Abstract
Background: The aims of this study were 1) to examine postural sway in the eyes open (EO) and eyes closed (EC) conditions in people with multiple sclerosis (MS) with moderate levels of disability compared with controls and 2) to examine relationships between postural sway and total Expanded Disability Status Scale (EDSS) scores, functional system subscores, and clinical measures of strength and spasticity in the MS group.
Methods: Thirty-four people with moderate MS and ten matched controls completed measures of postural sway with EO and EC, knee extension and ankle dorsiflexion isometric strength, EDSS total score and subscores, and spasticity levels.
Results: Participants with MS swayed significantly more with EO and EC and had reduced knee extension and ankle dorsiflexion strength compared with controls (P < .001). In the MS group, increased sway was associated with higher total EDSS scores and cerebellar function subscores, whereas increased sway ratio (EC/EO) was associated with reduced sensory function subscores. Postural sway was not significantly associated with strength or spasticity.
Conclusions: Participants with MS swayed more and were significantly weaker than controls. Cerebellar dysfunction was identified as the EDSS domain most strongly associated with increased sway, and sensory loss was associated with a relatively greater dependence on vision for balance control. These findings suggest that exercise interventions targeting sensory integration and cerebellar ataxia may be beneficial for enhancing balance control in people with MS.
Multiple sclerosis (MS) is a chronic neurodegenerative disease characterized by inflammation, demyelination, and axonal lesions in multiple areas of the central nervous system. Many people with MS have impaired balance, which has important clinical implications owing to associated loss of mobility and increased fall risk.1 More than 50% of people with MS fall in a 3- to 6-month period,2 with fall-related sequelae including injury,3 fear of falling, activity restriction,4 and death.5
In people with MS, it has been reported that impaired balance is associated with a variety of neurologic impairments, including reduced sensation in the feet,6 slower spinal somatosensory conduction,7 and lower-limb spasticity.8 Central nervous system lesions that delay sensory and motor integration can further impair balance control.9 In the clinic, the most commonly used formal examination of neurologic impairments is the Expanded Disability Status Scale (EDSS), which provides an overall indication of the level of clinical disability and functional system subscores for vision, brainstem, sensory, bladder/bowel, cerebral, pyramidal, and cerebellar functional systems.10 People with EDSS scores of 3.0 to 6.0 are able to walk but have a variety of mild-to-moderate neurologic impairments, as indicated by functional system subscores.11–14
Sensitive laboratory balance measures of postural sway may detect impairments that are not evident in standard neurologic assessments.12,15–21 In people with MS, increased postural sway, as measured using force plates or a trunk-mounted gyroscope, has been correlated with increased levels of disability, indicated by total EDSS scores.11,12 To date, no studies have investigated relationships between these sensitive balance measures and specific neurologic impairments identified by EDSS functional system subscores.
Examining associations between postural sway and distinct functional systems may help elucidate mechanisms that contribute to balance impairment in people with MS and may help guide research to target more specific impairment-based interventions. In neurologic rehabilitation, it is essential to target specific factors that contribute to poor balance control and, therefore, increase disability and fall risk. In this way, the functional system subscores of the EDSS might be used to direct people with MS toward specific referrals for rehabilitation. The aims of this study were, therefore, 1) to compare postural sway and visual dependence for postural sway by examining the eyes open (EO) and eyes closed (EC) conditions in people with MS with moderate levels of disability and in controls and 2) to examine relationships between sway and total EDSS scores, functional system subscores, and additional clinical measures of strength and spasticity in the MS group.
