Abstract
The Study to Explore Early Development (SEED), a multisite investigation addressing knowledge gaps in autism phenotype and etiology, aims to: (1) characterize the autism behavioral phenotype and associated developmental, medical, and behavioral conditions and (2) investigate genetic and environmental risks with emphasis on immunologic, hormonal, gastrointestinal, and sociodemographic characteristics. SEED uses a case–control design with population-based ascertainment of children aged 2–5 years with an autism spectrum disorder (ASD) and children in two control groups—one from the general population and one with non-ASD developmental problems. Data from parent-completed questionnaires, interviews, clinical evaluations, biospecimen sampling, and medical record abstraction focus on the prenatal and early postnatal periods. SEED is a valuable resource for testing hypotheses regarding ASD characteristics and causes.
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders characterized by qualitative impairments in social interaction and communication, and repetitive stereotyped behaviors and interests. Although previously reported to be rare (e.g., prevalence of 1–4 per 10,000 children), a number of systematic population surveys and routine monitoring systems in the United States and other countries since the 1990s have indicated the childhood prevalence to be from 0.7% to 1% (Fombonne 2009; CDC 2009). In comparison, the childhood prevalence of intellectual disability typically ranges from 1%–2% (Leonard and Wen 2002) and 13% of school-aged children in the United States have some type of developmental disability (Boulet et al. 2009). Changes in diagnostic criteria and development of improved clinical methods of autism screening and diagnosis have accompanied improvement in population-based monitoring of ASD prevalence. Yet, apart from the identification of some rare genetic conditions that commonly are associated with ASDs, causal mechanisms for this spectrum of disorders remain largely unknown.
The Study to Explore Early Development (SEED) was designed by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) Network to address gaps in understanding of the ASD phenotype and etiology. The CADDRE Network comprises study sites in six states (California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania), a data coordinating center (DCC) (in Michigan), and a central laboratory and biosample repository (CLBR) (in Maryland). SEED was conceived to be among the largest multisite epidemiologic investigations of multiple genetic and environmental (broadly defined as nongenetic) risk factors and causal pathways contributing to different ASD phenotypes. SEED’s primary scientific aims were to: (1) characterize the ASD behavioral phenotype and associated developmental, medical, and behavioral conditions, with a special focus on identifying distinct symptom profiles to guide etiologic analysis, and (2) investigate genetic and environmental risk factors, with emphasis on immunologic, hormonal, gastrointestinal, and sociodemographic characteristics.
The purpose of this paper is to provide details about SEED’s:
Scientific background, including its specific aims and the knowledge gaps it was designed to fill;
Study design and implementation, with a focus on methodological features that might be informative for the design of other ASD investigations; and
Strengths and expected contributions to the understanding of the ASD phenotype and associated risk factors.
SEED Scientific Background and Aims
ASD Behavioral Phenotype and Associated Conditions Addressed in SEED
Although the ASD behavioral phenotype is defined by deficits in three core behavioral domains, current diagnostic subtypes within the autism spectrum are distinguished by the number and level of impairments across the three domains. Adding to this diagnostic complexity are other developmental features that affect phenotypic diversity (such as cognitive ability and developmental trajectory), including the timing of achievement of behavioral milestones or appearance of a developmental plateau, or even the loss of acquired language or social skills, or both (McGovern and Sigman 2005; Rogers 2004; Turner et al. 2006). Further, other developmental (e.g., intellectual disability and inattention), medical (e.g., epilepsy and gastrointestinal, sensory, and sleep abnormalities), behavioral (e.g., aggression and hyperactivity), and psychiatric (e.g., attention-deficit/hyperactivity disorder, anxiety, and depression) conditions commonly co-occur with an ASD, thereby adding further complexity to the clinical phenotype (Gillot et al. 2001; Levy et al. 2010; Polimeni et al. 2005; Spence and Schneider 2009; Yeargin-Allsopp et al. 2003). Whether these conditions arise independently of ASDs as a consequence of the core ASD deficits or as an endophenotype that predisposes a child to have an ASD or can be attributed to the underlying neuropathologic abnormalities is not clear. Individuals with an ASD are more likely than the general population to have major congenital anomalies, especially those individuals with a co-occurring intellectual or other disability (Hultman et al 2002; Schendel et al 2009; Wier et al 2006). Children with autism who have dysmorphic features also are more likely to have a known genetic syndrome or a structurally abnormal brain, compared with children with autism who do not have dysmorphic features (Miles and Hillman 2000). A number of genetic, neurologic, and metabolic conditions have been identified as either causative for an ASD (in about 10% of cases) or related to ASD-like characteristics (Cohen et al. 2005; Fombonne 2003; Zecavati and Spence 2009). The pathogenetic mechanisms that link these conditions to expression of the ASD behavioral phenotype are not known. Key aims for SEED regarding the phenotypic profile are to derive quantitative and qualitative descriptions, investigate which aspects might be unique to ASDs, map the diversity of ASDs into consistent patterns of symptoms, and investigate the association of symptom profiles with underlying risk factors for ASDs.
ASD Risk Factors Addressed in SEED
The extreme heterogeneity of behavioral, developmental, and associated medical features across the autism spectrum suggests that multiple causal factors, perhaps producing separate but overlapping phenotypic profiles, contribute to ASDs. Evidence of a genetic component in ASDs was established from studies indicating higher pairwise concordance among monozygotic (36%–95%) than among dizygotic (0%–31%) twin pairs (Bailey et al. 1995; Folstein and Rutter 1977; Ronald et al. 2006; Rosenberg et al. 2009; Taniai et al. 2008; Hallmeyer et al. 2011) and a 2%–18% recurrence rate among siblings of children with an ASD (compared with a rate of 0.7%–1% among the general population) (Chakrabarti and Fombonne 2001; Icasiano et al. 2004; Lauritsen et al. 2005; Sumi et al. 2006; Ozonoff et al. 2011). Relatives of individuals with an ASD also might have qualitatively similar but less severe variations of the core features of ASDs, now referred to as the broader autism phenotype (Piven et al. 1997). Strong associations have been found between ASD and a variety of single-gene and chromosomal abnormalities such as tuberous sclerosis and fragile X, Rett, and Down syndromes (DiGuiseppi et al. 2010; Hall et al. 2008; Kielinen et al. 2004; Wulffaert et al 2009; Wassink et al. 2001; Wiznitzer 2004). Recent findings have implicated particular genes, as well as both inherited and de novo copy number variants (Freitag et al. 2010; Bremer et al. 2010). Yet, the fact that monozygotic twin concordance is less than 100% suggests that factors other than DNA sequence might play a causative role, thereby possibly contributing to the recent reports of increases in ASD prevalence.
Specific environmental factors that have been associated with ASDs include prenatal exposure to thalidomide and valproate (Moore et al. 2000; Rasalam et al. 2005; Stromland et al. 1994). Less specific markers of possible prenatal risk include associations with preterm birth, low birth weight or older parental age and obstetric conditions such as bleeding or general indices of a suboptimal perinatal situation (Gardener et al. 2009; Kolevzon et al. 2007; Moster et al. 2008; Schendel and Bhasin 2008; Stein et al. 2006; Durkin et al. 2008; King et al. 2009), although it is unknown if adverse fetal or obstetric characteristics are causal, or reflect epiphenomenon of autism or increased familial liability (Zweigenbaum et al. 2002). Various prenatal viral infections and elevated rates of postnatal childhood infection also have been associated with ASDs (Atladottir et al. 2010a, b; Chess et al. 1978; Deykin and MacMahon 1979; Ivarsson et al. 1990; Rosen et al. 2007; Sweeten et al. 2004). While available evidence does not support a causal link between ASDs and the ethyl mercury-based vaccine preservative thimerosal (DeStefano, 2007; Gerber and Offitt 2009; Price et al. 2010), there is growing evidence that exposure to chemicals in the environment might contribute to ASD risk (Kalkbrenner et al. 2010; Roberts et al. 2007; Volk et al. 2010; Windham et al. 2006), possibly mediated through immune or hormonal pathways, or both.
A variety of immune system abnormalities involving cytokines, immunoglobulins, inflammation, cellular activation, and autoimmunity have been reported in association with ASDs (Ashwood et al. 2006; Goines and Van de Water 2010; Pardo et al. 2005), suggesting that immune dysfunction might play an etiologic role. A possibly separate but related immune pathway is based on reported elevated rates of gastrointestinal symptoms and immunopathology among children with an ASD that might lead to a heightened systemic immune response and neuroinflammation and damage (Ashwood et al.2004; Ashwood et al. 2006; Jyonouchi et al. 2005). Hormonal influences also have been implicated in ASD etiology. A key indicator is the male sex bias associated with ASDs and accompanying hypotheses for a prenatal imbalance in sex hormones, especially testosterone levels (Baron-Cohen et al. 2005). A potential role for abnormal maternal reproductive hormone function is reflected in associations with older maternal age (Durkin et al. 2008; King et al. 2009) and, less consistently, with maternal assisted reproduction (Hvidtjørn et al. 2009; Hvidtjørn et al. 2011). Other potential hormonal candidates include abnormal maternal prenatal thyroid and stress hormone levels and exogenous exposures to pitocin or oxytocin for labor induction (Beversdorf et al. 2005; Colborn 2004; Gale et al. 2003; Li et al. 2009; Soldin et al. 2003; Wahl 2004).
