Abstract
Background: Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease of the central nervous system of potential autoimmune origin that is frequently associated with psychological disorders and cognitive deficits, as well as with fatigue, stress, and psychosocial burden. These factors often cause decreased quality of life, social withdrawal, and unemployment. We describe the development of a cognitive-behavioral group intervention based on the concept of metacognition and evaluation of the feasibility and acceptance of the program as a rehabilitation tool.
Methods: Metacognitive Training in MS (MaTiMS) consists of six modules, each 90 minutes in duration. We tested acceptance and design of the program in six focus groups (entire sample, n = 27). Framework analysis of transcripts was used to identify key topics and categories. Program modules were revised in accordance with appropriate recommendations of focus group members. We subsequently evaluated MaTiMS in two groups (n = 5, n = 6) in a rehabilitation center. Neuropsychological functioning as well as coping self-efficacy, depression, stress, perceived cognitive deficit, fatigue, and quality of life were assessed. Acceptance of MaTiMS from the patient perspective was also studied.
Results: The modules were highly accepted by patients. Pre-post assessments showed significant improvements in the Coping Self Efficacy Scale (P = .007), the Würzburger Fatigue Inventory for MS Score (P = .028), and the Hamburg Quality of Life Questionnaire in Multiple Sclerosis Mood subscale (P = .046).
Conclusions: These preliminary results suggest that MaTiMS represents a feasible psychological group training program that may foster improvements in self-efficacy, fatigue, and mood. The next step will be an evaluation of the program in a randomized controlled trial.
Multiple sclerosis (MS) is an inflammatory and degenerative disease that usually starts in young adulthood. Disease progression is largely unpredictable, and available therapies are only moderately effective,1 resulting in considerable coping challenges. Besides physical impairment, cognitive difficulties are common and substantially affect daily life.2 More than 50% of people with MS are reported to develop neuropsychological deficits.3 Importantly, many people with MS feel more restricted by cognitive impairments than by limited mobility,4 highlighting the need for interventions tackling these symptoms. Beyond cognitive deficits, impaired social cognitive functions compromise social interaction and social support systems that are crucial to coping, for example, buffering stress.5,6 We recently found deficits in social cognition early in the disease independent of other cognitive decline.7 Therefore, MS often has a negative impact on social life.8
Other psychological factors account for additional burdens: people with MS are at high risk for major depressive disorders,9 and they experience fatigue with a considerable impact on participation in life, socioeconomic status, and quality of life.10 Additionally, psychological stress is a relevant disease modulator; for example, associations between stressful life events and relapses have repeatedly been shown.11
Therapeutic concepts for interventions designed to improve cognitive performance and everyday life abilities, as well as proper assessments of these concepts, are scarce. Thus far, drug treatments have not shown convincing effects.12 Evidence for beneficial effects of psychological and neuropsychological interventions has recently been reviewed. Although effects are modest, Thomas et al.13 concluded that cognitive-behavioral approaches are beneficial in the treatment of depression and in helping people adjust to and cope with MS. Other meta-analytic work14 showed that cognitive remediation or specific functional training for impaired cognitive domains, such as memory or information processing, have only limited positive effects. It is largely unknown whether training effects are relevant to patients' daily life. Researchers have concluded that new interventions are needed to treat cognitive and psychological dysfunction.
We hypothesized that neuropsychological performance in a chronic progressive disease like MS might be best addressed by trying to change cognition, behavior, and coping styles. The aim of this study was to develop a metacognitive training program based on the cognitive-behavioral approach aimed at raising patients' awareness of common cognitive deficits and biases while taking into account MS-specific psychological and neuropsychological disturbances.
In this article, we describe the development of the Metacognitive Training in MS (MaTiMS) program and present pilot data on its feasibility and effectiveness.
