Abstract
A major focus of research in alcohol-related disorders is to identify the genes and pathways that modulate alcohol-seeking behavior. In light of this, animal models have been established to study various aspects of alcohol dependence. The selectively bred alcohol-preferring (P) and -nonpreferring (NP) lines were developed from Wistar rats to model high and low voluntary alcohol consumption, respectively. Using inbred P and NP strains, a strong QTL (LOD-9.2) for alcohol consumption was identified on rat chromosome 4. To search for candidate genes that underlie this chromosomal region, complementary molecular-based strategies were implemented to identify genetic targets that likely contribute to the linkage signal. In an attempt to validate these genetic targets, corroborative studies have been utilized including pharmacological studies, knock-out/transgenic models as well as human association studies. Thus far, three candidate genes, neuropeptide Y (Npy), α-synuclein (Snca), and corticotrophin-releasing factor receptor 2 (Crhr2), have been identified that may account for the linkage signal. With the recent advancements in bioinformatics and molecular biology, QTL analysis combined with molecular-based strategies provides a systematic approach to identify candidate genes that contribute to various aspects of addictive behavior.
Keywords: Alcoholism, selective breeding, QTL mapping, candidate gene, neuropeptide Y (Npy), alpha-synuclein (Snca), corticotrophin-releasing factor receptor 2 (Crhr2)
INTRODUCTION
The identification of genes that influence alcohol dependence represents an important facet of drug abuse research. These genes can provide insight into the neurobiological and metabolic components that influence addictive behaviors. In addition, genetic factors can be targeted for pharmaceutical development and can likely be used to screen predisposed individuals and to better diagnose comorbid psychiatric disorders. For almost two decades, quantitative trait locus (QTL) analysis has been applied to various rodent models resulting in only limited success [1]. This article will review the research strategies that have been applied to the selectively bred alcohol-preferring (P) and –nonpreferring (NP) model in order to target and attempt to validate candidate genes from a QTL for alcohol consumption [2].
Alcoholism is a complex psychiatric disorder influenced by both genes and the environment. Selective breeding has been implemented to study the various aspects of alcohol dependence [3]. By applying selective breeding, one can increase the frequency of trait-relevant alleles that are associated with a specific phenotype (e.g. alcohol consumption) in a population resulting in consistent and reliable phenotypic differences. Likewise, the P and NP lines were selectively bred from Wistar rats for high and low alcohol consumption, respectively [4]. In this model, P rats exhibit several features that are consistent with alcoholism in humans [5]. For example, P rats (1) orally self-administer ethanol in pharmacologically relevant amounts; (2) consume EtOH for its pharmacological effects (not caloric value or taste); (3) show positive reinforcement; (4) develop tolerance; and (5) exhibit withdrawal symptoms [6, 7]. To date, a plethora of correlative studies have been conducted to study the behavioral and neurobiological differences between the P and NP lines [7]. However, these studies have only provided limited information about the primary genetic factors that underlie these phenotypic differences. Therefore, the primary purpose of the candidate gene approach is to identify the genes that either cause or contribute to a disease or related phenotype (e.g., alcohol dependence).
To conduct genetic studies, inbred P (iP) and inbred NP (iNP) strains were developed from the selectively bred P and NP lines by performing brother-sister mating for over 20 generations [8]. The resulting iP and iNP strains maintain highly discordant alcohol consumption scores [2]. These inbred animals are virtually genetically homogeneous, and phenotypic differences within the inbred strain can largely be attributed to environmental factors. By decreasing the genomic variability within a population, inbreeding improves the likelihood of identifying the genetic factor(s) that are associated with a heritable trait like alcohol consumption.
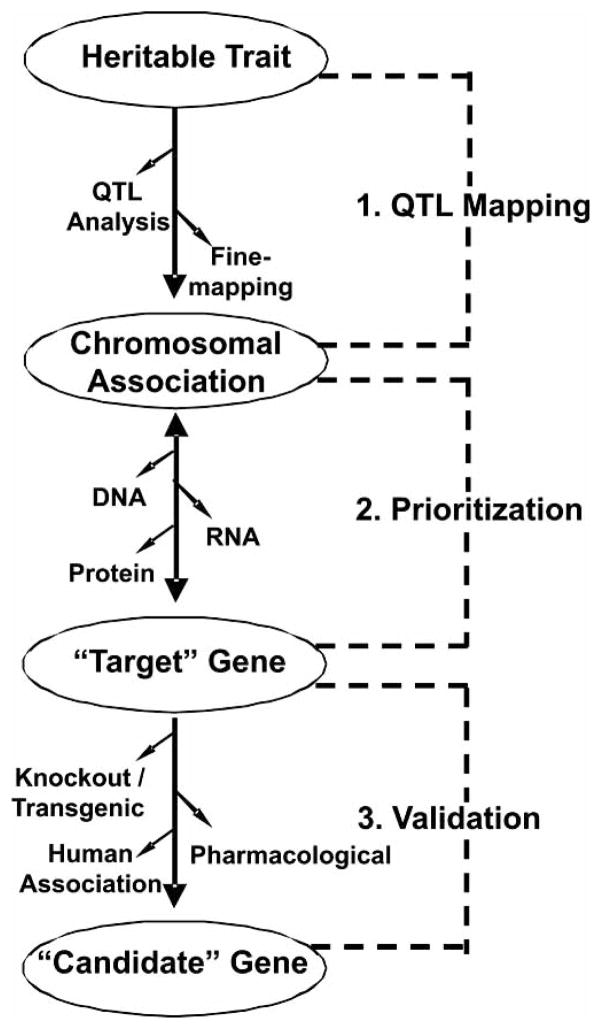
Several complementary approaches have been applied to the iP and iNP strains to identify candidate genes that contribute to the differences in alcohol-seeking behavior (Fig. 1). First, chromosomal regions (QTLs) associated with alcohol consumption in the iP and iNP strains were mapped through linkage analysis and then bred into congenic strains using marker-assisted breeding strategies. Second, complementary molecular techniques [i.e. sequence analysis, gene expression (mRNA, protein)] were implemented to screen and prioritize “target” genes that may underlie the observed QTL. Third, independent studies including genetic (knockout, transgenic) [9, 10], pharmacological [11–13], and human studies of association [14–17] have provided corroborative evidence that has been used in an attempt to validate individual “candidate” genes of interest identified in the P and NP model.