Neural, immune, and endocrine functions are integrated closely, both chemically and structurally, and work synchronously in the regulation of central nervous system and peripheral organ development and function. The molecular constituents of these systems (e.g., neurotransmitters, cytokines, and hormones), which play important roles in normal development, have pleiotropic functions and, therefore, potentially have widespread effects on neuronal function, including behavioral effects. Perturbation in any one system during development—through intrinsic abnormalities or from external exposures—can initiate a cascade of events with adverse neurodevelopmental consequences. Further, many genetic candidate genes for ASDs include those involved in central nervous system development, function and regulation (e.g., reelin, serotonin transporter gene, and brain derived neurotrophic factor, and immune (e.g., complement C4B) and hormonal expression (e.g., oxytocin receptor) (Abrahams and Geschwind 2008; Ashwood et al. 2006).
Thus, the growing body of evidence suggesting an etiologic role for one or more neural, immune or infectious, or hormonal factors in ASDs is the foundation for selecting these areas as the primary risk factors of interest for SEED.
Finally, there are disparities in ASD prevalence across racial and ethnic groups. These include lower reported rates among Hispanic and non-Hispanic Black or African-American children compared with non-Hispanic White children (CDC 2009; Windham et al. 2011), higher reported rates among Somali children in the United States (Minnesota Department of Health 2009), higher rates among immigrant populations in Northern European countries (Hultman et al. 2002; Maimberg and Vaeth 2006), and variations in ASD prevalence by socioeconomic status (defined by household education and income) (Durkin et al. 2010). These observations suggest that sociodemographic factors might be important markers of risk for ASDs through unrecognized links to underlying etiologic factors. On the other hand, some of these disparities might reflect differences in ascertainment and diagnosis of affected people among different racial and ethnic groups.
SEED Study Design and Implementation Methodology
Study design
A case–control study design was adopted for SEED with multiple-source, population-based ascertainment of case and comparison groups. Case patients (ASD) were those children meeting study definitions for several ASDs (autistic disorder, pervasive developmental disorder-not otherwise specified, and Asperger syndrome). Two different control groups were defined for SEED: a group of children sampled from the general population (POP) and a group of children with developmental delays or disorders other than ASDs (DD). Comparisons between the ASD and POP groups were designed to identify risk factors in children with ASDs relative to children among the general population. Future comparisons between the ASD and DD groups might provide the opportunity to distinguish risk factors for ASDs independent of risks common to other developmental disorders. The DD group also might provide a means of controlling for recall bias that might be associated with having a child with developmental problems.
Study population and eligibility
The population characteristics of each SEED recruitment catchment area are described in Table 1. The use of multiple catchment areas across the United States has provided a study population characterized by diverse racial, ethnic, and socioeconomic distributions. [Place Table 1 about here]
Table 1.
Population characteristics of the Study to Explore Early Development catchment areas
| California | Colorado | Georgia | Maryland | North Carolina | Pennsylvania | |
|---|---|---|---|---|---|---|
| Catchment Area | 2 Counties, San Francisco Bay Area | 7 Counties, Denver | 5 Counties, metropolitan Atlanta | 7Jurisdictions, northeast Maryland | 10 Counties, central North Caroline | 3 Counties, Philadelphia |
| Live births/yr (N) (2005) | 47,454 | 40,253 | 53,276 | 35,037 | 36,964 | 36,663 |
| Maternal race/ethnicity (%) | ||||||
| Black/African | 6.7 | 5.9 | 38.2 | 35.7 | 23.2 | 32.3 |
| American | ||||||
| White | 59.1 | 89.0 | 55.1 | 61.5 | 71.4 | 49.3 |
| Non-Hispanic | 25.6 | 55.5 | 32.3 | 56.2 | 53.6 | 46.1 |
| Hispanic | 33.5 | 33.5 | 22.7 | 5.3 | 17.8 | 2.9 |
| Asian | 16.6 | 4.5 | 6.2 | 5.1 | 4.7 | 6.3 |
| Mixed/Other | 14.5 | 0.6 | 0.1 | 0.0 | 0.7 | 9.3 |
| Unknown | 3.1 | 0.0 | 0.5 | 0.0 | 0.0 | 2.8 |
| Non-Hispanic | 65.8 | 65.9 | 70.5 | 94.3 | 82.0 | 85.6 |
| Hispanic | 34.2 | 34.1 | 22.7 | 5.7 | 18.0 | 12.9 |
| Maternal education | ||||||
| < 12 years | 20.0 | 22.9 | 22.0 | 14.5 | 23.2 | 19.2 |
| 12 years | 20.6 | 24.5 | 27.3 | 27.1 | 22.0 | 25.4 |
| >12 and < 16 years | 18.1 | 15.1 | 18.4 | 18.8 | 18.4 | 23.0 |
| >= 16 years | 41.3 | 34.9 | 32.3 | 38.6 | 36.1 | 31.2 |
| Proportion of children under 18 years living in poverty (%) | 12.2 (2005–07) | 15.7 (2005) | 17.6 (2005–07) | 14.9 (2005) | 20.1 (2005) | 22.1 (2005–07) |
Children were eligible for SEED if: (1) they were born in a study catchment area during the period from September 1, 2003, through August 31, 2006; (2) resided there at the time of first study contact; and (3) lived with a knowledgeable caregiver (family member or caregiver at least 18 years of age at enrollment who had resided with and consistently cared for the child since he or she was 6 months of age or younger) who was competent to communicate orally in English (or, at two sites, in English or Spanish) and gave consent. Enrolled children also had to be between 30–68 months of age at the completion of the in-person clinical developmental assessment to maintain the appropriate age range for validated study instruments. Children with circumstances (e.g., adoption) that prevented access to birth certificates or legal consent were not eligible to participate.
Ascertainment and Enrollment
Ascertainment of ASD and DD children
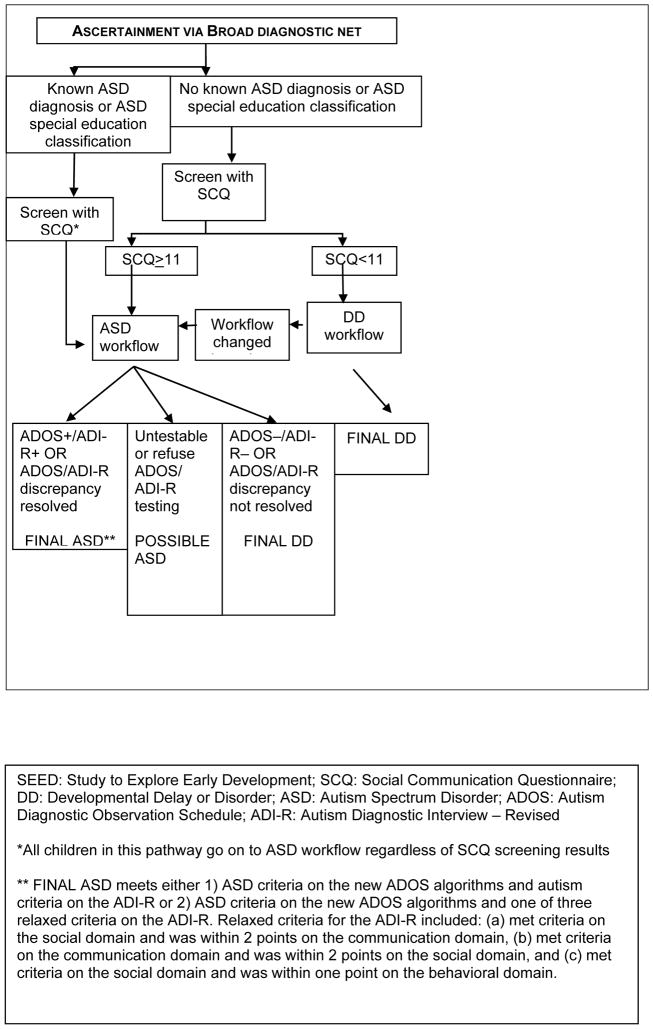
Both potential ASD and DD children were ascertained through multiple sources serving or evaluating children with developmental problems, including early intervention, special education, and related service programs for toddlers and young children; hospitals; clinics; and individual providers. Potential participants had to have received an ASD or related diagnosis (e.g., conduct disorder, intellectual disability, or significant developmental delay) from a clinical provider, or received early intervention or special education services for an ASD or related condition (e.g., intellectual disability or severe emotional disorder). This “broad diagnostic net” for ascertainment of potential ASD children ensured that both previously diagnosed and undiagnosed ASD children were identified (a full list of broad net diagnoses is available upon request). Parents who had a child with a documented ASD or ASD-related diagnosis also might have contacted the study directly and the child enrolled if eligible. [Place Figure 1 about here]
Figure 1.