Methods
Development of the Training Program
Based on previous metacognitive training for patients with psychiatric disorders,15 developed at the University Medical Center Hamburg-Eppendorf, an MS-specific training program, MaTiMS, has been developed. The term metacognition was developed by Flavell and Wellmann in the 1970s16 and means “thinking about thinking.” The goal was to translate the current understanding of cognitive disorders into an action-oriented training program. Metacognitive training programs have been evaluated in randomized controlled trials (RCTs) in various psychiatric disorders associated with neuropsychological impairments.17
MaTiMS is highly standardized and consists of six presentation modules based on Microsoft PowerPoint (Microsoft Inc, Redmond, WA) presentations, a manual, and moderation cards. Each session lasts about 90 minutes. To enhance interaction and learning from peers, MaTiMS is performed in small-group sessions of six to eight patients. The major modules of the program are memory, attention, depression, fatigue, stress, and social cognition.
MaTiMS combines three fundamental components: communication of the evidence about cognitive deficits in MS and their treatment based on principles of evidence-based patient information,18 concrete examples and interactive reflection addressing cognitive biases, and information on alternative coping strategies and how to avoid cognitive traps. The aims are to transfer current research knowledge to patients in order to unveil compromised coping strategies when handling impairments, to collect and present better strategies within the group, and to provide corrective experiences to patients. The ultimate goal is to promote a change in the patient's metacognition and resulting behavior, thereby facilitating daily life. Our metacognitive approach refers to knowledge about one's own cognition and addresses metacognitive learning strategies—that is, processes of planning, controlling, and regulating learning. It also includes self-reflection on cognitive processes, experiences of exhaustion, and depression.
First, in each module, a teaser introduces the main topics of the training. Current research findings are then displayed to the patients, and examples demonstrate the link to MS. Patients are invited to discuss their own experiences. Examples and exercises—the core of the program—are introduced and put into practice. The relevance of each module to MS is pointed out at the beginning and the end of each session. A slide entitled “Transfer to Everyday Life” collects the main contents of the module at the end of the session (an overview of the contents of each module is presented in Supplementary Table 1 (101.5KB, pdf) ).
The exercise part addresses examples, highlights possibly ineffective coping strategies, and suggests more appropriate thoughts and concepts to act upon. Furthermore, participants can explain their own strategies and their experiences in coping with typical problems, as well as anticipating potential behavioral alternatives.
The encounter with the experiences and strategies of other participants provides corrective alternatives in a fun and supportive atmosphere. To increase the effects of the training program, participants receive a booklet at the end of every training module containing information on training goals that facilitate the transfer of newly acquired skills to everyday life.
This study was approved by the Ethics Committee of the Hamburg Chamber of Physicians (PV3745). All participants received an explanation of the study, and written informed consent was obtained prior to participation.
Focus Groups
The pilot study aimed to improve the training modules and to adapt content to the needs of MS patients. Each module was performed once. We chose settings (semicircular table) for the sessions that encouraged participants to feel relaxed, facilitating the expression of ideas and thoughts. The training session was conducted by a psychologist. For each module, five to six patients were selected to participate in the session, discuss it, and offer suggestions for improvement. Inclusion criteria for focus groups were the following: diagnosis of MS, age of 18 years or older, and perceived problems with cognition and depression or fatigue (preselected from the University Medical Center Hamburg MS database). MS patients were contacted by telephone and invited to participate.
