Abstract
Objectives
To describe the differences in goals for their usual practice for various medical therapies from a number of international centers for children with severe traumatic brain injury.
Design
A survey of the goals from representatives of the international centers.
Setting
Thirty-two pediatric traumatic brain injury centers in the United States, United Kingdom, France, and Spain.
Patients
None.
Interventions
None.
Measurements and Main Results
A survey instrument was developed that required free-form responses from the centers regarding their usual practice goals for topics of intracranial hypertension therapies, hypoxia/ischemia prevention and detection, and metabolic support. Cerebrospinal fluid diversion strategies varied both across centers and within centers, with roughly equal proportion of centers adopting a strategy of continuous cerebrospinal fluid diversion and a strategy of no cerebrospinal fluid diversion. Use of mannitol and hypertonic saline for hyperosmolar therapies was widespread among centers (90.1% and 96.9%, respectively). Of centers using hypertonic saline, 3% saline preparations were the most common but many other concentrations were in common use. Routine hyperventilation was not reported as a standard goal and 31.3% of centers currently use Pbo2 monitoring for cerebral hypoxia. The time to start nutritional support and glucose administration varied widely, with nutritional support beginning before 96 hours and glucose administration being started earlier in most centers.
Conclusions
There were marked differences in medical goals for children with severe traumatic brain injury across our international consortium, and these differences seemed to be greatest in areas with the weakest evidence in the literature. Future studies that determine the superiority of the various medical therapies outlined within our survey would be a significant advance for the pediatric neurotrauma field and may lead to new standards of care and improved study designs for clinical trials.
Keywords: cerebral hypoperfusion, hypoxia, intracranial hypertension, pediatric neurocritical care, pediatric traumatic brain injury, secondary Injuries
Over the past decade, a number of international efforts have been made to improve the care and outcomes of children with severe traumatic brain injury (TBI)—the leading cause of death of children and responsible for billions of dollars of medical and societal costs each year (1–6). In 2003, the first edition of the Guidelines for the Medical Management of Traumatic Brain Injury in Infants, Children and Adolescents was published (7), which included evidence-based recommendations along with expert opinions. This landmark work was updated in 2012, with slight modifications of the topics reviewed and a diminished emphasis on expert opinion (8). Although influential, these guidelines emphasize that the neurotrauma literature is insufficient to provide firm recommendations regarding a host of medical decisions (Table 1) that are important in caring for children with severe TBI—including the superiority/inferiority of cerebrospinal fluid (CSF) diversion strategies, the relative effectiveness of various hyperosmolar therapies, the ability of hypoxia/ischemia monitoring strategies to improve outcomes, the timing and quantity of nutritional support, and the decisions on glucose administration and management. All of these decisions are likely to have some effect on overall outcomes, yet the current literature cannot discern their relative importance or impact on the overall outcomes.
Table 1.
Summary of Evidence and Recommendations Generated From the 2012 Pediatric Traumatic Brain Injury Guidelines (26)
| Topic | Level of Evidence |
Recommendation |
|---|---|---|
| Indications for ICP monitoring | Level III | “Use of ICP monitoring may be considered…” |
| Threshold for treatment of intracranial hypertension | Level III | “Treatment of ICP may be considered at a threshold of 20 mm Hg” |
| Cerebral perfusion pressure thresholds | Level III | “A minimum CPP of 40 mm Hg may be considered… A CPP threshold of 40–50 mm Hg may be considered…” |
| Advanced neuromonitoring | Level III | “If brain oxygenation monitoring is used, maintenance of Pbo2 ≥ 10 mm Hg may be considered” |
| Neuroimaging | Level III | “In the absence of neurological deterioration…routine repeat CT scan…may not be indicated…” |
| Hyperosmolar therapy | Level II Level III |
“Hypertonic saline should be considered…for intracranial hypertension…effective doses…range between 6.5 and 10 mL/kg” “Hypertonic saline may be considered…effective doses as a continuous infusion of 3% saline range between 0.1 and 1.0 mL/kg/hr administered on a sliding scale…” |
| Temperature control | Level II Level III |
“Moderate hypothermia…for only 24 hr duration should be avoided….moderate hypothermia starting within 8 hr after injury and lasting for 48 hr duration should be considered to reduce ICP…rewarming at a rate of 0.5°C/hr should be avoided” “Moderate hypothermia…for 48 hr duration may be considered.” |
| Cerebrospinal fluid drainage | Level III | “Cerebrospinal fluid drainage through an externalized ventricular drain…may be considered…The addition of a lumbar drain may be considered…” |
| Barbiturates | Level III | “High-dose barbiturate therapy may be considered in hemodynamically stable patients with refractory intracranial hypertension….continuous arterial blood pressure monitoring and cardiovascular support to maintain adequate CPP are required” |
| Decompressive craniectomy for the treatment of intracranial hypertension | Level III | “Decompressive craniectomy with duraplasty…may be considered for pediatric patients….showing early signs of neurological deterioration or herniation or are developing intracranial hypertension refractory to medical management…” |
| Hyperventilation | Level III | “Avoidance of prophylactic severe hyperventilation to a Paco2 < 30 mm Hg may be considered within the first 48 hr…If hyperventilation is used…advanced neuromonitoring for evaluation of cerebral ischemia may be considered” |
| Corticosteroids | Level II | “The use of corticosteroids is not recommended to improve outcome or lower ICP…” |
| Glucose and nutrition | Level II Level III |
“The evidence does not support the use of an immune-modulating diet…to improve outcome” “…glycemic control…should be left to the treating physician” |
| Antiseizure prophylaxis | Level III | “Prophylactic treatment with phenytoin may be considered to reduce the prevalence of early posttraumatic seizures…” |
ICP = intracranial pressure (levels of evidence based on current traumatic brain injury guidelines with levels I–III [“must be considered,” “should be considered,” and “may be considered,” respectively]), CPP = cerebral perfusion pressure (mean arterial blood pressure minus mean ICP), Pbo2 = partial pressure of interstitial brain oxygen.