Methods
Participants
Participants with MS were recruited from MS and physiotherapy clinics and the local MS society via e-mail and newsletter advertisements. To be included, participants were required to have a diagnosis of MS made by a neurologist (any type—relapsing-remitting, primary progressive, or secondary progressive MS) and a moderate level of disability13 and the ability to walk, as indicated by an EDSS score of 3.0 to 6.0. Participants with MS were excluded if they had a disease exacerbation or relapse in the past 3 months to ensure that the sample was as medically stable as possible. Participants were also excluded if they were taking medications prescribed for fatigue (amantadine or modafinil) or mobility (fampridine) in line with the exclusion criteria for the controlled trial component of this study examining fatigue effects on mobility and related measures.22 Ten controls well matched in terms of age, sex, height, and weight were recruited from the Flinders University and Repatriation General Hospital staff. The study was approved by the Repatriation General Hospital Research and Ethics Committee, and participants provided written informed consent.
Neurologic Function and Spasticity
All the clinical tests (including the EDSS examination) were completed by the same researcher (JM), a certified neurostatus investigator (http://www.neurostatus.net). The EDSS is a comprehensive clinical neurologic examination that includes subscores of vision, brainstem, bladder/bowel, sensory, pyramidal, cerebellar, and cerebral functions.10 Higher scores indicate poorer function. Because the pyramidal subscores included only very basic scales of limb spasticity and muscle strength, further objective clinical measures of lower-limb spasticity and strength were used. The modified Ashworth Scale was used to measure spasticity with the participant lying in the supine position on a large neurologic plinth, in the order of gastrocnemius, soleus, quadriceps, and hamstring muscle groups in the weaker lower limb, with higher scores indicating more spasticity.23 Hip, knee, and ankle joint range of motion was also examined to ensure that participants' ability to complete the testing protocol would not be compromised by poor joint mobility.
Lower-Limb Strength
Knee extension and ankle dorsiflexion muscle strength were determined as maximum isometric force produced, measured using a strain gauge linear to the direction of force production.24 Strength tests with the participant in a seated position were conducted in the weaker lower limb as determined in the EDSS examination. For knee strength testing, the hip and knee were positioned in 90° of flexion, with the strain gauge attached to the lower leg 10 cm above the malleoli. The test of ankle strength was conducted with the ankle at 30° of plantarflexion and the strap placed just proximal to the base of the fifth toe. Each strength test was repeated three times, with sufficient rest between trials (a minimum of 30 seconds) to minimize muscle fatigue, and the maximum force was recorded.24 Strength scores were adjusted for body height, and this measure was used in all the analyses.
Postural Sway
Participants stood with their feet 10 mm apart (measured from the medial malleoli), toes not touching, with each foot on adjacent force plates with reference points to ensure that foot placement was consistent between trials. Four 30-second trials under EO and EC conditions were presented in a randomized order using sealed, opaque envelopes. Path length was calculated as the summed displacements of a reflective marker attached over the seventh cervical (C7) spinous process over 30 seconds using transverse plane coordinates sampled at 100 Hz and measured using an eight-camera Vicon MX3 system (Vicon Motion Systems Ltd, Los Angeles, CA). We chose to use C7 path length because it provides a measure of body motion indicative of postural sway as observed in clinical examinations, such as the EDSS. This method of measuring standing postural sway has been used in other clinical populations and in previous studies of people with MS.25–27 Sway ratio (EC/EO) was calculated by dividing EC sway path length by EO sway path length as a measure of visual dependence for balance control, with higher ratios indicating a greater reliance on vision for controlling postural sway.