Fig. 1.
An illustration of a stepwise approach to target and attempt to validate candidate genes associated with a heritable trait. Following selective breeding and inbreeding, QTL mapping strategies were applied to the inbred P and NP strains in order to identify chromosomal regions that are correlated with alcohol consumption, the quantitative trait of interest. To target specific genes of interest, molecular-based strategies were implemented to screen for genetic targets that map to the chromosome 4 QTL region and display differences in gene expression and DNA sequence polymorphism. These complementary molecular-base strategies were utilized to identify and prioritize genetic targets for hypothesis-driven research. In an attempt to validate and further characterize the genes of interest, genetic (e.g., knockout, transgenic), pharmacological interventions (e.g., targeting associated receptors, enzymes and or transporters), and human association studies can provide corroborative evidence, ultimately defining the relevance of each candidate gene to alcohol-seeking behavior.
QUANTITATIVE TRAIT LOCUS (QTL) MAPPING
Mapping a QTL often represents the first step in a long process with the ultimate goal of identifying the gene(s) underlying a QTL of interest. QTL analysis was developed to identify chromosomal regions that are associated with a quantitative phenotype [18]. Unlike knockout and transgenic strategies, QTL analysis detects natural variability captured either by selective breeding or inbreeding. Derived from two parent populations, F1 animals are genetically identical and heterozygous at every locus throughout the genome. In the F2 population, a random distribution of genetic variability inherent in the parent strains is generated as a result of chromosomal recombination. Therefore, a QTL indicates that a significant correlation exists between polymorphic markers and a phenotypic measure determined for the F2 population. Applied to an iP x iNP F2 population, linkage analysis detected a highly significant QTL [logarithm of the odds (LOD) score = 9.2] for alcohol consumption on rat chromosome 4 (Fig. 2); [2, 19]. Due to the strong association, this chromosomal region likely harbors genes that influence alcohol consumption in the iP and iNP strains.
Fig. 2.
A QTL for alcohol consumption identified on rat chromosome 4 in iP and iNP rats. Boxes and arrows illustrate the basic methodologies that were incorporated to conduct QTL analysis in the P and NP strains. Using bidirectional selective breeding, the alcohol-preferring (P) and –nonpreferring (NP) rat strains were developed from a closed colony of Wistar rats (top box). The inbred P (iP) and NP (iNP) strains were later developed from the P and NP strains using brother-sister mating for over 20 generations. To perform QTL analysis, the iP and iNP strains were crossed to generate iP x iNP F1 animals, and these F1 animals were subsequently bred to generate the iP x iNP F2 population. In the F2 animals, free choice alcohol consumption (g/kg/day) and alcohol preference (v/v) were measured, and polymorphic microsatellite markers were genotyped. A genome screen was then performed to identify chromosomal regions that segregate with the alcohol consumption phenotype. Linkage analysis detected a highly significant QTL (lod score = 9.2) for alcohol consumption on rat chromosome 4. Thus far, three candidate genes have been identified that underlie the peak of this linkage signal including neuropeptide Y (Npy), α-synuclein (Snca), corticotrophin releasing factor receptor 2 (Crhr2).
QTL regions are often broad [e.g., 20 centiMorgans (cM)] and can contain hundreds of genes. The generation of congenic strains represents an extension of QTL analysis that is commonly employed to confirm and fine-map a QTL region. This strategy involves transferring a QTL interval from one inbred strain (i.e. donor strain) into another inbred strain (i.e. recipient strain) using marker-assisted breeding over ten generations of backcrossing [20]. With each generation, the chromosomal interval (QTL) of the donor strain is preserved, while approximately one half of the donor genome is replaced by the genome of the recipient strain. After ten generations, heterozygotes for the donor region are intercrossed to generate congenic strains. The resulting congenic strains are homozygous at the QTL locus, but contain 99.9% of the genome from the recipient strain.