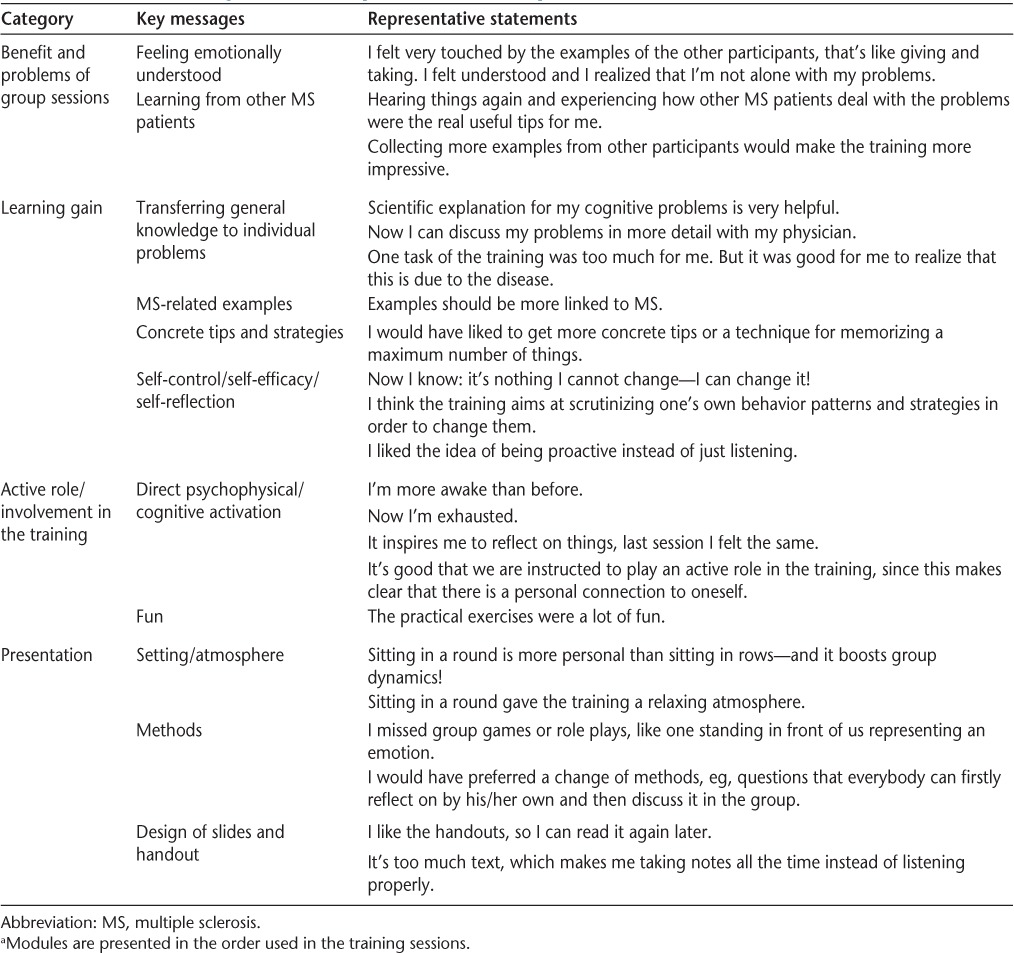
Notes at the training session were taken by an instructor/interviewer (SL) and included descriptions of nonverbal expressions (eg, body language or behaviors). Subsequently, patients were instructed to reflect on their individual needs and whether the training module addressed these needs adequately. The group interview was conducted in an open conversation. The focus groups were audiorecorded. After each session, the program modules were revised according to the comments of the participants. Data collected for analysis consisted of a full transcription of the interviews, notes taken during the sessions, and observations of the interviewer. Framework analysis methods19 were applied to all collected data. Transcripts were systematically reviewed and categorized. The categories were refined and adjusted where appropriate. Theoretical and practical interpretations of the findings were derived, and a summary table compiled. The contents of the summary were re-reviewed by an MS-specialized neurologist (CH) and another psychologist not involved in the performance of the focus groups. A final catalog was produced (Table 1).
Table 1.
Derived categories and representative MS patient statements a
Pilot Groups
The pilot study aimed to evaluate the feasibility of MaTiMS in a rehabilitation context, to assess participants' acceptance of the training curriculum, and to pilot possible outcome parameters for a subsequent trial. We hypothesized that targeting people during inpatient or outpatient rehabilitation offers the opportunity to teach cognitive and behavioral strategies in a convenient learning environment, and facilitates a program comprising a small number of sessions, which might be difficult in a pure outpatient setting.
All six MaTiMS modules were presented to each of two session groups. A total intervention cycle took 3 weeks, with two modules performed per week. Inclusion criteria were a definite diagnosis of MS according to the McDonald criteria20 and patients' perception that the training aim might be meaningful for them. At the beginning of each group session, we explained the aim of the session and emphasized that identification of difficulties and misunderstandings was vital for the training development process.