During this time span, randomized controlled trials (RCTs) have failed to provide more substantive evidence for the guidelines. The Canadian Critical Care Trials Group and the Pediatric Traumatic Brain Injury Consortium: Hypothermia carried out a total of three studies to determine the efficacy of therapeutic hypothermia in children with severe TBI (9, 10)—all of which failed to demonstrate efficacy. Significant variability in medical goals between centers has been suggested as a reason for the failure of these trials, and this pattern was previously observed in an RCT of hypothermia for adult TBI victims (11). For example, the use of hypertonic saline for intracranial hypertension was significantly greater in children randomized to normothermia in the Hyp-HIT study—potentially introducing a bias into the study that was uncontrollable by the study investigators.
We hypothesize substantial variations in medical goals for children with severe TBI in the overall pediatric neurotrauma community and have performed a survey of these goals for this article. We speculate that these variations may play a role in differences in outcomes observed between centers in routine care as well as in clinical studies. We assembled an international consortium of clinical centers that also agreed to participate in a planned comparative effectiveness study of medical therapies for pediatric TBI.
METHODS
This study was submitted to the Institutional Review Board (IRB) of the University of Pittsburgh for their consideration and deemed to be exempt from IRB review. An international consortium of pediatric TBI participants was recruited from a variety of sources (members of the recently formed Pediatric Neurocritical Care Research Group [http://www.pncrg.org], site principal investigators from the “Cool Kids” Trial and the Hyp-HIT study, and investigators within the ESPNIC [European Society of Pediatric and Neonatal Intensive Care] Neurocritical Care section). All potential participants were asked to report on the usual practices employed at their institution.
A survey instrument to determine current practices regarding intracranial hypertension therapies, hypoxia/ischemia monitoring strategies, and metabolic issues was generated (Table 2). In order to estimate how many children the participating institutions cared for with severe TBI—and therefore, to approximate the relative value of the survey—respondents were asked to audit the number of cases of severe TBI their institution had admitted in the past 3 years. For this audit, inclusion criteria (age ≤ 18 yr, severe TBI with Glasgow Coma Scale score ≤ 8, and placement of intracranial pressure [ICP] monitor) and exclusion criteria (pregnancy, penetrating injury, and unknown mechanism) were applied. Respondents were asked to describe the goals for the usual practices that occur at their centers regarding the questions listed in Table 2. These answers were given in free form and respondents were free to explain their practices without interpretation and did not represent any form of medical record review. We chose the topics for the survey for a number of reasons. All of the topics were aspects of the most recent version of the pediatric TBI guidelines—with the requisite requirement that the topics within the guideline were deemed to be important in TBI outcomes. We were primarily considering medical therapies that were necessary for routine care of all children with severe TBI and tailored our inquiries to those topics. We considered inquiring about sedation and neuromuscular blockade usage within the survey, but felt that the existing literature demonstrating the relationship between these agents and outcomes was still extremely preliminary. Large intracenter variation was likely for these agents, making the information provided by the participants less reliable than those for the other topics. Similarly, we did not study antiseizure prophylaxis due to lack of evidence that it may change overall outcomes. For other topics, particularly corticosteroids and hypothermia, sufficient data exist within the literature to generate substantive recommendations, and therefore, these topics were not surveyed. Lastly, we did not study neurological imaging and decompressive surgery, as these are not medical therapies for pediatric TBI. Results were collated and reported for the 18 different questions. Responses were analyzed overall and stratified based on institution size and location (United States vs international center).
Table 2.
