et al. (unpubl. data) in wheat demonstrated that the lateral roots consumed O2 which restrict O2 movement through aerenchyma in the primary root; thus restrict root penetration into the deeper zone. However, in the current experiment plants maintained growth during the stress periods; presumably, the lateral roots become functional roots as demonstrated for pasture legumes (Gibberd et al. 1999). It is promising that during the recovery period the shallow root resumed growth and reached the same length as in the drained control, allowing access to soil moisture at depth as the soil profile dries later in the season.
Conclusion
The present study with a limited number of legume genotypes identified variations in tolerance to transient waterlogging and its recovery between legume crops and also intra-species variation in pea and lentil—associated with seed mass. Waterlogging-tolerant legume genotypes had high root porosity, were relatively unaffected in shoot nitrogen content under waterlogging and in recovery could resume root growth and rapidly regain chlorophyll concentration to control levels. Clearly, there is substantial potential to select in a wider range of both pea and lentil germplasm for increased levels of waterlogging tolerance. Further investigation should first focus on evaluation of a large number of genotypes within each species of legumes to determine the genetic variation, followed by physiological assessment using contrasting genotypes.
Sources of Funding
Our work was supported by the Centre for Plant Genetics and Breeding (PGB), University of Western Australia and project CIM-2009-038 funded by the Australian Centre for International Agriculture Research (ACIAR).
Contributions by the Authors
T.I.A., A.I.M. and W.E. designed the research and analysed data; T.I.A. performed the experimentation in partial fulfilment of her MSc thesis project and A.I.M. drafted the manuscript. All Authors agreed to the final version of the manuscript.
Conflict of Interest Statement
None declared.
Acknowledgements
T.I.A. gratefully acknowledges the support of the Department of Foreign Affairs and Trade (DFAT) (formerly AusAID) for her MSc scholarship award. The authors gratefully acknowledge the above funding.
Literature Cited
- Aguilar EA, Turner DW, Gibbs DJ, Armstrong W, Sivasithamparam K. 2003. Oxygen distribution and movement, respiration and nutrient loading in banana roots (Musa spp. L.) subjected to aerated and oxygen-depleted environments. Plant and Soil 253:91–102. 10.1023/A:1024598319404 [DOI] [Google Scholar]
- Ali M, Singh KK, Pramanik SC, Ali MO. 2009. Cropping systems and production agronomy. In: Erskine W, Muehlbauer FJ, Sarker A, Sharma B, eds. The lentil: botany, production and uses. Oxford, UK: CABI, 213–228. [Google Scholar]
- Ali MO. 2011. Enhancing lentil (Lens culinaris Medik.) production through relay cropping in transplant aman rice in medium low lands of Bangladesh. PhD Thesis, University of Rajshahi, Bangladesh. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7:226–332. [Google Scholar]
- Armstrong W, Healy MT, Lythe S. 1983. Oxygen diffusion in pea II. Oxygen concentrations in the primary pea root apex as affected by growth, the production of laterals and radial oxygen loss. New Phytologist 94:549–559. 10.1111/j.1469-8137.1983.tb04864.x [DOI] [Google Scholar]
- Ashraf M, Mehmood S. 1990. Effects of waterlogging on growth and some physiological parameters of four Brassica species. Plant and Soil 121:203–209. 10.1007/BF00012313 [DOI] [Google Scholar]
- Awadhwal N, Gowda C, Chauhan Y, Flower D, Haware M, Rego T, Pande S, Saxena N, Shanower T, Johansen C. 2001. Establishment of legumes following rice–a review. Natural Resource Management Program Report Vol. 2. pp 55.