Following QTL analysis, reciprocal congenic strains were developed by transferring the chromosome 4 QTL into the respective iP or iNP backgrounds [21]. Using genomic DNA, four microsatellite markers (D4Mgh16, D4Rat30, D4Arb21, and D4Rat55) were genotyped to determine the donors for each subsequent generation. Following ten generations of backcrossing, the resulting animals were intercrossed to produce two homozygous congenic strains termed P.NP and NP.P. The P.NP strain contains the iNP chromosome 4 QTL region introgressed into the iP background, while the NP.P strain contains the iP chromosome 4 QTL region introgressed into the iNP background. Compared to their respective iP and iNP background strains, the P.NP and NP.P congenic strains exhibit the expected phenotypic differences in alcohol consumption that are consistent with a successful capture of the chromosome 4 QTL [21]. The P.NP strain displayed a significant decrease in alcohol consumption relative to the iP strain (p=0.001), while the NP.P strain exhibited a significant increase in alcohol consumption relative to the iNP strain (p=0.0005) [21]. However, the introgressed region in the P.NP and NP.P congenic strains is approximately 20–25 cM and still contains hundreds of genes.
Following the development of congenic strains, marker-assisted breeding can subsequently be applied to generate interval specific congenic strains (ISCSs). This breeding strategy identifies and then exploits chromosomal recombination within the QTL region. To develop ISCSs, the congenic strain (e.g., NP.P) is again bred with its background strain (e.g., iNP) to generate heterozygous animals. These heterozygous animals are again crossed with the background strain, ultimately producing some animals that encode smaller segments of the QTL region than the original congenic strain. These animals are subsequently bred to create stable ISCSs that can be tested to determine whether the QTL of interest is conserved. To date, this strategy has been successfully applied in various genetic studies of alcohol-related behaviors [22–24].
As an extension of QTL analysis, the development of ISCSs provides important advantages for candidate gene identification. First, marker-assisted breeding clearly defines and can reduce the chromosomal region associated with the QTL. By reducing the size of the QTL interval, fine-mapping through the development of ISCSs limits the number of possible genes that underlie the QTL interval. Thereby, fine-mapping improves the likelihood of candidate gene identification. In addition, a linkage signal can potentially result from a cumulative effect of multiple loci. These loci can independently contribute to the phenotypic variance associated with QTL region [25, 26]. The generation of ISCSs can be used to dissect the QTL region in order to segregate the effects of these specific loci. In the P and NP model, the development and testing of ISCSs is nearing completion and is expected to reduce the chromosome 4 QTL interval to roughly 1–2 cM. Ultimately, fine-mapping through the development of ISCSs represents the final step in QTL mapping and can be utilized to help delineate the effect(s) (e.g., biochemical, neurobiological, behavioral) of the gene(s) associated with a linkage signal.
CANDIDATE GENE PRIORITIZATION
QTL mapping has been widely used to identify chromosomal regions that are associated with a quantitative trait like alcohol consumption. However, the strategies to localize candidate genes underlying a QTL are still being developed. The task of identifying specific genes that contribute to a linkage signal is difficult because QTLs can often encompass broad chromosomal regions with hundreds of possible candidate genes. For example, the 95% confidence interval of the QTL identified on rat chromosome 4 spans 12.5 cM, and a significant LOD score (above 4) is detected for roughly 25 cM [19]. To screen for likely genetic targets, complementary molecular-based techniques were applied to iP and iNP strains to target specific genes that may contribute to the linkage signal identified on rat chromosome 4.
Bioinformatics & Literature
Until recently, the specific location of a gene in the genome was often poorly localized. For instance, the exact location of alpha-synuclein (Snca) in the rat was unknown until it was mapped using recombination-based methods in the iP and iNP strains [27]. Due to the advancement in bioinformatics and mapping techniques, the location of almost every gene throughout the genome can now be determined. While often broad, a QTL region defines a finite chromosomal interval with a limited number of genes. With current bioinformatics tools, all possible genes underlying a QTL region can now be identified. Combined with other bioinformatics resources, each of these genes can theoretically be screened and prioritized for their potential relevance to the quantitative trait of interest, thus, providing targets for molecular-based research [28]. Therefore, the literature and bioinformactics affords an additional, complementary tool that can help demarcate a specific gene of interest from a broad QTL region with a multitude of differentially expressed genes [29].
Several online databases were employed to map the genes that reside in the chromosome 4 QTL region. These databases include Ensembl Genome Browser (www.ensembl.org), Rat Genome database (www.rgd.mcw.edu), and Ratmap (www.ratmap.gen.gu.se). Following gene mapping, search engines like Pubmed (www.ncbi.nlm.nih.gov) were utilized to sort and prioritize genes based on their known biology as well as their relevance to alcohol-related phenotypes (e.g. biochemical, physiological, comorbidity, etc.). Ultimately, neuropeptide Y (Npy), Snca and corticotrophin-releasing factor repector 2 (Crhr2) were prioritized for further characterization using molecular-based research because they: (1) exhibit a close proximity to the peak of the linkage signal; (2) show biological correlates that are relevant to alcohol dependence or an alcohol-related phenotype; and (3) display differences in gene expression (RNA, protein) between the P and NP strains.