Sample Size
Thirty-five participants with MS were recruited, in line with an a priori power calculation for an associated study of fatigue and mobility in which these participants were also involved.22 The recruitment of ten age- and sex-matched controls was considered sufficient because variance in the test measures conducted is relatively low in healthy people.24,28
Statistical Analysis
Independent-samples t tests were used to compare the MS and control group demographic characteristics and strength measures, and Mann-Whitney U tests were used to compare sway measures because these were not normally distributed. Spearman correlations were used to examine relationships between the sway measures and total EDSS scores, EDSS functional system subscores, strength, and spasticity in the MS group and the relationship between EDSS cerebellar subscores and total EDSS scores. Analysis was performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY), and for all the statistical analyses, alpha was set to .05. Despite the multiple comparisons made, P values were not adjusted in this exploratory study because such adjustments may increase type II errors, especially in studies with small sample sizes.29
Results
Of the 35 people recruited, one was excluded owing to an exacerbation of MS. The remaining sample consisted of 34 people (26 women) with moderate MS (EDSS score: mean [SD], 3.7 [0.7]; median, 3.5; and range, 3.0–6.0; mean [SD] time since diagnosis, 8.2 [7.9] years; mean [SD] body-mass index [BMI], 27.6 [6.0]; mean [SD] age, 49.8 [10.4] years) and a sample of ten controls matched for age (mean [SD], 45.1 [14.0] years), sex (7 women), and BMI (mean [SD], 27.4 [3.9]). Table 1 presents participant characteristics for the MS and control groups and EDSS scores for the MS group. The MS group EDSS scores indicate that the sample had moderate levels of disability.13 The median score of 0 for vision indicates that the MS group had no measurable visual impairment.
Table 1.
Participant demographic, strength, and sway measures for the MS and control groups and EDSS neurologic examination and spasticity results for the MS group
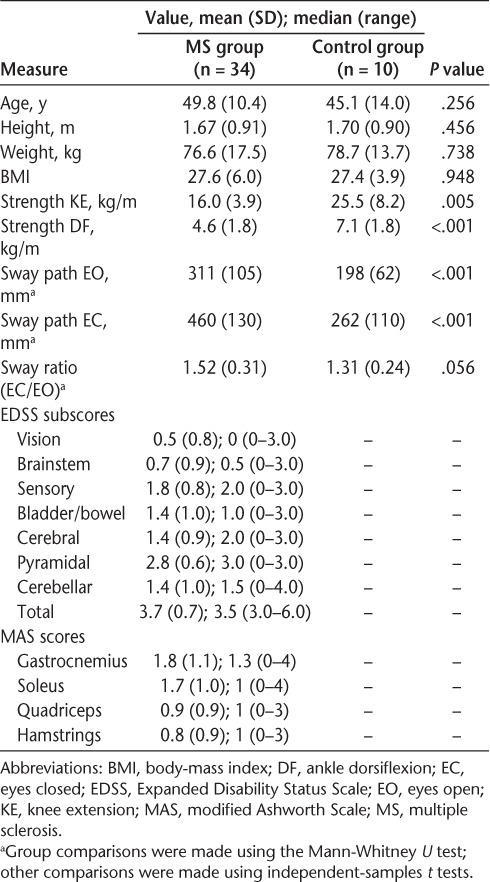
The MS group had significantly lower knee extension (P < .01) and ankle dorsiflexion strength (P < .001) and significantly larger sway path length for the EO and EC conditions compared with the control group (P < .001). There was a trend indicating that the MS group had a higher EC/EO sway ratio than the control group (P = .056).
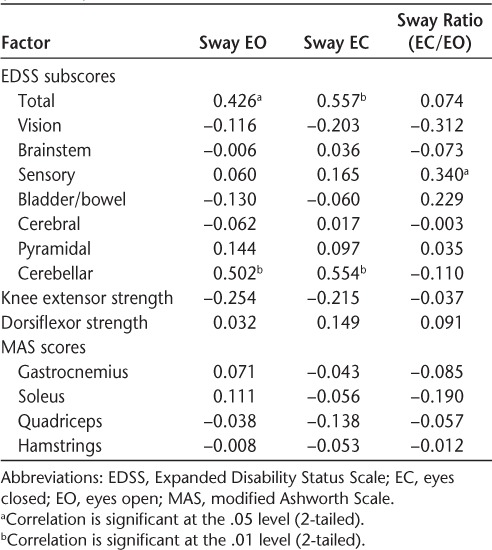
Table 2 displays the Spearman correlation coefficients of the sway parameters with the EDSS score, strength, and spasticity measures in the MS group. Total EDSS scores were significantly correlated with sway path length in the EO (P < .05) and EC (P < .01) conditions. Cerebellar subscores were also significantly correlated with the EO (P < .01) and EC (P < .01) conditions, and the sway ratio (EC/EO) was significantly correlated with the EDSS sensory subscore. There were no significant correlations between the sway measures and knee extension and ankle dorsiflexion strength or spasticity in the gastrocnemius, soleus, quadriceps, and hamstring muscle groups. Twenty-seven participants had an EDSS cerebellar subscore of 1 or greater, and cerebellar subscores and total EDSS scores were significantly correlated (r = 0.544, P < .01).
