Abstract
Background: Dalfampridine extended-release (ER) tablets, 10 mg twice daily, have been shown to improve walking in people with multiple sclerosis. We evaluated the safety and efficacy of dalfampridine-ER 5 mg compared with 10 mg.
Methods: Patients were randomized to double-blind treatment with twice-daily dalfampridine-ER tablets, 5 mg (n = 144) or 10 mg (n = 143), or placebo (n = 143) for 4 weeks. Primary efficacy endpoint was change from baseline walking speed by the Timed 25-Foot Walk 3 to 4 hours after the last dose. At 40% of sites, 2-week change from baseline walking distance was measured by the 6-Minute Walk test.
Results: At 4 weeks, walking speed changes from baseline were 0.363, 0.423, and 0.478 ft/s (placebo, dalfampridine-ER 5 mg, and dalfampridine-ER 10 mg, respectively [P = NS]). Post hoc analysis of average changes between pretreatment and on-treatment showed that relative to placebo, only dalfampridine-ER 10 mg demonstrated a significant increase in walking speed (mean ± SE): 0.443 ± 0.042 ft/s versus 0.303 ± 0.038 ft/s (P = .014). Improvement in 6-Minute Walk distance was significantly greater with dalfampridine-ER 10 mg (128.6 ft, P = .014) but not with 5 mg (76.8 ft, P = .308) relative to placebo (41.7 ft). Adverse events were consistent with previous studies. No seizures were reported.
Conclusions: Dalfampridine-ER 5 and 10 mg twice daily did not demonstrate efficacy on the planned endpoint. Post hoc analyses demonstrated significant increases in walking speed relative to placebo with dalfampridine-ER 10 mg. No new safety signals were observed.
Dalfampridine (Ampyra; Acorda Therapeutics Inc, Ardsley, NY) extended-release (ER) tablets, 10 mg twice daily (known as prolonged-release fampridine in Europe and as fampridine modified- or sustained-release elsewhere), have been shown to improve walking in people with multiple sclerosis (MS).1,2 In two phase 3 clinical trials, dalfampridine-ER resulted in a significantly greater proportion of patients who qualified as timed-walk responders relative to placebo: 35% versus 8% (P < .0001)1 and 42.9% versus 9.3% (P < .0001).2 The response criterion was prospectively defined as having a faster walking speed on the Timed 25-Foot Walk test (T25FW) for at least three of the four visits during the double-blind treatment period compared with the maximum speed for any of the five off-drug visits.
In both trials, dalfampridine-ER had a favorable tolerability and safety profile at the recommended therapeutic dosing regimen,1,2 although a dose-dependent increase in the risk of seizure has been observed at higher doses.3 Postmarketing safety data for 1 and 2 years suggested a safety profile in clinical practice similar to that observed in the clinical trials.4,5 As part of the postmarketing commitment, evaluation of the efficacy and safety of a lower 5-mg dose of dalfampridine-ER tablets was required. The purpose of the present study was to evaluate the efficacy and tolerability of dalfampridine-ER 5 mg twice daily.
Methods
Study Design and Participants
This randomized, placebo-controlled, double-blind, three-arm, parallel-group study was performed between April 7, 2011, and April 30, 2012, at 65 sites in the United States. Key inclusion criteria were age 18 to 70 years, a clinical diagnosis of MS defined by the 2005 revision of the McDonald criteria,6 and the presence of MS-related walking impairment, as determined by the clinician, but with sufficient ambulatory ability to complete all evaluations of the T25FW. Patients who had previously taken any formulation of dalfampridine-ER were also required to have been withdrawn from the drug for at least 1 month before screening, and women of childbearing potential were required to use adequate contraception during the study. Pregnant or lactating women were excluded from the study, and other key exclusion criteria included a history of seizures; the presence or history of moderate or severe renal impairment, defined by a calculated creatinine clearance of 50 mL/min or less; the presence of an active urinary tract infection at screening or within 4 weeks before screening; initiation of an MS disease-modifying therapy within 90 days before screening or a change in regimen of these drugs within 30 days before screening; and onset of an MS exacerbation within 60 days before screening.