Survey Questions
| General | |
| Audit of eligible patients over the past 3 yr | |
| What is the ICP threshold routinely used in your institution? | |
| Is the ICP threshold age-related? If so, how? | |
| What is the CPP threshold routinely used in your institution? | |
| Is the CPP threshold age-related? If so, how? | |
| Intracranial hypertension therapies | |
| CSF diversion | Do you use CSF diversion (via an externalized ventricular drain) in children with severe TBI? |
| If you use CSF diversion, do you drain CSF continuously or intermittently? | |
| If you drain CSF intermittently, do you have a goal amount of CSF to drain or a goal ICP that you will drain CSF to achieve? | |
| Hyperosmolar therapies | Do you use mannitol, hypertonic saline solutions, or both during intracranial hypertension crises? |
| If you use hypertonic saline solutions, what concentration(s) do you use? | |
| If you use hypertonic saline solutions, do you use it as a bolus administration or as a continuous infusion or both? | |
| Hypoxia/ischemia detection | |
| Hyperventilation | Do you intentionally use hyperventilation during periods of normal ICP? |
| Pbo2 monitoring | Do you use Pbo2 monitoring in all children? If only a portion, approximately what percentage? |
| If you use Pbo2 monitoring, what is your Pbo2 target? | |
| Metabolic support | |
| Nutritional support | When do you start nutritional support to patients? |
| Do you use enteral nutrition, parenteral nutrition, or both? | |
| Glucose | When do you start to administer glucose to patients? |
| Do you use insulin to control blood glucose? If so, do you use it by a defined protocol or by the bedside clinician’s judgment? | |
ICP = intracranial pressure, CPP = cerebral perfusion pressure (mean arterial blood pressure minus mean ICP), CSF = cerebrospinal fluid, Pbo2 = partial pressure of interstitial brain oxygen.
RESULTS
A total of 32 centers were recruited from the United States, United Kingdom, Spain, and France (Appendix 1), and all centers that agreed to participate completed the survey. To demonstrate the number of children generally seen at these centers, their audit revealed that approximately 557 children per year with severe TBI are cared for at these centers. With respect to their goals for medical therapies routinely employed at these centers, there was some unanimity for the results of various thresholds for cerebrohemodynamics. All centers report that they use an ICP threshold of 20 mm Hg for the majority of subjects. Eight of the centers (25%) report that they use slightly lower ICP thresholds for the youngest children (10 mm Hg at one site, 15 mm Hg at four centers, and 18 mm Hg at three centers). For cerebral perfusion pressure (CPP), one site does not target CPP thresholds, whereas all others report use of a minimum CPP threshold. Three centers (9.4%) use a single CPP threshold (one site at 70 mm Hg, the other two at 60 mm Hg) for all subjects admitted with severe TBI. Nine centers report two different thresholds for subjects based on age, whereas the others report at least three different age-related thresholds. The lowest CPP threshold from any site was 35 mm Hg, whereas the highest was 75 mm Hg.
