Abstract
Background
Air pollution is associated with cardiovascular disease, and systemic inflammation may mediate this effect. We assessed associations between long- and short-term concentrations of air pollution and markers of inflammation, coagulation, and endothelial activation.
Methods
We studied participants from the Multi-Ethnic Study of Atherosclerosis from 2000 to 2012 with repeat measures of serum C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, D-dimer, soluble E-selectin, and soluble Intercellular Adhesion Molecule-1. Annual average concentrations of ambient fine particulate matter (PM2.5), individual-level ambient PM2.5 (integrating indoor concentrations and time–location data), oxides of nitrogen (NOx), nitrogen dioxide (NO2), and black carbon were evaluated. Short-term concentrations of PM2.5 reflected the day of blood draw, day prior, and averages of prior 2-, 3-, 4-, and 5-day periods. Random-effects models were used for long-term exposures and fixed effects for short-term exposures. The sample size was between 9,000 and 10,000 observations for CRP, IL-6, fibrinogen, and D-dimer; approximately 2,100 for E-selectin; and 3,300 for soluble Intercellular Adhesion Molecule-1.
Results
After controlling for confounders, 5 µg/m3 increase in long-term ambient PM2.5 was associated with 6% higher IL-6 (95% confidence interval = 2%, 9%), and 40 parts per billion increase in long-term NOx was associated with 7% (95% confidence interval = 2%, 13%) higher level of D-dimer. PM2.5 measured at day of blood draw was associated with CRP, fibrinogen, and E-selectin. There were no other positive associations between blood markers and short- or long-term air pollution.
Conclusions
These data are consistent with the hypothesis that long-term exposure to air pollution is related to some markers of inflammation and fibrinolysis.
Inflammation plays an important role in the initiation and development of atherosclerosis and in precipitation of cardiovascular events. Animal, epidemiologic, and controlled exposure studies have provided evidence that air pollution causes an inflammatory response in the vasculature, which stimulates the process of atherosclerosis.1,2 Air pollution-induced inflammation can occur through autonomic nervous system imbalance (ie, sympathetic nervous system activation and/or parasympathetic nervous system withdrawal) or through localized inflammation in the lungs that spills over into the bloodstream.1 However, the exact biological mechanisms are still unclear.
The role of coagulation in cardiovascular disease has also been well established.3,4 Moreover, the relation between inflammatory markers and the coagulation cascade in cardiovascular disease5 makes these markers relevant to the study of air pollution health effects. However, the evidence supporting the hypothesis that air pollution increases concentrations of coagulation-related blood markers is mixed.6–10
The endothelial cell layer of blood vessels is dynamic, changing with factors such as age, altitude, exercise and diet,11 smoking, hypertension,12,13 and various disease states.14,15 The endothelium is also actively involved in regulating blood coagulation and inflammatory response.16–18 A negative association between long-term, but not short-term, concentrations of particulate matter <2.5 µm in aerodynamic diameter (PM2.5) and flow-mediated dilation—a marker of endothelial function—has been documented.19
Much of the existing literature has focused on associations between recent air pollution exposure and markers of inflammation and coagulation.1,2 A review of the association between C-reactive protein (CRP) and particulate matter concluded that epidemiologic evidence is inconsistent at best.2 The potential for air pollution to contribute to long-term inflammatory responses may be more relevant to the development of cardiovascular disease. The studies that have explored the association between long-term air pollution and blood markers have focused on markers of inflammation and coagulation and not on markers of endothelial activation.20–23
This study examined the association between long-term pollutant concentrations and several blood markers that may be biologically relevant to the mechanism by which air pollution exposure results in cardiovascular disease. The blood markers of interest are CRP, interleukin-6 (IL-6), fibrinogen and D-dimer, and markers of endothelial activation soluble E-selectin and soluble intercellular adhesion molecule-1 (sICAM-1). As a secondary aim, we examined the association between short-term PM2.5 concentrations and blood markers.
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal epidemiologic study designed to examine the progression of subclinical and clinical cardiovascular disease among adults free from such disease at baseline.24 From July 2000 to August 2002 (baseline examination), the study recruited 6,814 white, African-American, Hispanic, and Chinese men and women age 45 to 84 years from six US communities (Baltimore, MD; Chicago, IL; Winston-Salem, NC; Los Angeles, CA; New York, NY; and St. Paul, MN). Four follow-up exams were conducted between 2002 and 2012. Exam 2 was held between Fall 2002 and Winter 2004, the third examination between Spring 2004 and Fall 2005, the fourth between Fall 2005 and Spring 2007, and the fifth exam was from Spring 2010 to Winter 2012. All examinations included a blood draw, anthropometric measurements, and the collection of questionnaire data. Institutional review board approval was granted at each study site, and written informed consent was obtained from participants.