SEED recruitment and final classification of ASD and DD participants
Ascertainment of POP children
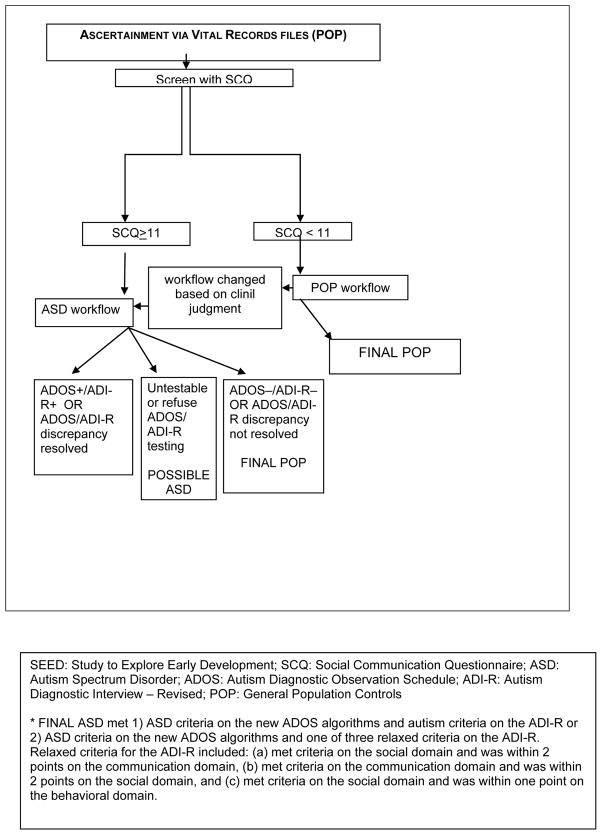
POP children were identified by randomly sampling state vital records of children born in the specified birth date range to mothers resident in a study catchment area at delivery. The number of POP children sampled by site was based on very conservative estimates of the expected enrollment and completion rates of invited POP families with the aim of obtaining a final 1:1ratio of complete ASD and POP families. Thus, each site randomly selected an oversample of POP children that ranged from about 20- to 90-fold higher than the final target sample size and randomized this total oversample into smaller batches for study invitation. Birth records are linked to state death certificate files to remove deceased children from the contact list. [Place Figure 2 about here]
Figure 2.
SEED recruitment and final classification of POP participants
Invitation to potential participants and assessment of eligibility
Invitations to participate in SEED were issued first in the autumn 2007. An invitation packet, written in English (and Spanish at the Colorado and California sites), was mailed to the primary caregiver of all potentially eligible participants. The name Study to Explore Early Development was intentionally designed to avoid the use of the word “autism,” in order to reduce participation bias by families with a particular interest in autism. The invitation described broad study objectives and provided an informational brochure, contact information, cash or a small toy of nominal value, and a prepaid response card to accept or decline further contact. Invitees who returned a positive response card received an invitation telephone call to assess their eligibility for the study (using previously cited criteria). Nonresponders received a second invitation letter in 2–6 weeks (at four sites) or a follow-up invitation telephone call (at two sites: nine attempts over different days of the week and times of day, leaving messages on most attempts). If the invitee expressed willingness to hear more about the study during an invitation call, the study staff proceeded to assess the invitee’s eligibility. To date, among a total of 22,100 combined ASD and DD families and 24,646 POP families sampled for whom the invitation process has been finalized: 64% of combined ASD and DD families and 68% of POP families never responded to the written invitation or invitation call (in 76% of combined ASD and DD families and 80% of POP families who never responded, the accuracy of the contact information was unknown); a combined 9% of ASD and DD children and 12% of POP children exceeded the eligible age limit for the standardized study instruments before contact could be made; and 26% (N=5,678) of combined ASD and DD families and 21% (N=5,064) of POP families were contacted by telephone (either the invitation call to assess study eligibility following a positive response card or, at some sites, the study invitation call for nonresponders). Among invited families for whom telephone contact was made, 34% of combined ASD and DD families and 40% of POP families refused; 21% of combined ASD and DD families and 34% of POP families were determined to be ineligible; and 43% of combined ASD and DD families and 25% of POP families consented to participate.
Screening for ASD
At the end of the study eligibility assessment call, the Social Communication Questionnaire (SCQ), a 5–10 minute interview, was administered to the primary caregiver of all eligible participants to identify children who required clinical diagnostic evaluation to determine final ASD status. Although the published SCQ was validated for children 4 years of age or older, several recent studies have found that a reduced cut-off score improves the sensitivity and specificity at younger ages, maximizing them at a cut-off score of 11 among children younger than 4 years of age (Lee et al 2007; Wiggins et al. 2007; Allen et al. 2006). For SEED, a positive screen was defined as an SCQ score ≥11. Regardless of ascertainment source (i.e., “broad diagnostic net” or vital records), any eligible children who had a positive screen or a previous ASD diagnosis or who were receiving special education services for an ASD were assigned to the ASD workflow which determined which instruments were administered and the type of diagnostic evaluation the child received during the data collection phase. DD and POP children with negative SCQ screens were assigned to the DD or POP workflow, respectively. If, during the clinical evaluation of a child in the DD or POP workflow, the clinician suspected an ASD, the child immediately was moved into the ASD workflow.
To date, 34% of children in the ASD workflow who had completed the clinical evaluation did not have a previous ASD diagnosis, but were assigned to the ASD workflow after a positive SCQ screen (32%) or clinical suspicion after a limited developmental evaluation (2%). Eighty-three percent of children in the ASD workflow without a previous ASD diagnosis were ascertained via the “broad diagnostic net” and 17% were ascertained via vital records (of whom 42% and 22%, respectively, received an ASD final classification; see final classification description below). It is expected that a larger proportion of children with a positive SCQ and consequent direction into the ASD workflow would be ascertained on the basis of a diagnosis of a developmental delay or disability (the “broad diagnostic net”) than would be children ascertained from the general population (via vital records), although there are no published date on the yield of the SCQ screen comparable to these results because of differences in ascertainment methods and ages of participant children.
Data Collection
Extensive data were collected via multiple ways to address the SEED scientific aims. Table 2 lists each instrument and the version used, with references, and the mode of collection, target participant, primary purpose, and analytic domains addressed. Data collection was uniform across all study groups except that those in the ASD workflow completed additional instruments to assess specific ASD features (see Table 2). Participants in the DD or POP workflow completed the Vineland Adaptive Behavior Scales if they scored <1.5 standard deviations below the mean on the Mullen Scales of Early Learning (i.e., an Early Learning Composite score of 78 points or less). [Place Table 2 about here]
Table 2.