Molecular-Based Research
Molecular-based strategies can be effectively used to screen and target genes that contribute to a QTL. A QTL represents the effect(s) of a specific biologically active variant(s) on a phenotypic measure. These allelic variants likely include the following: (1) coding polymorphisms that alter protein structure or enzyme function; (2) regulatory polymorphisms that affect gene expression (RNA, protein) or mRNA stability; (3) structural genomic variation (copy number variants); (4) DNA methylation polymorphisms; and (5) mitochondrial polymorphisms. Thus, the underlying biological mechanisms that drive a QTL are distinctly molecular in nature. Molecular-based techniques provide a means to detect relevant genetic artifacts and to characterize their effects on gene expression and function.
Applied to the P and NP model, QTL analysis and molecular-based research were utilized as complementary strategies to filter and target specific genes of interest to alcohol-seeking behavior. Gene expression studies were utilized as a functional measure to characterize the effects of regulatory polymorphisms on gene expression (mRNA, protein). In addition, sequence analysis was used to screen differentially expressed genes for underlying genetic polymorphism (coding, regulatory). These studies were followed by in vitro methods (i.e. luciferase) to assess the functional significance of individual polymorphisms. By inserting specific DNA sequence variants into a luciferase (luc) reporter vector, their effects on luciferase gene expression can be determined. Luciferase reporter gene assays can provide valuable insight into the regulatory polymorphism that may underlie differences in gene expression, establishing whether a polymorphism is functional in cell lines (e.g., neuroblastoma) that express the gene of interest.
Screening a QTL region for genes that display differences in mRNA expression provides an important strategy that has been utilized to target “potential” candidate genes [29–32]. However, gene expression profiling studies have several limitations that can make the results difficult to interpret [31]. First, the specificity of tissue dissection can significantly influence the resolution of gene expression studies, and gene expression is often not uniformly distributed or static, but can exhibit differences in spatial and temporal regulation. Second, inherent limitations in sensitivity can hamper the detection of differentially expressed genes that exhibit lower levels of expression (i.e. receptors). Third, RNA expression data from gene expression profiling can also be influenced by the data analysis and filtering methods. Finally, small sample sizes can also limit the resolution and power of gene expression studies.
Comparing the iP and iNP strains using analytic tools available from the Bioconductor Project [33], mRNA expression was measured using several techniques including real-time quantitative reverse transcription PCR (qRT-PCR), total gene expression analysis (TOGA) [34], and microarray analysis. These expression studies primarily targeted brain tissues associated with the mesocorticolimbic dopamine (DA) system and included the frontal cortex, hippocampus, hypothalamus, caudate-putamen, amygdala, and nucleus accumbens [27, 28, 35]. These brain regions were primarily selected due to their involvement in the mesocorticolimbic DA system, a system that modulates the rewarding properties of drugs of abuse and functions through reciprocal interactions between the ventral tegmental area (VTA) and various limbic structures [6].
Three candidate genes (Npy, Snca, Crhr2) were initially screened and selected based on their proximity to the peak of the QTL and their gene expression profile. For the three candidate genes, three independent mRNA expression studies resulted in some inconsistent findings. For example, significant differences in Npy mRNA expression were detected between the iP and iNP strains in mesolimbic regions using microarray analysis and qRT-PCR [35, 36], but were not detected using TOGA [27]. For Snca, TOGA and qRT-PCR detected differences in mRNA expression when comparing the iP and iNP strains [27]; however, these differences were not seen using microarray analysis [35]. Finally, qRT-PCR detected differences in Crhr2 mRNA expression [37], but these differences were not detected between the iP and iNP strains using microarray analysis or TOGA [27, 35].
These inconsistencies suggest that additional independent molecular-based measures (RNA, protein) are essential to limit both false positives and false negatives that can result from gene expression profiling. For example, both NPY and SNCA protein levels exhibited significant differences between the iP and iNP strains providing evidence that these genes are differentially expressed between the P and NP strains and may be functionally significant [27, 38]. In addition, the inconsistencies seen in Crhr2 mRNA expression (qRT-PCR, microarray, TOGA) may have resulted from a low level of Crhr2 mRNA expression detected by qRT-PCR in both the iP and iNP strains, suggesting that limitations in sensitivity may be an important factor to consider [37].
To target candidate genes, congenic strains potentially provide an advantage over inbred strains for gene expression studies. Gene regulation can be influenced by both cis-acting elements as well as transcription factors (trans-acting factors). Cis-acting elements are present on the same strand and chromosome as the gene they regulate and are binding sites for transcription factors that regulate gene transcription. A cis-element may be located in the promoter region 5′ to the gene it controls, in an intron, or in the 3′ untranslated region (3′UTR). Unlike cis-acting elements, transcription factors regulate genes distant from the gene from which they were transcribed.
In congenic strains, regulatory polymorphism in cis-acting elements (within the gene/QTL) is preserved, while the genetic variation outside the gene/QTL is significantly decreased because the background and congenic strains are virtually identical outside the QTL region. As a result, genes differentially expressed in the congenic animals outside the QTL region compared to the background strain are likely due to a regulatory gene located within the chromosome 4 region, and the development of congenic strains should theoretically decrease the effects of polymorphism that reside outside the region (trans-acting effects) on gene expression within the QTL. Therefore, the congenic gene expression profile (NP.P) within the QTL should be more consistent with that of the progenitor (iP), while the expression profile of genes outside the QTL region should be more minimized and more consistent with that of the background strain (iNP).