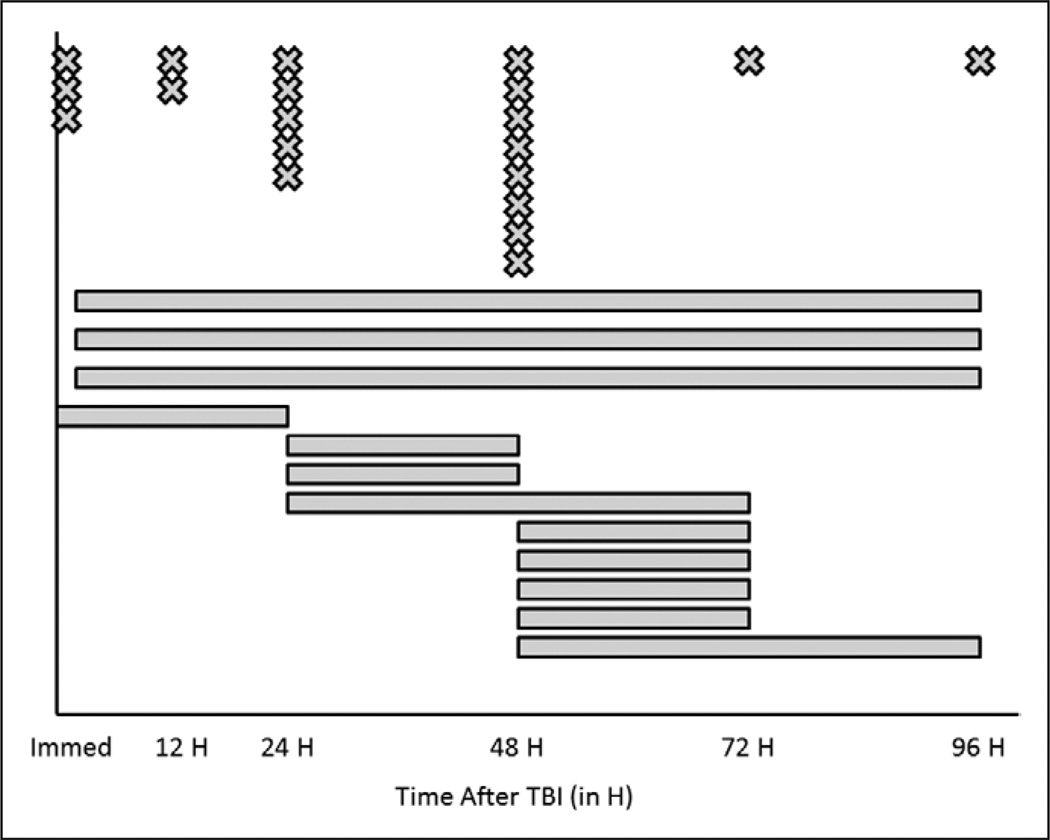
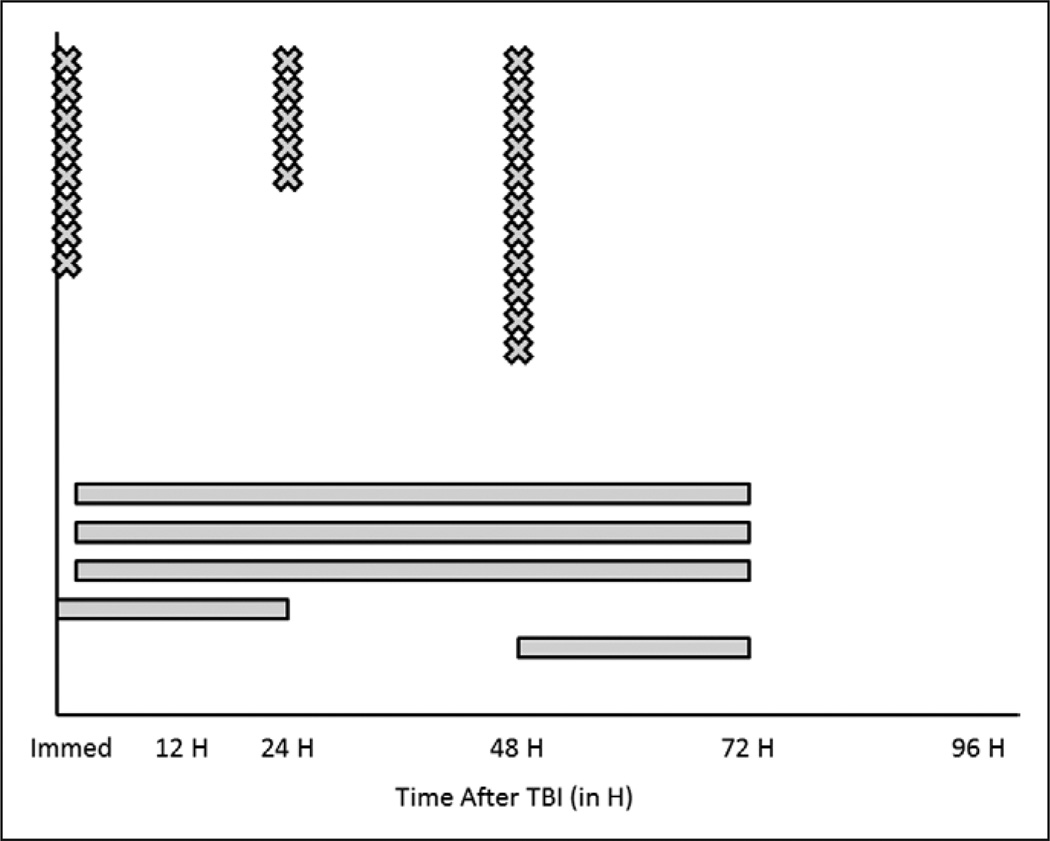
For intracranial hypertension therapies, CSF diversion was stratified into continuous drainage, intermittent drainage, or no drainage and a wide variety of strategies were identified (Table 3). For hyperosmolar therapies, one center does not use hypertonic saline while three do not use mannitol. The concentrations of hypertonic saline solutions used within centers are also striking, with one center reporting use of six different concentrations of such agents. In addition, three centers use the highest commercially available saline concentration of 23.4% saline. In four centers, only bolus doses of hypertonic saline solutions are used, whereas others use both boluses and continuous infusions. For hypoxia/ischemia prevention and detection, none of the centers report intentionally using hyperventilation during periods of normal ICP. Ten centers (31.3%) monitor partial pressure of interstitial brain oxygen (Pbo2) with threshold values ranging from 20 to 35 mm Hg. For metabolic support, the distributions regarding timing of nutritional support and glucose administration are seen in Figures 1 and 2, respectively. For nutritional support, the most common time to start nutritional support was 48 hours after TBI, but the distribution varied widely. Within centers, there was significant variability in this goal as well (as indicated by the bars on the figure), starting from within the first 24 hours to starting from 48 to 96 hours after TBI. As for choices of nutritional support, 20 centers routinely use enteral feedings, eight parenteral prescriptions, and four use a combination of both. For glucose, the distribution of timing of administration is also variable, but earlier than for the start of nutritional support (Fig. 2). Interestingly, one site provides glucose only when the patient becomes hypoglycemic, one site administers glucose on an “age-dependent” basis, and one site begins glucose administration when ICP is less than 20 mm Hg. Nineteen centers (59.4%) use insulin regularly to control hyperglycemia, of which 10 use a locally derived standard protocol for its administration. Thirteen centers do not use insulin regularly to control hyperglycemia after TBI.
Table 3.
Summary of Cerebrospinal Fluid Diversion Strategies and Hyperosmolar Therapies
| CSF Diversion Strategies | n of 32 (% [95% CI]) |
|---|---|
| Never use CSF diversion | 11 (34.4% [18.6–53.2]) |
| Intermittent CSF diversion only | 0 (0% [0–10.9]) |
| Continuous CSF diversion only | 11 (34.4% [18.6–53.2]) |
| Combinations | |
| No CSF diversion + continuous CSF diversion | 2 (6.3% [0.8–20.8]) |
| No CSF diversion + intermittent CSF diversion | 2 (6.3% [0.8–20.8]) |
| Intermittent CSF diversion + continuous CSF diversion | 6 (18.8% [7.2–36.4]) |
| Hyperosmolar therapy strategies | |
| Never use mannitol | 3 (9.4% [2.0–25.0]) |
| Never use hypertonic saline solutions | 1 (3.1% [0.1–16.2]) |
| Concentrations of hypertonic saline solutions | |
| Only use one concentration | 22 (68.8% [50–83.9]) |
| 3% only | 18 |
| 2.7% only | 2 |
| 6.4% only | 1 |
| 7.4% only | 1 |
| Use more than one concentration | 10 (31.3% [16.1–50.0]) |
CSF = cerebrospinal fluid.