The study was performed in accordance with the revised Declaration of Helsinki. The protocol was approved by the appropriate institutional review boards or independent ethics committees, and all patients provided written informed consent.
Treatments
After a 1-week screening period, patients were randomized equally to treatment with oral dalfampridine-ER 5 or 10 mg or placebo to be taken twice daily at approximately 12-hour intervals for 4 weeks. Randomization was assigned through a centralized interactive voice response system according to a computer-generated block randomization schedule. Active treatment and placebo tablets were identical in appearance, and placebo tablets contained the same inactive ingredients as the dalfampridine-ER tablets.
Outcomes
Assessment of the T25FW was performed at screening, randomization (visit 1), 2 weeks after treatment initiation (visit 2), and the end of treatment at 4 weeks (visit 3). Theprimary efficacy outcome was the change in walking speed from baseline using the T25FW 3 to 4 hours after administration of the last dose of dalfampridine-ER at week 4 (visit 3), which was a previously untested single endpoint analysis, different from the consistent response criterion that was used in the pivotal studies.1,2 Two T25FW evaluations were made at week 4, the first approximately 12 hours after the previous dose and the second designed to correspond approximately to the time of peak steady-state plasma concentration (CmaxSS)7 after a dose taken in the clinic. Given that this was a postmarketing commitment to explore the relative effects of dalfampridine-ER doses versus placebo and the potential maintenance effect during the 12-hour dosing interval, the study design, including the use of a single endpoint analysis as the primary efficacy outcome, was based on discussion and agreements with the US Food and Drug Administration.
Secondary efficacy endpoints assessed at visit 3 included the change from baseline in walking speed at the approximate time of the minimum steady-state plasma concentration (CminSS) and the change from baseline on the 12-item Multiple Sclerosis Walking Scale (MSWS-12). The MSWS-12, consisting of 12 questions with Likert-type responses, is a disease-specific, patient-reported outcome that evaluates the impact of MS on walking.8 The MSWS-12 has a recall period of 2 weeks and a range in score from 12 to 60 that is converted for convenience to a scale from 0 to 100, with higher scores indicating greater impact of disease on walking; estimates suggest that the minimal clinically important difference on the MSWS-12 is 6.2 points.9
Walking distance was also evaluated at visit 2 (2 weeks after treatment initiation) using the 6-Minute Walk (6MW) test10 as a prespecified secondary endpoint for the 26 sites that had the capability of performing this test of the 65 sites at which the study was conducted. The 6MW test measures the distance that an individual can walk on a flat, hard surface during a 6-minute period. For the purpose of the study, the 6MW test was administered using a straight 100-ft hallway. The starting line and turnaround points were clearly marked, and the 6MW test was administered using the protocol modified for MS, which instructs patients to walk as far and as fast as possible back and forth in a hallway for 6 minutes without rest or encouragement.11
Safety was evaluated based on the incidence of treatment-emergent adverse events (TEAEs) and on findings from physical examination, vital sign measurements, 12-lead electrocardiography, and clinical laboratory testing.
Statistical Analysis
This study was powered to detect differences between dalfampridine-ER 10 mg and placebo on change from baseline in walking speed at CmaxSS. A sample size of 135 patients in each group was expected to provide approximately 90% power to detect a difference of 0.16 ft/s between dalfampridine-ER 10 mg twice daily and placebo. This determination was based on previously observed differences between dalfampridine-ER 10 mg twice daily and placebo and used an assumed standard deviation of 0.40 ft/s. To maintain an α ≤ .05, a hierarchical analysis was performed using a step-down sequential testing procedure that permitted subsequent comparisons only if the previous comparison demonstrated statistical significance.
Treatment effects for the primary endpoint were compared using analysis of variance, with terms for treatment and baseline, for the full-analysis population, defined as all randomized patients who took at least one dose of double-blind treatment and who had a baseline and at least one postbaseline T25FW assessment. The 6MW test was also analyzed using analysis of variance.