Comparing the NP.P congenic strain to the iNP background strain, microarray analysis detected mRNA expression differences in 13 known genes that reside in the QTL interval [28]. In addition, various genes in the PKC signal transduction pathway were identified [28]. Both Npy and Snca mRNA displayed significant differences in expression when the data from all five discrete brain regions from each animal were averaged. However, Npy mRNA expression differences were not confirmed using qRT-PCR. For Snca, mRNA expression was increased in the iNP relative to the NP.P strain indicating that the transfer of the QTL region did not significantly affect the direction of Snca mRNA expression. These results suggest that Snca mRNA expression is more prominently regulated by trans- than cis-regulating factors. A difference in Crhr2 mRNA expression was not detected by microarray analysis.
Protein content has not yet been measured in the NP.P congenic strain to confirm these mRNA expression results. While gene expression profiling when applied to inbred and congenic strains represents an important tool in the search for the gene(s) and biochemical pathway(s) that contributes to a linkage signal, inherent limitations predicate the use of independent molecular-based measures (mRNA, protein) for gene confirmation and prioritization. Gene expression profiling is on-going using the newly developed P.NP strain and will likely provide further insight.
Correlative Studies Using Congenic Strains
While many correlative studies (behavioral, neurobiological) have been conducted to study the differences between the P and NP lines [7], these genotypic correlations have not yet been examined in the newly developed P.NP and NP.P congenic strains. Hypothesis-driven research can be utilized to extend these correlative findings to congenic strains. For example, these correlative studies can yield important information regarding how the transfer of the QTL affects phenotypic measures (biochemical, neurobiological, behavioral) associated with quantitative trait as well as a specific genetic target(s). Furthermore, strong correlations established between selectively bred or inbred strains can be studied in congenic strains in order to provide evidence that these genotypic correlations are likely to be trait-relevant. In short, phenotypes that are inherited with a QTL and are consistent with the known biology of a genetic target can significantly strengthen the case for the nomination of an individual candidate gene.
The P.NP and NP.P congenic strains were primarily developed to confirm and narrow the chromosome 4 QTL interval, thereby, limiting the number of possible candidate genes. However, the P.NP and NP.P congenic strains were also utilized to study the effect of the transfer of the chromosome 4 QTL on other phenotypes. In addition to alcohol consumption [21], the transfer of the chromosome 4 QTL significantly affects body weight and bone phenotypes. For example, the congenic NP.P strain weighs significantly less than the iNP strain, and the P.NP strain weighs significantly more than the iP strain [39]. Using the iP x iNP F2 population [2], a QTL for body weight (LOD-5.4) was mapped to rat chromosome 4 in males. Likewise, bone phenotypes were measured in the congenic and inbred strains. Relative to the iP strain, the congenic P.NP strain exhibit decreased bone mass and strength [40]. These phenotypic correlates (i.e., body weight, bone) are consistent with the expected differences in Npy expression (mRNA, protein). Thus, these results may be indicative of a pleiotropic effect(s) associated with Npy or the effects of distinct biologically active variants that were co-selected due to genetic linkage.
CORROBORATIVE EVIDENCE AND CANDIDATE GENE VALIDATION
While no formal criteria have yet been established, a “candidate gene” is principally defined by the body of evidence supporting a gene’s candidacy. The identification of promising genetic targets (i.e. Npy, Snca, Crhr2) that map to a QTL and show strain-specific molecular and phenotypic correlations can provide a starting point for hypothesis-driven research. Hypothesis-driven research can ultimately yield important corroborative evidence that can be used in an attempt to further validate each candidate gene of interest. For example, transgenic and knockout strains can be used to determine how the direct genetic manipulation of gene expression affects alcohol-related phenotypes. In addition, pharmacological and human association studies can be conducted to further characterize the relationship between the genetic factor and the alcohol-related phenotypes. Applied to the three candidate genes that are most likely to contribute to the chromosome 4 QTL, these approaches represented independent, corroborative measures that better define the relevance of each candidate gene (Npy, Snca, Crhr2) to alcohol dependence.
Neuropeptide Y (Npy)
NPY is considered an important neuromodulator in the central nervous system, particularly in the amygdala and the hypothalamus. To date, studies have implicated Npy in the regulation of emotion, anxiety, consummatory behavior as well as alcohol dependence [41]. An inverse relationship has been documented between Npy levels (mRNA, protein) and alcohol-seeking behavior. For example, lower levels of Npy mRNA and protein were detected in P relative to NP rats in various regions in the mesolimbic system including the hypothalamus and amygdala [36, 38, 42]. Several variants in Npy’s DNA sequence were detected between the iP and iNP strains [36]. Using neuroblastoma cells, in vitro expression studies yielded a significant decrease in luciferase expression of the iP-(2nd intron)-luc construct relative to the NP-(2nd intron)-luc construct (p<0.05) suggesting that Npy expression may be regulated at the transcriptional level [36]. In addition, intracerebroventricular infusion of NPY has been shown to reduce ethanol intake in the P rat [11]. Mice deficient in NPY exhibit increased alcohol consumption compared to wild-type mice, and an over-expression of NPY decreases ethanol consumption in transgenic mice [9]. In humans, a polymorphism in Npy (Leu7Pro) was significantly associated with alcohol dependence in human alcoholics [14, 15].