Table 2.
Spearman correlations between sway and total EDSS score, functional system subscore, strength, and spasticity measures (N = 34)
Discussion
This study provides further confirmation that postural sway is increased and lower-limb strength is reduced in people with MS with moderate disability compared with controls. In the MS group, increased sway was associated with higher EDSS cerebellar subscores and total EDSS scores, and a greater need for visual control of postural sway was associated with higher EDSS sensory function subscores.
These results suggest that balance control is reduced and is associated with total EDSS scores in people with MS with moderate disability.11,12 Corporaal et al.12 also found a moderate correlation between total EDSS scores and trunk sway with EC (r = 0.542). However, unlike in the present study, they did not find a significant correlation with EO sway. This inconsistency may be explained by the different measures of postural sway (pitch and roll angle and velocity range vs. total path length), the different devices (digital gyroscope worn on the lumbar spine vs. three-dimensional motion capture marker at C7), and the different standing positions (feet shoulder-width apart vs. feet together).12
Analysis of the EDSS subscores revealed that cerebellar subscores were associated with increased postural sway in the EO and EC conditions. These results are not surprising, as patients with cerebellar ataxia have difficulties scaling their postural responses in stance, resulting in overcorrective, hypermetric movements.30 Demyelination in the cerebellum is prominent in people with MS,31 and lesions in the brainstem and cerebellum have been shown to be associated with increased sway and number of falls over a 6-month period.32 Standing sway measures have previously been shown to be positively correlated with gray and white matter lesions in the cerebellum and its connections as measured using magnetic resonance imaging in people with MS.33 Sway with EO has also been shown to be associated with cerebellar atrophy in people with MS.34 These studies confirm that cerebellar dysfunction plays a major role in the observed increase in EO and EC postural sway in people with MS, with the present study indicating that cerebellar dysfunction is likely to have a major influence on the relationship between increased postural sway and total EDSS scores.
It has been reported that a strong reliance on vision for balance control is evident in people at increased risk for falls, such as those with previous polio and weak older people.35 The near significant difference in sway ratio (EC/EO) between the MS and control groups and the significant association between the EDSS sensory subscore and the sway ratio suggests a progressive loss of balance control with MS severity and a significantly greater need for vision to maintain stability. The present findings are consistent with other studies that have demonstrated visual dependence in people with MS36 and may be explained by reduced lower-limb sensation. Delayed somatosensory evoked potentials in the spinal cord have been shown to correlate with delayed postural responses to standing backward translation,7 and vibration perception at the great toe and sway amplitude have been associated with dorsal column gray matter integrity.37 However, the fact that sensory subscores did not correlate with postural sway in the present study suggests that other mechanisms may have played a greater role in controlling postural sway in the MS group. Many participants had a variety of impairments that could influence standing balance. For example, 27 of the 34 participants with MS had both sensory and cerebellar dysfunction. More sensitive measures of central nervous system structures may help determine the various multifactorial roles influencing postural sway. A recent study involving in vivo magnetic resonance imaging has shown an association of sway with EO with cerebellar atrophy, and sway with EC was associated with spinal cord atrophy.33
We found no significant correlations between the EDSS pyramidal subscores (which consisted of upper- and lower-limb reflexes, muscle strength, functional tests, and spasticity measures) or lower-limb spasticity and postural sway. In contrast, a previous cluster analysis of high/low spasticity as measured by the H-reflex in people with MS found that those with higher spasticity had increased velocity, area, and mediolateral range of postural sway.8 It is possible that the null findings reported herein relate to the different measure of postural sway, the measurement of spasticity in the weaker leg only, and use of the modified Ashworth Scale clinical measure of muscle tone compared with the more sensitive H-reflex measurement of spasticity.38
Previous studies have shown that reduced muscle strength is associated with reduced mobility in people with MS39,40 and that muscle weakness is a predictor of poor balance and falls in older people.24,41 In the present study, knee extensor and ankle dorsiflexion strength were not significantly associated with sway path length in the EO and EC conditions, which suggests that strength is not as important as cerebellar and sensory impairments in the maintenance of postural sway in people with MS. Given these findings, interventions that target sensory and motor strategies,42 visuoproprioceptive training,43 and rehabilitation approaches for cerebellar ataxia44 may be indicated for this clinical group.