The T25FW data were also analyzed post hoc in a manner with similarities to the analyses used in the pivotal studies in that it considered data from all the study visits rather than single measures at baseline and at the end of treatment.1,2 This analysis combined all T25FW assessments before treatment by averaging screening and visit 1 as the baseline and all on-treatment assessments (visits 2 and 3) as the on-drug value, and it compared the change from baseline and percentage change from baseline in walking speed between dalfampridine-ER and placebo. The proportions of patients achieving an average improvement in walking speed of at least 20% from baseline were also compared between treatments using the Cochran-Mantel-Haenszel test; a 20% change in walking speed is considered clinically relevant.12–14
Results
Disposition and Baseline Characteristics
Of the 429 patients who were randomized and took at least one dose of study drug, 31 withdrew from the study: 10 (7.0%) in the placebo group, 6 (4.2%) in the dalfampridine-ER 5-mg group, and 15 (10.5%) in the dalfampridine-ER 10-mg group (Figure 1). Adverse events were the most common reason for withdrawal across all groups: 5 (3.5%), 3 (2.1%), and 14 (9.8%) in the placebo, dalfampridine-ER 5-mg, and dalfampridine-ER 10-mg groups, respectively.
Figure 1.
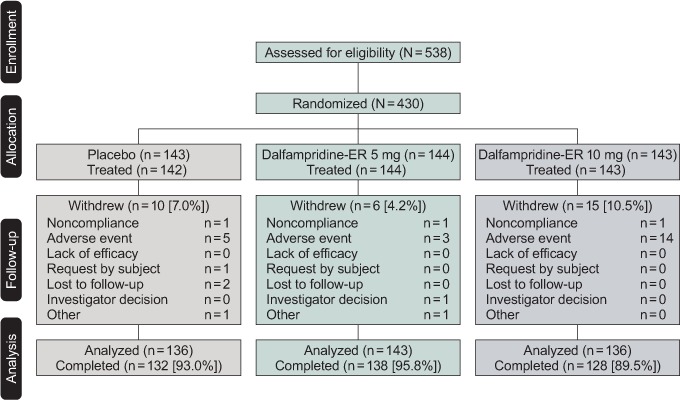
Patient disposition
Safety was analyzed for all patients who took at least one dose of double-blind treatment, and the analyzed population for efficacy analysis represents a modified intention-to-treat population that included all randomized patients who took at least one dose of double-blind treatment and who had baseline and at least one postbaseline assessment of the Timed 25-Foot Walk. ER, extended release.
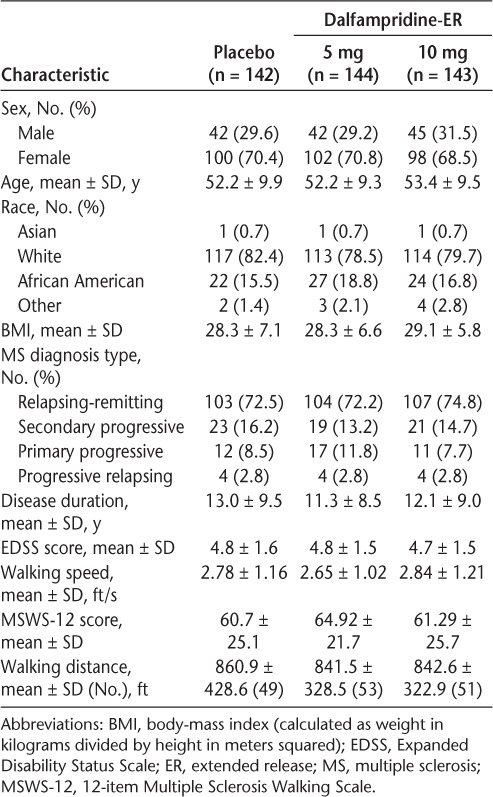
The demographic and clinical characteristics were balanced across treatments (Table 1). Overall, the population was primarily white (80.2%) and female (69.9%) and had relapsing-remitting MS (73.2%). The mean ± SD age of the study cohort was 52.6 ± 9.5 years, with a mean ± SD Expanded Disability Status Scale (EDSS) score of 4.8 ± 1.5 and a mean baseline walking speed of 2.75 ft/s.
Table 1.
Demographic and clinical characteristics of the treatment groups at baseline
Efficacy
In the full-analysis population, 136 patients were included in the placebo group, 143 in the dalfampridine-ER 5-mg group, and 136 in the 10-mg group. One patient was randomized to receive dalfampridine-ER 10 mg but received placebo instead; following the intention-to-treat principle, this patient was classified in the dalfampridine-ER 10-mg group for efficacy analyses.