It has been well-documented that the P and NP strains exhibit marked differences in anxiety-like behavior with studies suggesting that P rats are more “anxious” than NP rats. Compared with NP rats, P rats: (1) showed greater foot-shock-induced suppression of operant responding in an approach-avoidance conflict test; (2) spent less time in the open arms of an elevated plus maze; and (3) took longer in a passive avoidance test to step down from a platform to a grid floor where footshock was received 24 hours earlier [43]. In addition, both acoustic startle and potentiated startle response were consistently greater in P than NP rats, and only P rats showed significant fear-conditioned startle [44]. Therefore, due to NPY’s role in the modulation of behavioral effects of stress, particularly anxiety-like behavior ([45] see following reviews [46; 47]), Npy may represent an important biologic target for alcohol-seeking behavior, especially in the P and NP model.
Alpha-Synuclein (Snca)
Snca has primarily been implicated in neurodegenerative disorders, especially in Parkinson’s disease [48]. Studies suggest that SNCA plays an important role in the regulation of dopaminergic neurotransmission including dopamine synthesis, storage, release and re-uptake [49–54]. For Snca, a direct relationship has been established between Snca expression (mRNA, protein) and alcohol-seeking behavior. For example, the iP rats exhibit higher Snca mRNA & protein levels in the hippocampus relative to iNP rats [27]. Comparing the Snca DNA sequence of the iP and iNP strains, five variants were detected in the Snca promoter region and two in the 3′-untranslated region (3′UTR) [27, 55]. While the promoter polymorphisms did not affect luciferase gene expression, the 3′UTR (+679) polymorphism appears to be functional and luciferase expression levels are consistent with the mRNA and protein findings [27]. Subsequently, a study found that the iP polymorphism (+679) is also associated with a significantly longer mRNA half-life suggesting that mRNA stability may contribute to the differences seen in Snca expression (mRNA) between the iP and iNP strains [55].
Consistent with the P and NP findings, the deletion of Snca in mice significantly decreases alcohol consumption relative to wild-type [10]. Furthermore, compared to alcohol-naïve controls, monkeys that chronically self-administered alcohol for 18 months exhibit a 3-fold increase in Snca mRNA measured in peripheral blood [56]. Likewise, elevated Snca (mRNA, protein) levels were detected in alcoholics [57], and Snca DNA sequence variants have been linked to craving for alcohol in humans [16, 17]. In addition to alcohol-related phenotypes, elevated SNCA levels in midbrain dopaminergic neurons were detected in both cocaine abusers and mice in withdrawal following morphine treatment [58, 59]. Therefore, due to its effects on dopaminergic neurotransmission, Snca is a candidate gene of considerable interest to alcohol-seeking behavior.
Corticotrophin-Releasing Factor Receptor 2 (Crhr2)
Hypothalamic-pituitary-adrenal (HPA) axis regulation is an important component of stress response and has been implicated in both anxiety as well as alcohol-seeking behavior (see following review [60]). Studies suggest that CRHR2 functions in the hypothalamus and amygdala as an inhibitory or modulatory receptor to dampen HPA activation; thereby, CRHR2 is thought to promote adaptation and recovery from environmental stressors [61]. An inverse relationship has been detected between Crhr2 expression and alcohol-seeking behavior. The iP relative to iNP rats exhibit decreased Crhr2 mRNA expression in various regions of the limbic system [37]. Crhr2’s DNA sequence exhibits two polymorphisms (3′UTR, coding) between iP and iNP rats that could potentially influence gene expression or CRHR2 binding affinity [37]. These polymorphisms are currently being tested for their functional significance.
In addition to the P and NP findings, the administration of the CRHR2 selective agonist urocortin 3 (UCN 3) reverses the increases in ethanol self-administration during the early stages of ethanol withdrawal [12], and the infusion of UCN 3 into the central amygdala (CeA) decreases ethanol self-administration in ethanol dependent Wistar rats [13]. This reduction in alcohol self-administration seen during CRHR2 activation within the CeA may be related to a decrease in anxiety-like behavior [13]. Therefore, due to its involvement in the modulation of anxiety as well as stress responsivity (HPA-axis regulation), Crhr2 represents an interesting target for alcohol-seeking behavior.
CONCLUSION
The identification of the genetic factors that influence addictive behavior can provide important targets for both basic and clinical research. In the P and NP model, QTL mapping (QTL analysis, fine-mapping) and molecular-based research were implemented as complementary approaches to identify and prioritize promising genetic targets for alcohol-seeking behavior. In an attempt to validate the resulting genes of interest, hypothesis-driven research has been used to independently corroborate results. With the recent advancements in molecular biology and bioinformatics, QTL mapping and molecular-based strategies applied to selectively bred and inbred strains provide a multifaceted approach to target high-priority genes that contribute to alcohol dependence.
Key Learning Objectives.
The overall objective of this research is to identify genes that influence alcohol-drinking behavior.
To target high-priority genes, QTL mapping and molecular techniques were applied to selectively bred and inbred strains developed from Wistar rats.
Following their initial identification, these genetic targets can be validated using several independent measures.
These complementary strategies represent a step-wise approach that can be used to identify and confirm candidate genes for alcohol-drinking behavior.
Future Research Questions.
Future studies will test how alcohol affects the candidate gene expression in the iP and iNP strains.