Psychological and Neuropsychological Measures
MaTiMS primarily aims to alter coping behavior. To assess this goal, we used the Coping Self Efficacy Scale (CSES),21 which provides a measure of a person's perceived ability to select the adequate coping strategy for a given challenge and has recently been applied to MS.22
For assessment of psychological status, quality of life, fatigue, and stress, we used the Hospital Anxiety and Depression Scale (HADS),23 neuropsychiatric scales of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS),24 the Würzburger Fatigue Inventory for MS (WEIMUS),25 and the Perceived Stress Scale (PSS).26
Self-perceived deficits in attention were measured with the Scale to Assess Attention Deficits (SEA).27 To assess self-perceived cognitive abilities we used the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ).28 We compared subjectively perceived cognitive deficits with objective measures. We used the Symbol Digit Modalities Test (SDMT)29 as an overall estimate of information processing ability. We applied the Test Battery for Attention Performance (TAP) of Zimmermann and Fimm30 to assess attention, that is, alertness (tonic and phasic), divided attention (simultaneous processing of visual and acoustic stimuli), and selective attention (response selection and inhibition). For learning and memory testing we used the Verbal Learning and Memory Test (VLMT)31 and the Wechsler Memory Scale (WMS-R)32 both forward and backward to assess short-term and working memory. The whole test battery was administered within a week both before starting and after finishing the intervention. The examination time for each assessment date was 2 hours.
Quality Evaluation
To assess satisfaction with the program, we used a 17-item questionnaire that was developed by the research group. Items were rated on a 5-point Likert scale ranging from 0 (“not at all”) to 4 (“extensively”). Additional items were asked in an open format to collect positive and negative perceptions of the program and recommendations. Finally, patients were asked to give an overall rating for the entire program on a scale from 1 to 6.
Statistical Analysis
In the single-site, uncontrolled, pre-post group evaluation of the pilot group training, we used the nonparametric Wilcoxon signed rank test. Statistical analysis was completed using SPSS for Windows version 15.0 (IBM, Armonk, NY).
Results
Focus Groups
Overall, 27 patients participated in the six MaTiMS modules. Five to six patients participated in each module. Twenty-five of the 27 patients participated in only one module, one patient attended three modules, and one patient four modules. The mean age of participants was 46.2 years (SD 9.18), and the mean disease duration was 9.1 years (range, 1–31). Twenty-three (85%) participants were female. Eighteen (67%) had a relapsing-remitting disease course, and nine had a primary or secondary progressive disease course.
Participants' statements about the training allocated to the derived categories for each module are presented in Table 1. Comments include direct psychological and behavioral reactions during the training, mediated key and take-home messages, and overall assessments of the training. No participant judged the training unnecessary or unsuitable. All participants pointed out that there is a dearth of training programs to improve psychological and cognitive impairment in MS in Germany, including rehabilitation centers. Regarding the formal training presentation, all participants approved the curriculum, including the theoretical framework, the exercises, and the interactive group involvement. Participants assessed the background information on the evidence of cognitive and psychological impairments in MS as being very important. The exchange with other group members was highly valued; participants felt relaxed and accepted by the group while realizing that others had the same problems. They also valued being exposed to experiences and strategies of other group members.
The social cognition module drew the most attention. Participants were not aware of the difficulties in social cognition in MS patients, while, on the other hand, most of them experienced problems in social interaction. In this module, in particular, the exercises were very well received and assessed.
For the other modules, the most often mentioned shortcoming was the lack of functional training exercises. Additionally, participants felt that not enough time was dedicated to the group discussions and that there was not enough room for the presentation of personal experiences. Concerning the presented material, participants suggested that sentences be shortened and more pictures included.
Pilot Study
The complete program, with all six modules, was presented to 11 patients (5 female and 6 male) from an outpatient rehabilitation center in Hamburg. Two session groups were conducted. The mean age of participants was 40.27 years (SD 14.42) and mean disease duration was 7.14 years (SD 5.93). At baseline, patients showed high anxiety and moderate depression values. The mean HADS anxiety score was 9.82 (SD 5.96), and the mean HADS depression score was 8.36 (SD 6.02). Seven out of 11 patients showed anxiety scores above the cutoff of 9, and four patients showed a HADS depression score above 9.0, which indicated a clinically relevant anxiety/depression disorder. Fatigue was highly prevalent, with a mean score of 44.09 (SD 13.62). Patients reported high values for perceived cognitive deficits (the mean MSNQ score was 28.55, SD 11.24), but the objective screening measure (SDMT) did not confirm the perceived impairment (the mean SDMT score was 52.46, SD 12.32).