For the primary endpoint, no significant differences were observed between placebo and active treatment for the changes from baseline in walking speed: placebo, 0.363 ft/s; dalfampridine-ER 5 mg, 0.423 ft/s (P = .457); and dalfampridine-ER 10 mg, 0.478 ft/s (P = .107) (Figure 2).
Figure 2.
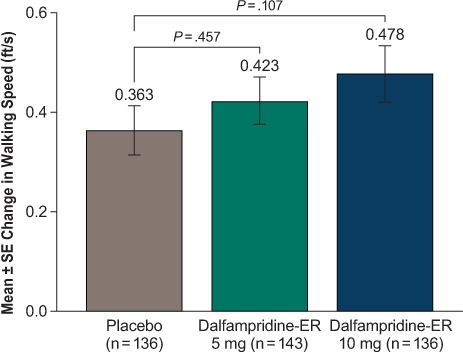
Primary efficacy endpoint: change from baseline in walking speed 3 to 4 hours after administration of the last dose at visit 3 (4 weeks)
The timing of the Timed 25-Foot Walk evaluation was designed to correspond approximately to the peak steady-state plasma concentration of dalfampridine. ER, extended release.
Similarly, for the key secondary endpoints of change from baseline at visit 3 (end of treatment at 4 weeks) in walking speed at CminSS and the MSWS-12 scores, no significant differences relative to placebo were observed for either of the dalfampridine-ER doses (Figure 3).
Figure 3.
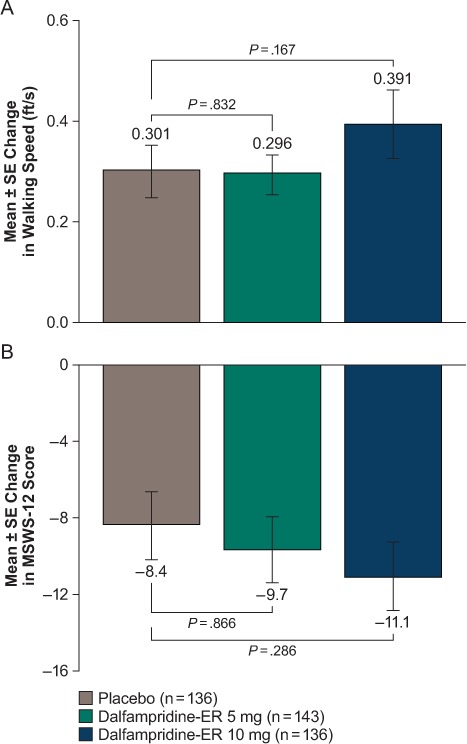
Change from baseline at 4 weeks (visit 3) for the key secondary endpoints of (A) walking speed at time of minimum steady-state plasma concentration and (B) 12-item Multiple Sclerosis Walking Scale (MSWS-12) score
ER, extended release.
In the post hoc analysis, the mean ± SE change from baseline in walking speed averaged over all visit measurements with dalfampridine-ER 10 mg (0.443 ± 0.042 ft/s) was greater than the change with placebo (0.303 ± 0.038 ft/s, nominal P = .014), but the difference observed between placebo and dalfampridine-ER 5 mg was small and without nominal significance (0.336 ft/s, P = .292) (Figure 4A). Similarly, only for dalfampridine-ER 10 mg was the mean percentage change from baseline in walking speed different from that for placebo (19.9% vs. 12.8%, nominal P = .010) (Figure 4B).
Figure 4.