The chromosome 4 QTL region will be fine-mapped to 2 cM using interval specific congenic strains (ISCSs).
Microarray analysis will be used to determine how the transfer of the chromosome 4 QTL interval affects mRNA expression by comparing the iP and congenic P.NP strains.
Acknowledgments
Supported by U.S. Public Service Grants AA010707, AA07611 to LC, MH65702 and MH52619 to AS.
ABBREVIATIONS
- P
Alcohol-preferring
- NP
Alcohol-nonpreferring
- iP
Inbred P
- iNP
Inbred NP
- QTL
Quantitative trait locus
- cM
centiMorgan
- LOD
Logarithm of the odds
- ISCS
Interval specific congenic strain
- TOGA
Total gene expression analysis
- qRT-PCR
Real-time quantitative reverse transcription PCR
- 3′UTR
3′-Untranslated region
- luc
Luciferase
- DA
Dopamine
- HPA
Hypothalamic-pituitary-adrenal
- CeA
Central amygdala
- Npy
Neuropeptide Y
- Snca
Alpha-synuclein
- Crhr2
Corticotrophin-releasing factor receptor 2
- Ucn 3
Urocortin 3
References
- 1.Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev Genet. 2005;6:271–86. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- 2.Carr LG, Foroud T, Bice P, et al. A quantitative trait locus for alcohol consumption in selectively bred rat lines. Alcohol Clin Exp Res. 1998;22:884–7. [PubMed] [Google Scholar]
- 3.Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Li TK, Lumeng L, Doolittle DP, Carr LG. Molecular associations of alcohol-seeking behavior in rat lines selectively bred for high and low voluntary ethanol drinking. Alcohol Alcohol Suppl. 1991;1:121–4. [PubMed] [Google Scholar]
- 5.Cicero TJ. In: Biochemistry and Pharmacology of Ethanol, Vol 2. A critique of animal analogues of alcoholism. Majchrowicz E, Noble EP, editors. New York: Plenum Press; 1979. pp. 533–60. [Google Scholar]
- 6.McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 7.Murphy JM, Stewart RB, Bell RL, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- 8.Grahame NJ. Selected lines and inbred strains. Tools in the hunt for the genes involved in alcoholism. Alcohol Res Health. 2000;24:159–63. Review. [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–9. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 10.Miranda C, Walker D, Alva H, Blednov YA, Harris RA. Deletion of alpha-synuclein decreases ethanol consumption in mice. Alcohol Clin Exp Res. 2003;27:84A. [Google Scholar]
- 11.Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–94. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- 12.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 13.Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–8. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lappalainen J, Kranzler HR, Malison R, et al. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–31. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- 15.Zhu G, Pollak L, Mottagui-Tabar S, et al. NPY Leu7Pro and alcohol dependence in Finnish and Swedish populations. Alcohol Clin Exp Res. 2003;27:19–24. doi: 10.1097/01.ALC.0000050642.62233.44. [DOI] [PubMed] [Google Scholar]
- 16.Bonsch D, Greifenberg V, Bayerlein K, et al. Alpha-synuclein protein levels are increased in alcoholic patients and are linked to craving. Alcohol Clin Exp Res. 2005;29:763–5. doi: 10.1097/01.alc.0000164360.43907.24. [DOI] [PubMed] [Google Scholar]
- 17.Foroud T, Wetherill LF, Liang T, et al. Association of alcohol craving with alpha-synuclein (SNCA) Alcohol Clin Exp Res. 2007;31:537–45. doi: 10.1111/j.1530-0277.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 18.Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–99. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bice P, Foroud T, Bo R, et al. Genomic screen for QTLs underlying alcohol consumption in the P and NP rat lines. Mamm Genome. 1998;9:949–55. doi: 10.1007/s003359900905. [DOI] [PubMed] [Google Scholar]
- 20.Bennett B. Congenic strains developed for alcohol- and drug-related phenotypes. Pharmacol Biochem Behav. 2000;67:671–81. doi: 10.1016/s0091-3057(00)00412-3. [DOI] [PubMed] [Google Scholar]
- 21.Carr LG, Habegger K, Spence JP, Liu L, Lumeng L, Foroud T. Development of congenic rat strains for alcohol consumption derived from the alcohol-preferring and nonpreferring rats. Behav Genet. 2006;36:285–90. doi: 10.1007/s10519-005-9021-z. [DOI] [PubMed] [Google Scholar]
- 22.Bennett B, Carosone-Link P, Beeson M, Gordon L, Phares-Zook N, Johnson TE. Genetic dissection of quantitative trait locus for ethanol sensitivity in long- and short-sleep mice. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 23.Kozell L, Belknap JK, Hofstetter JR, Mayeda A, Buck KJ. Mapping a locus for alcohol physical dependence and associated withdrawal to a 1.1 Mb interval of mouse chromosome 1 syntenic with human chromosome 1q23.2–23. 3. Genes Brain Behav. 2008;7:560–7. doi: 10.1111/j.1601-183X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 24.Ruf C, Carosone-Link P, Springett J, Bennett B. Confirmation and genetic dissection of a major quantitative trait locus for alcohol preference drinking. Alcohol Clin Exp Res. 2004;28:1613–21. doi: 10.1097/01.alc.0000145693.58448.95. [DOI] [PubMed] [Google Scholar]
- 25.Legare ME, Frankel WN. Multiple seizure susceptibility genes on chromosome 7 in SWXL-4 congenic mouse strains. Genomics. 2000;70:62–5. doi: 10.1006/geno.2000.6368. [DOI] [PubMed] [Google Scholar]
