Abstract
Drugs of abuse elicit dopamine release in the ventral striatum, possibly biasing dopamine-driven reinforcement learning towards drug-related reward at the expense of non-drug related reward. Indeed, reactivity in dopaminergic target areas of patients with alcohol dependence is shifted from non-drug related stimuli towards drug-related stimuli. Such ‘hijacked‘ dopamine signals may impair flexible learning from non-drug related rewards and thus promote craving for the drug of abuse.
Here, we used fMRI to measure ventral striatal activation by reward prediction errors (RPEs) during a probabilistic reversal learning task in recently detoxified alcohol-dependent patients and healthy controls (N=27). The same subjects also underwent FDOPA PET to assess ventral striatal dopamine synthesis capacity.
Neither ventral striatal activation by RPEs, nor striatal dopamine synthesis capacity differed between patients and controls. However, the ventral striatal coding of RPEs was negatively correlated with craving in patients. Furthermore, we found a negative correlation between ventral striatal coding of RPEs and dopamine synthesis capacity in healthy controls, but not in alcohol-dependent patients. Moderator analyses showed that the magnitude of the association between RPE coding and dopamine synthesis capacity depended on the amount of chronic-habitual alcohol intake.
Using a multimodal imaging approach, this study suggests that dopaminergic modulation of neural learning signals is disrupted in alcohol dependence and this is linked to long-term alcohol intake of patients. Drug intake may thus perpetuate itself by interfering with dopaminergic modulation of neural learning signals in the ventral striatum, thus increasing craving for habitual drug intake.
Keywords: fMRI, PET, dopamine, alcohol dependence, prediction error
Introduction
Alcohol along with most classes of drugs of abuse stimulates dopamine release in the ventral striatum. This provides a conduit for reinforcing drug consumption and assigning the value of stimuli associated with it (Di Chiara, 1995; Heinz et al., 2004; Volkow et al., 2004). In alcohol-dependent patients, ventral striatal activation to drug-associated stimuli is greater than to non-drug-associated stimuli (Wrase et al., 2007; Beck et al., 2012). Exaggerated activation induced by drug-cues indicates a ‘hijacked‘ state of the brain’s so-called ‘reward system’ and is related to clinical measures of alcohol addiction, especially acute craving for alcohol (Wrase et al., 2007), as well as individual alterations of the dopamine system on a neurochemical level (Heinz et al., 2005; Martinez et al., 2005). This suggests a model of addiction in which dopamine dysfunction and the associated shift in salience processing (Robinson and Berridge, 1993) impairs flexible learning from non-drug-related rewards (Park et al., 2010; Ersche et al., 2011). However, this has not yet been shown directly. Here, we therefore examined reward prediction error (RPE) signals in a reversal learning task requiring flexible adaptation to non-drug rewards and related these directly to both craving and presynaptic dopamine.
Phasic dopamine signals have previously been shown to be commensurate with temporal RPEs that are causally involved in learning the expected reward associated with environmental cues and are thus critical for certain types of learning (Schultz et al., 1997; Bayer and Glimcher, 2005; Steinberg et al., 2013). This is mirrored in human imaging studies using functional Magnetic Resonance Imaging (fMRI) where ventral striatal activation covaries with RPEs derived from computational models of reinforcement learning (e.g. O'Doherty et al., 2004). Although the hemodynamic fMRI activation is not dopamine-specific, such fMRI-derived phasic signals were indeed found to relate to measures and manipulations of the dopamine system (Pessiglione et al., 2006; Schlagenhauf et al., 2013). In healthy volunteers, we have recently found evidence for a regulation of phasic, event-related RPEs (measured via fMRI) by rather long-term, tonic dopamine synthesis capacity (assessed with 6-[18F]-fluoro-DOPA (FDOPA) positron emission tomography (PET; Schlagenhauf et al., 2013)). This may be disrupted in early alcohol abstinence (Heinz et al., 2005; Kumakura and Cumming, 2009).
In alcohol-dependent patients, impaired learning of novel, non-drug related rewards may result in a dominance of inflexible behavioural patterns associated with chronic, habitual alcohol intake and potentially triggered by drug-cue induced craving (Everitt and Robbins, 2005). Indeed, the ability to flexibly adapt behaviour to changing reward contingences is impaired in drug-dependent patients (Park et al., 2010; Ersche et al., 2011). A better understanding of the dopaminergic regulation of non-drug reward-related learning signals can provide insight into the neural processes underlying this impaired flexible behavioural adaption. In the present study, we examined the relationship between PET-derived dopamine synthesis capacity and fMRI-derived RPEs in controls and recently detoxified alcohol-dependent patients during reversal learning.
In accordance with the idea that chronic alcohol intake impairs the neurobiological correlates of flexible, non-drug reward learning, thereby promoting craving for alcohol, two hypotheses were examined in the present study. We found, first, that the individual coding of RPEs in ventral striatal activation during flexible behavioural adaptation to non-drug rewards correlates negatively with the patients’ level of craving for alcohol. Second, dopamine synthesis capacity failed to covary with ventral striatal activation elicited by RPEs reflecting a disrupted dopaminergic regulation of phasic learning signals in alcohol-dependent patients.