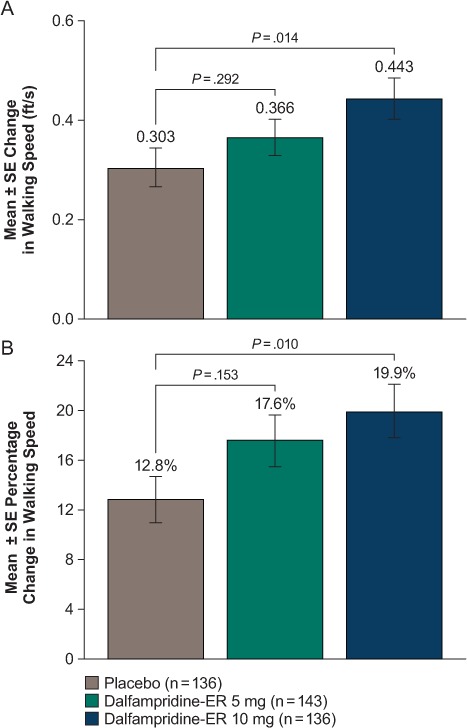
Post hoc analysis of walking speed using all Timed 25-Foot Walk (T25FW) assessments before treatment (screening and visit 1) as baseline and all on-treatment T25FW assessments (visits 2 and 3; 2 and 4 weeks, respectively) as the on-drug value
A, Mean change from baseline; B, mean percentage change from baseline. ER, extended release.
Relative to the placebo group, the proportion of patients achieving at least 20% improvement from baseline in average walking speed was significantly greater in the dalfampridine-ER 10-mg group (44.1% vs. 27.2%, nominal P = .004) but not in the dalfampridine-ER 5-mg group (32.2% vs. 27.2%, nominal P = .366) (Figure 5A). In all patients who achieved at least 20% improvement in walking speed regardless of treatment allocation, the mean change from baseline in MSWS-12 score was greater than that in patients whose change in walking speed was less than 20% (−15.8 vs. −6.6, nominal P < .001) (Figure 5B).
Figure 5.
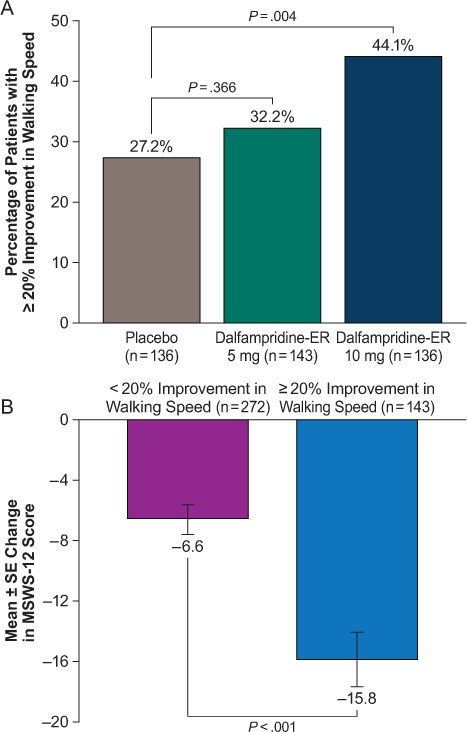
Post hoc analysis of (A) proportion of patients achieving at least 20% improvement in average walking speed and (B) change from baseline in 12-item Multiple Sclerosis Walking Scale (MSWS-12) score in patients achieving at least 20% improvement in average walking speed
ER, extended release.
The change from baseline in walking distance on the 6MW test at 2 weeks was approximately three times greater with dalfampridine-ER 10 mg relative to placebo (128.6 ft vs. 41.7 ft, nominal P = .014) (Figure 6). Although the change from baseline with the dalfampridine-ER 5-mg dose (76.8 ft) was greater than that with placebo, it did not achieve nominal significance (P = .308).
Figure 6.
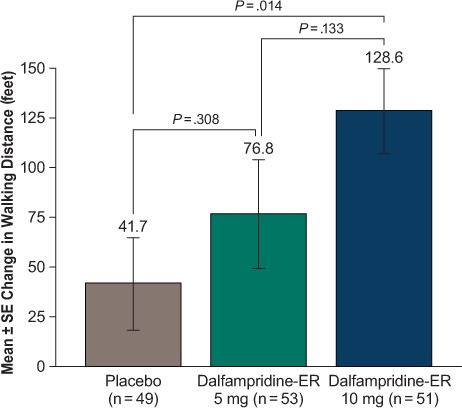
Change from baseline in walking distance at 2 weeks assessed using the 6-Minute Walk test
ER, extended release.
