Abstract
Purpose
To optimize isolation of viable bovine corneal endothelial cells (BCECs), we evaluated the effectiveness of various preparation protocols. This entailed comparing the effects of collagenase A and trypsin in the presence and absence of a Rho kinase inhibitor, Y-27632, on proliferation and tight junctional and cytoskeletal integrity during their expansion.
Methods
5-bromo-2'-deoxyuridine (BrdU) incorporation evaluated cell proliferation. Western blot analysis evaluated F-actin, zonule occludin, and ZO-1 associated nucleic acid binding protein (ZONAB) and RhoA expression. Rho A pulldown assay evaluated Rho A activity.
Results
In the trypsin (TrypLE)-prepared BCECs, BrdU incorporation decreased whereas nuclear ZONAB expression increased and became stable from day 3 to 7. In contrast, in the collagenase-A-prepared BCECs, we observed preserved ZO-1 integrity, invariant nuclear ZONAB expression, and dense cortical F-actin expression, and BrdU incorporation was invariant from days 1 to 7. Y-27632 did not increase BrdU incorporation and nuclear ZONAB expression in the TrypLE-prepared and the collagenase-A-prepared BCECs. Moreover, Y-27632 increased irregular cellular morphology and downregulated the expression of ZO-1 in the collagenase-A-prepared BCECs from days 1 to 7. Y-27632 inhibited RhoA activation irrespective of whether the cells were isolated with trypsin or collagenase A.
Conclusions
It is preferable to isolate BCECs with collagenase A and expand them without Y-27632. With this protocol, proliferative activity and tight junctional and cytoskeletal integrity are better preserved than if trypsin is used in the presence or absence of Y-27632.
Introduction
Tissue engineering and increased understanding of corneal endothelial functions have enabled the transplant of cultivated corneal endothelial cell (CEC) sheets and the use of medications to treat corneal endothelial dysfunction [1-3]. Recently, a novel therapeutic concept involving the use of p120 siRNA or Y-27632 (applied topically, injected intracamerally, or expanded ex vivo) has prompted numerous studies on treating corneal endothelial dysfunction in clinical scenarios [1,4-6]. For example, cell injection of rabbit CECs in combination with Y-27632 in the anterior chamber of an animal model helped reconstruct CECs in a monolayer and recover corneal transparency [7]. In a primate model that exhibited endothelial dysfunction after mechanical scraping, transplanting Y-27632-treated CEC sheets resolved corneal edema [8]. Although these studies have reported promising outcomes for the use of p120 siRNA or Y-27632, the individual effects on corneal endothelial proliferation and TJ restoration merit further investigation.
The TJ, a multifunctional complex, and its regulation by Rho GTPases, are often overlooked in mediating endothelial renewal. Rho-associated kinase (ROCK), a putative serine/threonine kinase target for Rho, modulates the actin–myosin cytoskeleton dynamics [9,10]. Disrupting ROCK activity markedly reduces polymerized actin and cytoskeletal rearrangement [10]. In the corneal endothelium, a thick band of actin cytoskeleton, called the perijunctional actomyosin ring, is located proximal to the apical junctional complexes [11,12]. Activating Rho A and its effector ROCK leads to perijunctional actomyosin ring contraction, reduces cell–cell tethering, and subsequently interrupts the TJ barrier [12-14]. Calcium depletion and readdition have been reported to cause disassembly and reformation of TJ in corneal endothelial cells, respectively; however, pretreatment with Y-27632 prevents the normal redistribution of zonula occludens-1 (ZO-1) on the calcium add-back in these cells [15].
Transmembrane proteins, such as ZO-1, localize to the TJ domain and function as a barrier in the endothelial monolayer [11,16,17]. ZO-1 organizes the TJ components and links the transmembrane protein occludin to the actin cytoskeleton [18]. ZO-1 has been demonstrated to stabilize TJ by coupling to the perijunctional cytoskeleton in Madin–Darby canine kidney cells [19]. Several ZO-1-binding proteins have been discovered to interact with ZO-1 through various specific binding sites. Filamentous actin (F-actin) attaches to ZO-1 in the actin-binding region and influences ZO-1 through actin-cytoskeleton interaction with adjacent proteins [20,21]. Furthermore, the ZO-1-associated nucleic acid binding protein (ZONAB), a Y-box transcription factor binds to ZO-1 through an SH3 domain, activates proliferating cell nuclear antigen (PCNA) and cyclin D1 expression, and regulates the morphogenesis and homeostasis of proliferation in a Rho-dependent manner after nuclear localization [22-25]. Previous studies reported that disintegration of the junctional ZO-1 leads to nuclear translocation of ZONAB and increases cell proliferative ability [23,26,27]. Although these findings strongly suggest profound interactions between ROCK and ZO-1, the effect of ROCK inhibition on cell proliferation has led to varying conclusions for numerous cell types [5,28,29].