We acknowledge that this study has certain limitations. First, we assessed postural sway in quiet stance only, and we appreciate that balance control requires many other movements that involve leaning, reaching, head turning, and responding to external perturbations. Second, the measure of postural sway using path length at C7 cannot be directly compared with other studies investigating associations of disability with standing balance using digital gyroscope movements of trunk sway12 and center of pressure measures.11 Third, strength was tested only in the knee extensors and ankle dorsiflexors in the weaker leg, and, consequently, we have not been able to elucidate the roles of other muscle groups or muscle strength in the stronger leg in controlling standing balance. Fourth, we used simple clinical assessments of spasticity with uncertain validity and reliability45 that may not be sensitive to uncovering relationships with balance control in people with MS. Fifth, because the sample size is relatively small, it is possible that some important associations may not have been uncovered owing to type I errors.
Conclusion
The results of this study are in line with previously reported findings demonstrating increased postural sway in people with MS compared with controls17,18,21 and a correlation of increased sway with total EDSS scores in the MS group.11,12 Further analysis revealed that EDSS cerebellar subscores correlate with postural sway in the EO and EC conditions. People with MS also showed a greater reliance on vision for control of sway in standing balance, a problem likely exacerbated by lower-limb proprioceptive loss. Postural sway seems to increase with disease severity in people with MS, and further investigation into its relationship with clinical progression and response to therapeutic interventions is warranted.
PracticePoints.
People with MS with moderate levels of disability have significantly increased postural sway compared with controls.
In people with MS, increased postural sway is associated with increased disability levels and clinical measures of cerebellar dysfunction but not with reduced strength or increased spasticity.
Sensory loss is associated with a relatively greater dependence on vision for balance control in people with MS.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This work was supported by Multiple Sclerosis Research Australia (grant 00045) and Foundation Daw Park.
Trial Registration: This trial was registered with the Australian New Zealand Clinical Trials Registry, registration number ACTRN12612000218897.