- Bacanamwo M, Purcell LC. 1999. Soybean dry matter and N accumulation responses to flooding stress, N sources and hypoxia. Journal of Experimental Botany 50:689–696. 10.1093/jxb/50.334.689 [DOI] [Google Scholar]
- Bedard-Haughn A. 2009. Managing excess water in Canadian prairie soils: a review. Canadian Journal of Soil Science 89:157–168. 10.4141/CJSS07071 [DOI] [Google Scholar]
- Bramley H, Tyerman SD, Turner DW, Turner NC. 2011. Root growth of lupins is more sensitive to waterlogging than wheat. Functional Plant Biology 38:910–918. 10.1071/FP11148 [DOI] [PubMed] [Google Scholar]
- Buwalda F, Barrett-Lennard EG, Greenway H, Davies BA. 1988. Effects of growing wheat in hypoxic nutrient solutions and of subsequent transfer to aerated solutions. II. Concentrations and uptake of nutrients and sodium in shoots and roots. Functional Plant Biology 15:599–612. [Google Scholar]
- Cannell RQ, Belford RK. 1980. Effects of waterlogging at different stages of development on the growth and yield of winter oilseed rape (Brassica napus L.). Journal of the Science of Food and Agriculture 31:963–965. 10.1002/jsfa.2740310915 [DOI] [Google Scholar]
- Cannell RQ, Gales K, Snaydon RW, Suhail BA. 1979. Effects of short-term waterlogging on the growth and yield of peas (Pisum sativum). Annals of Applied Biology 93:327–335. 10.1111/j.1744-7348.1979.tb06549.x [DOI] [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26:17–36. 10.1046/j.1365-3040.2003.00846.x [DOI] [Google Scholar]
- Crawford RMM. 1977. Tolerance of anoxia and ethanol metabolism in germinating seeds. New Phytologist 79:511–517. 10.1111/j.1469-8137.1977.tb02235.x [DOI] [Google Scholar]
- Davies CL, Turner DW, Dracup M. 2000. Yellow lupin (Lupinus luteus) tolerates waterlogging better than narrow-leafed lupin (L. angustifolius) I. Shoot and root growth in a controlled environment. Australian Journal of Agricultural Research 51:701–709. 10.1071/AR99073 [DOI] [Google Scholar]
- Erskine W. 2009. Global production, supply and demand. In: Erskine W, Muehlbauer FJ, Sarker A, Sharma B, eds. The lentil: botany, production and uses. Oxford, UK: CABI, 4–12. [Google Scholar]
- Erskine W, Tufail M, Russell A, Tyagi MC, Rahman MM, Saxena MC. 1994. Current and future strategies in breeding lentil for resistance to biotic and abiotic stresses. Euphytica 73:127–135. 10.1007/BF00027189 [DOI] [Google Scholar]
- Food and Agriculture Organization (FAO). 2013. Food and Agriculture Organisation of the United Nations. Rome, Italy: http://faostat.fao.org. [Google Scholar]
- Gibberd MR, Colmer TD, Cocks PS. 1999. Root porosity and oxygen movement in waterlogging-tolerant Trifolium tomentosum and -intolerant Trifolium glomeratum. Plant, Cell and Environment 22:1161–1168. 10.1046/j.1365-3040.1999.00472.x [DOI] [Google Scholar]
- Gibberd M, Gray JD, Cocks PS, Colmer TD. 2001. Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation and ‘aerotropic rooting’. Annals of Botany 88:579–589. 10.1006/anbo.2001.1506 [DOI] [Google Scholar]
- Henshaw TL, Gilbert RA, Scholberg JMS, Sinclair TR. 2007. Soya bean (Glycine max L. Merr.) genotype response to early-season flooding: I. Root and nodule development. Journal of Agronomy and Crop Science 193:177–188. 10.1111/j.1439-037X.2007.00257.x [DOI] [Google Scholar]
- Huang B, Johnson JW, Nesmith S, Bridges DC. 1994. Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. Journal of Experimental Botany 45:193–202. 10.1093/jxb/45.2.193 [DOI] [Google Scholar]
- Jackson MB. 1979. Rapid injury to peas by soil waterlogging. Journal of the Science of Food and Agriculture 30:143–152. 10.1002/jsfa.2740300208 [DOI] [Google Scholar]