Safety
In the safety population, 143 patients were included in the placebo group, 144 in the dalfampridine-ER 5-mg group, and 142 in the dalfampridine-ER 10-mg group. One patient was randomized to receive dalfampridine-ER 10 mg but received placebo instead; this patient was classified in the placebo group for safety analyses.
The incidence of TEAEs was similar among the treatment groups (Table 2), and these events were generally mild or moderate in severity. No seizures were reported, and there were no deaths. Six serious TEAEs occurred in four patients, including a breast abscess with associated cellulitis, and a case of urosepsis in the dalfampridine-ER 5 mg group, and in the dalfampridine-ER 10 mg group, vertigo and a loss of consciousness (syncope) in one patient 4 days after drug discontinuation, and ovarian adenoma in another patient. The vertigo and loss of consciousness and the case of urosepsis were deemed by the investigators as possibly related to treatment.
Table 2.
Summary of treatment-emergent adverse events (TEAEs)
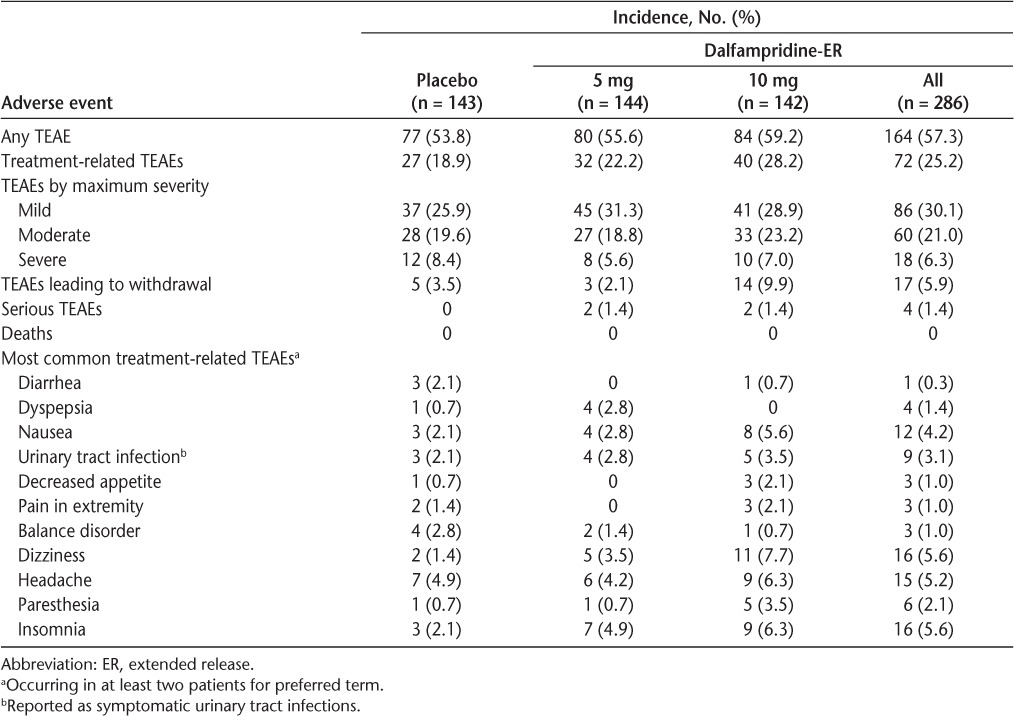
The most common TEAEs leading to withdrawal were those related to gastrointestinal and musculoskeletal disorders, and they were more frequent with dalfampridine-ER; the highest rate of TEAE-related withdrawals was in the dalfampridine-ER 10-mg group (9.9%). The most frequent TEAEs in dalfampridine-treated patients were dizziness, insomnia, and headache (Table 2).
There were no clinically meaningful changes in vital signs by treatment group and no treatment differences by type or frequency of abnormal vital sign values.
Discussion
Using a previously untested endpoint that was required as part of a postmarketing commitment to evaluate dalfampridine-ER 5 and 10 mg relative to placebo, neither of the dalfampridine-ER doses demonstrated efficacy that was statistically significant. This endpoint was a single-point assessment of walking speed relative to baseline to evaluate the effects at the time of CmaxSS.