Blood Markers
The blood markers of interest were measured at baseline for most MESA participants, and in subsets of individuals at follow-up through three ancillary studies. The MESA abdominal body composition study, conducted over exams 2 and 3, assessed CRP, fibrinogen, and IL-6 in 1970 existing MESA participants. The MESA and Air Pollution (MESA Air) assessed CRP, D-dimer, fibrinogen, E-selectin, and sICAM-1 in approximately 715 participants at exams 4 and 5. Two-hundred fifty-seven of these participants were newly recruited into the MESA cohort during follow-up exam 4 (September 2005 to May 2007) and followed through exam 5. These new participants were recruited from the Los Angeles and New York areas to increase the range of air-pollution exposures among the study population.25 Finally, the MESA Stress examined D-dimer and IL-6 in 1,002 participants over exams 3 and 4. A follow-up study to the MESA Stress was conducted during exam 5 that measured CRP, D-dimer, fibrinogen, and IL-6 in about 1,300 participants. Because these ancillary studies did not collect each blood marker at each visit, our final sample of participants are missing a D-dimer measurement from exam 2 and E-selectin and sICAM-1 measurements from exams 2 and 3. In addition, only a subset of baseline participants had measurements of E-selectin and sICAM-1. Table 1 provides sample sizes by examination for each blood marker. Participants in our study had up to four measures of biomarkers collected between 2000 and 2012 (mean 1.6 observations).
TABLE 1.
Sample Size for Each Blood Marker at Each Exam, After Exclusion of Missing and Outlier Outcome Data
| Exam 1 (2000–2002), No. |
Exam 2 (2002–2004), No. |
Exam 3 (2004–2005), No. |
Exam 4 (2005–2007), No. |
Exam 5 (2010–2012), No. |
Total No. of Observations |
% of Participants with ≥2 Observations |
No. of Unique Participantsa |
|
|---|---|---|---|---|---|---|---|---|
| CRP | 6,713 | 760 | 1,160 | 687 | 1,870 | 11,190 | 47 | 6,889 |
| IL-6 | 6,617 | 754 | 1,683 | 335 | 1,265 | 10,654 | 42 | 6,663 |
| D-dimer | 6,761 | - | 651 | 1,017 | 1,879 | 10,308 | 30 | 7,029 |
| Fibrinogen | 6,765 | 779 | 1,187 | 711 | 1,876 | 11,318 | 48 | 7,037 |
| E-selectin | 989 | - | - | 704 | 711 | 2,404 | 11 | 1,251 |
| sICAM-1 | 2,594 | - | - | 701 | 712 | 4,007 | 11 | 2,865 |
| Total no. | 6,814 | 780 | 1,753 | 1,019 | 1,882 | 12,248 |
Empty cells indicate that the blood marker was not measured at this exam.
Reflects participants from MESA parent study (n = 6,814) and MESA Air new recruits (n = 257).
At all exams, blood was drawn after 12 hours of fasting. All plasma assays were performed at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT) with test characteristics as previously described.26 High-sensitivity CRP and fibrinogen antigen assays were performed using BNII nephelometers (N High Sensitivity CRP and N Antiserum to Human Fibrinogen, respectively; Dade Behring Inc., Deerfield, IL). Both IL-6 and sICAM-1 were measured by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS Human IL-6 Immunoassay and Parameter Human sICAM-1 Immunoassay, respectively; R&D Systems, Minneapolis, MN, respectively). Fibrin fragment D-dimer was measured using an immuno-turbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ). E-selectin was measured using a high-sensitivity quantitative sandwich enzyme (Parameter Human sE-selectin Immunoassay; R&D Systems, Minneapolis, MN). With the exception of E-selectin, all analytes have been previously evaluated for within- and between-individual variation.27–29
Air Pollution Concentrations
Several ambient pollutant concentrations were analyzed, including: PM2.5, ambient-origin individual-level PM2.5 (integrating infiltration into the home and time-location data), oxides of nitrogen (NOx), nitrogen dioxide (NO2), and light absorption coefficient, which represents black carbon (BC). NOx, NO2, and BC are considered markers of traffic-related air pollution (TRAP). All pollution concentrations were predicted at the participant’s residential address. Participants who moved during the year had data incorporated for their new address when possible.