Materials and Methods
Participants and instruments
A total of 27 participants, consisting of 13 recently detoxified, male alcohol-dependent patients and 14 matched male healthy controls were included in the study (Table 1). Patients fulfilled DSM-IV and ICD-10 criteria for alcohol dependence, had no other psychiatric axis I disorder and no current drug abuse other than nicotine consumption (SCID interview, First et al., 2001). Patients were recruited at the Department of Psychiatry and Psychotherapy (Campus Charité Mitte) of the Charité – Universitätsmedizin Berlin. Disease severity and alcohol craving were assessed using the Alcohol Dependence Scale (ADS; Skinner and Sheu, 1982) and the Obsessive Compulsive Drinking Scale (OCDS; Anton, 2000) at the time of imaging data collection. The amount of alcohol intake was evaluated with the Lifetime Drinking History (LDH; Skinner and Sheu, 1982). Based on the LDH and a clinical interview, the age of onset, the duration of illness as well as the number of previous detoxifications and relapses were evaluated (Table 1). At the time of imaging data collection, patients were withdrawn from any previous medication for at least four plasma half-lives.
Table 1.
Sample Characteristics. Group means with standard deviations and range in brackets are reported; for group comparisons two-sample t-test were used.
|
Alcohol-dependent
patients (N=13) |
Healthy controls
(N=14) |
Sig. | |
|---|---|---|---|
| Age (years) | 45.08 ± 5.97 (33-55) | 43.86 ± 9.23 (28-61) | .69 |
| Sex | all male | all male | - |
| EHI (12/13) |
95.00 ± 7.977 (80-100) | 83.69 ± 36.20 (-30-100) | .30 |
| Verbal IQ (13/14) |
104.85 ± 10.35 (92-125) | 105.21 ± 10.48 (92-125) | .93 |
| D2 attention (13/12) |
142.69 ± 26.92 (89-185) | 148.50 ± 36.75 (97-202) | .66 |
| WCST perseveration score (13/14) |
35.19 ± 14.95 (11.80-64.40) | 28.82 ± 21.48 (.00-68.70) | .38 |
| LDH last year (kg) (13/14) |
48.28 ± 42.86 (2.10-157.38) | 7.18 ± 17.89 (.12-68.88) | <.01 |
| OCDS sum (13/14) |
19.62 ± 8.19 (8-33) | 2.57 ± 2.79 (.00-11) | <.001 |
| OCDS mean craving (13/14) |
39.39 ± 42.44 (.00-100) | 7.50 ± 11.20 (.00-40) | <.05 |
| ADS | 15.62 ± 7.91 (3-29) | - | - |
| Age of onset (years) | 29.62 ± 7.89 (19-43) | - | - |
| Duration of illness (years) | 15.46 ± 9.91 (1-36) | - | - |
| Number of detoxifications | 3.38 ± 2.14 (1-7) | - | - |
EDI = Edinburgh Handedness Inventory, WCST = Wisconsin Card Sorting Test, LDH = Lifetime Drinking History, OCDS = Obsessive Compulsive Drinking Scale, ADS = Alcohol Dependence Scale
Healthy controls had no axis I or II psychiatric disorder, no family history of psychiatric disorders in first degree relatives and no current drug abuse or a past history of drug dependence other than nicotine consumption (SCID Interview; First et al., 1997; First et al., 2001). Controls were matched to patients for age and handedness (Table 1). 13 of 14 healthy controls were already published in a previous fMRI-PET study focusing on controls only (Schlagenhauf et al., 2013). To further characterize the two samples, verbal IQ was assessed with a German vocabulary test (Schmidt and Metzler, 1992). Neuropsychological functioning was assessed to analyze cognitive deficits as possible confounds of reversal learning. Therefore, the Wisconsin Card Sorting Test (Grant and Berg, 1993) and the D2-Test (Brickenkamp, 2001) for attention were applied (Table 1). The local ethics committee approved the study, which was in accordance with national radiation safety regulation. After complete description of the study to the participants, written informed consent was obtained.
Reversal learning task
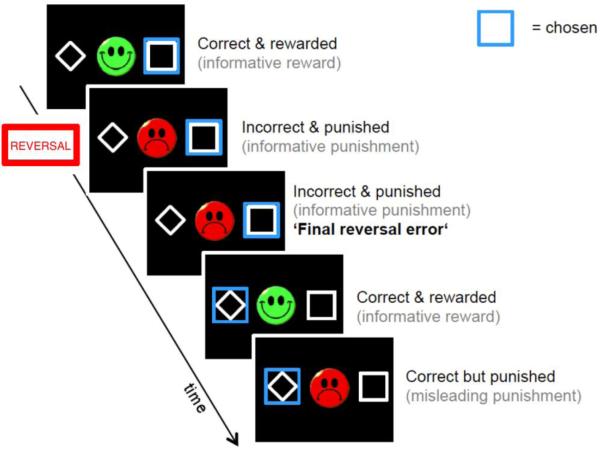
Reversal learning was examined as in previous studies (Park et al., 2010; Schlagenhauf et al., 2013). During fMRI acquisition, participants performed two sessions of 100 trials with three types of blocks: in block type 1, for the right-hand stimulus a reward (green smiley) was delivered in 80% of the recent right-hand choices, and a punishment (red frowny) delivered otherwise. Conversely, a punishment was delivered for choosing the left-hand stimulus in 80% of the recent left-hand choices, and a reward was delivered otherwise. In block type 2, the contingencies were simply reversed for the left and right sides. In block type 3, the probabilities were 50/50 instead of 80/20. Reversals always occurred after 16 trials, or any time after 10 trials once subjects reached 70% correct choices. Participants were instructed to respond as quickly as possible (response window: 2s). The chosen option and feedback were presented simultaneously for 1s. The trials were separated with a jittered interval of 1-6.5s. Before entering the scanner, subjects performed a practice version of the task (without reversal component), so as to be introduced to the probabilistic nature of the task. Furthermore, participants were instructed that reversals would occur and that they should try to adapt their behaviour accordingly.