Figure 1.
Summary of the goals for starting of nutritional support from an international pediatric neurotrauma consortium consisting of 32 centers from the United States and Europe. Centers were asked, “When do you start nutritional support to patients?” and were able to respond in freeform text. Centers responded with discrete time points (“X” in the figure) or by describing a range of time periods where nutritional support was intended to be started (“line” in the figure). All times are expressed as time after traumatic brain injury (TBI).
Figure 2.
Summary of the goals for starting of glucose from an international pediatric neurotrauma consortium consisting of 32 centers from the United States and Europe. Centers were asked, “When do you start to administer glucose to patients?” and were able to respond in freeform text. Centers responded with discrete time points (“X” in the figure) or by describing a range of time periods where nutritional support was intended to be started (“line” in the figure). Three centers responded with goals that were not time-based (when intracranial pressure < 20, when hypoglycemic, based on the age of patient) and are excluded from this figure. All times are expressed as time after traumatic brain injury (TBI).
DISCUSSION
We report that the medical goals, as derived from the self-described usual practices at centers that care for hundreds of children with severe TBI each year, vary greatly between institutions within our international consortium. This variability appears to be most prominent in areas where the literature is the least conclusive, including CSF diversion, metabolic goals, and Pbo2 monitoring. For topics with more evidence—hyperosmolar therapy and perhaps hyperventilation—there was a more unified approach to the medical goals. One comparable survey of these medical goals was performed by Segal et al (12) more than a decade ago. In this U.K. survey, they found that all centers practiced hyperventilation and mannitol use, whereas a subset of centers used furosemide, corticosteroids, fluid restriction, barbiturates, and hypothermia. Although the specific therapies in question were different than ours, this likely reflects the changes in TBI practice as new evidence has emerged over the past decade. Intriguingly, the ICP and CPP goals adopted by these institutions were quite similar to the ones that we observed. More recently, a survey of 194 U.S. clinicians found that approximately 60% of recommendations made within the contemporary guidelines (13) were part of their clinical goals. These results were similar to ours in that age-dependent ICP and CPP goals were reported and approximately 36% of respondents did not use CSF diversion. However, the use of hyperosmolar therapies was significantly less than in our survey (57% for mannitol and 68% for hypertonic saline). Other efforts to understand medical decision making in pediatric TBI have used data collected from either databases or retrospective registries, generally concentrating on decisions for placement of ICP monitors (14, 15) or prospective studies discerning variations in practices between centers (16). Our survey is unique in outlining the variables that a large number of international institutions use as a part of their usual practice in children with severe TBI in three specific categories— intracranial hypertension therapies, hypoxia/ischemia detection and prevention, and metabolic support—that have significant implications for outcomes and clinical trials.
Intracranial Hypertension Therapies
The decision regarding CSF diversion is quite unique, in that a clinician must decide whether or not to drain CSF in all cases. This binary question arises because ICP monitoring technology necessarily involves an externalized ventricular drain (which drains CSF) or a strain-gauge monitor within the parenchymal space (with cannot drain CSF). Currently, no studies are of sufficient quality to demonstrate the superiority of any CSF diversion strategy. The best evidence supporting CSF diversion is derived from a series of 23 children with refractory ICP—of which 20 children achieved control of intracranial hypertension with CSF diversion (17). Conversely, the evidence for the use of hyperosmolar therapies for intracranial hypertension is among the strongest for any topic within the guidelines. Based on two small RCTs (18, 19), the utility of hypertonic saline solutions to lower ICPs generated a level II recommendation that hypertonic saline “should” be considered. Although similar studies have not shown beneficial effects of mannitol, it has been used for decades for treatment of intracranial hypertension (20). Our findings reflect these differences in level of evidence. There was wide disparity in CSF diversion goals between the various centers—and even within clinicians within centers themselves. This is quite consistent with the lack of conclusive evidence of the effectiveness of CSF drainage within the literature—as proponents may argue for its therapeutic potential and others may be concerned with complications of device placement. On the other hand, our study demonstrated wide adoption of hyperosmolar therapies, with only a few centers opting to not use either mannitol or hypertonic saline solutions. The variability we observed in this aspect of TBI care was focused more on the wide variety of concentrations of hypertonic saline solutions which was surprising.