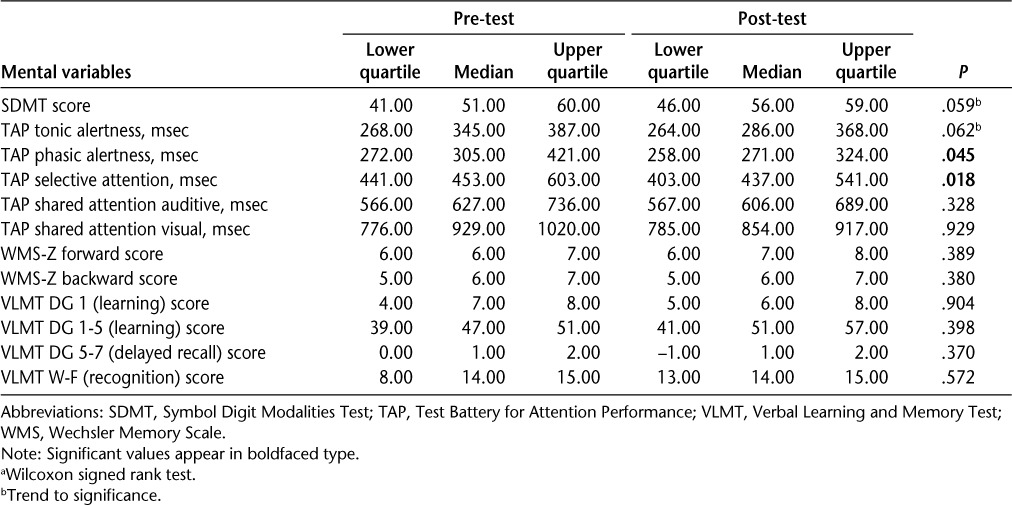
We found statistically significant improvements for coping (CSES), fatigue (WEIMUS), and mood as measured within the HAQUAMS (Table 2). In addition, we found trends toward improvements in the SEA and the PSS. No significant changes were found in HADS depression and anxiety scores and subjective neuropsychological measures. For objective neuropsychological measures we found significant improvements in TAP phasic alertness and TAP selective attention (Tables 2 and 3).
Table 2.
Pre-post differences in both study groups (n = 11) a
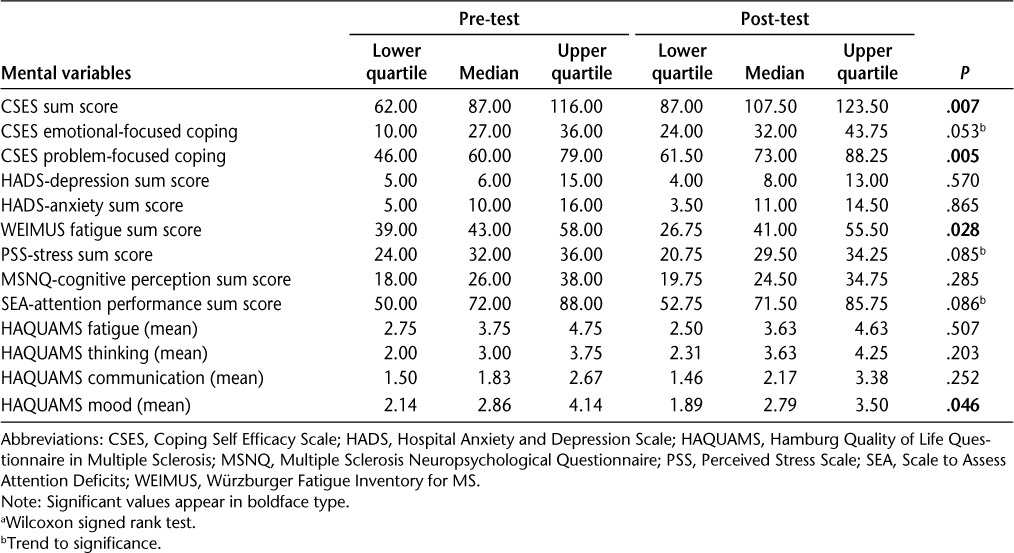
Table 3.