Collagenase, an enzyme derived from Clostridium histolyticum [30], has been widely used to disaggregate various tissues and prepare cell suspensions for establishing primary cell culture systems, such as murine retinal endothelial cells [31] and the porcine nonpigmented ciliary epithelium [32]. Engelmann et al. introduced collagenase for isolating human corneal endothelial cells from the Descemet membrane in cell cultures [33]. In limbal epithelial cells, intercellular junctions are preserved, cellular degeneration is absent during cell isolation, and basal epithelial progenitor cells isolated with collagenase digestion are more numerous than those isolated with trypsin digestion [34]. Li et al. demonstrated that human CECs could be isolated without keratocyte contamination with collagenase A digestion (2 mg/ml) treated for 16 h [35]. Nevertheless, the effects of collagenase A in the presence or absence of Y-27632 on TJ reformation and proliferation warrant further investigation. This study compared the side by side effects of isolating BCECs with either collagenase or trypsin and expanding them in the presence or absence of Y-27632 on the proliferation and restoration of tight junctional integrity.
Methods
Materials
Dulbecco’s modified Eagle’s medium, Ham’s F12 medium, PBS (1X; 120 mM NaCl, 20 mM, KCl, 10 mM NaPO4, 5 mM KPO4, pH 7.4)., amphotericin B, penicillin, streptomycin, fetal bovine serum, 2 ng/ml of human recombinant epidermal growth factor, sodium bicarbonate, insulin-transferrin-sodium selenite media supplement, trypsin (TrypLE) express dissociation enzyme, and monoclonal ZO-1 antibodies, including Alexa Fluor 546 Phalloidin (F-actin), Alexa Fluor 488, and 546 goat anti-mouse or anti-rabbit immunoglobulin G (IgG), were purchased from Invitrogen (Carlsbad, CA). Collagenase A was purchased from Roche (Indianapolis, IN). Hydrocortisone, dimethyl sulfoxide, cholera toxin, propidium iodide, Triton X-100, bovine serum albumin (BSA), human basic fibroblast growth factor, paraformaldehyde, and diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO); the ZONAB (CSDA monoclonal) antibody was purchased from Abnova Corporation (Taoyuan, Taiwan). Y-27632 dihydrochloride was purchased from Tocris Bioscience (Bristol, UK). The protease inhibitor was purchased from Cytoskeleton (Denver, CO). The prestained protein ladder and Thermo Scientific Halt Phosphatase Inhibitor Cocktail were purchased from Thermo Scientific (Rockford, IL). The WesternBright enhanced chemiluminescence (ECL) horseradish peroxidase (HRP) substrate was purchased from Advansta (Menlo Park, CA).
Bovine corneal endothelial cell isolation using collagenase A and TrypLE
Primary BCEC cultures were prepared according to previous protocols with some modifications [35]. After the Descemet membranes were stripped from the posterior surface of the peripheral corneoscleral tissue under a dissecting microscope, endothelial cell digestion was performed at 37 °C for 1.5 to 16 h with 2 mg/ml of collagenase A in a supplemented hormonal epithelial medium (SHEM). The digestion solution was centrifuged for 3 min at 2,555 ×g to isolate the BCEC aggregates, which were directly seeded in 24-well plates coated with type IV collagen. For the TrypLE preparation, bovine endothelial cell dissociation was performed at 37 °C for 45 min with the TrypLE dissociation enzyme in the SHEM. After the dissociation solution was centrifuged, the BCECs were seeded in 24-well, type IV collagen-coated plates with a seeding density of 104 cells per well.
Cellular proliferation and Rho kinase inhibition
On average, a resultant confluent monolayer was observed 7 days after expansion. The cell growth pattern was observed and determined using crystal violet staining from Sigma-Aldrich (St. Louis, MO). Primary BCEC cultures were treated with and without 10 μM Y-27632 to evaluate the effects of Rho kinase inhibition on cell proliferation in the collagenase-A- and TrypLE-digested cell cultures. Cell proliferative ability was determined in the BCEC cultures with a 5-bromo-2'-deoxyuridine (BrdU) incorporation assay, the BrdU enzyme-linked immunosorbent assay (ELISA) kit from Roche (Indianapolis, IN), according to the manufacturer’s instructions. Three independent samples in each group were assessed in quintuplicate (mean ± standard error of the mean [SEM], n=5).
Immunostaining
The BCECs were fixed in 4% formaldehyde (pH 7.0) for 15 min at room temperature. They were subsequently washed three times with PBS for 5 min each, incubated with 0.2% Triton X-100 and 10% BSA for 30 min to block nonspecific staining, and then rinsed three times with PBS for 5 min each. The BCECs were subsequently incubated with monoclonal anti-ZO-1, anti-F-actin, and anti-ZONAB (all at 1:200 dilution) antibodies for 16 to 24 h at 4 °C. After three washes with PBS, the BCECs were incubated with fluorescein isothiocyanate (FITC)-conjugated (Alexa Fluor 488) or rhodamine-conjugated (Alexa Fluor 546) goat anti-mouse or anti-rabbit IgG (all at 1:200 dilution) for 60 min at room temperature, followed by counterstaining with DAPI (blue; 1:5,000 dilution) for 15 min at room temperature. After three washes with PBS, the cells were analyzed under a fluorescence microscope (Leica).