- 26.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98:1787–92. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang T, Spence J, Liu L, et al. alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and -nonpreferring rats. Proc Natl Acad Sci USA. 2003;100:4690–5. doi: 10.1073/pnas.0737182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr LG, Kimpel MW, Liang T, et al. Identification of candidate genes for alcohol preference by expression profiling of congenic rat strains. Alcohol Clin Exp Res. 2007;31:1089–98. doi: 10.1111/j.1530-0277.2007.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spence J, Liang T, Foroud T, Lo D, Carr L. Expression profiling and QTL analysis: a powerful complementary strategy in drug abuse research. Addict Biol. 2005;10:47–51. doi: 10.1080/13556210412331308958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebicke-Haerter PJ, Sommer WH. DNA microarrays and expression profiling in drug abuse research. Addict Biol. 2005;10:1–3. doi: 10.1080/13556210412331308967. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman P, Tabakoff B. Gene expression in animals with different acute responses to ethanol. Addict Biol. 2005;10:63–9. doi: 10.1080/13556210412331308985. [DOI] [PubMed] [Google Scholar]
- 32.Spanagel R, Heilig M. Addiction and its brain science. Addiction. 2005;100:1813–22. doi: 10.1111/j.1360-0443.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 33.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutcliffe JG, Foye PE, Erlander MG, et al. TOGA: an automated parsing technology for analyzing expression of nearly all genes. Proc Natl Acad Sci USA. 2000;97:1976–81. doi: 10.1073/pnas.040537997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimpel MW, Strother WN, McClintick JN, et al. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spence JP, Liang T, Habegger K, Carr LG. Effect of polymorphism on expression of the neuropeptide Y gene in inbred alcohol-preferring and -nonpreferring rats. Neuroscience. 2005;131:871–6. doi: 10.1016/j.neuroscience.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang T, Watts V, Carr LG. Polymorphism in the Corticotrophin-releasing factor 2 receptor of alcohol-preferring and alcohol-nonpreferring rats. Alcohol Clin Exp Res. 2006;30:124A. [Google Scholar]
- 38.Ehlers CL, Li TK, Lumeng L, et al. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–82. [PubMed] [Google Scholar]
- 39.Spence JP, Liu L, Foroud T, Carr LG, Shekhar A. Quantitative trait loci for body weight identified in male inbred alcohol-preferring (iP) and –nonpreferring (iNP) rats. Alcohol Clin Exp Res. 2007;31:134A. [Google Scholar]
- 40.Alam I, Carr LG, Lumeng L, Edenberg HJ, Econs MJ, Turner CH. NPY, SNCA, Spr and Gtrap3-18 genes are strongly correlated with bone mass and strength phenotypes on congenic P/NP rats. J Bone Miner Res. 2006;21:S145. [Google Scholar]
- 41.Carvajal C, Dumont Y, Quirion R. Neuropeptide y: role in emotion and alcohol dependence. CNS Neurol Disord Drug Targets. 2006;5:181–95. doi: 10.2174/187152706776359592. Review. [DOI] [PubMed] [Google Scholar]
- 42.Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li TK. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–30. [PubMed] [Google Scholar]
- 43.Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- 44.McKinzie DL, Sajdyk TJ, McBride WJ, et al. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–6. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- 45.Sajdyk TJ, Johnson PL, Leitermann RJ, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–34. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–24. doi: 10.1016/j.npep.2004.05.002. Review. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–6. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 49.Conway KA, Rochet JC, Bieganski RM, Lansbury PT. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–9. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 50.Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–26. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 51.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–9. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–92. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 53.Sidhu A, Wersinger C, Moussa CE, Vernier P. The role of {alpha}-synuclein in both neuroprotection and neurodegeneration. Ann NY Acad Sci. 2004;1035:250–70. doi: 10.1196/annals.1332.016. [DOI] [PubMed] [Google Scholar]
- 54.Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–70. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang T, Carr LG. Regulation of alpha-synuclein expression in alcohol-preferring and -non preferring rats. J Neurochem. 2006;99:470–82. doi: 10.1111/j.1471-4159.2006.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker SJ, Grant KA. Peripheral blood alpha-synuclein mRNA levels are elevated in cynomolgus monkeys that chronically self-administer ethanol. Alcohol. 2006;38:1–4. doi: 10.1016/j.alcohol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Bonsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol Psychiatry. 2004;56:984–6. doi: 10.1016/j.biopsych.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Ziolkowska B, Gieryk A, Bilecki W, et al. Regulation of alpha-synuclein expression in limbic and motor brain regions of morphine-treated mice. J Neurosci. 2005;25:4996–5003. doi: 10.1523/JNEUROSCI.4376-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mash DC, Adi N, Duque L, Pablo J, Kumar M, Ervin FR. Alpha synuclein protein levels are increased in serum from recently abstinent cocaine abusers. Drug Alcohol Depend. 2008;94:246–50. doi: 10.1016/j.drugalcdep.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 61.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. Review. [DOI] [PubMed] [Google Scholar]