Hypoxia/Ischemia Prevention and Detection
Hyperventilation—which lowers ICP by lowering cerebral blood flow and thereby cerebral blood volume in regions with intact Co2 reactivity—has been among the more controversial topics in pediatric neurotrauma care. Although a common practice in older studies, Skippen et al (21) demonstrated a significant decrease of cerebral blood flow with incremental decreases in Paco2, and other studies suggested that hypocarbia may be associated with unfavorable outcome (22). In our survey, centers reported that they did not intentionally use hyperventilation during periods of normal ICP, but it is likely to be a goal that the centers strive to achieve. In the Hyp-HIT study, more than 40% of subjects in the study were severely hyperventilated to PaCo2 less than 30 mm Hg (9). For Pbo2 monitoring of cerebral hypoxia, a number of reports have been published describing the utility of this system in children investigations have emerged in the past several years. Figaji et al (23, 24) have published several series describing the integration of Pbo2 into the neuromonitoring milieu of children with severe TBI, and it appears that a threshold of Pbo2 may be possible to determine in a large series. Our survey reflects the adoption of this technique by several centers, but the penetration of use for this technology is still limited—likely reflecting the relatively small amount of information currently available.
Metabolic Support
Although believed to be extremely important for wound healing, growth, and providing energy for physiologic/pathological processes, evidence for providing metabolic support for children with severe TBI is still rudimentary. Within the guidelines, only a single RCT of 40 children who were fed an immune-enhanced diet was sufficiently rigorous to be included, and this study demonstrated that this diet was not associated with any meaningful outcomes (25). In adult TBI victims, the timing of nutritional support appears to play a role in outcomes (26). Specifically, in an audit of 797 adult TBI victims from New York State, those patients who did not get fed until the fifth or seventh day after trauma had a 2.4-fold and four-fold increase in mortality, respectively. Data from our survey suggest that the pediatric neurotrauma community is cognizant of this association, as most centers have the goal of starting nutritional support within the first several days after TBI. As for glucose, the data are similarly nebulous. Although several series have found that hyperglycemia is associated with adverse outcome in both the acute and more delayed time periods after TBI (27–29), there is little evidence to guide the clinician on when glucose administration should start and how hyperglycemia should be managed. This is reflected by the data from our survey, where the range of time for starting glucose administration and insulin usage is quite wide across centers.
Limitations and Implications
There are several limitations to this study. First, this survey was performed in a relatively small number of centers in the United States, United Kingdom, and Europe and the results may not reflect the pediatric neurotrauma community as a whole. However, the purpose of this survey was to determine the variations that currently exist in a number of important treatments that are standardly applied for children, and the centers within this study treat more than 500 such children each year. Second, the respondents stated that the answers they gave were reflective of the overall care of children at their center. Obviously, actual practices at centers might deviate significantly from the stated goals— either because patient care dictates that a given goal could not be achieved or based on variability of care provided by clinicians at the centers. Lastly, this survey cannot address which goals are associated with the best outcomes for patients—an obvious goal for any future study. We believe that a prospective, observational, and cohort study with sufficient statistical power and detailed data collection could inform the pediatric neurotrauma community on the relative effects of these strategies on patient outcomes.
In conclusion, clinicians reported marked differences in medical goals for children with severe TBI across our international consortium. We believe that our data have significant implications. First, we speculate that there are superior strategies to achieve the medical goals outlined within our survey and believe that a study such as the one outlined above may yield important advances for the field. Second, given our data, it is conceivable that the widespread variability in clinical goals across institutions would yield marked variations in patient outcomes at the various centers. This may explain, in part, the failure of multicentered RCTs—as the effect of variability in medical therapies outlined in our survey may lead to variations in outcomes that obscure observable treatment effects of experimental interventions. We believe that future studies that determine the superiority of the various medical therapies outlined within our survey would be a significant advance for the pediatric neurotrauma field.
ACKNOWLEDGMENTS
We acknowledge the invaluable assistance of Ms. Marci Provins in preparing this manuscript.
Dr. Bell (HD0499893, HD08003, and NS072308), Dr. Adelson (NS052478), Dr. Kochanek (NS070324, T32HD040686, W81XWH-09-2-0187, and W81XWH-10-0623), Dr. Vavilala (NS072308), Dr. Beers (MH56612 and MH085722), Dr. Fabio (CE001630), and Dr. Wisniewski (NS052478 and NS069247) received federal grants. Dr. Bell’s institution received grant support (HD0499893, HD08003, and NS072308). Dr. Bell received support for article research from NIH and HD0499893, HD08003, and NS072308. Dr. Adelson is employed by the Phoenix Children's Hospital and provided expert testimony for various medical legal cases. Dr. Adelson’s institution received grant support from Arizona Biomedical Research Commission, Baxter Foundation, and Codman. Dr. Hutchison is employed by SickKids, Toronto; provided expert testimony for CMPA; and lectured for the Japanese Intensive Care Society and as visiting professor. Dr. Hutchinson’s institution received grant support from Ontario Neurotranining, NIH, and CIHR. Dr. Kochanek received grant support from the National Institutes of Health (NIH), the US Army, DARPA, Laerdal Foundation, and AHA (NIH NS070324 and T32HD040686 US Army W81XWH-09-2-0187 and W81XWH-10-0623); is a co-provisional patent holder on 3 patents; and received support for article research from NIH and the US Army. Dr. Tasker received royalties from Oxford University Press (Oxford Handbook of Paediatrics). Dr. Vavilala received support for article research from NIH. Dr. Beers is employed by the University of Pittsburgh and University of Pittsburgh Physicians, provided expert testimony for various institutions, received royalties from John Wiley & Sons and Springer, and received support for travel from the National Academy of Neuropsychology (airfare to Board meeting). Dr. Beers’ institution received grant support from Family Pathways to Early Onset Suicide Attempt, Psychiatric Outcomes of Children with High- and Low-Risk for Depression, Oxidative Lipidomics in Pediatric Traumatic Brain Injury, TBI Biological Diagnosis via High Definition Tractography Asymmetry Screening, High Definition Fiber Tracking Biological Diagnosis of TBI Providing Actionable Clinical Report of Quantified Damage. Dr. Wisniewski consulted for Cyberonic Inc. (2005–2009); Ima RX, Therapeutics, Inc. (2006); Bristol-Myers Squibb Company (2007–2008); Organon (2007); Case-Western University (2007); Singapore Clinical Research Institute (2009); Dey Pharmaceuticals (2010); Venebio (2010); and Dey (2010) and received grant support from Eli Lilly (2012).