The development and maturation of the TJ protein ZO-1 were observed in cultures incubated with and without Y-27632 for 4 days after seeding. Double immunofluorescent staining was performed for F-actin and ZO-1. In addition, double staining was performed for ZO-1 and ZONAB. The dynamic changes in ZO-1 were recorded after the Y-27632 treatment was withdrawn for 24, 48, and 72 h.
Western blotting
Proteins were isolated using a lysis buffer with a 1× protease inhibitor and a 1× phosphatase inhibitor. The protein content was quantified using spectrophotometry. Samples with equal protein content were electrophoresed on 10% polyacrylamide gels and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were incubated with 5% milk in PBST (1× PBS, 0.1% Triton X-100) to block nonspecific binding sites, and then incubated for 16 to 24 h at 4 °C with monoclonal anti-ZO-1 (1:1,000 dilution) antibodies. The PVDF membranes were washed three times with PBST and subsequently hybridized with horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,000) as the secondary antibody at room temperature for 1 h. After three washes with PBST, immune complexes were visualized by adding the WesternBright ECL HRP substrate and detecting the luminescent signal with an X-ray film. The molecular size of the immunoreactive bands was determined by comparing them with a prestained protein ladder (Thermo-Pierce, Rockford, lL). The relative band intensity was analyzed using LabWorks software (Version 4.6) from UVP Bioimaging Systems (Upland, CA).
Rho A pulldown assay
Rho A activation was assessed using a Rho-guanosine triphosphate (GTP) pulldown assay kit purchased from Cytoskeleton (Denver, CO). The pulldown assay involves the use of the Rho-A-binding domain from the effector protein Rhotekin as a probe to isolate the active forms of Rho A. ROCK phosphorylates the myosin phosphatase target subunit 1 (MYPT-1) at Thr696 and is involved in the measurement of ROCK activity by detecting phospho-MYPT1 (Thr696). A ROCK activity immunoblot kit was purchased from Cell Biolabs (San Diego, CA), which helped detect ROCK activity by using western blot analysis with antiphospho-MYPT1 (Thr696). The cell lysates were analyzed using western blotting with an antiphospho-Ser19 myosin light chain 2 antibody (Cell Signaling, #3671, dilution 1:250) or antimyosin light chain 2 antibodies (Cell Signaling, #3672, dilution 1:500).
Statistical analysis
Data in Figure 1B,D are reported as means ± standard deviation. To compare the differences between the two groups, a statistical analysis of BrdU incorporation was performed using an unpaired Student t test (n=5); p<0.05 was considered statistically significant.
Figure 1.
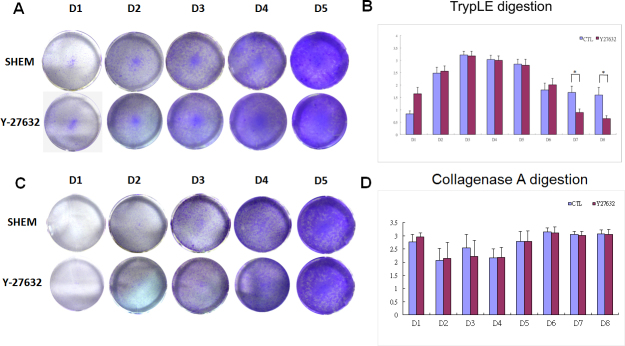
Gross pattern of cell growth was observed and determined using crystal violet staining. The effects of Y-27632 on the proliferative ability of bovine corneal endothelial cells (BCECs) were assessed according to 5-bromo-2'-deoxyuridine (BrdU) cell incorporation. After BrdU was incubated for an additional 24 h, the proliferation was assessed based on BrdU incorporation. Photographs of multiwell plates stained with crystal violet exhibited a cellular growth pattern similar to that of the TrypLE-preparations. The number of colonies increased from day 1 to day 7 in the BCECs with or without Y-27632, prepared with either TrypLE or collagenase A (A, C). The BrdU incorporation rates increased from day 1 to day 3 and decreased after day 4. There was no statistical difference between the groups prepared with TrypLE from day 1 to day 6. However, Y-27632 significantly decreased the BrdU incorporation rates on day 7 and day 8 (B). In contrast, the BrdU incorporation rates remained essentially constant in the BCECs with or without Y-27632 prepared with collagenase A from day 1 to day 7, and differed from those in the TrypLE-prepared BCECs (B,D). * indicates p<0.05.
Results
Effects of different cell preparation methods on cell proliferation, tight junction formation, and cytoskeleton
The number of colonies increased from days 1 to 7 in BCECs with or without Y-27632, prepared either with TrypLE or with collagenase A (Figure 1A,C). The BrdU incorporation increased from day 1 to day 3 and decreased after day 4. There was no statistical difference from day 1 to day 6 between the groups prepared with TrypLE alone. However, Y-27632 significantly decreased BrdU incorporation on day 7 and day 8 (Figure 1B). In contrast, the BrdU incorporation remained essentially constant in the BCECs with or without Y-27632 prepared with collagenase A from day 1 to day 6, whereas they declined in the TrypLE- prepared cells during days 7 and 8 compared to the cells isolated with TrpyLE and not treated with Y-27632 (Figure 1B,D).