Concentrations of pollutants were calculated from a spatiotemporal prediction model which used a unified modeling approach for all pollutants.30,31 To derive these predictions, we used data from several sources: regulatory monitoring stations from the US Environmental Protection Agency’s Air Quality System (AQS), monitors deployed by MESA Air at fixed sites throughout the study area, outdoor monitors at participant’s homes, and monitors placed to better capture roadway concentration gradients,32 with the exception of the BC model, which did not utilize AQS monitoring data. These different sources of monitoring data provided detailed spatial and temporal data to better characterize within-site variability. Further investigation revealed no systematic differences between the different monitors used to derive our predictions.32,33 Models used geographic covariates such as roadway density, land use, and outputs from dispersion models. Over 150 geographic covariates were available for use in these models. To select the most relevant ones, we employed a partial least squares approach, which is similar to principal-components analysis. Smoothed time trends were extracted from AQS and fixed site monitors to assess temporal trends. Models were built separately for each pollutant and each city in the study. Cross validation was used to select the best final model. In eTable 1 (http://links.lww.com/EDE/A886), we report the site-specific leave-one-out cross-validated R2 based on fit to the 1-1 line and R2 based on fit to the regression line. The cross-validated R2 based on the 1-1 line are the most relevant measure of prediction accuracy, but they are generally lower than those based on the regression line, which we also report to facilitate comparison with research that uses R2-based regression line.30
PM2.5, NOx, and NO2 pollutant concentrations were time varying and estimated the annual average concentration for the year before the participant’s date of blood draw. The individual level PM2.5 predictions were of ambient origin (ie, they do not include indoor sources). They integrate data from time–activity questionnaires kept by the participant to assess the amount of time spent indoors versus outdoors by season during the course of a typical weekday and weekend. The season-specific residential infiltration fraction is also incorporated into this individual-level metric and is derived from questionnaires about characteristics of the participant’s home as well as indoor–outdoor residential pollution sampling in a subset of homes (5% from each study site).34 BC was not time varying because predictions were only available from 2006 to 2008.
Short-term concentrations of PM2.5 reflected several averaging periods before the date of blood draw, including: day of blood draw, prior day and moving average of prior 2, 3, 4, and 5 day periods. Because the dominant variability in short-term PM2.5 concentrations is temporal rather than spatial, short-term PM2.5 concentrations are based on daily observations from a single central site monitor in each region. We did not assess short-term exposures for NOx, NO2, and BC because daily central site measurements were not available or did not adequately capture the spatio-temporal variation. To control for temporal confounding, the short-term PM2.5 concentrations were pre-adjusted using splines for calendar time (12 degrees of freedom [df]/year), temperature (6 df/year), and relative humidity (6 df/year), and an indicator variable for day of the week. This approach has been demonstrated to be an effective alternative to standard semi-parametric adjustment that can increase precision for epidemiologic models assessing health effects of short-term pollutant exposures.31
Covariates
The following covariates were included in the analysis: age, race/ethnicity (white, black, Hispanic, or Chinese), gender, study exam (one through five), site, individual socioeconomic status (SES) defined as education (less than or equal to high school, some college, greater than or equal to college graduate), income (specified continuously as permanent income, which is the average of all reported income over all exams), and employment status (working outside the home or not), neighborhood SES (summary index derived from factor analysis),35 smoking status (current, former, never smoker), second-hand smoke exposure (yes or no), current alcohol consumption (yes or no), body mass index (weight in kilograms/height in meters squared), waist–hip ratio (waist circumference/hip circumference), diabetes (normal, borderline, or treated/untreated as defined by the 2003 American Diabetes Association fasting blood glucose criteria algorithm),36 hypertension (yes/no as defined by the criteria in the sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure),37 and use of any one of the following anti-inflammatory medications: aspirin, NSAIDs or oral anti-inflammatory medications, hormone replacement therapy for women, and lipid-lowering medications. For the sICAM-1 models, we included the single nucleotide polymorphism RS4591 (specified as dichotomous for those with and without the T-allele), which may affect the assays ability to accurately detect circulating levels of sICAM-1.38
In addition, we included city-specific trends in calendar time, modeled with splines that use 4 df for each year the blood marker was collected; therefore CRP, IL-6, and fibrinogen models included 48 df (4 df × 12 years), D-dimer 40 df (4 df × 10 years), and E-selectin and sICAM-1 20 df (4 df × 5 years). In MESA, there is clustering of participants within neighborhood and participants from the same neighborhoods came in for examinations at about the same time, resulting in an association between time and long-term air pollution concentrations. Furthermore, there is a documented seasonality in several of the blood markers under study (CRP, IL-6, fibrinogen, and sICAM-1),39–41 thus necessitating the adjustment for calendar time.
Analysis
The outcomes, main exposure, and most covariates in the analytic models were time varying. A few covariates were fixed or treated as such, including: race/ethnicity, gender, education, neighborhood SES, and income. CRP, IL-6, and D-dimer were log-transformed for analysis. We excluded some extreme outlying values for each blood marker: CRP ≥ 30 mg/liter (n = 76), IL-6 ≥ 10 pg/ml (n = 64), D-dimer ≥ 18 µg/ml (n = 12), fibrinogen ≥ 1,000 mg/dl (n = 6), E-selectin > 132 ng/ml (n = 27), and sICAM-1 > 537 ng/ml (n = 44). Outliers were identified by plotting the distribution of the outcomes. Both log-transformation and exclusion of outliers helped reduce excessive skewness and kurtosis of the outcomes.