Behavioural data analysis
The number of learned blocks was calculated for each individual and this count of achieved reversal stages was compared between groups using a two-sample t-test. This was tested one-tailed based on previous reports of reversal learning impairments in alcohol-dependent and cocaine-dependent patients (Park et al., 2010; Ersche et al., 2011). A learned block was defined as in the study by Park et al. (Park et al., 2010): over a sliding window of five trials, subjects had to choose the correct response a minimum of four times indicating 80% correct instrumental behaviour (Park et al., 2010). Analyses were carried out using IBM SPSS 21 (Statistical Package for the Social Sciences, Stanford, USA).
Computational modeling
As it was the main goal of the present study to examine the neural coding of RPEs and its relation to dopamine synthesis capacity, we applied a standard reinforcement learning model, a Rescorla-Wagner model, to each participants behavioural task sequence as reported in previous studies of alcohol-dependent patients (Park et al., 2010) and healthy participants in a combined fMRI-PET study (Schlagenhauf et al., 2013). The likelihood of a subject’s choice for action a on trial t is represented by the action’s value Qt(a) and expressed by the softmax rule
(1) p(a|Qt)=exp(Qt(a))/(Σa’exp(Qt(a’)))
The value Qt(a) of a chosen action is iteratively updated using the following equation:
(2) Qt(a)=Qt−1(a)+ε(Rt–Qt−1(a))
Here, ε is the individual learning rate that weights the difference between the delivered reward in trial t and the expected outcome. The obtained reward (1 or −1) is scaled by the variable R to depict the individual’s effective reinforcement sensitivity (β). This variable was assigned with value Rt=βrew if a reward was obtained and βpun if a punishment was obtained, thus resulting in a total of three free parameters θ=[ε’,βpun’,βrew’]. Here, we report the maximum a posteriori estimates of these parameters using a Gaussian prior with mean and variance parameters, μ□ and σ. Using Expectation Maximization, the priors were set empirically as described in more detail elsewhere (Huys et al., 2011). Both groups did not differ in terms of the inferred parameters or with respect to the likelihood that the observed data is actually described by the parameters (all p’s > .2). Based on the individually fitted parameters θi for each of the subjects, a time series of RPEs was computed for each subject i:
| (3) |
Positron emission tomography
Subjects were positioned within the aperture of the PET/CT (Siemens Biograph 16) scanner in 3-D mode. After a low dose transmission CT-scan, a dynamic 3-D ‘list-mode’ emission recording lasting 124 minutes started immediately after intravenous bolus administration of 200 MBq FDOPA. After CT-based tissue attenuation correction and scatter correction, list-mode data were iteratively reconstructed (OSEM, 16 iterations with 6 subsets) and framed (30 frames: 3×20s, 3×1min, 3×2min, 3×3min, 15x×5min, 3×10min). Arterial blood samples were collected during the emission recording with continuous on-line measurements for the first 6min and with manual sampling thereafter. The total radioactivity concentration in plasma samples was measured using a well counter cross-calibrated to the PET. The fractions of untransformed FDOPA and the main metabolite, O-methyl-[18F]-fluoro-L-DOPA (OMFD), were measured by reversed phase high performance liquid chromatography (HPLC) in plasma extracts from blood collected at 5, 15, 30, 45, and 60min post injection and the continuous arterial input functions were calculated by bi-exponential fitting of the measured fraction (Gillings et al., 2001).
Analysis of PET data
PET data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). The emission recording frames and the individual T1 image were coregistered to frame 12. The individual anatomical T1 image was spatially normalized using the unified segmentation approach of SPM (Ashburner and Friston, 2005), and the computed normalization parameters were applied to all frames.
For statistical analysis, dopamine synthesis capacity was quantified as FDOPA Kinapp (ml g−1 min−1) in voxel-by-voxel. FDOPA Kinapp was estimated using the frames from 20min to 60min of the emission recording (Kumakura and Cumming, 2009) by applying Gjedde-Patlak linear graphic analysis (Patlak and Blasberg, 1985) after subtraction of the total radioactivity concentration measured in a standard cerebellum mask as defined in the WFU Pick Atlas (Wake Forest University; http://fmri.wfubmc.edu/software/PickAtlas), as a partial to correction for OMFD concentrations throughout the brain. Finally, the FDOPA Kinapp images were spatially smoothed with a Gaussian kernel of 8mm full width at half maximum and mean values were extracted from the voxelwise FDOPA Kinapp maps using a literature-based volume of interest (VOI) (see Magnetic Resonance Imaging).
Magnetic Resonance Imaging
MRI was performed using a 3 Tesla GE Signa scanner with a T2*-weighted sequence (29 slices with 4mm thickness, TR=2.3s, TE=27ms, flip=90°, matrix size=128×128, FOV=256×256mm2, in-plane voxel resolution of 2x2mm2) and a T1-weighted structural scan (TR=7.8ms, TE=3.2ms, flip=20°, matrix size 256×256, 1mm slice thickness, voxel size of 1mm3).