Study to Explore Early Development data collection
| Data collection | Reference; version | Collection mode (time to complete) | Participanta | Primary purpose for SEED | Analytic domainsb |
|---|---|---|---|---|---|
| Autism-related assessments | |||||
| Social Communication Questionnaire (SCQ) | Rutter et al. 2003; 2003 WPS Current Version | Telephone (10 min) | C | Screen for autism spectrum disorders | B |
| Autism Diagnostic Observation Scale (ADOS) | Lord et al. 1999; Lord et al. 2000; Gotham et al, 2007; 2001 WPS Version | Clinic (40 min) | C (ASD) | Assess ASD symptoms; determine final ASD status | B |
| Autism Diagnostic Interview-Revised (ADI-R) | Lord et al. 1994; Rutter et al. 2003; 2003 WPS Version | Clinic (120 min) | C (ASD) | Assess ASD symptoms; determine final ASD status | B |
| Ohio State University (OSU) Autism Rating Scale (OARS) | OSU Research Unit, 2005; adapted for SEED | Staff review (n/a) | C (ASD) | Assess ASD symptoms and comprehensive clinical impression | B |
| Early Development Questionnaire (EDQ) | Ozonoff et al. 2005; Version 3 | Filled out by parent (20 min) | C (ASD) | Assess regression of language and social skills, and functional behavior | B |
| Social Responsiveness Scale (SRS) | Constantino 2002; 2006 WPS Preschool Version & 2005 WPS Adult Version | Filled out by parent (15–30 min) | C, M, F | Assess social behavior | B |
| Other behavioral and developmental assessments | |||||
| Mullen Scales of Early Learning | Mullen, 1995; 1995 AGS/Pearson Version | Clinic (45 min) | C | Assess cognitive function | B |
| Vineland Adaptive Behavior Scales | Sparrow et al. 1984; 2005 AGS/Pearson Version | Clinic (45 min) | C | Assess adaptive behavior | B |
| Child Behavior Checklist (CBCL) | Achenbach, 1992; 2000 ASBE Version | Filled out by parent (15 min) | C | Assess behavior problems and social competencies | B |
| Carey Temperament Scale | Carey and McDevitt, 1995; 1995 Behavioral /Developmental Initiatives Version, | Filled out by parent (10 min) | C | Assess temperament | B |
| Health and medical information | |||||
| Physical examination | CADDRE-derived | Clinic (15 min) | C | Anthropometry and dysmorphology exam | D |
| Services and treatments questionnaire | CADDRE-derived | Filled out by parent (5 min) | C (ASD) | Assess types and frequency of current services and treatments | B |
| Child Sleep Habits Questionnaire | Owens 2000 | Filled out by parent (10 min) | C | Assess behaviors and medical disorders of sleep | B |
| Gastrointestinal questionnaire | CADDRE-derived | Filled out by parent (10 min) | C | Assess past and current gastrointestinal disturbances | GI |
| Paternal medical history form | CADDRE-derived | Self-administered (10 min) | F | Assess medical and psychiatric conditions | B |
| Maternal medical history form | CADDRE-derived | Self-administered (10 min) | M | Assess medical and psychiatric conditions | B, H, I |
| Autoimmune disease survey | CADDRE-derived | Self-administered (20 min) | Nuclear family | Assess history of autoimmune disease | I |
| Medical records for the preconceptionc, prenatal, and labor/delivery periods | CADDRE-derived | Staff review (n/a) | M | Prenatal, perinatal, and postnatal medical information related to risk factors of interest | S,H,I,GI,O |
| Medical records for the neonatal and pediatric3 periods | CADDRE-derived | Staff review (n/a) | C | Prenatal, perinatal-, and postnatal medical information related to risk factors of interest | S,H,I,GI,O |
| 7-day stool diary | Bristol Stool Form Scale (Lewis, 1997) | Filled out by parent (40 min) | C | Assess bowel movement and stool patterns | GI |
| 3-day diet diary | CADDRE-derived | Filled out by parent (20 min) | C | Assess diet patterns during stool diary period | GI |
| Biologic specimens | |||||
| Buccal cells (cheek swab) | Kit mailed to family (15 min) | C, M, F | For analysis of genetic biomarkers | G | |
| Blood | Clinic (15 min) | C, M, F | For analysis of genetic and non-genetic biomarkers | H,I,G,E | |
| Hair | Clinic (1 min) | C | For analysis of environmental exposures | E | |
| Guthrie card bloodspots | Clinic (4 min) | C | For analysis of genetic and non-genetic biomarkers | H,I,G,E | |
| Other data | |||||
| Paternal occupational history form | CADDRE-derived | Self-administered (5 min) | F | Paternal occupational history during index pregnancy | E |
| Multidimensional | |||||
| Birth certificate | Data linkage | C | S | ||
| Caregiver interview | CADDRE-derived | Telephone (60 min) | M | Biologic mother as informant: biologic mother’s full reproductive and pregnancy history and diagnosed developmental outcomes in all children; medical, therapeutic, obstetric and lifestyle characteristics of pregnancy (considered 3 months before conception through breastfeeding) with index child (including infertility treatments of either biological parent); maternal occupational history during index pregnancy | B,GI,H,I,O,E, S |
| Biologic mother or other primary caregiver as informant: index child’s postnatal developmental and medical history; parent/caregiver demographics; | |||||
C = Child M = Mother F = Father
B = Behavioral Phenotype; D = Dysmorphology; GI = Gastrointestinal disorders; H = Hormonal; I = Infection and immune function; O = Obstetric; G = Genetic; E = Environmental; S = Sociodemographic
Preconception records consist of specialists for infertility, immune, and psychiatric conditions in 3 years before conception; Pediatric records consist of pediatrician and specialists records in 3 years after birth (pediatric genetics clinic records consist of all evaluations prior to the date of the Study to Explore Early Development physical examination)
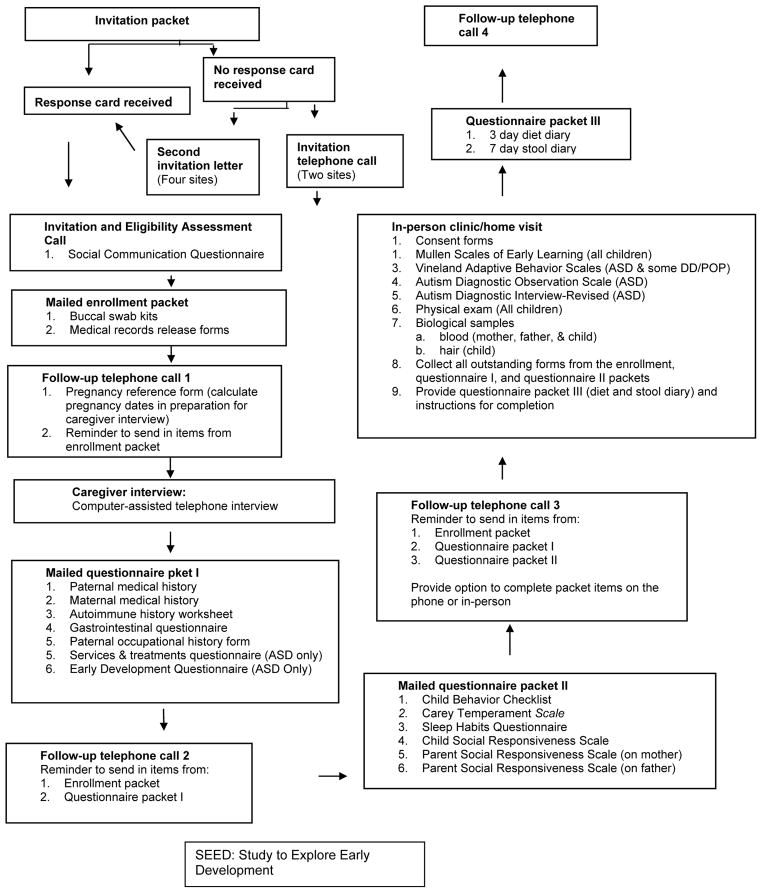
Due to the large number of items and the complexity of the data collection protocol, a number of strategies were incorporated to facilitate implementation and completion. Data collection was organized into multiple stages (Figure 3) to more evenly distribute the study burden over time, as well as to enhance the completion of data collection at one stage before proceeding to the next. The stages are made up of the mailed enrollment packet, three different packets of self-administered forms, a telephone-assisted caregiver interview, and one or two in-person visits (at a clinic or, by one site, offered at the family’s home). Scheduling of the stages was determined with input from participants to enhance compliance. Support in completing self-administered forms was provided as needed to overcome literacy barriers. Periodic contacts by telephone, email, or biannual newsletter served as reminders for data item completion or scheduled data events.
Figure 3.
SEED participant data collection steps
Quality Control for Data Collection
The need for appropriate staff training and oversight, including systematic quality control (QC), was considered carefully for each contact with participants. QC steps included a variety of QC training exercises, initial “in-the-field” QC standards, and ongoing QC standards. For all instruments, high thresholds were set for “acceptable” QC, so that even slight variations from established protocols could be identified early and remediation strategies implemented (such as retraining and continued monitoring until acceptable standards were reached). QC details and results to date for each instrument or contact are presented in Table 3. [Place Table 3 about here]
Table 3.