APPENDIX 1. THE MULTIPLE MEDICAL THERAPIES FOR PEDIATRIC TRAUMATIC BRAIN INJURY WORKGROUP (LISTED ALPHABETICALLY BY CENTER)
Laura Loftis, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX; Kevin Morris, Birmingham Children’s Hospital, Birmingham, UK; Kerri LaRovere, Boston Children’s Hospital and Harvard University, Cambridge, MA; Philippe Meyer, Centre Hospitalier Universitaire Necker Enfantes Malades, Paris, France; Karen Walson, Children’s Healthcare of Atlanta, Atlanta, GA; Jennifer Exo, Children’s Hospital of Colorado, Denver, CO; Ajit Sarnaik, Children’s Hospital of Michigan and Wayne State University, Detroit, MI; Todd Kilbaugh, Children’s Hospital of Philadelphia, Philadelphia, PA; Darryl Miles, Children’s Medical Center, Dallas, TX; Mark Wainwright, Children’s Memorial Hospital, Chicago, IL; Nathan Dean, Children’s National Medical Center, Washington, DC; Ranjit Chima, Cincinnati Children’s Hospital, Cincinnati, OH; Katherine Biagas, Columbia University Medical Center and the Children’s Hospital of New York Presbyterian, New York, NY; Mark Peters, Great Ormond Street Hospital and University College Hospital, London, UK; Joan Balcells and Joan Sanchez del Toledo, Hospital Vall d’Hebron, Barcelona, Spain; Courtney Robertson, Johns Hospital University, Baltimore, MD; Dwight Bailey, Lauren Piper and William Tsai, Levine Children’s Hospital of Carolinas Medical Center, Charlotte, NC; John Ragheb, Miami Children’s Hospital and University of Miami Miller School of Medicine, Miami, FL; Rachel Agbeko, Newcastle Upon Tyne Hospitals NHS Trust, Newcastle Upon Tyne, UK; Nicole O’Brien, Nationwide Children’s Hospital and Ohio State University, Columbus, OH; Amber Young, North Bristol NHS Trust, Bristol, UK; Neal Thomas, Pennsylvania State University, Hershey, PA; Sandra Buttram, Phoenix Children’s Hospital, Phoenix, AZ; Santiago Borasino, University of Alabama, Birmingham, AL; JoAnne Natale, University of California, Davis, Sacramento, CA; Christopher Giza, University of California, Los Angeles, CA; David Shellington, University of California, San Diego, CA; Deborah Stein, University of Maryland and R Adams Cowley Shock Trauma Center, Baltimore, MD; Robert Clark and Alicia Au, University of Pittsburgh, Pittsburgh, PA; Jerry Zimmerman, University of Washington, Seattle, WA; Jose Pineda, University of Washington, St. Louis, MO; and Peter Ferrazzano, University of Wisconsin, Madison, WI.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Corso P, Finkelstein E, Miller T, et al. Incidence and lifetime costs of injuries in the United States. Inj Prev. 2006;12:212–218. doi: 10.1136/ip.2005.010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faul MD, Xu L, Wald MM, et al. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Death 2002–2006. Atlanta GA: Centers for Disease Control and Prevention, National Center for Injury Prevention; 2010. [Google Scholar]
- 3.Stocchetti N, Conte V, Ghisoni L, et al. Traumatic brain injury in pediatric patients. Minerva Anestesiol. 2010;76:1052–1059. [PubMed] [Google Scholar]
- 4.Tasker RC, Fleming TJ, Young AE, et al. Severe head injury in children: Intensive care unit activity and mortality in England and Wales. Br J Neurosurg. 2011;25:68–77. doi: 10.3109/02688697.2010.538770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tude Melo JR, Di Rocco F, Blanot S, et al. Mortality in children with severe head trauma: Predictive factors and proposal for a new predictive scale. Neurosurgery. 2010;67:1542–1547. doi: 10.1227/NEU.0b013e3181fa7049. [DOI] [PubMed] [Google Scholar]
- 6.Zaloshnja E, Miller T, Langlois JA, et al. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 7.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the management of severe traumatic brain injury in infants, children and adolescents. Pediatr Crit Care Med. 2003;4(3) Suppl:S1–S73. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 8.Kochanek PK, Carney NA, Adelson PD, et al. American Academy of Pediatrics-Section on Neurological Surgery; American Association of Neurological Surgeons/Congress of Neurological Surgeons; Child Neurology Society; European Society of Pediatric and Neonatal Intensive Care; Neurocritical Care Society; Pediatric Neurocritical Care Research Group; Society of Critical Care Medicine; Paediatric Intensive Care Society; Society for Neuroscience in Anesthesiology and Critical Care; World Federation of Pediatric Intensive and Critical Care Societies: Guidelines for the acute medical management of severe traumatic brain injury in infants, children and adolescents: Second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 9.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group: Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 10.Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- 11.Clifton GL, Choi SC, Miller ER, et al. Intercenter variance in clinical trials of head trauma—Experience of the National Acute Brain Injury Study: Hypothermia. J Neurosurg. 2001;95:751–755. doi: 10.3171/jns.2001.95.5.0751. [DOI] [PubMed] [Google Scholar]