Because the major differences in BrdU incorporation among these groups were the preparation methods of BCECs, we compared the expression of ZO-1, ZONAB, and F-actin in BCECs prepared with TrypLE or collagenase-A on days 1, 3, and 7 (Figure 2 and Figure 3). The expression of nuclear ZONAB in the TrypLE-prepared BCECs gradually increased after day 1 and became stable in the nucleus from days 3 to 7, whereas a continuously band-shaped ZO-1 was expressed at the cellular junctions only on day 7, determined with immunostaining (Figure 2A,B,D,E,G,H). In contrast, following collagenase A isolation, a characteristic band-shaped ZO-1 was restored after day 1, and increases in ZONAB nuclear localization were evident during the 7 days of expansion (Figure 3A,B,D,E,G,H). We observed a strong correlation between the BrdU incorporation results and the nuclear ZONAB expression in the collagenase-A-prepared BCECs from days 1 to 7 (Figure 1D and Figure 3B,E,H).
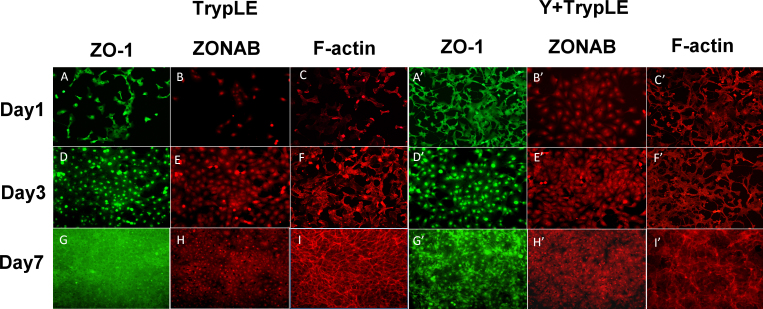
Figure 2.
ZO-1, ZONAB, and F-actin expression in TrypLE-prepared BCECs with or without Y-27632 on days 1, 3 (subconfluence), and 7 (confluence). In the TrypLE-prepared bovine corneal endothelial cells (BCECs) without Y-27632, TJ protein ZO-1 developed gradually from the nucleus to the cell–cell contact and formed a continuous hexagonal pattern. ZONAB expression exhibited increasing staining in the nucleus, as in ZO-1 expression at the cellular borders (A, B: day 1; D, E: day 3; G, H: day 7). The F-actin cytoskeleton was gradually arranged into a dense peripheral band at the cell margin and cortical actin mat with prominent perinuclear staining from days 1 to 7 (C, F, I). The effect of Y-27632 on the expression of the ZO-1 (A’, D’, G’), ZONAB (B’, E’, H’), and F-actin cytoskeleton (C’, F’, I’) was similar to that without Y-27632. The cellular morphology became irregular (G, G’), and there was a marked decrease in the amount of F-actin in the presence of Y-27632 compared with the BCECs without Y-27632 on day 7 (I, I’). However, the ZONAB expression patterns were similar in the BCECs with and without Y-27632 (B, E, H, B’, E’, H’).
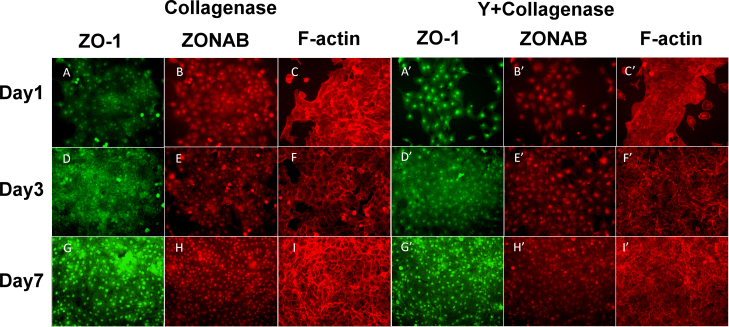
Figure 3.
ZO-1, ZONAB, and F-actin expression in collagenase A-prepared BCECs with or without Y-27632 on days 1, 3 (subconfluence), and 7 (confluence). In the collagenase A-prepared bovine corneal endothelial cells (BCECs) without Y-27632, the TJ protein ZO-1 developed gradually from the nucleus to the cell–cell contact and formed a continuous hexagonal pattern, as it did in the TrypLE-prepared BCECs without Y-27632. The ZONAB expression exhibited staining in the nucleus that was stronger that in the TrypLE-prepared BCECs in the beginning, as in the ZO-1 expression at the cellular borders (A, B: day 1; D, E: day 3; G, H: day 7). The F-actin cytoskeleton was arranged into a dense peripheral band at the cell margin and cortical actin mat with prominent perinuclear staining in the beginning (C, F, I). The effect of Y-27632 on the expression of the ZO-1 (A’, D’, G’), ZONAB (B’, E’, H’), and F-actin cytoskeleton (C’, F’, I’) was similar to that without Y-27632. Similarly, the cellular morphology became irregular (G, G’), and there was a marked decrease in the amount of F-actin in the presence of Y-27632 compared with the BCECs without Y-27632 in the beginning (I, I’). The ZONAB expression patterns were similar in the BCECs with and without Y-27632 (B, E, H, B’, E’, H’).