References
- 1.Cameron MH, Lord S. Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. 2010;10:407–412. doi: 10.1007/s11910-010-0128-0. [DOI] [PubMed] [Google Scholar]
- 2.Gunn HJ, Newell P, Haas B, Marsden JF, Freeman JA. Identification of risk factors for falls in multiple sclerosis: a systematic review and meta-analysis. Phys Ther. 2013;93:504–513. doi: 10.2522/ptj.20120231. [DOI] [PubMed] [Google Scholar]
- 3.Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil. 2008;89:1031–1037. doi: 10.1016/j.apmr.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler. 2007;13:1168–1175. doi: 10.1177/1352458507079260. [DOI] [PubMed] [Google Scholar]
- 5.Brønnum-Hansen H, Hansen T, Koch-Henriksen N, Stenager E. Fatal accidents among Danes with multiple sclerosis. Mult Scler. 2006;12:329–332. doi: 10.1191/135248506ms1280oa. [DOI] [PubMed] [Google Scholar]
- 6.Citaker S, Gunduz AG, Guclu MB, Nazliel B, Irkec C, Kaya D. Relationship between foot sensation and standing balance in patients with multiple sclerosis. Gait Posture. 2011;34:275–278. doi: 10.1016/j.gaitpost.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res. 2008;25:113–122. doi: 10.1080/08990220802131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosnoff JJ, Shin S, Motl RW. Multiple sclerosis and postural control: the role of spasticity. Arch Phys Med Rehabil. 2010;91:93–99. doi: 10.1016/j.apmr.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Diener H, Dichgans J, Hülser PJ, Buettner UW, Bacher M, Guschbauer B. The significance of delayed long-loop responses to ankle displacement for the diagnosis of multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1984;57:336–342. doi: 10.1016/0013-4694(84)90156-1. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.Cao H, Peyrodie L, Boudet S et al. Expanded Disability Status Scale (EDSS) estimation in multiple sclerosis from posturographic data. Gait Posture. 2012;37:242–245. doi: 10.1016/j.gaitpost.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Corporaal SHA, Gensicke H, Kuhle J, Kappos L, Allum JHJ, Yaldizli O. Balance control in multiple sclerosis: correlations of trunk sway during stance and gait tests with disease severity. Gait Posture. 2013;37:55–60. doi: 10.1016/j.gaitpost.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–390. doi: 10.1177/1352458507082607. [DOI] [PubMed] [Google Scholar]
- 14.Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil. 2013;95:480–486. doi: 10.1016/j.apmr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Findling O, Sellner J, Meier N et al. Trunk sway in mildly disabled multiple sclerosis patients with and without balance impairment. Exp Brain Res. 2011;213:363–370. doi: 10.1007/s00221-011-2795-8. [DOI] [PubMed] [Google Scholar]
- 16.Martin CL, Phillips BA, Kilpatrick TJ et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006;12:620–628. doi: 10.1177/1352458506070658. [DOI] [PubMed] [Google Scholar]
- 17.Fanchamps MHJ, Gensicke H, Kuhle J, Kappos L, Allum JHJ, Yaldizli Ö. Screening for balance disorders in mildly affected multiple sclerosis patients. J Neurol. 2012;259:1413–1419. doi: 10.1007/s00415-011-6366-5. [DOI] [PubMed] [Google Scholar]
- 18.Fjeldstad C, Pardo G, Bemben D, Bemben M. Decreased postural balance in multiple sclerosis patients with low disability. Int J Rehabil Res. 2011;34:53–58. doi: 10.1097/MRR.0b013e32833d6ccb. [DOI] [PubMed] [Google Scholar]
- 19.Kalron A, Dvir Z, Achiron A. Effect of a cognitive task on postural control in patients with a clinically isolated syndrome suggestive of multiple sclerosis. Eur J Phys Rehabil Med. 2011;47:579–586. [PubMed] [Google Scholar]
- 20.Karst GM, Venema DM, Roehrs TG, Tyler AE. Center of pressure measures during standing tasks in minimally impaired persons with multiple sclerosis. J Neurol Phys Ther. 2005;29:170–180. doi: 10.1097/01.npt.0000282314.40230.40. [DOI] [PubMed] [Google Scholar]
- 21.Fjeldstad C, Pardo G, Frederiksen C, Bemben D, Bemben M. Assessment of postural balance in multiple sclerosis. Int J MS Care. 2009;11:1–5. [Google Scholar]