Pre-post neuropsychological values (n = 11) a
Further data demonstrate feasibility and high patient compliance. With the evaluation questionnaire, patients assessed the training program as positive, with a mean overall score of 55.8 of 68.0 points. The mean overall grade was 2.1 (range, 1–3).
Discussion
We developed a metacognitive group treatment approach for psychological and cognitive impairment in MS. The study showed feasibility and positive effects of MaTiMS on coping self-efficacy even though the training was not performed by a psychologist, possibly as a result of the high degree of standardization.
The main difference of metacognition compared with other cognitive-behavioral therapy approaches is the specific focus on changes in restrictive thoughts. “Thinking about thinking” and changes in detected cognitive traps are the main aims of the training. In MaTiMS, we included parts of the four approaches to create self-efficacy as suggested by Bandura.33 People need to 1) experience success in overcoming obstacles, 2) observe others like themselves being successful, 3) be persuaded by others that they are capable of performing the task, and 4) learn how to manage the stress and anxiety they feel about performing a new task. MaTiMS contents, its presentation in group sessions, and the invitation to participants to share their own experiences in coping with cognitive and psychological deterioration address those four elements.
One of the most important features of MaTiMS is the highly standardized curriculum realized with modules in a PowerPoint presentation style. A comprehensive manual gives the trainer instructions for presentation and conduct but also elaborate background knowledge regarding each module, including the scientific basis and explanation of the mode of action.
Participants' evaluation of the training program with a mean of 55.8 out of 68.0 points indicates a high level of acceptance. While in general the modules were highly appreciated, more examples with a closer link to MS were asked for and subsequently included. In addition, functional training exercises were requested. This was not the aim of MaTiMS, but it may indicate that MaTiMS might be combined with focused functional training depending on neuropsychological assessments to achieve additional activity gains. Formal changes of the presentation style were also conducted, for example, shortening sentences, using more colors, and including more pictures.
The two pilot groups showed high acceptance of MaTiMS. Patients receiving MaTiMS showed higher levels of coping self-efficacy and less fatigue after the intervention. We regarded those findings as highly encouraging, as we believe that coping self-efficacy is a concept that is highly relevant for daily functioning. Self-efficacy might be best addressed by self-management–enhancing interventions. Mikula et al.22 found significant correlations between coping and mental health–related quality of life in MS patients. Therefore, improving coping skills might substantially improve neuropsychiatric symptoms in MS.
This study was a pilot/feasibility work with a small sample size, and there are no follow-up data. One major limitation of this study is the absence of a control group. Therefore, we cannot exclude the possibility that social attention was the major effective factor. Depression, which was addressed explicitly in only one of our modules, did not change in this pilot study. The 3-week training period might have been too short to induce changes in depressive symptoms. In addition, at baseline, only four patients had HADS scores above the clinical cutoff, with a mean score of 8.36, indicating only moderate levels of depression in both groups.
One major problem with neuropsychological interventions is the selection of a clinically relevant primary outcome. Improvement in a neuropsychological measure may not necessarily be relevant for the patient's daily life, while, conversely, lack of change may not exclude enhanced ability of patients to cope. However, to the best of our knowledge, studies in the area of neuropsychological abnormalities and daily functioning are scarce. In our cohort, we found some improvements in the attentional domains measured by TAP, but no differences in memory tasks or in learning and recognition. Change scores may be partially explained by practice effects.
We did demonstrate improvements in fatigue. Fatigue management is one of the unmet needs of highest relevance for MS patients. No effective drug treatments have yet been developed, and the most compelling evidence comes from behavioral interventions.34,35 Just recently, Induruwa et al.36 concluded that enhancing self-management techniques, which is a crucial aspect of MaTiMS, is of the highest relevance.
Neuropsychological problems are largely overlooked and even more often undertreated in MS, although they are often described as highly relevant.2 Studies on cognitive rehabilitation in MS must account for several methodological problems. On one hand, MS is a chronic progressive disease. Thus, the main goals of any intervention are maintenance of abilities and compensation for deficits. Furthermore, cognitive problems in MS are heterogeneous, sometimes progressive, and sometimes quite stable, which complicates a standardized intervention and assessment procedure.37
Functional cognitive training interventions to treat cognitive impairment in MS are moderately effective.14 However, it is not clear whether specific deficit training is able to improve a patient's daily life. Also, psychological interventions have been proven to be only moderately effective, and cognitive-behavioral approaches seem the most promising.13 Therefore, there is an urgent need to further develop standardized concepts applicable in daily routines, like MaTiMS, so that people other than highly skilled MS psychologists can apply them.