We compared the influence of Y-27632 on ZO-1 and ZONAB expression in the TrypLE- and collagenase-A-prepared BCECs. The TrypLE-prepared BCECs exhibited similar gradual formation of ZO-1 at the cellular junction, and nuclear ZONAB expression with and without Y-27632 as the BCECs expanded on days 1 (Figures 2A, A’,B,B’), 3 (Figure 2D,D’,E,E’), and 7 (Figure 2G,G’,H,H’). However, ZO-1 did not form a characteristically continuous band in a confluent, hexagonal BCEC monolayer in both groups on day 7 (Figure 2G’). In the collagenase-A-prepared BCECs, we observed the characteristic expression of ZO-1 at the cellular junction with and without Y-27632 treatment since the cell clusters gradually expanded from days 1 to 7 (Figure 3A,D,G,A’,D’,G’). However, the cellular morphology with the Y-27632 treatment was more irregular than that of the BCECs without Y-27632 on day 7 (Figure 3G,G’). The nuclear ZONAB expression did not increase in the BCECs treated with Y-27632 (Figure 3B’,E’,H’) compared with those treated without Y-27632 (Figure 3B,E,H).
We evaluated the effects of Y-27632 on F-actin distribution in the TrypLE- and collagenase-A-prepared BCECs. F-actin was expressed in a weak peripheral band and cortical actin mat in the individual BCECs with Y-27632 compared with those without Y-27632 on day 7 (Figure 2I,I’), whereas normal and intact ZO-1 expression was observed at the cellular junctions of the BCECs without Y-27632 compared with the BCECs with Y-27632 on day 7 (Figure 2G,G’). In contrast, actin occurred in a dense peripheral band and cortical mat in the collagenase-prepared BCECs without Y-27632 on days 1, 3, and 7 (Figure 3C,F,I), and normal and intact ZO-1 expression was observed at the cellular junctions of these BCECs on day 7 (Figure 3G). The collagenase-prepared BCECs with Y-27632 revealed altered cell shapes and a substantial change in the organization and expression of F-actin (Figure 3C’,F’,I’). Therefore, TrypLE- or collagenase A-prepared BCECs with Y-27632 did not exhibit characteristic immunostaining of F-actin (Figure 2C’,F’,I’,C’ and Figure 3C’,F,I’).
Effects of Y-27632 on myosin light chain phosphorylation
A comparison of ROCK activity, Rho A activation, and myosin light chain phosphorylation between the TrypLE- and collagenase-A-prepared BCECs indicated that Y-27632 inhibited Rho A activation on days 1, 3, and 5 (Figure 4A,B). In addition, Y-27632 inhibited RhoA/ROCK activity irrespective of the cell isolation procedures (Figure 4C,D). For the TrypLE- and collagenase-A-prepared BCECs, the amount of phosphorylated myosin light chains decreased in the Y-27632-treated BCECs on days 3, 5, and 7 (Figure 4E,F).
Figure 4.
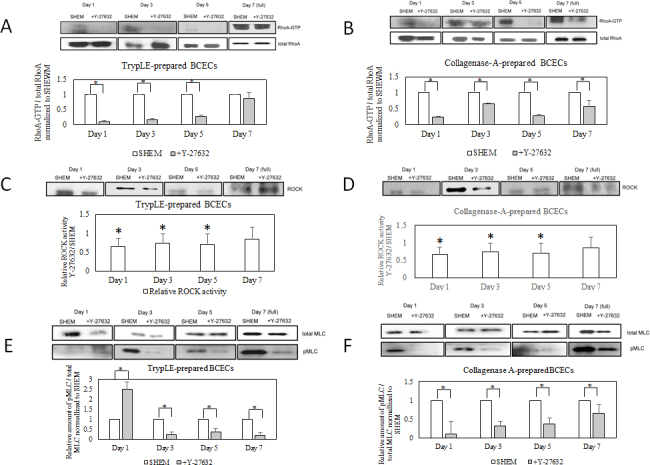
Rho A pulldown assay, ROCK activity assay, and myosin light chain phosphorylation in TrypLE-prepared and collagenase-A-prepared BCECs. In the TrypLE-prepared bovine corneal endothelial cells (BCECs), the Y-27632 treatment reduced Rho A activation on days 1, 3, and 5. Rho A activation decreased at confluence status on day 7 (A). In the collagenase-A-prepared BCECs, Y-27632 treatment reduced Rho A activation on days 1, 3, 5, and 7 (confluence status; B). In the TrypLE-prepared BCECs, the Y-27632 treatment reduced ROCK activity on days 1, 3, and 5 but exhibited no inhibitory effects at confluence on day 7 (C). In the collagenase-A-prepared BCECs, the Y-27632 treatment inhibited ROCK activity on days 1, 3, 5, and 7 (confluence status; D). The Y-27632 treatment reduced the amount of phosphorylated myosin light chain in the TrypLE-prepared BCECs on days 3, 5, and 7 (E), and reduced the amount of phosphorylated myosin light chain in the collagenase-A-prepared BCECs on days 1, 3, 5, and 7 (F). * indicates p<0.05.