Study to Explore Early Development quality control procedures and results
| Study contacts and instruments | Type of quality control assessment(s) and requirements | Specific quality control training requirementsa | Ongoing quality control (frequency) | Results ongoing quality controlb |
|---|---|---|---|---|
| Invitation telephone call, including eligibility screener and Social Communication Questionnaire | Semiqualitative call rating form—a priori criteria established for acceptable score.c | Acceptable scores on 4 role-playing (mock) calls and first 2 “live” calls. | 5% per interviewer | 88% acceptable rating scores overall, with improvements over time; >90% after first 6 months. |
| Follow-up call, including structured pregnancy dates questionnaire for caregiver interview | Semiqualitative call rating form—a priori criteria established for acceptable score.c | Acceptable scores on 3 role playing (mock) calls. | 5% per interviewer | 93% acceptable rating scores overall, with improvements over time; >90% after first 6 months and 100% after first 12 months. |
| Caregiver interview |
|
Acceptable scores for both assessments on 3 role-playing (mock) interviews and first 2 “live” calls. | 5% per interviewer | 99% acceptable call rating scores; 98% acceptable inter-rater reliability assessments throughout study. |
| Questionnaire packets I, II, and III (self-administered forms)d | No specific systematic QC requirements, but continual supervisor oversight and all forms reviewed for missing or illegible data or contradictory entries. Participants are recontacted as needed. | None. General training provided on forms and appropriate responses to participant queries. | NA | NA |
| Autism Diagnostic Observation Schedule (ADOS) | Intersite: Supervising clinicians establish reliability by scoring the same ADOS exam videotapes. Acceptable score is ≥80% concordance on algorithm items. Intrasite: All clinicians establish reliability with supervising clinician. Acceptable score is ≥80% concordance on algorithm items | Both intersite and intrasite reliability established in advance of study start. | Quarterly intersite and intrasite reliability exercises | Intersite: 99% acceptable scores on “first pass” quarterly exercises and 100% acceptable scores on “second pass” Intrasite: 99% acceptable scores on “first pass” quarterly exercises and 100% acceptable scores on “second pass”. |
| Autism Diagnostic Interview-Revised (ADI-R) | Intersite: Supervising clinicians establish reliability by scoring the same ADI-R interview videotapes. Acceptable score is ≥90% concordance on algorithm items. Intrasite: All clinicians establish reliability with supervising clinician. Acceptable score is ≥90% concordance on algorithm items. | Both intersite and intrasite reliability established in advance of study start. | Quarterly intersite and intrasite reliability exercises. | Intersite: 99% acceptable scores on “first pass” quarterly exercises and 100% acceptable scores on “second pass”. Intrasite: 87% acceptable rating scores on “first pass” quarterly exercises and 100% acceptable scores on “second pass”. |
| Mullen Scales of Early Learning | No specific systematic QC requirements, but continual supervisor oversight and all forms reviewed for missing or illegible data or contradictory entries. | 5 clinicians monitor initial assessments until competency determined. | NA | NA |
| Vineland Adaptive Behavioral Scales | No specific systematic QC requirements, but continual supervisor oversight and all forms reviewed for missing or illegible data or contradictory entries. | None. Supervising site clinicians monitor initial assessments until competency determined. | NA | NA |
| Dysmorphology physical examination: examination, photography, and anthropometric measurements. | Intersite: Examiners review common set of photos and compare measurements. Acceptable score is ≥80% concordance. Intrasite: Examiners certified as meeting acceptable levels of reliability (80% or higher depending on component) on performance standards in several areas. | Acceptable intrasite scores in all areas on 5 practice examinations and first 3 “live” examinations. | Intersite: 1 exercise /month Intrasite: 10% per examiner | Intersite: 100% acceptable scores on monthly exercises. Intrasite: 95% acceptable scores. |
| Biologic specimens: buccal swabs and blood specimens (child, mother, father) and hair specimen (child). | All: Central laboratory staff processes specimens upon receipt and performs preliminary QC (gross visual inspection). Sample of participants: Second blood specimen obtained for duplicate processing and analysis | None. Extensive staff training on study protocol for obtaining and processing biologic specimens | 2% sample of duplicate blood specimens | Will assess analyte concordance (e.g., genotypes) among duplicates |
| Medical record abstraction (4 forms: prenatal, labor and delivery, neonatal, pediatric). | Intersite: Common reliability abstraction exercises, each focusing on different sections/ forms. Acceptable score is ≥90% concordance across sites. Intrasite: Quantitative inter-rater reliability assessment of selected items on each form. Acceptable score is ≥90% concordance. | Acceptable intrasite scores on first 2 “live” abstractions for each form type (8 total abstractions). | Intersite: Quarterly reliability exercises. Intrasite: 5% per abstractor (across form types) | Intersite: Exercises revealed minor inconsistencies in abstraction process; no major substantive differences noted. Intrasite: 91–100% acceptable rating scores across the 4 form types |
Training QC requirements consisted of requirement for staff to pass formal reliability or other QC assessment on mock exercises in advance of “live” field work and initial QC requirement on first instruments/examinations once in the field.
For each instrument, if a study staff member did not meet criteria for acceptable score during ongoing QC, retraining and training QC requirements were instituted.
Semiqualitative call rating forms for invitation, follow-up, and caregiver interview calls included items such as use of call script, coverage of essential points, ability to respond to participant questions, probing on unclear or neutral responses, professionalism, and delivery and response recording for applicable study instruments (Social Communications Questionnaire, pregnancy reference form, or caregiver interview). For each item, QC supervisor rated interviewer as “good”, “fair”, or “poor”. Criteria for acceptable score consisted of: no item rated as “poor” and 20% or fewer rated as “fair”; and mandatory ratings of “good” for select items (dependent on type of call).
Questionnaire packet I included maternal medical history form, paternal medical history form, family autoimmune disease history form, paternal occupational exposure questionnaire, child services and treatments questionnaire, child early development questionnaire, and child gastrointestinal function questionnaire. Questionnaire packet II included Child Behavior Checklist, Carey Temperament Scale, Child Sleep Habits questionnaire, Child Social Responsiveness Scale, and Parent Social Responsiveness Scale. Questionnaire packet III included child diet diary and child stool diary.
Final Classification
To date, among families who consented to participate, 69% of children in the ASD workflow and 70% of children in the combined DD and POP workflow completed the clinical evaluation and were assigned a final study group classification. Final classifications were dependent on the child’s ascertainment source (i.e., “broad diagnostic net” or birth certificate); workflow classification; and, for children in the ASD workflow, the results from both the Autism Diagnostic Observation Schedule (ADOS; using new scoring algorithms) (Gotham et al. 2007; Lord et al. 1999; Lord et al. 2000) and the Autism Diagnostic Interview–Revised (ADI-R) (Lord et al. 1994; Rutter et al. 2003). As shown in Figures 1 and 2, the SEED rubric placed children into one of four final classifications: (1) Final ASD, (2) Possible ASD, (3) Final DD, and (4) Final POP. Children with Final DD, Final POP or Possible ASD final classifications who went through the ASD workflow were further divided into several subclassifications to characterize children by degree of observed or reported, or both, ASD behavioral symptoms based on the ADOS or ADI-R. To date, among children who completed the clinical evaluation, 27% were classified as a Final ASD, 2% as a Possible ASD, 37% as a Final DD, and 34% as a Final POP. Among children who were classified as a Final ASD, 18% did not have a previous ASD diagnosis.
Target Sample Size and Study Power
During SEED planning, the proposed size of the study birth cohort, and therefore the anticipated number of potential ASD, DD, and POP participants, was determined partly by time and resource considerations. Few data were available to estimate anticipated effect sizes for particular exposure–outcome relationships of interest. Given these constraints, we calculated the minimum detectable relative risk estimates with the anticipated sample size, which was based on an estimated prevalence of 3.4 ASD cases per 1,000 children aged 3–10 years in metropolitan Atlanta in 1996 (Yeargin-Allsopp et al. 2003). Because we were focusing on preschool children, we chose a more conservative estimate of 3.2 cases per 1,000 children. Therefore, based on about 485,000 births among the SEED study population over a 24-month period (September 1, 2003–August 31, 2005), we anticipated that there would be about 1,550 potential ASD case children to be ascertained, including previously diagnosed and study-identified children. Based on estimated rates of invitation contact, eligibility, enrollment, and data collection completion, in part from another large study of ASDs using similar recruitment methods (Hertz-Picciotto et al. 2006), we expected about 42% of all potential case children, or 650 children (families) with an ASD, to be contacted, enroll, and provide complete data. Due to lower invitation contact, enrollment, and completion rates than anticipated, an additional birth cohort year was added (September 1, 2005–August 31, 2006), expanding the SEED study population to about 750,000 births, in order to obtain complete data on 650 ASD case children. Applying the same birth date, residence, and other eligibility criteria as those used to identify case children, we implemented the approach described previously to ascertain and enroll children in each of the two control groups, in a 1:1 ratio with case children.
A number of estimations were made to gauge the effects on minimum detectable odds ratios (MDORs) of different case group sizes—total ASD group or specific ASD phenotypic subgroups—and exposure prevalence rates. For all estimates, conventional alpha and beta error tolerances were applied (0.05 and 0.20, respectively). The MDORs ranged from 1.37 when using the total ASD group in an analysis of any infection during pregnancy (estimated exposure prevalence 50%) to 5.28 when using an ASD subgroup (nonverbal with regression, 30% of all cases) in an analysis of oral contraceptive use during pregnancy (1% prevalence). For all exposures with a prevalence of at least 5%, the MDOR using the total ASD group was less than 1.87. For the majority of ASD subgroups and likely exposure prevalences, the MDOR was estimated to be less than 2.0.
Minimum detectable risk estimates for interaction (e.g., a perinatal infection and mode of delivery) also were calculated for a range of prevalence estimates, from 0.025 to 0.5 for one exposure (Exposure 1), and 0.05 to 0.5 for the other (Exposure 2). For the minimum detectable interaction odds ratio (assuming no stratification), the values ranged from about 5.0 to 12.0 for the lowest prevalences of Exposure 1 combined with different Exposure 2 prevalences; they improved to about 2.0 to 3.5 for the highest prevalence for Exposure 1 combined with different Exposure 2 prevalences.