Analysis of fMRI data
FMRI data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). ArtRepair was used to remove noise spikes and to repair bad slices within a particular scan by interpolation between adjacent slices (“Noise Filtering”, http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm). Preprocessing included correction for delay of slice time acquisition and scan-to-scan movement. The images were spatially normalized into the Montreal Neurological Institute (MNI) space using the normalization parameters generated during the segmentation of each subject’s anatomical T1 scan (Ashburner and Friston, 2005); spatial smoothing was applied with an isotropic Gaussian kernel of 8mm full width at half maximum.
An event-related analysis was applied to the images on two levels using the general linear model approach (GLM) as implemented in SPM8. At the first level, hemodynamic responses were modeled for win and loss feedback separately by stick functions. As a parametric modulator trial-by-trial RPEs from computational modeling were used at the trial related stick (Buchel et al., 1996). The modulated stimulus functions were convolved with the canonical hemodynamic response function (HRF) as provided by SPM8. Invalid trials (no choice within response window) were modelled separately. The six movement parameters from the realignment were included in the model as regressors of no interest. A single subject contrast of RPE modulated feedback (combining win and loss) was taken to the second level. At the second level, random-effects group-level analysis was performed using a one-sample t-test across the entire sample and a two-sample t-test to compare groups. This study focused primarily on neural correlates of RPEs in right ventral striatum for two reasons: 1) RPE time series have been reported to be more robustly correlated with BOLD-changes in right ventral striatum (Daw et al., 2011) and 2) we previously reported that dopamine synthesis capacity in right ventral striatum is negatively correlated with right ventral striatal RPEs in healthy controls (Schlagenhauf et al., 2013).
For correction of multiple comparisons, family wise error (FWE) correction was applied using small volume correction within ventral striatum. The ventral striatal VOI was constructed using an in-house-tool to create a literature-based probabilistic VOI as described in (Schubert et al., 2008): Coordinates from 16 previous, independent fMRI studies (containing data from 325 healthy participants) reporting ventral striatal RPEs were used (O'Doherty et al., 2003; O'Doherty et al., 2004; Cohen and Ranganath, 2005; Pessiglione et al., 2006; Rodriguez et al., 2006; Tobler et al., 2006; Bray and O'Doherty, 2007; Cohen, 2007; Schonberg et al., 2007; D'Ardenne et al., 2008; Murray et al., 2008; Gershman et al., 2009; Kahnt et al., 2009; Krugel et al., 2009; Palminteri et al., 2009; Valentin and O'Doherty, 2009). Based on the previous observation that ventral striatal activation by monetary reward is negatively associated with craving for alcohol (Wrase et al., 2007), mean beta-weights of the RPE contrast were extracted for the literature-based ventral striatal VOI and then correlated with the craving score from the OCDS as used in Wrase et al. (2007). To test for an association with dopamine synthesis capacity, each individual’s ventral striatal Kinapp value was entered as a covariate in the SPM random-effects analysis. A disturbed relationship of RPE related hemodynamic activity and dopamine synthesis capacity is expressed by the interaction of covariate by group (Figure 2A).
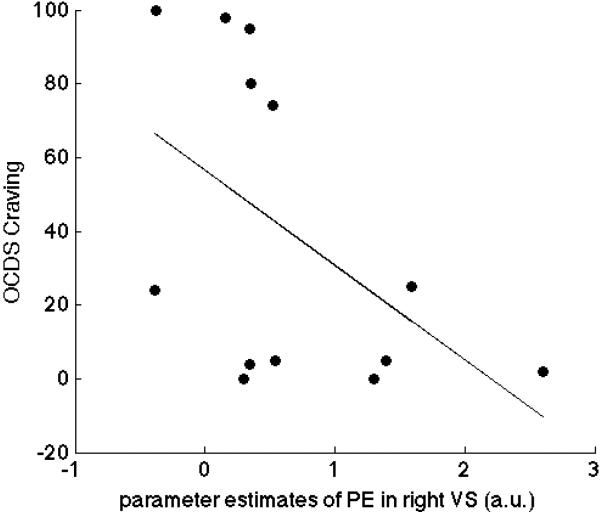
Figure 2. Negative correlation of ventral striatal reward prediction errors and craving in patients.
In alcohol-dependent patients, mean parameter estimates of the prediction error contrast were extracted for the literature-based volume of interest of the right ventral striatum and correlated with craving scores (r=−.53, p<.05 one-tailed).
To explore this interaction, we set up a moderation analysis in SPSS across the whole sample. This regression model included beta-weights of the RPE contrast in right ventral striatum as dependent variable and group, dopamine synthesis capacity in right ventral striatum, the amount of previous chronic alcohol intake in the last year (evaluated using the LDH) as well as craving (OCDS) as explanatory variables. The interaction of dopamine synthesis capacity and alcohol intake was additionally entered to the model (Hayes and Matthes, 2009). In order to meet variance homogeneity and sphericity assumptions, all variables were z-transformed, which also results in standardized, easy to interpret, regression coefficients. In addition, we tested for moderation by craving to demonstrate specificity of the observed moderation by chronic alcohol intake. To specify the interpretation of the moderation analysis, we split up the entire sample in two groups using the median of chronic alcohol intake as a cut-off point (6.02 kg).
Results
Behavioural performance
Performance on the WCST and the D2-attention-test did not differ between controls and patients (Table 1). During reversal learning and with respect to the criterion for learning (four correct responses over a sliding window of five trials), a group difference for successfully achieved reversal stages was observed (healthy controls: mean 10.71, SD 1.86; alcohol-dependent patients: mean 9.39, SD 1.76, t=1.91, p<.05, one-tailed). This is in line with results of our previous study in another, larger sample of alcohol-dependent patients with the same task (Park et al., 2010).