- Jackson MB, Colmer TD. 2005. Response and adaptation by plants to flooding stress. Annals of Botany 96:501–505. 10.1093/aob/mci205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James EK, Sprent JI. 1999. Development of N2-fixing nodules on the wetland legume Lotus uliginosus exposed to conditions of flooding. New Phytologist 142:219–231. 10.1046/j.1469-8137.1999.00394.x [DOI] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. 2001. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Functional Plant Biology 28:1121–1131. 10.1071/PP01089 [DOI] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M. 2002. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytologist 153:225–236. 10.1046/j.0028-646X.2001.00318.x [DOI] [Google Scholar]
- Malik AI, Ali MO, Zaman MS, Flower K, Rahman MM, Erskine W. 2015. Relay sowing of lentil (Lens culinaris subsp. culinaris) to intensify rice-based cropping. The Journal of Agricultural Science 10.1017/S0021859614001324. [DOI] [Google Scholar]
- Mano Y, Muraki M, Fujimori M, Takamizo T, Kindiger B. 2005a. Identification of QTL controlling adventitious root formation during flooding conditions in teosinte (Zea mays ssp. huehuetenangensis) seedlings. Euphytica 142:33–42. 10.1007/s10681-005-0449-2 [DOI] [Google Scholar]
- Mano Y, Omori F, Muraki M, Takamizo T. 2005b. QTL mapping of adventitious root formation under flooding conditions in tropical maize (Zea mays L.) seedlings. Breeding Science 55:343–347. 10.1270/jsbbs.55.343 [DOI] [Google Scholar]
- Mendoza R, Escudero V, García I. 2005. Plant growth, nutrient acquisition and mycorrhizal symbioses of a waterlogging tolerant legume (Lotus glaber Mill.) in a saline-sodic soil. Plant and Soil 275:305–315. 10.1007/s11104-005-2501-3 [DOI] [Google Scholar]
- Palta JA, Ganjeali A, Turner NC, Siddique KHM. 2010. Effects of transient subsurface waterlogging on root growth, plant biomass and yield of chickpea. Agricultural Water Management 97:1469–1476. 10.1016/j.agwat.2010.05.001 [DOI] [Google Scholar]
- Pang J, Zhou M, Mendham N, Shabala S. 2004. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Australian Journal of Agricultural Research 55:895–906. 10.1071/AR03097 [DOI] [Google Scholar]
- Patrick W, Gambrell R, Faulkner S, Sparks D, Page A, Helmke P, Loeppert R, Soltanpour P, Tabatabai M, Johnston C. 1996. Redox measurements of soils. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, eds. Methods of soil analysis. Part 3—chemical methods. Madison, WI: Soil Science Society of America, American Society of Agronomy, 1255–1273. [Google Scholar]
- Purseglove JW. 1968. Tropical crops dicotyledons. London, UK: Longman. [Google Scholar]
- Ramakrishna A, Gowda CLL, Johansen C. 2000. Management factors affecting legumes production in the Indo-Gangetic Plain. In: Legumes in rice and wheat cropping systems of the Indo-Gangetic Plain-constraints and opportunities. Patancheru, Andhra Pradesh: ICRISAT, 156–165. [Google Scholar]
- Raskin I. 1983. A method for measuring leaf volume, density, thickness, and internal gas volume. HortScience 18:698–699. [Google Scholar]
- Real D, Warden J, Sandral GA, Colmer TD. 2008. Waterlogging tolerance and recovery of 10 Lotus species. Australian Journal of Experimental Agriculture 48:480–487. 10.1071/EA07110 [DOI] [Google Scholar]
- Riche CJ. 2004. Identification of soybean cultivars tolerance to waterlogging through analyses of leaf nitrogen concentration. MSc Thesis Louisiana State University. [Google Scholar]