Management of Laboratory Specimens
SEED biospecimens were blood samples, dried blood spot cards, hair, and buccal (cheek cell) swabs. Blood sample collection tubes for children included EDTA (2 x 3 milliliters (mL)), CPT (2 x 4 mL]), SST (3.5mL), and Paxgene (2.5mL) and for parents include EDTA (6mL), CPT (8mL), and SST (5mL). All biologic specimens, other than buccal swabs, were collected by trained local staff, packaged, and shipped via overnight courier following standardized protocols to the CLBR in Maryland where they were received, processed, and stored. Samples were identified by laboratory-specific bar codes to enable tracking of receipt and inventory without direct links to other study information. All blood tubes were inspected visually upon receipt at the CLBR for tube integrity and volume, which were recorded in the laboratory database. Samples then were aliquoted into smaller portions (a variable number of aliquots depending on the type and volume of blood collection tube) for long-term storage to minimize thaws per aliquot. Blood spot cards were prepared both at the local recruitment sites and at the CLBR, using blood from one of the venipuncture tubes. All blood spot cards and hair samples were stored at room temperature. SEED buccal sample collection was self-administered (three brushes per person) and specimens were mailed directly to the CLBR by participants. DNA was extracted from buccal samples within 30 days. Recruitment sites were notified of samples with low DNA yield so that an additional buccal sample could be collected if needed; recollection was not prompted by familial inconsistencies which were detected later at genotyping and flagged for analysis. DNA from blood also was isolated as blood was received and stored in a standardized concentration in up to four aliquots. Integrated communication between the CLBR and the DCC allowed for inventory monitoring and for biosample requests to the CLBR to prepare specific participant samples for particular bioanalyses as needed.
Data Management and Protection
The primary functions of the SEED DCC in Michigan have been to develop, host, and support the CADDRE Information System (CIS), a web-based application developed exclusively for CADDRE. The CIS has provided the mechanisms for managing workflow and data processing. The CIS also has integrated with the Internet System for Assessing Autistic Children (ISAAC), a system built and maintained by Autism SpeaksTM for entry of standardized clinical assessments, and the Freezerworks database of biologic specimens housed at the SEED CLBR. All participant data have been entered into the CIS by SEED staff and securely stored in a Health Insurance Portability and Accountability Act-class facility. Personally identifiable information has been encrypted with access governed by site-defined roles.
The DCC also provided a medical coding function for all text fields in SEED instruments, including medical diagnoses (International Classification of Diseases (ICD) codes) and procedures (Current Procedural Terminology (CPT) codes), medications (Slone Drug Dictionary™) (Slone Epidemiology Center 2009), and a variety of additional categories (such as ethnicity). The coding process, implemented in batches, used both a computer-based autocoding process followed by manual coding, with an updating of the autocoding electronic dictionaries following each completed batch. Each batch of coded fields was subjected to a quality assurance procedure involving review by a physician (ICD and CPT codes), research pharmacist (medications), or epidemiologist.
The DCC data cleaning management functions included a comprehensive system for internal validity checks to enable identification and correction of data discrepancies as new data were entered into CIS. A log of all data edits was maintained. The data cleaning functions were applied to newly entered data on a 24-hour cycle and the resulting cleaned data were presented in a series of de-identified tables organized by study instrument. The tables were frozen nightly to reflect the previous day’s data entry and edits. Authorized users were granted access to the tables via a remote connection to a dedicated and secure DCC server for review and analysis.
Community Involvement
Each SEED site made extensive efforts to involve health and education professionals, voluntary organizations, and parents in the development and implementation of the project. Site investigators participated in voluntary organizations such as Autism Society of America and Autism SpeaksTM, where they had the opportunity to communicate information about the project as it proceeded and receive feedback. Outreach activities focused on professionals serving children with developmental disabilities; parents of children who participated in early intervention or special education programs; and support groups, events, and conferences for parents of children with an ASD. Communication about the project also has taken place through SEED’s biannual newsletter to participants, news articles, television profiles, and study websites.
SEED Scientific Contributions
Despite significant growth in ASD research funding in recent years, the number of large epidemiologic studies of ASD is still relatively small, due in part to their methodological complexity and expense. SEED was designed to provide unique insights into ASDs and other child development epidemiologic research at multiple levels: methodological, descriptive, and analytic (Table 4).
Table 4.
Study to Explore Early Development design-based research strengths
| Feature | Advantage |
|---|---|
| Multisite | Multiple U.S. sites located in diverse communities improves generalizability of SEED findings to the broad U.S. population compared with studies performed in single areas. |
| Multisource ascertainment | Identification of potential ASD and DD participants from multiple facilities at each site improves representativeness of the full range of children with autism in each study area by diminishing biases that could arise from limiting study families to those who are seen only at single facilities. |
| Ascertainment of diagnosed and undiagnosed ASD children | Actively recruiting children who did not have a previous autism diagnosis but met SEED’s rigorous autism case definition improves representativeness of ASD group in SEED’s target preschool age range because not all affected children might have received an autism diagnosis from community providers by the time of SEED enrollment. |
| Two comparison groups | The DD (children affected with developmental problems other than autism) and POP (children drawn from the study population of births, most of whom have typical development) comparison groups are designed to control for potential recall bias among ASD parents and to more accurately distinguish features of autism from both typical development and other developmental problems. |
| Uniform study protocol across sites | Uniform eligibility criteria and data collection protocols, including final participant classification procedures, and robust quality control measures:
|
| Large sample size | The final sample size will be among the largest of autism epidemiologic studies planned to date, resulting in:
|
| Data collection redundancy | Some data are being collected under different modalities (e.g., self-report and medical record abstraction). This will:
|
| Enrollment of participants who speak only Spanish | Two sites are actively recruiting families who speak only Spanish to accommodate the large Hispanic population in their study areas. This will greatly inform the future development of appropriate autism study methods and improvement of autism instruments for Spanish-only speakers. |
| Use of both existing and novel CADDRE-derived instruments | Assess feasibility and performance in large, population-based research setting of both existing standardized instruments (performance of many instruments has not been assessed outside smaller clinical research applications), and CADDRE-derived novel instruments and approaches for special data types (e.g., stool, diet, and dysmorphology assessment) |
Implementation of the population-based SEED study protocol in multiple, U.S. settings was complex. Protocol implementation has yielded information that will inform the design and conduct of future epidemiologic studies and provide insights into both the practical feasibility and performance of a variety of data collection instruments and approaches in a large field study setting. Specific anticipated methodological contributions relate to recruitment strategies and associated participation rates; data collection strategies and associated completeness; comparisons of data retrieved via caregiver interview versus medical records; biospecimen collection; informatics, data management, and quality control; performance of both ASD- and non–ASD-specific instruments and data collection approaches in population-based field studies, including instruments for special types of data such as characterization of dysmorphic features (methods to be reported elsewhere); diet and stool patterns; and autism research among Hispanic populations, with a special focus on Spanish-speaking participants.
Another important contribution of SEED research will be descriptive in nature. Previous evaluations of developmental features and risk factors for ASD often have been based on smaller, clinic-based samples and often have lacked data from comparison groups. Given its size and population-based ascertainment, information from SEED will be able to fill many descriptive data gaps and provide more reliable estimates of the distributions of these factors among children with different developmental profiles. Specific contributions will include results from standardized developmental assessments; prevalences and distributions of developmental, behavioral, biologic, physical and morphologic and associated medical features and conditions; and prevalences and distributions of demographic characteristics and environmental and genetic risk factors.
The primary goal of SEED was to test important hypotheses related to ASD phenotype and etiology. A key strength is that data have been collected to address multiple different but potentially interrelated hypotheses. Consequently, the type of data and targeted topics of information collection were diverse and will enhance SEED’s analytic breadth and potential. The richness of detailed phenotype, environment, genetic, and biomarker data, coupled with the large, well-defined study sample, will permit the simultaneous investigation of numerous genetic factors, environmental factors and phenotypic features, and their interplay—an analytic strength addressing a limitation of many studies to date.
While SEED does have limitations, efforts have been made to reduce their effects on its analytic potential. First, although a population-based method to ascertain potential invitees was implemented, the actual proportion of invitees with contact for the study invitation and eligibility assessment telephone call was relatively low (as described previously, 28% of combined ASD and DD families and 19% of POP families). This might have limited the representativeness of the enrolled sample. Characteristics of the SEED participants relative to the base population will be assessed and remedial analytic steps devised as needed. For example, we may perform a sensitivity assessment by comparing results from an analysis based on participant data to an analysis based on a simulated augmented data set with an assumed distribution of the risk factor in question among those who chose not to participate (Molenberghs and Kenward 2007).