For long-term air pollution exposures, we used hierarchical models with a person-level random intercept to account for within-person clustering of blood markers. All other variables were treated as fixed. For short-term exposures, we used fixed effects models specifying person as the fixed effect to handle within-person variability of the outcome. For both long- and short-term exposures, we evaluated three models, each with incrementally more covariates. Model 1 was adjusted for age, race/ethnicity, gender, site, and exam. Model 2 additionally included income, education, employment, neighborhood SES, health behaviors (smoking, second-hand smoke exposure and alcohol consumption), cardiovascular disease risk factors (BMI, waist–hip ratio, and physical activity) and splines for calendar time and was considered our primary model. Model 3 adjusted for variables from model 2 as well as diabetes, hypertension, and anti-inflammatory medications, which may lie on the causal pathway between air pollution and the blood markers of interest. In addition, included in the online supplement (http://links.lww.com/EDE/A886) is model 2a, which includes all the variables in model 1 (described above) as well as the SES variables (income, education, employment, and neighborhood SES). This model staging approach allows readers to ascertain which set of confounders influence parameter estimates the most. Parameter estimates were reported as 5 µg/m3 increase in both ambient and individual PM2.5, 40 parts per billion (ppb) increase in NOx, 17 ppb increase in NO2, and 0.7 10−6 m−1 increase in BC. These values are not interquartile ranges of the pollutants but are close to the interquartile range. Because interquartile ranges depend on site and time frame, these values were chosen to simplify interpretation of results. Short-term models were similar to long-term but did not include splines for calendar time because the short-term exposures were pre-adjusted to control for temporal confounding.31 When the associations between long-term concentrations of air pollution and blood markers were statistically significant, we further included short-term concentrations of PM2.5 in the analysis and conducted stratified analysis to assess effect modification by key covariates. In sensitivity analysis, we employed time-varying nearest monitor predictions and time-invariant predictions calculated for each participant’s baseline home address from Jan 1 to Dec 30, 2000 for all pollutants using the same model staging approach described above.
RESULTS
The maximum number of observations and participants for long-term models is provided in Table 1. For short-term models, each participant was required to have at least two observations, resulting in fewer observations for each pollutant-blood marker combination (eTable 2; http://links.lww.com/EDE/A886). Observations were excluded because of missing data on exposures, outcomes, or covariates and because of outliers for blood markers. The number of participants missing air pollution concentrations varied by pollutant resulting in different final sample sizes for each pollutant–blood marker combination.
The study population was on average 62 years old, 53% female and 27% African-American, 22% Hispanic, 11% Chinese and 39% white (Tables 2 and 3). Correlation coefficients between long-term pollutants were highest for NO2 and NOx (R2 = 0.93) and lowest between ambient PM2.5 and BC (R2 = 0.50; eTable 3; http://links.lww.com/EDE/A886). The distribution of site-specific long-term annual average pollutant concentrations are shown in eFigure1 (http://links.lww.com/EDE/A886). The 4- and 5-day average short-term air pollution concentrations were highly correlated (R2 = 0.97), whereas the day of blood draw and 5-day average were only moderately correlated (R2 = 0.47; eTable 4; http://links.lww.com/EDE/A886).
TABLE 2.
Number (%) of Demographic Characteristics and Cardiovascular Disease Risk Factors at Baseline Exam for All Study Participants
| Participant Characteristic | No. (%) |
|---|---|
| Female | 3,740 (53) |
| Blacka | 1,933 (27) |
| Hispanica | 1,547 (22) |
| Chinesea | 805 (11) |
| Current smokersb | 903 (13) |
| Former smokersb | 2,607 (37) |
| Exposed to second-hand smoke | 3,170 (46) |
| Current alcohol | 3,903 (56) |
| Hypertensive | 3,162 (45) |
| Treated and untreated diabeticc | 905 (13) |
| Borderline diabeticc | 990 (14) |
| Anti-inflammatory medications | 3,814 (54) |
| ≥1 acute infection | 2,277 (32) |
Category not shown is white.
Category not shown is non-smoker.
Category not shown is normal blood sugar.
TABLE 3.