- Sarlistyaningsih L, Sivasithamparam K, Setter TL. 1995. Influence of waterlogging on germination and survival of lupin seeds (Lupinus angustifolius L. cv. Gungurru) coated with calcium peroxide and streptomycin. Australian Journal of Experimental Agriculture 35:537–541. 10.1071/EA9950537 [DOI] [Google Scholar]
- Setter TL, Waters I. 2003. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil 253:1–34. 10.1023/A:1024573305997 [DOI] [Google Scholar]
- Shimamura S, Mochizuki T, Nada Y, Fukuyama M. 2003. Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant and Soil 251:351–359. 10.1023/A:1023036720537 [DOI] [Google Scholar]
- Siddique KHM, Sykes J. 1997. Pulse production in Australia past, present and future. Australian Journal of Experimental Agriculture 37:103–111. 10.1071/EA96068 [DOI] [Google Scholar]
- Siddique KHM, Walton GH, Seymour M. 1993. A comparison of seed yields of winter grain legumes in Western Australia. Australian Journal of Experimental Agriculture 33:915–922. 10.1071/EA9930915 [DOI] [Google Scholar]
- Smethurst CF, Garnett T, Shabala S. 2005. Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant and Soil 270:31–45. 10.1007/s11104-004-1082-x [DOI] [Google Scholar]
- Solaiman Z, Colmer TD, Loss SP, Thomson BD, Siddique KHM. 2007. Growth responses of cool-season grain legumes to transient waterlogging. Australian Journal of Agricultural Research 58:406–412. 10.1071/AR06330 [DOI] [Google Scholar]
- Striker GG, Izaguirre RF, Manzur ME, Grimoldi AA. 2012. Different strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biology 14:50–55. [DOI] [PubMed] [Google Scholar]
- Striker GG, Casas C, Manzur ME, Ploschuk RA, Casal JJ. 2014. Phenomic networks reveal largely independent root and shoot adjustment in waterlogged plants of Lotus japonicus. Plant, Cell and Environment 37:2278–2293. [DOI] [PubMed] [Google Scholar]
- Talbot RJ, Etherington JR, Bryant JA. 1987. Comparative studies of plant growth and distribution in relation to waterlogging. XII. Growth, photosynthetic capacity and metal ion uptake in Salix caprea and S. cinerea ssp. Oleifolia. New Phytologist 105:563–574. 10.1111/j.1469-8137.1987.tb00894.x [DOI] [Google Scholar]
- Thomas AL, Guerreiro SMC, Sodek L. 2005. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Annals of Botany 96:1191–1198. 10.1093/aob/mci272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. 1990. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell and Environment 13:395–403. 10.1111/j.1365-3040.1990.tb02144.x [DOI] [Google Scholar]
- Trought MCT, Drew MC. 1980. The development of waterlogging damage in young wheat plants in anaerobic solution cultures. Journal of Experimental Botany 31:1573–1585. 10.1093/jxb/31.6.1573 [DOI] [Google Scholar]
- Voesenek LACJ, Armstrong W, Colmer TD, Bögemann GM, McDonald MP. 1999. A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Functional Plant Biolology 26:87–93. [Google Scholar]
- White P, Seymour M, Burgess P, Harries M. 2005. Producing pulses in the southern agricultural region. South Perth, WA: Department of Agriculture, Western Australia; and Grains Research & Development Corporation, pp 132. [Google Scholar]
- Youn JT, Van K, Lee JE, Kim WH, Yun HT, Kwon YU, Ryu YH, Lee SH. 2008. Waterlogging effects on nitrogen accumulation and N2 fixation of supernodulating soybean mutants. Journal of Crop Science and Biotechnology 11:111–118. [Google Scholar]
- Yu Q, Rengel Z. 1999. Waterlogging influences plant growth and activities of superoxide dismutases in narrow-leafed lupin and transgenic tobacco plants. Journal of Plant Physiology 155:431–438. 10.1016/S0176-1617(99)80127-8 [DOI] [Google Scholar]