FMRI results
Collapsing across healthy controls and alcohol-dependent patients, a significant RPE signal in bilateral ventral striatum was observed (right: MNI space x=17, y=8, z=-5: t=3.83, FWE-corrected for VS VOI p<.05, left: MNI space x=−10.5, y=8, z=−5: t=3.51, FWE-corrected for VS VOI p<.05). No group difference was observed (FWE-corrected for VS VOI p>.60). Within the group of alcohol-dependent patients, beta weights of RPEs in right ventral striatum were negatively correlated with craving for alcohol (r=−.51, p<.05 one-tailed, Figure 1) but not chronic alcohol intake (LDH, p>.30). This finding in patients remained significant in a partial correlation analysis including dopamine synthesis capacity and chronic alcohol intake as nuisance variables (r=−.53, p<.05 one-tailed). To examine whether this correlation between RPE signalling and craving is specifically present in alcohol-dependent patients only, regression analysis was conducted across the entire sample with craving as dependent variable and right ventral striatal RPE, group and the interaction of group × RPE as independent variables. Indeed, the interaction of right ventral striatal RPE × diagnosis reached significance (β = .65, p <.05, one-tailed; R2 change = .10).
Figure 1. Reversal learning task.
During fMRI acquisition, participants performed two sessions of 100 trials with three types of blocks: in block type 1, for the right-hand stimulus a reward (green smiley) was delivered in 80% of the recent right-hand choices, and a punishment (red frowny) delivered otherwise. In block type 2, the contingencies were simply reversed for the left and right sides. Reversals always occurred after 16 trials, or any time after 10 trials once subjects reached 70% correct choices.
PET results
There was no significant voxelwise group difference in dopamine synthesis capacity in ventral striatum even at a low uncorrected threshold (p=.05, uncorrected), nor did the mean Kinapp values for the ventral striatal VOI differ between groups (p=.25). Finally, there was no significant correlation between Kinapp and craving or chronic alcohol intake in patients or controls (p’s > .2).
Combined fMRI and PET results
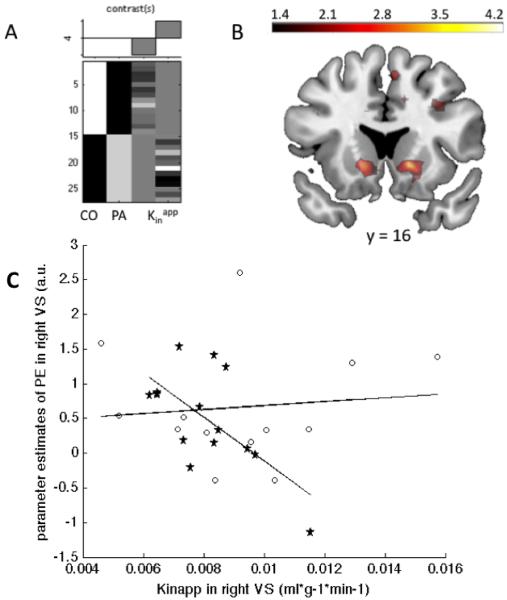
As previously reported, the RPEs in right ventral striatum were negatively correlated with dopamine synthesis capacity in right ventral striatum for the 14 healthy controls (MNI space x=14.5, y=13, z =−5: t=3.73, FWE-corrected for VS VOI p<.05) of whom 13 had been drawn from a previous publication (Schlagenhauf et al., 2013). This correlation was not significant in the 13 alcohol-dependent patients (FWE-corrected for right VS VOI p=.30). When assessing this difference directly, the interaction of group by covariate (Figure 2A) reached significance in right ventral striatum (MNI space x=14.5, y=13, z=−5: t=3.32, FWE-corrected for VS VOI p<.05, Figure 2B & 2C), indicating that there was a significant negative correlation between right ventral striatal RPEs and FDOPA Kinapp in healthy controls but not in patients.
We next tested for a moderation of the relation between ventral striatal RPEs and FDOPA Kinapp by chronic alcohol intake or craving across the entire sample (controls and patients). In this regression model, dopamine synthesis capacity in the ventral striatum was significantly and craving trendwise associated with RPEs (dopamine synthesis capacity β = −.47, p < .05; craving β = .36, p = .09), while group and chronic alcohol intake were not (group β = .46, p = .34, chronic alcohol intake β = .03, p = .89). Crucially, in an additional analysis the interaction of dopamine synthesis capacity and chronic alcohol intake reached significance (dopamine synthesis capacity × chronic alcohol intake β = .65, p = .02; R2 change = .21, p = .02), demonstrating a moderation of the relation of RPEs and dopamine synthesis capacity in ventral striatum by chronic alcohol intake. Splitting the entire sample in two groups about the median of chronic alcohol intake as a cut-off point (6.02 kg) resulted in high- and low-intake groups that closely mapped onto diagnostic groups (13 participants including one patient with low chronic alcohol intake versus 14 participants including two controls with high chronic alcohol intake). Nevertheless, correcting for group and craving as a covariate, the post-hoc partial correlations between dopamine synthesis capacity and RPE still reached significance in the group with low chronic alcohol intake (r=−.69, p<.05) but not in the group with high chronic alcohol intake (r=−.06, p=.86).