Mean (Standard Deviation) of Blood Markers, Short- and Long-term Air Pollution, and Cardiovascular Disease Risk Factors at Baseline Exam for All Study Participants
| Participant Characteristic | Mean (Standard Deviation) |
|---|---|
| Cardiovascular disease risk factors | |
| Age (years) | 62 (10) |
| Intentional exercise (MET-min/wk) | 1,576 (2,344) |
| BMI (kg/m2) | 28.4 (5.5) |
| Waist–hip ratio | 0.9 (0.1) |
| Annual average air pollution concentrationsa | |
| Ambient PM2.5 (µg/m3) | 16.5 (3.4) |
| Individual PM2.5 (µg/m3) | 10.9 (3.6) |
| NOx (ppb) | 49.8 (27.0) |
| NO2 (ppb) | 21.5 (9.1) |
| Black carbon (10−6 m−1) | 0.8 (0.4) |
| Short-term PM2.5 concentrations (µg/m3) a | |
| Day of blood draw | 17.2 (10.2) |
| Day prior | 16.7 (10.1) |
| 2-day average | 16.6 (9.1) |
| 3-day average | 16.7 (8.4) |
| 4-day average | 16.8 (7.9) |
| 5-day average | 16.9 (7.5) |
| Blood markers | |
| CRP (mg/liter) | 3.5 (4.3) |
| CRP (geometric mean) | 1.9 (3.1) |
| IL-6 (pg/ml) | 1.6 (1.2) |
| IL-6 (geometric mean) | 1.2 (1.9) |
| Fibrinogen (mg/dl) | 348.8 (74.9) |
| D-Dimer (µg/ml) | 0.4 (0.5) |
| D-Dimer (geometric mean) | 0.2 (2.5) |
| E-selectin (ng/ml) | 50.8 (23.2) |
| sICAM-1 (ng/ml) | 267.2 (71.3) |
Means calculated for 6,814 exam 1 participants recruited from 2000 to 2002. Excludes participants recruited at exam 4 (2005–2007) for MESA Air ancillary study (n = 257) because of declining trends in air pollution. Means (standard deviation) for 257 MESA Air participants at recruitment are as follows: ambient PM2.5 = 14.4 (3.6), individual PM2.5 = 10.3 (3.2), NOx = 37.3 (11.7), NO2 = 16.5 (4.3), BC = 0.9 (0.3), day of blood draw = 14.4 (8.8), day prior = 13.9 (7.9), 2-day average = 14.0 (7.0), 3-day average = 13.9 (6.3), 4-day average = 13.8 (5.8), and 5-day average = 14.1 (5.6).
BMI = body mass index; CRP = C-reactive protein; MET = metabolic equivalents.
For most blood markers, means across quartiles of air pollution concentrations did not vary (Table 4). Means for several of the blood markers for quartiles of BC appeared to increase monotonically, ie, the highest quartile of BC had the highest values of the blood markers. The pattern was similar for IL-6 and sICAM-1 for quartiles of NOx and sICAM-1 and NO2. The means of D-dimer and quartiles of PM2.5, however, were higher for the lowest quartile (0.36 µg/ml) but lower for the highest quartile of PM2.5 (0.29 µg/ml).
TABLE 4.
Adjusted Mean Concentrations (Standard Deviation) of Blood Markers by Quartile of Long-term Air Pollution Concentrations for Study Participants at Baseline Exama
| CRP (mg/liter) | IL-6 (mg/liter) | Fibrinogen (mg/dl) | D-Dimer (µg/ml) | E-Selectin (ng/ml) | sICAM-1 (ng/ml) | |