Compared with cost-intensive, psychotherapeutic, face-to-face interventions and their limited application in daily routine, highly standardized group training programs offer a cost-effective and feasible option that can be implemented in usual care.38
Based on these data, a larger RCT is needed with longer follow-up to clarify the specificity of treatment effects and their persistence in helping the patient cope with cognitive dysfunction, fatigue, and depression in order to improve quality of life.
Practice Points.
We developed a cognitive-behavioral group intervention program for people with MS based on the concept of metacognition, covering all major neuropsychiatric domains (attention, depression, stress, fatigue, social cognition, and memory) and addressing coping and compensation strategies.
The program consists of six small-group training sessions with a highly standardized curriculum, using Microsoft PowerPoint software, moderator quick-reference instruction cards, a comprehensive manual with scientific background, and instructions for presentation and conduct.
Preliminary testing of the intervention showed a high level of acceptance, as well as encouraging results on measures of coping, fatigue, and quality of life.
Footnotes
Note: Supplementary material for this article is available on IJMSC Online at ijmsc.org.
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This research was supported by a grant from the Werner Otto Stiftung, Germany.
References
- 1.Filippini G, Del Giovane C, Vacchi L et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2013;6:CD008933. doi: 10.1002/14651858.CD008933.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 3.Rao SM. Neuropsychology of multiple sclerosis. Curr Opin Neurol. 1995;8:216–220. doi: 10.1097/00019052-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245:41–46. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Mohr DC, Genain C. Social support as a buffer in the relationship between treatment for depression and T-cell production of interferon gamma in patients with multiple sclerosis. J Psychosom Res. 2004;57:155–158. doi: 10.1016/S0022-3999(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 6.Beadle JN, Brown V, Keady B, Tranel D, Paradiso S. Trait empathy as a predictor of individual differences in perceived loneliness. Psychol Rep. 2012;110:3–15. doi: 10.2466/07.09.20.PR0.110.1.3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pöttgen J, Dziobek I, Reh S, Heesen C, Gold SM. Impaired social cognition in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:523–528. doi: 10.1136/jnnp-2012-304157. [DOI] [PubMed] [Google Scholar]
- 8.Patti F, Amato MP, Trojano M et al. Longitudinal changes in social functioning in mildly disabled patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: results from the COGIMUS (COGnitive Impairment in MUltiple Sclerosis) study (II) Qual Life Res. 2012;21:1111–1121. doi: 10.1007/s11136-011-0021-6. [DOI] [PubMed] [Google Scholar]
- 9.Feinstein A. Multiple sclerosis and depression. Mult Scler. 2011;17:1276–1281. doi: 10.1177/1352458511417835. [DOI] [PubMed] [Google Scholar]
- 10.Bakshi R, Shaikh ZA, Miletich RS et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler. 2000;6:181–185. doi: 10.1177/135245850000600308. [DOI] [PubMed] [Google Scholar]
- 11.Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review. Neuroepidemiology. 2011;36:109–120. doi: 10.1159/000323953. [DOI] [PubMed] [Google Scholar]
- 12.DeLuca J, Nocentini U. Neuropsychological, medical and rehabilitative management of persons with multiple sclerosis. NeuroRehabilitation. 2011;29:197–219. doi: 10.3233/NRE-2011-0695. [DOI] [PubMed] [Google Scholar]
- 13.Thomas PW, Thomas S, Hillier C, Galvin K, Baker R. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;(1):CD004431. doi: 10.1002/14651858.CD004431.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosti-Otajarvi EM, Hamalainen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev. 2011;(11):CD009131. doi: 10.1002/14651858.CD009131.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Moritz S, Woodward TS. Metacognitive training in schizophrenia: from basic research to knowledge translation and intervention. Curr Opin Psychiatry. 2007;20:619–625. doi: 10.1097/YCO.0b013e3282f0b8ed. [DOI] [PubMed] [Google Scholar]