Discussion
To the best of our knowledge, this is the first molecular imaging study demonstrating that disrupted dopaminergic regulation of neural learning signals is linked to the amount of chronic alcohol intake. Combining FDOPA PET and fMRI, we observed that chronic alcohol intake abolishes a previously reported association between dopamine synthesis capacity and ventral striatal RPEs (Schlagenhauf et al., 2013). Also, dopamine-dysregulated ventral striatal RPEs correlated negatively with craving for alcohol in patients.
The observation of a disrupted modulation of ventral striatal RPEs, correlating with a long-term measure of dopamine synthesis capacity in controls (Kumakura and Cumming, 2009), sheds light on the dysregulation of ventral striatal dopaminergic neurotransmission in detoxified alcohol-dependent patients. We previously observed in healthy controls that levels of baseline dopamine synthesis capacity were inversely related with the encoding of event-related ventral striatal RPEs, a potential proxy of phasic dopamine release (Schlagenhauf et al., 2013). This is in keeping with the hypothesis that baseline, tonic (extracellular) dopamine levels reduce event-related, phasic dopamine release (Grace, 1991; Ito et al., 2011). We now demonstrate that this interaction is absent in detoxified alcohol-dependent patients, suggesting impaired interactions between different aspects (e.g. tonic and phasic) measures of dopamine neurotransmission in alcohol dependence.
Previous human PET studies support the hypothesis that acute and chronic alcohol intake alter dopaminergic neurotransmission in the ventral striatum: relative to orange juice consumption, acute alcohol intake reduced ventral striatal D2/3-receptor availability (Boileau et al., 2003), which is suggestive of greater dopamine release. Furthermore, baseline availability of ventral striatal D2/3-receptors predicted subjective responses to acute alcohol infusion (Yoder et al., 2005). Next, chronic consumption in alcohol dependence was characterised by reduced availability of (ventral) striatal D2/3-receptors (Volkow et al., 1996; Heinz et al., 2004), plausibly reflecting a (possibly counter-adaptive homeostatic) down-regulation in the face of long-term alcohol-induced dopamine release (Koob and Le Moal, 1997). In healthy controls, D2/3-receptor availability is inversely related to both dopamine synthesis capacity and amphetamine-induced dopamine release (Buckholtz et al., 2010; Ito et al., 2011). The latter observation confirms an interaction of D2/3-receptors and presynaptic dopamine function. Indeed, direct evidence for such an interaction is provided by recent animal research: D2-autoreceptor deficient mice exhibited elevated dopamine synthesis and disinhibited dopamine release in striatum (Bello et al., 2011). Thus, alcohol dependence might be expected to be characterised both by elevated synthesis and release of dopamine and reduced availability of dopamine striatal D2/3 receptors (Volkow et al., 1996; Heinz et al., 2004). Indeed, striatal dopamine synthesis capacity was reported in one FDOPA-PET study (Tiihonen et al., 1998). However, this was replicated neither in another study (Heinz et al., 2005) nor in the present sample. Presynaptic dopamine release evoked by psychostimulants was blunted in a PET depletion paradigm in recently detoxified alcohol-dependent patients (Martinez et al., 2005), suggesting that presynaptic dopamine storage and release is impaired in recently detoxified patients. Indeed, microdialysis experiments confirm substantial reductions of ventral striatal dopamine levels in detoxified rodents (Diana et al., 1993). Neurotoxic effects of chronic ethanol on dopamine neurons and their striatal terminals may help to explain these observations. This latter interpretation is supported by a few longitudinal studies indicating that reduced D2/3-receptor availability recovers slowly if at all (Volkow et al., 2002), imparting an increased risk for subsequent relapse (Heinz et al., 1996). Persistent reductions in dopamine release, receptor binding or synthesis can contribute to mood impairments (Chang and Grace, 2013) and impaired reward-associated learning (Schultz et al., 1997; Steinberg et al., 2013).
Based on these considerations, two questions arise: first, why is ventral striatal RPE signalling in the present sample and in a previous sample (Park et al., 2010) of alcohol-dependent patients still in the same range as in healthy controls? Second, what are the implications of the observed lack of an association between long-term ventral striatal dopamine synthesis capacity and phasic, event-related ventral striatal RPEs?
With respect to the first question, patients and controls displayed no group difference in ventral striatal RPE signals and this replicates findings in a previous sample (Park et al., 2010). Notably, the reinforcement learning model used to fit the observed choice behaviour and to generate regressors for the fMRI analysis explained both groups equally well. Thus, learning based on RPEs is equally well accounted for by this particular type of model, and this may be one reason why the neural correlates are not dissimilar. Despite the similar model fits, there were differences in behaviour differed between groups (patients exhibited impaired flexible behavioural adaptation). This in turn suggests the possibility that RPEs in alcohol-dependent patients are incorporated into behaviour in a manner that differs from healthy controls. One extant suggestion is that addiction involves an enhanced transfer of drug-related signals from ventral to dorsal striatal areas (Wong et al., 2006; Belin and Everitt, 2008) that is seen in the disrupted acquisition of new non-drug behavioural patterns (Park et al., 2010; Ersche et al., 2011). The converse of this explanation would suggest that RPEs determine behaviour less in patients because gating of non-drug-associated learning signals from ventral to dorsal striatum controlled by loops via the lateral prefrontal cortex is reduced (Haber and Knutson, 2010; Park et al., 2010).