|---|---|---|---|---|---|---|
| Ambient PM2.5 (µg/m3) | ||||||
| <14.5 | 3.09 (0.15) | 1.47 (0.04) | 340.68 (2.44) | 0.36 (0.02) | 57.03 (3.42) | 261.03 (7.42) |
| ≥14.5 to <15.8 | 3.32 (0.12) | 1.49 (0.03) | 344.42 (2.06) | 0.38 (0.02) | 54.23 (1.88) | 260.87 (3.65) |
| ≥15.8 to < 17.7 | 3.43 (0.12) | 1.57 (0.03) | 351.86 (2.07) | 0.35 (0.02) | 54.85 (1.69) | 261.63 (3.2) |
| ≥ 17.7 | 3.07 (0.2) | 1.55 (0.06) | 346.88 (3.37) | 0.29 (0.03) | 56.95 (2.78) | 271.82 (5.51) |
| Ptrendb | 0.95 | 0.21 | 0.08 | 0.03 | 0.98 | 0.30 |
| Black carbon (10−6 m−1) | ||||||
| <0.51 | 2.87 (0.16) | 1.38 (0.04) | 341.31 (2.68) | 0.34 (0.02) | 54.15 (2.11) | 252.11 (4.03) |
| ≥0.51 to <0.65 | 3.1 (0.13) | 1.46 (0.04) | 342.89 (2.11) | 0.34 (0.02) | 56.95 (1.85) | 259.49 (3.3) |
| ≥0.65 to <1.17 | 3.6 (0.12) | 1.58 (0.03) | 348.52 (2.03) | 0.38 (0.02) | 56.58 (1.91) | 266.69 (3.39) |
| ≥1.17 | 3.41 (0.18) | 1.65 (0.05) | 350.86 (2.96) | 0.34 (0.02) | 55.07 (2.52) | 274.01 (4.6) |
| Ptrendb | 0.03 | 0.0006 | 0.03 | 0.75 | 0.86 | 0.003 |
| NOx (ppb) | ||||||
| <26.0 | 3.01 (0.17) | 1.44 (0.05) | 343.2 (2.79) | 0.33 (0.02) | 53.42 (2.24) | 251.74 (4.29) |
| ≥26.0 to <42.4 | 3.31 (0.12) | 1.47 (0.03) | 346.48 (2.05) | 0.34 (0.02) | 55.86 (1.71) | 258.08 (3.07) |
| ≥42.4 to <71.9 | 3.36 (0.13) | 1.55 (0.03) | 345.65 (2.1) | 0.36 (0.02) | 55.61 (1.82) | 267.92 (3.3) |
| ≥71.9 | 3.28 (0.18) | 1.61 (0.05) | 347.98 (2.98) | 0.35 (0.02) | 57.53 (2.33) | 274.16 (4.33) |
| Ptrendb | 0.38 | 0.02 | 0.40 | 0.50 | 0.34 | 0.001 |
| NO2 (ppb) | ||||||
| <13.8 | 3.22 (0.17) | 1.51 (0.05) | 343.26 (2.88) | 0.34 (0.02) | 53.71 (2.35) | 253.41 (4.6) |
| ≥13.8 to <19.9 | 3.25 (0.13) | 1.49 (0.04) | 346.75 (2.19) | 0.35 (0.02) | 54.75 (1.84) | 264.19 (3.36) |
| ≥19.9 to <30.4 | 3.41 (0.14) | 1.57 (0.04) | 344.33 (2.28) | 0.37 (0.02) | 56.19 (2.07) | 265.86 (3.81) |
| ≥30.4 | 3.11 (0.19) | 1.51 (0.05) | 348.35 (3.13) | 0.34 (0.02) | 58.07 (2.58) | 267.42 (4.85) |
| Ptrendb | 0.87 | 0.75 | 0.46 | 0.89 | 0.31 | 0.11 |
Means are adjusted for age, race/ethnicity, gender, and site using linear regression models. Air pollution metrics are annual average concentrations before date of blood draw. Outliers for markers of inflammation, coagulation and endothelial activation have been excluded. Includes 6,814 exam 1 participants only, recruited from 2000 to 2002.
P values refer to tests for trend.
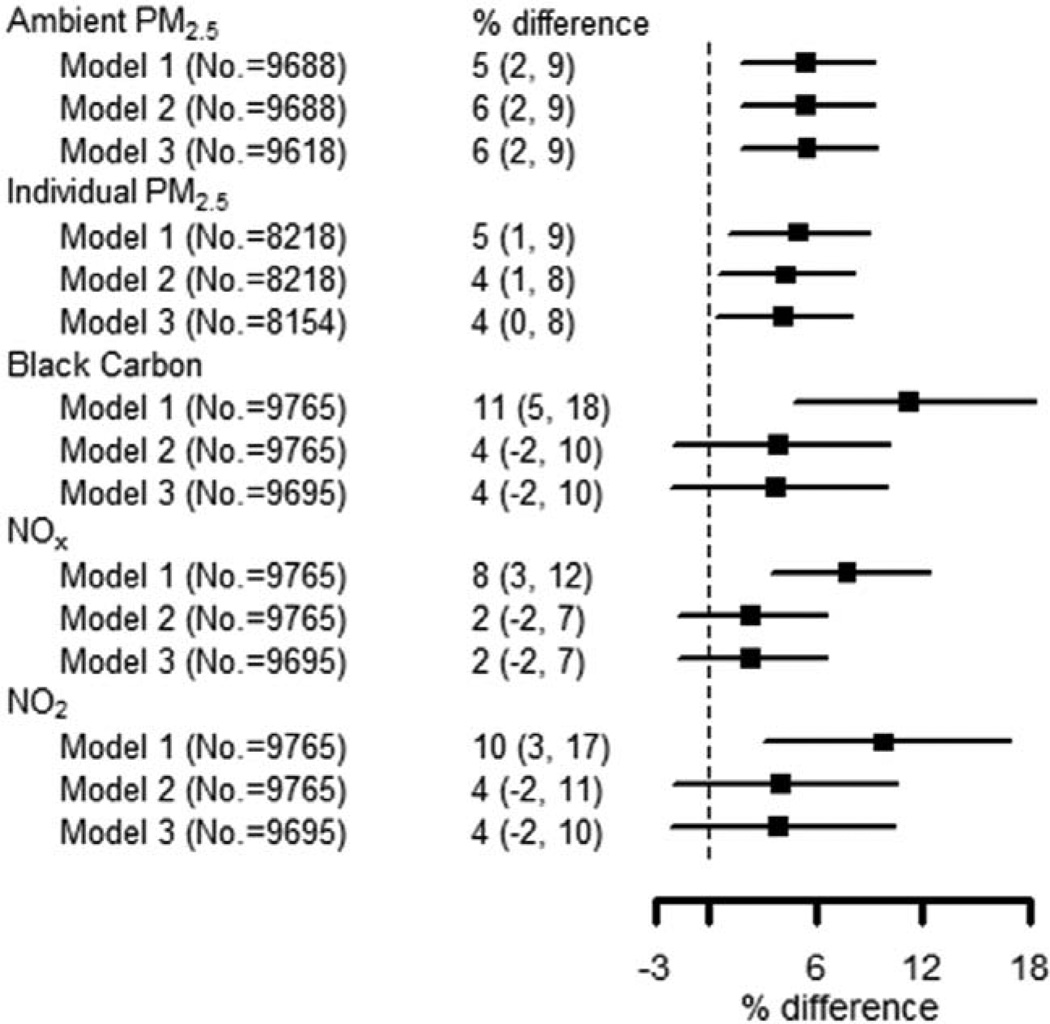