Because a single assessment typically has higher variability than an assessment averaged over multiple time points, the study was powered based on a sample size estimate derived from single-time-point data from previous studies. However, the variability at CmaxSS was higher than was seen in previous data, perhaps because of the long duration and complexity of procedures at visit 3 related to the need to measure, 3 to 4 hours apart, walking speeds at CminSS and CmaxSS.
The endpoint selected for the pivotal trials was designed to reduce the effect of variability in individual measurements of the T25FW by using an a priori consistent response analysis that incorporated data from all on-treatment and off-treatment visits.1,2 A post hoc analysis of the present study, which was more consistent with the method of analysis in the pivotal studies, showed that only the 10-mg dose demonstrated a significant effect on walking speed compared with placebo. Because of the limited number of visits in this study and its short duration, it was not possible to replicate the response analysis used in the pivotal studies. Nevertheless, using the post hoc criterion of average improvement in walking speed of at least 20% from baseline, which is considered clinically relevant,12–14 significant effects relative to placebo were observed for the 10-mg group (P = .004) but not for the 5-mg group (P = .366).
The clinical relevance of the 20% threshold was further supported in the present study by the observations that the average change from baseline in MSWS-12 score was significantly greater in those who achieved at least 20% improvement in walking speed relative to those with a change in walking speed less than 20% and that this difference comfortably exceeded estimates of the minimal clinically important difference for the MSWS-12 score.9
In addition to the primary endpoint and the short duration, the present study was different from the pivotal clinical trials with respect to several other characteristics, including lack of requirement for a minimum or maximum time on the T25FW test at screening. Hence, the range of walking speed was wider and the baseline average walking speed for all groups was slightly faster than in previous dalfampridine studies.
Treatment with dalfampridine-ER 10 mg also improved walking distance relative to placebo in the subset of patients assessed using the 6MW test (128.6 ft vs. 41.7 ft, nominal P = .014). These results, which mark the first time that dalfampridine-ER has been evaluated using a long-distance walk test, suggest that a measure of sustained physical activity and endurance (ie, the 6MW test) may be a more sensitive measure for assessing improvement in walking impairment than one of a shorter duration of physical activity, such as the T25FW test. The data also support the 6MW test as a more appropriate measure for use in patients with lower levels of disability, as has previously been suggested.11
No new safety signals were observed, and, overall, TEAEs were consistent with what has been observed in previous controlled trials and in postmarketing surveillance.1,2,4,5
In conclusion, this study provides evidence that dalfampridine-ER tablets, 5 mg twice daily, are not effective for improving walking in patients with MS. Although the primary endpoint of this study was not achieved, additional analyses with similarities to those used in previous trials showed that dalfampridine-ER 10 mg twice daily seems to be the minimally effective dose for improvement of walking. Post hoc analyses indicated improvements in walking speed and walking distance at this dose with a tolerability profile that was consistent with previous reports; there was no support in these analyses for efficacy of the 5-mg dose.
PracticePoints.
Dalfampridine extended-release (ER) tablets, 10 mg twice daily, are indicated to improve walking in people with MS.
This study provides evidence that dalfampridine-ER tablets, 5 mg twice daily, are not effective for improving walking in patients with MS.
Although dalfampridine-ER 5 and 10 mg twice daily did not demonstrate efficacy using the new primary endpoint selected for this trial, additional analyses with similarities to those used in previous pivotal trials showed that dalfampridine-ER 10 mg twice daily seems to be the minimally effective dose for improvement of walking.
Acknowledgments
Editorial assistance was provided by E. Jay Bienen, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY, and funded by Acorda Therapeutics, Inc, Ardsley, NY.