Effects of Y-27632 removal on ZO-1 distribution
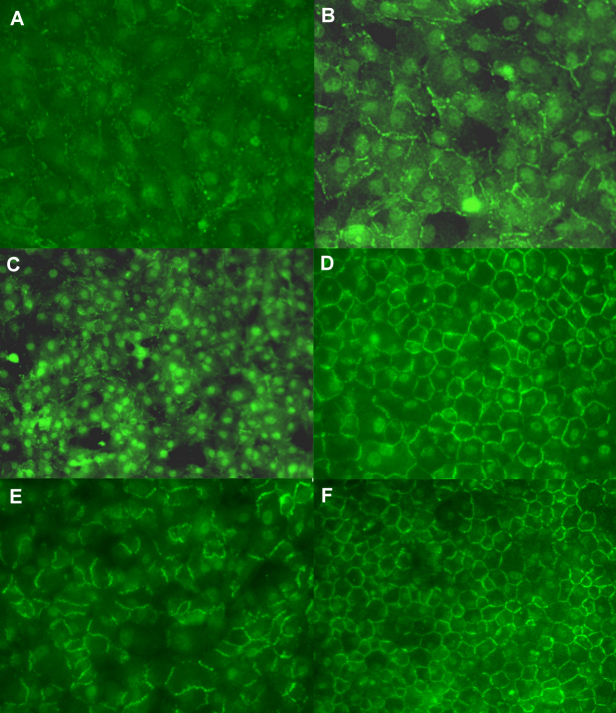
ZO-1 nuclear accumulation and junctional expression declined in TrypE-prepared cells cultured with Y-27632. However, the ZO-1 expression at the cellular junctions reappeared after Y-27632 withdrawal and subsequent incubation for days 1 (Figure 5A,B), 3 (Figure 5C,D), and 5 (Figure 5E, F).
Figure 5.
Reformation and distribution of TJ protein ZO-1 after Y-27632 was removed. Recovery of ZO-1 expression after pretreatment of cells with Y-27632 for 1 day resulted in the gradual development of ZO-1 in the contiguous cells (A, B). The continuous pattern of the ZO-1 protein reformed abruptly at cell junctions and aligned in a hexagonal shape, after the cells were pretreated with Y-27632 for 2 days (C, D) and 3 days (E, F).
Discussion
Previous studies have reported diverse results regarding the effects of Y-27632 on corneal endothelial proliferation. For example, Koizumi et al. demonstrated increased Ki67 staining of monkey CECs accompanied by an F-actin rearrangement after Y-27632 was added. The cellular proliferation at subconfluence substantially increased during the first 2 days. Accordingly, the researchers concluded that Y-27632 could promote cell adhesion and migration and enhance the proliferative ability of CECs [1]. Pipparelli et al. conducted proliferative assays in Y-27632-treated human CECs after cellular confluence was reached in two different media and observed few Ki67-positive cells at confluence under all conditions [36]. In our study, we found that adding Y-27632 to the TrypLE-prepared BCECs increased the BrdU incorporation rates from day 1 to day 3 and decreased them after day 4. There was no difference in the BrdU incorporation between the groups prepared with TrypLE from day 1 to day 6, whereas Y-27632 significantly decreased the BrdU incorporation rates on day 7 and day 8 (Figure 1B).
Furthermore, the TrypLE- and collagenase-A-prepared BCECs exhibited different proliferative patterns. The TrypLE-prepared BCECs exhibited contact-inhibited growth at saturation cell density, in which BrdU incorporation decreased after day 4, and nuclear ZONAB expression increased and became stable in the nucleus from days 3 to 7 (Figure 1B and Figure 2B,E,H). In contrast, the collagenase-A-prepared BCECs exhibited a consistently high level of BrdU incorporation and nuclear ZONAB expression from days 1 to 7 (Figure 1D and Figure 3B,E,H). However, the levels of nuclear accumulation of ZONAB between days 3 and 7 were the same with the two different isolation procedures. Our results are consistent with previous reports that collagenase A digestion removes interstitial collagens instead of basement membrane components, and trypsin digestion disrupts the intercellular junction and causes cellular degeneration [35,37]. Chen et al. reported that collagenase digestion of limbal progenitor cells isolated more basal epithelial progenitor cells and mesenchymal cells than that with trypsin/EDTA digestion because of the superior preservation of basement membrane matrices [34]. Disrupting the intercellular junctions of CECs through trypsin/EDTA digestion may activate canonical Wnt signaling and promote mesenchymal transition [5]. Our study results confirm the finding that the collagenase digestion method is superior to the trypsin digestion method for prolonging proliferative ability at cellular confluence.
Kameda et al. investigated the effects of Y-27632 on F-actin and ZO-1 in a monolayer of Schlemm’s canal endothelial cells [38]. The Y-27632-treated cells exhibited a loss of the normal F-actin polymerization pattern in conjunction with downregulated ZO-1 expression, which is consistent with our results (Figure 2). Furthermore, longitudinal F-actin fibers and ZO-1 formation were disrupted by Latrunculin B, a Rho-dependent inhibitor of actin polymerization, and rescued by Jasplakinolide, an actin filament stabilizer [38]. In addition, we demonstrated that myosin light chain phosphorylation decreased after Y-27632 treatment in TrypLE- and collagenase-prepared BCECs (Figure 4E,F). Similarly, Satpathy and D’Hondt revealed that Rho kinase promotes myosin light chain phosphorylation, increases actin cytoskeleton contractility, and disrupts cortical actin organization in BCECs; pretreatment with Y-27632 could interfere with the myosin light chain phosphorylation and alter its downstream actin cytoskeleton reorganization [39,40].