After controlling for confounders in model 2, a 5 µg/m3 increase in ambient and individual PM2.5 was associated with higher levels of IL-6 (6% [95% confidence interval {CI} = 2, 9] and 4% [95% CI = 1, 8], respectively). The parameter estimates for BC, NOx, and NO2 were substantially attenuated with the addition of confounders (Figure 1). The SES covariates were responsible for most of the confounding in these models (eTable 5; http://links.lww.com/EDE/A886). A 40 ppb increase in NOx was associated with 7% higher D-dimer level (95% CI = 2, 13; model 2, Figure 2). The association with NO2 was similar but attenuated. There was no association between D-dimer and ambient PM2.5, individual PM2.5 or BC. The inclusion of additional potential mediators (model 3) had little impact on the point estimates for the associations shown in Figures 1 and 2. We evaluated effect modification for IL-6-ambient PM2.5 and D-dimer-NOx associations (Table 5). In both cases, CIs overlapped between groups (eg, men and women), but the magnitude of parameter estimates was larger for older persons, those not working outside the home, smokers, and hypertensives. In the D-dimer–NOx association, estimates were larger for women, obese, and diabetic participants.
FIGURE 1.
Associations between IL-6 and long-term pollutant concentrations. Air pollution metrics are annual average concentrations. Percent differences in IL-6 are reported as 5 µg/m3 increase in PM2.5, 0.7·10−6 m−1 increases in black carbon, 40 ppb increase in NOx, and 17 ppb increase in NO2. Model 1 is adjusted for age, race/ethnicity, gender, exam, and site. Model 2 is adjusted for the covariates in Model 1 as well as for education, employment, income, neighborhood SES, recent infection, second-hand smoke exposure, smoking status, alcohol consumption, physical activity, BMI, waist–hip ratio, and splines for calendar time. Model 3 is adjusted for the covariates in Model 2 as well as for hypertension, diabetes, and anti-inflammatory medications.
FIGURE 2.
Associations between D-dimer and long-term pollutant concentrations. Air pollution metrics are annual average concentrations. Percent differences are reported as 5 µg/m3 increase in PM2.5, 0.7·10−6 m−1 increases in black carbon, 40 ppb increase in NOx, and 17 ppb increase in NO2. Model 1 is adjusted for age, race/ethnicity, gender, exam, and site. Model 2 is adjusted for the covariates in Model 1 as well as education, employment, income, neighborhood SES, recent infection, second-hand smoke exposure, smoking status, alcohol consumption, physical activity, BMI, waist–hip ratio, and splines for calendar time. Model 3 is adjusted for the covariates in Model 2 as well as for hypertension, diabetes, and anti-inflammatory medications. BMI indicates body mass index.
TABLE 5.