The results of the sensitivity assessment would guide analytic interpretation of SEED results. The low contact rates during the invitation process also might have increased the potential for biased measures of association if individuals in the case or comparison groups with certain exposures or sociodemographic characteristics responded disproportionately to the attempts to invite them, resulting in different enrollments. To reduce this possibility, study goals were described in broad terms to invitees so that parents with concerns about specific exposures would not have a heightened motivation to enroll.
A second study limitation was that much of the environmental risk factor data were collected retrospectively from parents via interview or questionnaire, long after critical prenatal or perinatal time periods; thus, the data were liable to recall error. The SEED target age range was a compromise between the need to gather risk factor information from parents as soon after pregnancy and infancy as possible, yet enroll families whose children were old enough for reliable developmental assessment. Further, the retrieval and extensive abstraction of medical records of mother and child, containing prospectively recorded data, were designed to complement and extend the array of information that parents provided and mitigate potential recall bias.
Finally, although minimum detectable interaction effect sizes are quite large when exposure prevalence is moderate to low, the power to detect causal effects also is influenced heavily by measurement error. Careful phenotyping of SEED participants reduced outcome misclassification and provided greater precision than less costly, but more error-prone, case confirmation protocols that afford collection of larger samples. Further, the detailed phenotyping in SEED will allow for the accurate identification of clinical subgroups with which it may be possible to detect interaction effects of sufficiently high magnitude. Last, to enhance SEED total sample size and analytic power, a second round of SEED enrollment and data collection will be initiated in 2012.
Conclusion
SEED was designed to collect a sufficient breadth and depth of data to make significant scientific contributions in several key research domains, while also filling important knowledge gaps in a variety of other areas. In addition to analytic hypothesis testing, SEED will contribute important methodological and descriptive information on many factors. Findings from the SEED study will inform the interpretation of results from previous studies, as well as the planning of the next generation of studies on ASDs and child development. Finally, SEED data, including the valuable biospecimen repository, will be a unique resource to test new hypotheses as they arise, reexamine old hypotheses from new perspectives, and contribute to future data pooling efforts for analyses requiring robust sample sizes beyond the reach of individual studies.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach T. Child Behavior Checklist. Burlington, VT: Achenbach System of Empirically based Assessment; 1992. [Google Scholar]
- Allen CW, Silove N, Williams K, Hutchins P. Validity of the social communication questionnaire in assessing risk of autism in preschool children with developmental problems. Journal of Autism and Developmental Disorders. 2007;37:1272–1278. doi: 10.1007/s10803-006-0279-7. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous Mucosal Lymphocyte Cytokine Profiles in Children with Autism and Gastrointestinal Symptoms: Mucosal Immune Activation and Reduced Counter Regulatory Interleukin-10. Journal of Clinical Immunology. 2004;24(6):664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: A new frontier for autism research. Journal of Leukocyte Biology. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Schendel DE, Ostergaard L, Lemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: A Danish cohort study. Archives of Pediatrics and Adolescent Medicine. 2010a;164(5):470–477. doi: 10.1001/archpediatrics.2010.9. [DOI] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010b;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine. 1995;25(1):63–78. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: Implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Beversdorf D, Manning S, Hillier A, Anderson S, Nordgren R, Walters S, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of Prenatal Stressors and Autism. Journal of Autism and Developmental Disorders. 2005;35(4):471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Boyle CA, Schieve LA. Health Care Use and Health and Functional Impact of Developmental Disabilities Among US Children, 1997–2005. Archives of Pediatrics and Adolescent Medicine. 2009;163(1):19–26. doi: 10.1001/archpediatrics.2008.506. [DOI] [PubMed] [Google Scholar]
- Bemer A, Giacobini M, Eriksson M, Gustavsson P, Nordin V, Fernell E, Gillberg C, Nordgren A, Uppströmer A, Anderlid B-M, Nordenskjöld M, Schoumans J. Copy number variation characteristics in subpopulations of patients with autism spectrum disorders. American Journal Medicine Genetics B Neuropsychiatric Genetic. 2010 doi: 10.1002/ajmg.b.31142. 2010 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Carey WB, McDevitt SC. The Carey temperament scales. Scottsdale, AZ: Behavioral-Developmental Initiatives; 1995. [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders-Autism and Developmental Disabilities Monitoring Network, United States, 2006. Morbid and Mortality Weekly Report. 2009;58(SS-10):1–20. [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive Developmental Disorders in Preschool Children. Journal of American Medical Association. 2001;285(24):3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. Journal of Pediatrics. 1978;93(4):699–703. doi: 10.1016/s0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, Lazar G, Mazet P, Pinquier C, Verloes A, Heron D. Specific Genetic Disorders and Autism: Clinical Contribution Towards their Identification. Journal of Autism and Developmental Disorders. 2005;35(1):103–116. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Colborn T. Neurodevelopment and endocrine disruption. Environmental Health Perspectives. 2004;112(9):944–949. doi: 10.1289/ehp.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- DeStefano F. Vaccines and autism: Evidence does not support a causal association. Clinical Pharmacology and Therapeutics. 2007;82(6):756–759. doi: 10.1038/sj.clpt.6100407. [DOI] [PubMed] [Google Scholar]
- Deykin EY, MacMahon B. Viral exposure and autism. American Journal of Epidemiology. 1979;109(6):628–638. doi: 10.1093/oxfordjournals.aje.a112726. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi C, Hepburn S, Davis JM, Fidler DJ, Hartway S, Lee NR, Nancy R, Miller L, Ruttenber M, Robinson C. Screening for autism spectrum disorders in children with Down syndrome: population prevalence and screening test characteristics. Journal of Developmental and Behavioral Pediatrics. 2010;31(3):181–191. doi: 10.1097/DBP.0b013e3181d5aa6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschaffer CN, Lee L, Cunniff CM, Daniels JL, Kirby RS, Leavitt L, Miller L, Zahorodny W, Schieve LA. Advanced Parental Age and the Risk of Autism Spectrum Disorder. American Journal of Epidemiology. 2008;168(11):1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Meaney FJ, Levy SE, DiGuiseppi C, Nicholas JS, Kirby RS, Pinto-Martin JA, Schieve LA. Socioeconomic inequality in the prevalence of autism spectrum disorder: Evidence from a U.S. cross-sectional study. PLoS ONE. 2010;5(7):1–8. e11551. doi: 10.1371/journal.pone.0011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. Journal of Autism and Developmental Disorders. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of Pervasive Developmental Disorders. Pediatric Research. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R. Genetics of autistic disorders: Review and clinical implications. European Child and Adolescent Psychiatry. 2010;19:169–178. doi: 10.1007/s00787-009-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S, Ozonoff S, Lainhart J. Brief report: Pitocin induction in autistic and nonautistic individuals. Journal of Autism and Developmental Disorders. 2003;33:205–208. doi: 10.1023/a:1022951829477. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for utism: comprehensive meta-analysis. British Journal of Psychiatry. 2009;195(1):7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber JS, Offit PA. Vaccines and autism: A tale of shifting hypotheses. Clinical Infectious Diseases. 2009;48(4):456–461. doi: 10.1086/596476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillott A, Furniss F, Walter A. Anxiety in high-functioning children with autism. Autism. 2001;5:277–286. doi: 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- Goines P, Van de Water J. The immune system's role in the biology of autism. Current Opinion in Neurology. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal on Mental Retardation. 2008;113:44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman C, Sparen P, Cnattingius S. Perinatal Risk Factors for Infantile Autism. Epidemiology. 2002;13(4):417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Hvidtjorn D, Schieve L, Schendel D, Jacobsson B, Svaerke C, Thorsen P. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted Conception: A Systematic Review and Meta-analysis. Archives of Pediatrics & Adolescent Medicine. 2009;163(1):72–83. doi: 10.1001/archpediatrics.2008.507. [DOI] [PubMed] [Google Scholar]
- Hvidtjørn D, Grove J, Schendel D, Schieve L, Svaerke C, Ernst E, Thorsen P. Risk of Autism Spectrum Disorders in children born after assisted conception. A population-based follow-up study. Journal of Epidemiology Community Health. 2011;65(6):497–502. doi: 10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- Icasiano F, Hewson P, Machet P, Cooper C, Marshall A. Childhood autism spectrum disorder in the Barwon region: A community based study. Journal of Pediatrics and Child Health. 2004;40(12):696–701. doi: 10.1111/j.1440-1754.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. Immunization Safety Review: Vaccines and Autism. 2004 Retrieved from: http://iom.edu/Reports/2004/Immunization-Safety-Review-Vaccines-and-Autism.aspx. [PubMed]
- Ivarsson SA, Bjerre I, Vegfors P, Ahlfors K. Autism as one of several disabilities in two children with congenital cytomegalovirus infection. Neuropediatrics. 1990;21(2):102–103. doi: 10.1055/s-2008-1071471. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: Their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51(2):77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631–641. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielinen M, Rantala H, Timonen E, Linna SL, Moilanen I. Associated medical disorders and disabilities in children with autistic disorder: A population study. Autism. 2004;8(1):49–60. doi: 10.1177/1362361304040638. [DOI] [PubMed] [Google Scholar]
- King MD, Fountain C, Dakhlallah D, Bearman PS. Estimated autism risk and older maternal age. American Journal of Public Health. 2009;99:1673–1679. doi: 10.2105/AJPH.2008.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and Perinatal Risk Factors for Autism: A Review and Integration of Findings. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. Journal of Child Psychology and Psychiatry. 2005;46(9):963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- Lee L, David AB, Rusyniak J, Landa R, Newschaffer CJ. Performance of the Social Communication Questionnaire in children receiving preschool special education services. Research in Autism Spectrum Disorders. 2007;1:126–138. [Google Scholar]
- Leonard H, Wen X. The epidemiology of mental retardation: Challenges and opportunities in the new millennium. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:117–134. doi: 10.1002/mrdd.10031. [DOI] [PubMed] [Google Scholar]
- Levy S, Giarelli E, Lee L, Schieve L, Kirby R, Cunniff C, Nichols J, Rice C. Autism Spectrum Disorder and Co-occurring Developmental, Psychiatric, and Medical Conditions Among Children in Multiple Populations of the United States. Journal of Developmental and Behavioral Pediatrics. 2010;31:267–275. doi: 10.1097/DBP.0b013e3181d5d03b. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 2009;123:1102–1107. doi: 10.1542/peds.2008-1734. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur AL. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Maimburg R, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatrica Scandinavica. 2006;114(4):257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- McGovern C, Sigman M. Continuity and change from early childhood to adolescence in autism. Journal of Child Psychology and Psychiatry. 2005;46(4):401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Miles JH, Hillman RE. Value of a clinical morphology examination in autism. American Journal of Medical Genetics. 2000;91:245–253. [PubMed] [Google Scholar]
- Minnesota Department of Health. Autism spectrum disorders among preschool children participating in the Minneapolis public schools early childhood special education programs. 2009 Retrieved from http://www.health.state.mn.us/ommh/projects/autism/report090331.pdf.