At this point, it is important to note that in the present study craving negatively correlated with ventral striatal RPEs signals in detoxified patients. Here, we suggest that reduced coding of new, reward-related information via RPEs facilitates craving for the habitual consumption of alcohol. Previous studies have shown that craving severity reflects drug-associated cue reactivity (Volkow et al., 2006; Wong et al., 2006), and is inversely related to non-drug-associated cue reactivity (Wrase et al. 2007). The latter observation is consistent with the negative relationship between craving and ventral striatal RPE signals reported here. Overall, this suggests that craving for a habitually consumed drug of abuse (thought to be associated with the dorsal striatum) is increased when an individual’s ability to encode RPEs in other tasks not related to drugs is low.
With respect to the second question, our results suggest that (phasic) ventral striatal learning signals are rather intact in alcohol-dependent patients, while their relation to (tonic) dopamine synthesis capacity is disrupted. Based on animal research, it has been proposed that tonic extracellular dopamine concentrations inhibit presynaptic (phasic) dopamine release (Grace, 1991) and recent work gives evidence for a crucial involvement of D2-autoreceptors in this autoregulatory process (Bello et al., 2011). The present study shows that the association of dopamine synthesis capacity and RPEs is disrupted in the ventral striatum of alcohol-dependent patients and that the degree of this impairment was moderated by the amount of chronic alcohol intake. It is conceivable that this disrupted balance of different aspects of dopamine neurotransmission can impair the propagation of feedback-driven learning signals to the prefrontal cortex (Braver and Cohen, 1999; Frank, 2011). This notion is supported by a previous study, which also reported intact ventral striatal RPE coding but observed diminished functional connectivity between ventral striatum and dorso-lateral prefrontal cortex in patients which was related to the observed behavioural impairment in patients (Park et al., 2010). Indeed, a profound decrease of prefrontal metabolism was reported in alcohol-dependent patients compared to controls (Volkow et al., 2007). Future studies should explore whether a lack of (tonic) dopaminergic regulation of phasic learning signals impairs striatal-prefrontal connectivity and executive behavioural control.
Limitations of our study include the correlational nature of our results and the relatively small sample size arising from the requirement to scan patients with PET and fMRI. The restriction to men was intended to avoid variance due to gender differences in PET dopamine measures (Laakso et al., 2002). Also, it would be desirable to measure the entire triad of D2/3-receptors, dopamine synthesis capacity and fMRI-PE to test more definitely the relationship between these variables within subjects rather than across studies. Future studies could also benefit from longitudinal designs to examine temporal dynamics in the dopaminergic system during withdrawal. Dopamine is not the sole mediator of striatal circuits and recent animal research suggests that associative learning signals in ventral striatum are also modulated by cholinergic inputs and the activation of GABAergic neurons in the ventral tegmental area (Brown et al., 2012). The interaction of these neurotransmitter systems and their contribution to dysfunctional flexible learning in alcohol dependence is also an important target for future studies.
In conclusion, we observed that an association between ventral striatal dopamine synthesis capacity and RPEs, while prominent in healthy individuals, is practically abolished in alcohol-dependent patients. This disruption was modulated by chronic alcohol intake, resulting in a lack of an association between the two measures at high levels of alcohol intake. Furthermore, we observed that weaker ventral striatal coding of RPEs predicts higher craving for alcohol. Together, these two findings support the hypothesis that abolished interactions between tonic dopamine measures and phasic learning signals interfere with ability of recently detoxified patients to flexibly adapt behaviour to non-drug rewards and pursue reinforcers other than the habitually consumed drugs of abuse.
Figure 3. Disrupted dopaminergic regulation of reward prediction errors in right ventral striatum of alcohol-dependent patients.
A SPM design matrix and contrast weights for the group × covariate (right ventral striatal FDOPA Kinapp) interaction. B Voxelwise map of the group × covariate contrast at y = 16, thresholded at T > 2.5 for display purposes; this reached significance in right ventral striatum (x=14.5, y=13, z=−5: t=3.32, FWE-corrected for ventral striatum p<.05). C Scatterplot of the group x covariate interaction for visualization with controls as asterisks and patients as circles. Reward prediction errors (mean parameter estimates) and dopamine synthesis capacity were extracted using the literature-based right ventral striatal volume of interest of interest.
Acknowledgments
The study was supported by a grant from the German Research Foundation to Andreas Heinz (DFG HE2597/4-3&7-3, DFG Exc 257 and as part of DFG FOR 1617) and by the German Ministry for Education and Research (BMBF 01QG87164 & 01GS08159). The authors thank M. Keitel, A. Goldmann and B. Neumann for assistance during data acquisition; N. Fonyuy and E. Jaeschke for organization and assistance during FDOPA PET; R. Michel and A. Gerhardt for radiochemical analysis.
Drs. Rapp and Huys have received funding from the German Research Foundation (DFG RA1047/2-1). Dr. Rapp has received funding from the German Federal Ministry of Education and Research (BMBF 01ET1001A, BMBF BFNL 01GQ0914) and lecture fees from Merz, Glaxo Smith Kline, Servier, and Johnson & Johnson. Drs. Schlagenhauf and Buchert report having received funding from the German Research Foundation (SCHL 1969/1-1 & 2-1). Dr. Schlagenhauf has received lecture fees from BMS and was supported by the Max-Planck-Society. Dr. Plotkin has received research funding from the German Research Foundation (HE 2597/4-3; 7-3). Dr. Heinz has received research funding from the German Research Foundation (HE 2597/4-3; 7-3; FOR 16/17; Excellence Cluster Exc 257 & STE 1430/2-1) and the German Federal Ministry of Education and Research (01GQ0411; 01QG87164; NGFN Plus 01 GS 08152 and 01 GS 08159).