- 22.McLoughlin J, Barr C, Sturnieks D, Lord S, Crotty M. Effect of wearing a dorsiflexion assist orthosis on mobility, perceived fatigue and exertion during the six-minute walk test in people with multiple sclerosis: a randomised cross-over protocol. BMC Neurol. 2012;12:27. doi: 10.1186/1471-2377-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 24.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 25.Marsden J, Playford D, Day B. The vestibular control of balance after stroke. J Neurol Neurosurg Psychiatry. 2005;76:670–679. doi: 10.1136/jnnp.2004.046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramdharry GM, Marsden JF, Day BL, Thompson AJ. De-stabilizing and training effects of foot orthoses in multiple sclerosis. Mult Scler. 2006;12:219–226. doi: 10.1191/135248506ms1266oa. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds RF. The ability to voluntarily control sway reflects the difficulty of the standing task. Gait Posture. 2010;31:78–81. doi: 10.1016/j.gaitpost.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Lord SR, Ward JA. Age-associated changes in sensori-motor function and balance in community dwelling women. Age Ageing. 1994;23:452–460. [PubMed] [Google Scholar]
- 29.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horak F, Diener H. Cerebellar control of postural scaling and central set in stance. J Neurophysiol. 1994;72:479–493. doi: 10.1152/jn.1994.72.2.479. [DOI] [PubMed] [Google Scholar]
- 31.Kutzelnigg A, Faber-Rod JC, Bauer J et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prosperini L, Kouleridou A, Petsas N et al. The relationship between infratentorial lesions, balance deficit and accidental falls in multiple sclerosis. J Neurol Sci. 2011;304:55–60. doi: 10.1016/j.jns.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Prosperini L, Sbardella E, Raz E et al. Multiple sclerosis: white and gray matter damage associated with balance deficit detected at static posturography. Radiology. 2013;268:181–189. doi: 10.1148/radiol.13121695. [DOI] [PubMed] [Google Scholar]
- 34.Prosperini L, Petsas N, Raz E et al. Balance deficit with opened or closed eyes reveals involvement of different structures of the central nervous system in multiple sclerosis. Mult Scler. 2014;20:81–90. doi: 10.1177/1352458513490546. [DOI] [PubMed] [Google Scholar]
- 35.Butler AA, Lord SR, Rogers MW, Fitzpatrick RC. Muscle weakness impairs the proprioceptive control of human standing. Brain Res. 2008;1242:244–251. doi: 10.1016/j.brainres.2008.03.094. [DOI] [PubMed] [Google Scholar]
- 36.Daley ML, Swank RL. Changes in postural control and vision induced by multiple sclerosis. Agressologie. 1983;24:327–329. [PubMed] [Google Scholar]
- 37.Zackowski KM, Smith SA, Reich DS et al. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transfer-imaging abnormalities in the spinal cord. Brain. 2009;132:1200–1209. doi: 10.1093/brain/awp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandyan AD, Price CIM, Barnes MP, Johnson GR. A biomechanical investigation into the validity of the modified Ashworth Scale as a measure of elbow spasticity. Clin Rehabil. 2003;17:290–294. doi: 10.1191/0269215503cr610oa. [DOI] [PubMed] [Google Scholar]
- 39.DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85:290–297. doi: 10.1016/j.apmr.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 40.White L, McCoy S, Castellano V et al. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler. 2004;10:668–674. doi: 10.1191/1352458504ms1088oa. [DOI] [PubMed] [Google Scholar]
- 41.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 42.Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil. 2007;21:771–781. doi: 10.1177/0269215507077602. [DOI] [PubMed] [Google Scholar]
- 43.Prosperini L, Leonardi L, De Carli P, Mannocchi ML, Pozzilli C. Visuo-proprioceptive training reduces risk of falls in patients with multiple sclerosis. Mult Scler. 2010;16:491–499. doi: 10.1177/1352458509359923. [DOI] [PubMed] [Google Scholar]
- 44.Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195–216. doi: 10.1177/0269215510382495. [DOI] [PubMed] [Google Scholar]
- 45.Pandyan A, Johnson G, Price C, Curless R, Barnes M, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil. 1999;13:373–383. doi: 10.1191/026921599677595404. [DOI] [PubMed] [Google Scholar]