- Molenberghs G, Kenward MG. Missing Data in Clinical Studies. Chichester, England: John Wiley & Sons, Ltd; 2007. [Google Scholar]
- Moore S, Turnpenny P, Quinn A, Glover S, Lloyd D, Montgomery T, Dean JCS. A clinical study of 57 children with fetal anticonvulsant syndromes. Journal of Medical Genetics. 2000;37(7):489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster D, Lie RT, Markestad T. Long term medical and social consequences of reterm birth. New England Journal Medicine. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. San Antonio, TX: Pearson; 1995. [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: The delays-plus regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zweigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011;128:e488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics. 2004;114(3):793–804. doi: 10.1542/peds.2004-0434. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader Autism Phenotype: Evidence From a Family History Study of Multiple Incidence Autism Families. American Journal of Psychiatry. 1997;154(2):185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Polimeni M, Richdale A, Francis A. A survey of sleep problems in autism, Asperger's disorder and typically developing children. Journal of Intellectual Disability Research. 2005;49(4):260–268. doi: 10.1111/j.1365-2788.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Price CS, Thompson WW, Goodson B, Weintraub ES, Croen LA, Hinrichsen VL, Marcy M, Robertson A, Eriksen E, Lewis E, Bernal P, Shay D, Davis RL, DeStefano F. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126(4):656–664. doi: 10.1542/peds.2010-0309. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. International Review of Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JCS. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Developmental Medicine and Child Neurology. 2005;47(8):551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environmental Health Perspectives. 2007;115(10):1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ. Developmental regression in autism spectrum disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(2):139–43. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic Heterogeneity Between the Three Components of the Autism Spectrum: A Twin Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(6):691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- Rosen NJ, Yoshida CK, Croen LA. Infection in the first 2 years of life and autism spectrum disorders. Pediatrics. 2007;119:61–69. doi: 10.1542/peds.2006-1788. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Law K, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and Concordance of Autism Spectrum Disorders Among 277 Twin Pairs. Archives of Pediatrics and Adolescent Medicine. 2009;163:907–914. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R: The Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008;121:1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- Schendel DE, Autry A, Wines R, Moore C. The co-occurrence of autism and birth defects: Prevalence and risk in a population-based cohort. Developmental Medicine and Child Neurology. 2009;51:779–786. doi: 10.1111/j.1469-8749.2009.03310.x. [DOI] [PubMed] [Google Scholar]
- Slone Epidemiology Center. Slone Drug Dictionary 2009 [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland Adaptive Behavior Scales. San Antonio, TX: Pearson; 1984. [Google Scholar]
- Spence SJ, Schneider MT. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatric Research. 2009;65(6):599–606. doi: 10.1203/01.pdr.0000352115.41382.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin OP, Lai S, Lamm SH, Mosee S. Lack of a relation between human neonatal thyroxine and pediatric neurobehavioral disorders. Thyroid. 2003;13(2):193–198. doi: 10.1089/105072503321319503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D, Weizman A, Ring A, Barak Y. Obstetric complications in individuals diagnosed with autism and in healthy controls. Comprehensive Psychiatry. 2006;47:69–75. doi: 10.1016/j.comppsych.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in thalidomide embryopathy: A population study. Developmental Medicine and Child Neurology. 1994;36(4):351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- Sumi S, Taniai H, Miyachi T, Tanemura M. Sibling risk of pervasive developmental disorder estimated by means of an epidemiologic survey in Nagoya, Japan. Journal of Human Genetics. 2006;51:518–522. doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, McDougle CJ. Brief report: autistic disorder in three children with cytomegalovirus infection. Journal of Autism and Developmental Disorders. 2004;34(5):583–586. doi: 10.1007/s10803-004-2552-y. [DOI] [PubMed] [Google Scholar]
- Taniai H, Nishiyama T, Miyachi T, Imaeda M, Sumi S. Genetic influences on the broad spectrum of autism: study of proband-ascertained twins. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. 2008;147B(6):844–849. doi: 10.1002/ajmg.b.30740. [DOI] [PubMed] [Google Scholar]
- The OSU Autism Rating Scale. Ohio State University Research Unit on Pediatric Psychopharmacology; Retrieved August 30, 2010. http://psychmed.osu.edu/resources.htm. [Google Scholar]
- Turner LM, Stone WL, Pozdol SL, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism. 2006;10:243–265. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and Autism in the CHARGE study. Environmental Health Perspective 2010. 2010 doi: 10.1289/ehp.1002835. Online December 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl RUR. Could oxytocin administration during labor contribute to autism and related behavioral disorders? - A look at the literature. Medical Hypotheses. 2004;63:456–460. doi: 10.1016/j.mehy.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Wassink T, Piven J, Patil S. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatric Genetics. 2001;11(2):57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Wier ML, Yoshida CK, Odouli R, Grether JK, Croen LA. Congenital anomalies associated with autism spectrum disorders. Developmental Medicine and Child Neurology. 2006;48(6):500–507. doi: 10.1017/S001216220600106X. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Bakeman R, Adamson LB, Robins DL. The utility of the Social Communication Questionnaire in screening for autism in children referred for early intervention. Focus on Autism and Other Developmental Disabilities. 2007;22:33–38. [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environmental Health Perspectives. 2006;114(9):1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Anderson MC, Croen LA, Smith KS, Collins J, Grether JK. Birth prevalence of autism spectrum disorders in the San Francisco Bay Area by demographic and ascertainment source characteristics. Journal Autism Developmental Disorders 2011. 2011 doi: 10.1007/s10803-010-1160-2. in press. [DOI] [PubMed] [Google Scholar]
- Wiznitzer M. Autism and tuberous sclerosis. Journal of Child Neurology. 2004;19(9):675–679. doi: 10.1177/08830738040190090701. [DOI] [PubMed] [Google Scholar]
- Wulffaert J, Van Berckelaer-Onnes IA, Scholte EM. Autistic disorder symptoms in Rett syndrome. Autism. 2009;13(6):567–581. doi: 10.1177/1362361309338184. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of Autism in a US Metropolitan Area. Journal of American Medical Association. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Zecavati N, Spence S. Neurometabolic disorders and dysfunction in autism spectrum disorders. Current Neurology and Neuroscience Reports. 2009;9:129–136. doi: 10.1007/s11910-009-0021-x. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Szatmari P, Jones MB, Bryson SE, MacLean JE, Mahoney WJ, Bartolucci G, Tuff L. Pregnancy and birth complications in autism and liability to the broader autism phenotype. J Amer Acad Child Adolesc Psychiatry. 2002;41(5):572–579. doi: 10.1097/00004583-200205000-00015. [DOI] [PubMed] [Google Scholar]