Small Rho GTPase and its downstream enzyme Rho kinase are known to regulate occludin expression and TJ function in other cell types [41-43]. For example, a study reported that occludin phosphorylation through activated Rho kinase is associated with increased permeability in brain microvascular endothelial cells and that Y-27632 could partially inhibit this phosphorylation and reduce the permeability of these vascular endothelial cells [44]. Moreover, Rho/ROCK influenced the adherent junction-associated proliferation through modulating catenin components. In human corneal endothelial cells, P120-catenin nuclear translocation and endothelial proliferation induced by p120 siRNA relied on RhoA–ROCK signaling. Inhibiting RhoA through CT-04 (a RhoA inhibitor) or Y-27632 eliminated p120 nuclear translocation and prevented proliferation [5]. The effects of Y-27632 on actin cytoskeleton, TJ, and cell proliferation have been discussed separately in relevant studies [45-47]. In the current study, Y-27632 increased the ZONAB activity of cells at confluence (Figure 2 and Figure 3). However, adding Y-27632 did not increase BrdU incorporation in the BCECs in the SHEM (Figure 1B,D).
Senoo et al. considered the use of trypsin/EDTA for disrupting contact inhibition and manipulating TJ to enhance endothelial proliferation at confluence [48]. Balda et al. reported that decreased ZONAB expression is associated with decreased cell division kinase 4, which regulates cell division, primarily in the G1 phase [23]. Y-27632 was found to promote cyclin D expression and facilitate degradation of p27Kip1 (p27) in the G1 phase [49]. Therefore, according to these studies, an abundant F-actin cytoskeleton would stabilize the formation and distribution of ZO-1 and subsequently inhibit the nuclear translocation of ZONAB and cell proliferation at confluence in the SHEM. Adding Y-27632 released ZONAB from ZO-1 and translocated ZONAB into the nucleus. However, our results indicated that Y-27632 modulates ZO-1 distribution through F-actin polymerization in TrypLE- and collagenase-A-prepared BCECS. Adding Y-27632 did not substantially increase the BrdU incorporation in BCECs using TrypLE or the collagenase A culture system. Moreover, Y-27632 did not increase nuclear ZONAB expression in the TrypLE- and collagenase-A-prepared BCECs. Nevertheless, the control mechanisms with which Y-27632 promoted the cells in the G1 phase but reduced their entry into the S phase during BrdU incorporation requires further elucidation.
Although several studies have proposed using collagenase A in cell isolation and preserving TJ during cell expansion, collagenase A has been infrequently adopted because of the difficulty of using it to process tissues [30]. Our study demonstrates that collagenase A isolation preserves nuclear ZONAB expression and maintains consistently higher BrdU incorporation rates in the subconfluent and confluent states. Y-27632 did not increase BrdU incorporation in either the TrypLE- or collagenase-A-prepared BCECs. In summary, instead of using Y-27632 to disrupt ZO-1 expression, preserving intercellular junctions during cell growth may be a more favorable option than disrupting TJ integrity during this process.
Acknowledgments
This study was partially supported by grants from the National Science Council, Taiwan (NSC 983112B002040, 993112B002029, and 992314B002039MY3) and National Taiwan University Hospital (NTUH 98S-1105, 102-CGN01). Shwu-Huey Lee, MD is the co-corresponding author for this study and an affiliate at the Department of Ophthalmology, Cathay General Hospital, 280 Section 4, Jen-Ai Road, 106, Taipei, Taiwan.