Footnotes
Financial Disclosures. Mr. Deserno, Dr. Beck, Dr. Huys, Mr. Lorenz, Dr. Buchert, Dr. Buchholz, Dr. Plotkin, Dr. Kumakura, Dr. Cumming, Dr. Heinze and Dr. Grace reported no biomedical financial interests or potential conflicts of interest.
Reference List
- Anton RF. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95(Suppl 2):S211–217. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Prog Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Bray S, O'Doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. J Neurophysiol. 2007;97:3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R. Hogrefe; Göttingen: 2001. Test d2 - Aufmerksamkeits-Belastungstest.Überarbeitete und neu normierte Auflage. [Google Scholar]
- Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Buchel C, Wise RJ, Mummery CJ, Poline JB, Friston KJ. Nonlinear regression in parametric activation studies. Neuroimage. 1996;4:60–66. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Grace AA. Amygdala-Ventral Pallidum Pathway Decreases Dopamine Activity After Chronic Mild Stress in Rats. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Individual differences and the neural representations of reward expectation and reward prediction error. Soc Cogn Affect Neurosci. 2007;2:20–30. doi: 10.1093/scan/nsl021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Behavioral and neural predictors of upcoming decisions. Cogn Affect Behav Neurosci. 2005;5:117–126. doi: 10.3758/cabn.5.2.117. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans' choices and striatal prediction errors. Neuron. 2011;69:1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Muller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. American Psychiatric Press; Washington D.C.: 1997. Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. New York State Psychiatric Institute; New York: 2001. Structured Clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) [Google Scholar]
- Frank MJ. Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol. 2011;21:381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Gershman SJ, Pesaran B, Daw ND. Human reinforcement learning subdivides structured action spaces by learning effector-specific values. J Neurosci. 2009;29:13524–13531. doi: 10.1523/JNEUROSCI.2469-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings NM, Bender D, Falborg L, Marthi K, Munk OL, Cumming P. Kinetics of the metabolism of four PET radioligands in living minipigs. Nucl Med Biol. 2001;28:97–104. doi: 10.1016/s0969-8051(00)00187-6. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA. WCST-Wisconsin Card Sorting Test. In: Hogrefe, editor. Göttingen: 1993. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Heinz A, Dufeu P, Kuhn S, Dettling M, Graf K, Kurten I, Rommelspacher H, Schmidt LG. Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Arch Gen Psychiatry. 1996;53:1123–1128. doi: 10.1001/archpsyc.1996.01830120061011. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Huys QJ, Cools R, Golzer M, Friedel E, Heinz A, Dolan RJ, Dayan P. Disentangling the roles of approach, activation and valence in instrumental and pavlovian responding. PLoS Comput Biol. 2011;7:e1002028. doi: 10.1371/journal.pcbi.1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, Suhara T. Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. J Neurosci. 2011;31:7886–7890. doi: 10.1523/JNEUROSCI.6024-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci. 2009;21:1332–1345. doi: 10.1162/jocn.2009.21092. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Krugel LK, Biele G, Mohr PN, Li SC, Heekeren HR. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci U S A. 2009;106:17951–17956. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, Salokangas RK, Hietala J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52:759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;131:239. doi: 10.1038/sj.mp.4002058. 267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Palminteri S, Boraud T, Lafargue G, Dubois B, Pessiglione M. Brain hemispheres selectively track the expected value of contralateral options. J Neurosci. 2009;29:13465–13472. doi: 10.1523/JNEUROSCI.1500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodriguez PF, Aron AR, Poldrack RA. Ventral-striatal/nucleus-accumbens sensitivity to prediction errors during classification learning. Hum Brain Mapp. 2006;27:306–313. doi: 10.1002/hbm.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Rapp MA, Huys QJ, Beck A, Wustenberg T, Deserno L, Buchholz HG, Kalbitzer J, Buchert R, Bauer M, Kienast T, Cumming P, Plotkin M, Kumakura Y, Grace AA, Dolan RJ, Heinz A. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 2013;34:1490–1499. doi: 10.1002/hbm.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K-H, Metzler P. Beltz Test GmbH; Weinheim: 1992. Wortschatztest (WST) [Google Scholar]
- Schonberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007;27:12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R, Ritter P, Wustenberg T, Preuschhof C, Curio G, Sommer W, Villringer A. Spatial attention related SEP amplitude modulations covary with BOLD signal in S1--a simultaneous EEG--fMRI study. Cereb Cortex. 2008;18:2686–2700. doi: 10.1093/cercor/bhn029. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Vilkman H, Rasanen P, Ryynanen OP, Hakko H, Bergman J, Hamalainen T, Laakso A, Haaparanta-Solin M, Solin O, Kuoppamaki M, Syvalahti E, Hietala J. Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry. 1998;3:156–161. doi: 10.1038/sj.mp.4000365. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O'Doherty JP, Dolan RJ, Schultz W. Human neural learning depends on reward prediction errors in the blocking paradigm. J Neurophysiol. 2006;95:301–310. doi: 10.1152/jn.00762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin VV, O'Doherty JP. Overlapping prediction errors in dorsal striatum during instrumental learning with juice and money reward in the human brain. J Neurophysiol. 2009;102:3384–3391. doi: 10.1152/jn.91195.2008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Wong DF, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O'Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor avxailability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]