- 12.Segal S, Gallagher AC, Shefler AG, et al. Survey of the use of intracranial pressure monitoring in children in the United Kingdom. Intensive Care Med. 2001;27:236–239. doi: 10.1007/s001340000717. [DOI] [PubMed] [Google Scholar]
- 13.Dean NP, Boslaugh S, Adelson PD, et al. Physician agreement with evidence- based recommendations for the treatment of severe traumatic brain injury in children. J Neurosurg. 2007;107(5) Suppl:387–391. doi: 10.3171/PED-07/11/387. [DOI] [PubMed] [Google Scholar]
- 14.Keenan HT, Nocera M, Bratton SL. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatr Crit Care Med. 2005;6:537–541. doi: 10.1097/01.PCC.0000164638.44600.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilford JM, Simpson PM, Yeh TS, et al. Variation in therapy and outcome for pediatric head trauma patients. Crit Care Med. 2001;29:1056–1061. doi: 10.1097/00003246-200105000-00037. [DOI] [PubMed] [Google Scholar]
- 16.Morris KP, Forsyth RJ, Parslow RC, et al. UK Paediatric Traumatic Brain Injury Study Group. Paediatric Intensive Care Society Study Group: Intracranial pressure complicating severe traumatic brain injury in children: Monitoring and management. Intensive Care Med. 2006;32:1606–1612. doi: 10.1007/s00134-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 17.Jagannathan J, Okonkwo DO, Yeoh HK, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2:240–249. doi: 10.3171/PED.2008.2.10.240. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4:4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Simma B, Burger R, Falk M, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: Lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26:1265–1270. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 20.Wise BL, Chater N. Use of hypertonic mannitol solutions to lower cerebrospinal fluid pressure and decrease brain bulk in man. Surg Forum. 1961;12:398–399. [PubMed] [Google Scholar]
- 21.Skippen P, Seear M, Poskitt K, et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402–1409. doi: 10.1097/00003246-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 22.Curry R, Hollingworth W, Ellenbogen RG, et al. Incidence of hypoand hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines. Pediatr Crit Care Med. 2008;9:141–146. doi: 10.1097/PCC.0B013e318166870e. [DOI] [PubMed] [Google Scholar]
- 23.Figaji AA, Fieggen AG, Argent AC, et al. Does adherence to treatment targets in children with severe traumatic brain injury avoid brain hypoxia? A brain tissue oxygenation study. Neurosurgery. 2008;63:83–91. doi: 10.1227/01.NEU.0000335074.39728.00. [DOI] [PubMed] [Google Scholar]
- 24.Figaji AA, Zwane E, Thompson C, et al. Brain tissue oxygen tension monitoring in pediatric severe traumatic brain injury. Part 1: Relationship with outcome. Childs Nerv Syst. 2009;25:1325–1333. doi: 10.1007/s00381-009-0822-x. [DOI] [PubMed] [Google Scholar]
- 25.Briassoulis G, Filippou O, Kanariou M, et al. Temporal nutritional and inflammatory changes in children with severe head injury fed a regular or an immune-enhancing diet: A randomized, controlled trial. Pediatr Crit Care Med. 2006;7:56–62. doi: 10.1097/01.pcc.0000192339.44871.26. [DOI] [PubMed] [Google Scholar]
- 26.Härtl R, Gerber LM, Ni Q, et al. Effect of early nutrition on deaths due to severe traumatic brain injury. J Neurosurg. 2008;109:50–56. doi: 10.3171/JNS/2008/109/7/0050. [DOI] [PubMed] [Google Scholar]
- 27.Chiaretti A, Piastra M, Pulitanò S, et al. Prognostic factors and outcome of children with severe head injury: An 8-year experience. Childs Nerv Syst. 2002;18:129–136. doi: 10.1007/s00381-002-0558-3. [DOI] [PubMed] [Google Scholar]
- 28.Michaud LJ, Rivara FP, Grady MS, et al. Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery. 1992;31:254–264. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Smith RL, Lin JC, Adelson PD, et al. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012;13:85–91. doi: 10.1097/PCC.0b013e3182192c30. [DOI] [PMC free article] [PubMed] [Google Scholar]