References
- 1.Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res. 2012;95:60–7. doi: 10.1016/j.exer.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95:16–23. doi: 10.1016/j.exer.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimoto M, Shima N, Yamaguchi M, Hiraoka Y, Amano S, Yamagami S. Development of a bioengineered corneal endothelial cell sheet to fit the corneal curvature. Invest Ophthalmol Vis Sci. 2014;55:2337–43. doi: 10.1167/iovs.13-13167. [DOI] [PubMed] [Google Scholar]
- 4.Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Hamuro J, Kinoshita S. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30(Suppl 1):S54–9. doi: 10.1097/ICO.0b013e3182281ee1. [DOI] [PubMed] [Google Scholar]
- 5.Zhu YT, Chen HC, Chen SY, Tseng SC. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J Cell Sci. 2012;125:3636–48. doi: 10.1242/jcs.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okumura N, Koizumi N, Kay EP, Ueno M, Sakamoto Y, Nakamura S, Hamuro J, Kinoshita S. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54:2493–502. doi: 10.1167/iovs.12-11320. [DOI] [PubMed] [Google Scholar]
- 7.Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Tsuchiya H, Hamuro J, Kinoshita S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181:268–77. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi N. Cultivated corneal endothelial cell sheet transplantation in a primate model. Nippon Ganka Gakkai Zasshi. 2009;113:1050–9. [PubMed] [Google Scholar]
- 9.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Street CA, Bryan BA. Rho kinase proteins–pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011;31:3645–57. [PMC free article] [PubMed] [Google Scholar]
- 11.Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Invest Ophthalmol Vis Sci. 1995;36:1115–24. [PubMed] [Google Scholar]
- 12.Turner JR. 'Putting the squeeze' on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol. 2000;11:301–8. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- 13.Madara JL, Moore R, Carlson S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol. 1987;253:C854–61. doi: 10.1152/ajpcell.1987.253.6.C854. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran C, Srinivas SP. Formation and disassembly of adherens and tight junctions in the corneal endothelium: regulation by actomyosin contraction. Invest Ophthalmol Vis Sci. 2010;51:2139–48. doi: 10.1167/iovs.09-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–36. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 17.Matter K, Balda MS. Functional analysis of tight junctions. Methods. 2003;30:228–34. doi: 10.1016/s1046-2023(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 18.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–53. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 19.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–40. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–7. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- 22.Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–98. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–32. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K, Balda MS, Ali RR. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS ONE. 2010;5:e15730. doi: 10.1371/journal.pone.0015730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penes MC, Li X, Nagy JI. Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB)-MsY3 in glial cells and colocalization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur J Neurosci. 2005;22:404–18. doi: 10.1111/j.1460-9568.2005.04225.x. [DOI] [PubMed] [Google Scholar]
- 26.Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. doi: 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–8. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Roovers K, Assoian RK. Effects of rho kinase and actin stress fibers on sustained extracellular signal-regulated kinase activity and activation of G(1) phase cyclin-dependent kinases. Mol Cell Biol. 2003;23:4283–94. doi: 10.1128/MCB.23.12.4283-4294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Swant JD, Rendon BE, Symons M, Mitchell RA. Rho GTPase-dependent signaling is required for macrophage migration inhibitory factor-mediated expression of cyclin D1. J Biol Chem. 2005;280:23066–72. doi: 10.1074/jbc.M500636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kin T, Johnson PR, Shapiro AM, Lakey JR. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation. 2007;83:7–12. doi: 10.1097/01.tp.0000243169.09644.e6. [DOI] [PubMed] [Google Scholar]
- 31.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–8. [PubMed] [Google Scholar]
- 32.Shahidullah M, Tamiya S, Delamere NA. Primary culture of porcine nonpigmented ciliary epithelium. Curr Eye Res. 2007;32:511–22. doi: 10.1080/02713680701434899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 1988;29:1656–62. [PubMed] [Google Scholar]
- 34.Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–48. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, Tseng SC. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007;48:614–20. doi: 10.1167/iovs.06-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pipparelli A, Arsenijevic Y, Thuret G, Gain P, Nicolas M, Majo F. ROCK Inhibitor Enhances Adhesion and Wound Healing of Human Corneal Endothelial Cells. PLoS ONE. 2013;8:e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004;78:573–8. doi: 10.1016/s0014-4835(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 38.Kameda T, Inoue T, Inatani M, Fujimoto T, Honjo M, Kasaoka N, Inoue-Mochita M, Yoshimura N, Tanihara H. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest Ophthalmol Vis Sci. 2012;53:3092–103. doi: 10.1167/iovs.11-8018. [DOI] [PubMed] [Google Scholar]
- 39.Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79:477–86. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 40.D'Hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest Ophthalmol Vis Sci. 2007;48:120–33. doi: 10.1167/iovs.06-0770. [DOI] [PubMed] [Google Scholar]
- 41.Srinivas SP. Dynamic regulation of barrier integrity of the corneal endothelium. Optom Vis Sci. 2010;87:E239–54. doi: 10.1097/OPX.0b013e3181d39464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivas SP. Cell signaling in regulation of the barrier integrity of the corneal endothelium. Exp Eye Res. 2012;95:8–15. doi: 10.1016/j.exer.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda) 2010;25:16–26. doi: 10.1152/physiol.00034.2009. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–33. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalimarada SS, Shivanna M, Kini V, Mehta D, Srinivas SP. Microtubule disassembly breaks down the barrier integrity of corneal endothelium. Exp Eye Res. 2009;89:333–43. doi: 10.1016/j.exer.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campos SB, Ashworth SL, Wean S, Hosford M, Sandoval RM, Hallett MA, Atkinson SJ, Molitoris BA. Cytokine-induced F-actin reorganization in endothelial cells involves RhoA activation. Am J Physiol Renal Physiol. 2009;296:F487–95. doi: 10.1152/ajprenal.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okumura N, Ueno M, Koizumi N, Sakamoto Y, Hirata K, Hamuro J, Kinoshita S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50:3680–7. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 48.Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:2930–5. [PubMed] [Google Scholar]
- 49.Okumura N, Nakano S, Kay EP, Numata R, Ota A, Sowa Y, Sakai T, Ueno M, Kinoshita S, Koizumi N. Involvement of Cyclin D and p27 in Cell Proliferation Mediated by ROCK Inhibitors Y-27632 and Y-39983 During Corneal Endothelium Wound Healing. Invest Ophthalmol Vis Sci. 2014;55:318–29. doi: 10.1167/iovs.13-12225. [DOI] [PubMed] [Google Scholar]