- 16.Flavell JH, Wellman HM. Metamemory. In: Hagen JW, editor. Perspectives on the Development of Memory and Cognition. Hillsdale, NJ: Erlbaum; 1977. pp. 3–33. [Google Scholar]
- 17.Moritz S, Jelinek L, Hauschildt M, Naber D. How to treat the untreated: effectiveness of a self-help metacognitive training program (myMCT) for obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12:209–220. doi: 10.31887/DCNS.2010.12.2/smoritz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunge M, Muhlhauser I, Steckelberg A. What constitutes evidence-based patient information? Overview of discussed criteria. Patient Educ Couns. 2010;78:316–328. doi: 10.1016/j.pec.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess R, editors. Analysing Qualitative Data. London, UK: Routledge; 1994. pp. 173–194. [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesney MA, Neilands TB, Chambers DB, Taylor JM, Folkman S. A validity and reliability study of the coping self-efficacy scale. Br J Health Psychol. 2006;11(pt 3):421–437. doi: 10.1348/135910705X53155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikula P, Nagyova I, Krokavcova M et al. Coping and its importance for quality of life in patients with multiple sclerosis. Disabil Rehabil. 2014;36:732–736. doi: 10.3109/09638288.2013.808274. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Schäffler N, Schönberg P, Stephan J, Stellmann JP, Gold SM, Heesen C. Comparison of patient-reported outcome measures in multiple sclerosis. Acta Neurol Scand. 2013;128:114–121. doi: 10.1111/ane.12083. [DOI] [PubMed] [Google Scholar]
- 25.Flachenecker P, Müller G, König H, Meissner H, Toyka KV, Rieckmann P. “Fatigue” in multiple sclerosis: development and validation of the “Würzburger Fatigue Inventory for MS.”. Nervenarzt. 2006;77:165–166. 168–170, 172–174. doi: 10.1007/s00115-005-1990-x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 27.Volz-Sidiropoulou E, Boecker M, Niemann H, Privou C, Zimmermann P, Gauggel S. Skala zur Erfassung von Aufmerksamkeitsdefiziten (SEA): Erste psychometrische Evaluation mit einer Rasch-Analyse. Zeitschrift für Neuropsychol. 2007;18:299–309. [Google Scholar]
- 28.Benedict R, Munschauer F, Linn R et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler. 2003;9:95–101. doi: 10.1191/1352458503ms861oa. [DOI] [PubMed] [Google Scholar]
- 29.Smith A. Symbol Digit Modalities Test (SDMT) Manual. Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- 30.Zimmermann P, Fimm B. Ergänzungsmanual zur Testbatterie zur Aufmerksamkeitsprüfung (TAP) Version 1.7 Teil 1. Herzogenrath, Germany: Psytest; 2002. [Google Scholar]
- 31.Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest (VLMT) Göttingen, Germany: Hogrefe; 2001. [Google Scholar]
- 32.Haerting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K, Kessler J. WMS-R Wechsler Gedächtnis Test—Revidierte Fassung. Bern, Switzerland: Hans Huber; 2000. [Google Scholar]
- 33.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman; 1997. [Google Scholar]
- 34.Van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70:205–213. doi: 10.1097/PSY.0b013e3181643065. [DOI] [PubMed] [Google Scholar]
- 35.Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50:415–421. doi: 10.1016/j.brat.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis—a brief review. J Neurol Sci. 2012;323:9–15. doi: 10.1016/j.jns.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Calabrese P. Neuropsychology of multiple sclerosis—an overview. J Neurol. 2006;253(suppl):110–115. doi: 10.1007/s00415-006-1103-1. [DOI] [PubMed] [Google Scholar]
- 38.Peterson CB, Mitchell JE, Engbloom S, Nugent S, Mussell MP, Miller JP. Group cognitive-behavioral treatment of binge eating disorder: a comparison of therapist-led versus self-help formats. Int J Eat Disord. 1998;24:125–136. doi: 10.1002/(sici)1098-108x(199809)24:2<125::aid-eat2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