Footnotes
Financial Disclosures: Dr. Yapundich has received research support from Acorda Therapeutics, Inc, Biogen Idec, Roche, Serono, and UCB. He is on the speakers' bureau for EMD Serono, Pfizer, Teva, and US WorldMeds. Dr. Applebee currently is involved in clinical trials supported by Acorda Therapeutics, Inc, Actelion, Biogen Idec, Genentech, Novartis, Opexa Therapeutics, and Sanofi Aventis. She currently serves on advisory boards for Acorda Therapeutics, Inc, Genzyme, and Teva Neuroscience. Dr. Bethoux has received research support from Acorda Therapeutics, Inc, the Consortium of Multiple Sclerosis Centers, Innovative Neurotronics, Merz Pharmaceuticals, and the National Multiple Sclerosis Society. He has served on the speakers' bureaus of Acorda Therapeutics, Inc and Allergan; has been a member of the faculty at Consortium of Multiple Sclerosis Centers–sponsored events; has consulted for or served on scientific advisory boards for Acorda Therapeutics, Inc, Concert Pharmaceuticals, GW Pharmaceuticals, Impax Pharmaceuticals, and Merz Pharmaceuticals; and is editor-in-chief of the International Journal of MS Care. Dr. Goldman has received research support from Biogen Idec, the National Institutes of Health, and Novartis. She has consulted for Concert Pharmaceuticals and has served as an advisor to Acorda Therapeutics, Inc. Dr. Hutton has received research support from Acorda Therapeutics, Inc, Avanir, Biogen Idec, Genzyme, Hoffman La Roche, Novartis, Opexa, and the National Institutes of Health. He has served on the scientific advisory boards of Biogen Idec, Genzyme, and Novartis and has received compensation as a consultant for Bayer, Biogen Idec, EMD Serono, Pfizer, and Teva Neuroscience. Dr. Mass has served on the speakers' bureau of Novartis. Dr. Pardo has received research support from Acorda Therapeutics, Inc. He has served on the scientific advisory boards of Acorda Therapeutics, Inc, Biogen Idec, Novartis, and Teva Neuroscience and on the speakers' bureaus of Acorda Therapeutics, Inc, Bayer, Biogen Idec, EMD Serono, Genzyme, Novartis, Pfizer, and Teva Neuroscience. Mr. Klingler and Drs. Blight and Carrazana are employees and stockholders of Acorda Therapeutics, Inc. Dr. Henney was an Acorda Therapeutics, Inc, employee and stockholder at the time of the study.
Funding/Support: This study was sponsored by Acorda Therapeutics, Inc.
References
- 1.Goodman AD, Brown TR, Krupp L et al. Sustained release of oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- 2.Goodman AD, Brown TR, Edwards KR et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68:494–502. doi: 10.1002/ana.22240. [DOI] [PubMed] [Google Scholar]
- 3.Haut SR, Bienen EJ, Miller A. Clinical overview of the seizure risk of dalfampridine. Expert Opin Drug Saf. 2012;11:651–657. doi: 10.1517/14740338.2012.697896. [DOI] [PubMed] [Google Scholar]
- 4.Jara M, Barker G, Henney HR., III. Dalfampridine extended-release tablets: 1 year of postmarketing safety experience in the US. Neuropsychiatr Dis Treat. 2013;9:365–370. doi: 10.2147/NDT.S41596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jara M, Adera M, Adedeji A, Henney HR, III, Carrazana EJ. Dalfampridine extended release tablets: safety profile after 2 years of postmarketing experience in the United States. Poster presented at: 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 12. 2012; Lyon, France.
- 6.Polman CH, Reingold SC, Edan G et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 7.Vollmer T, Blight AR, Henney HR., III. Steady-state pharmacokinetics and tolerability of orally administered fampridine sustained release 10-mg tablets in patients with multiple sclerosis: a 2-week, open-label, follow-up study. Clin Ther. 2009;31:2215–2223. doi: 10.1016/j.clinthera.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60:31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Hobart J. Prolonged-release fampridine for multiple sclerosis: was the effect on walking ability clinically significant [abstract]? Mult Scler. 2010;16(suppl):S72. [Google Scholar]
- 10.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman M, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000;6:286–290. doi: 10.1177/135245850000600411. [DOI] [PubMed] [Google Scholar]
- 13.Schwid SR, Goodman AD, McDermott MP, Bever CF, Cook SD. Quantitative functional measures in MS: what is a reliable change? Neurology. 2002;58:1294–1296. doi: 10.1212/wnl.58.8.1294. [DOI] [PubMed] [Google Scholar]
- 14.Hobart J, Blight AR, Goodman A, Lynn F, Putzki N. Timed 25-Foot Walk: direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology. 2013;80:1509–1517. doi: 10.1212/WNL.0b013e31828cf7f3. [DOI] [PubMed] [Google Scholar]