Percent Differences in Concentrations of Blood Markers (IL-6 and D-Dimer) Associated with Incremental Increase in Long-term Pollutant Concentration (Ambient PM2.5 and NOx), in Models Stratified by Individual Demographic and Health Characteristicsa
| % Difference in IL-6 Associated with Ambient PM2.5 (95% CI) |
% Difference in D-Dimer Associated with NOx (95% CI) |
|
|---|---|---|
| Women | 4 (−1, 9) | 10 (3, 19) |
| Men | 8 (3, 14) | 3 (−4, 11) |
| <65 years old | 3 (−2, 9) | 5 (−2, 12) |
| ≥65 years old | 9 (3, 14) | 11 (3, 21) |
| White | 5 (−3, 13) | 11 (1, 23) |
| Non-white | 4 (0, 9) | 4 (−3, 11) |
| ≥Some college | 8 (3, 14) | 8 (1, 16) |
| ≤High school | 3 (−2, 9) | 8 (−1, 17) |
| Working outside home | 4 (−1, 10) | 5 (−2, 12) |
| Not working outside home | 7 (1, 13) | 12 (3, 21) |
| Not current smoker | 5 (1, 9) | 6 (0, 12) |
| Current smoker | 9 (−2, 22) | 16 (0, 35) |
| Not obese | 6 (1, 11) | 6 (−1, 13) |
| Obese | 5 (−1, 11) | 11 (2, 21) |
| Not diabetic | 5 (1, 10) | 6 (0, 12) |
| Diabetic | 6 (−3, 17) | 23 (5, 43) |
| Not hypertensive | 4 (−1, 9) | 3 (−4, 10) |
| Hypertensive | 9 (3, 15) | 12 (4, 22) |
Air pollution metrics are annual average concentrations. Percent differences in IL-6 and D-dimer are relative to 5 µg/m3 increase in ambient PM2.5 and 40 ppb increase in NOx and are derived from random effects models. Models are adjusted for age, race, gender, exam, education, employment, income, neighborhood SES, second-hand smoke exposure, smoking status, alcohol consumption, physical activity, BMI, waist–hip ratio, recent infection and for calendar time splines, except when model is stratified by one of the aforementioned variables. IL-6 models include 48 df calendar time splines (4 df * 12 years) and D-dimer models include 40 df splines (4df * 10 years).
CRP, E-selectin, and sICAM-1 showed little association with any of the long-term air pollutants (Table 6). The data suggested a negative association between fibrinogen and several air pollutant concentrations, whereas higher pollutant levels were associated with lower levels of fibrinogen (eg, 17 ppb increase in NO2 was associated with 13.1 mg/dl decrease in fibrinogen (95% CI = −19.82, −6.37). We performed sensitivity analysis using nearest monitor PM2.5 data and year 2000 estimates and found largely similar results with improved precision for the time-varying exposures (eTable 6; http://links.lww.com/EDE/A886).
TABLE 6.
Percent Differences and Difference from Mean Blood Markers per Increment of Long-term Pollutant Concentrations (5 µg/m3 Increase in PM2.5, 0.7 10−6 m−1 Increase in Black Carbon, 40 ppb Increase in NOx, and 17 ppb Increase in NO2) from Random-effects Modelsa
| N | CRP, % Difference | N | Fibrinogen, mg/dl | N | E-Selectin, ng/ml | N | sICAM-1, ng/ml | |
|---|---|---|---|---|---|---|---|---|
| Ambient PM2.5 | ||||||||
| Model 1b | 10,120 | 3 (−2, 9) | 10,155 | −0.42 (−4.33, 3.49) | 2,182 | 2.3 (0.54, 4.05) | 3,374 | 0.97 (−5.11, 7.06) |
| Model 2c | 10,120 | 1 (−4, 6) | 10,232 | −3.45 (−7.43, 0.52) | 2,182 | 1.08 (−0.66, 2.82) | 3,374 | −2.07 (−7.69, 3.56) |
| Model 3d | 10,045 | 0 (−5, 6) | 10,232 | −3.29 (−7.28, 0.7) | 2,160 | 0.87 (−0.88, 2.63) | 3,349 | −2.07 (−7.72, 3.59) |
| Individual PM2.5 | ||||||||
| Model 1 | 8,651 | 0 (−6, 6) | 8,741 | −0.34 (−4.54, 3.86) | 2,026 | 1.64 (−0.28, 3.57) | 2,985 | −0.58 (−6.58, 5.42) |
| Model 2 | 8,651 | −2 (−8, 4) | 8,741 | −2.31 (−6.46, 1.84) | 2,026 | 0.71 (−1.2, 2.62) | 2,985 | −3.02 (−8.47, 2.43) |
| Model 3 | 8,581 | −2 (−8, 4) | 8,669 | −2.25 (−6.41, 1.9) | 2,004 | 0.53 (−1.4, 2.46) | 2,960 | −2.84 (−8.32, 2.64) |
| Black carbon | ||||||||
| Model 1 | 10,197 | 5 (−4, 15) | 10,310 | −3.51 (−10, 2.98) | 2,202 | −0.89 (−4.26, 2.49) | 3,461 | 0.94 (−8.2, 10.08) |
| Model 2 | 10,197 | 2 (−7, 12) | 10,310 | −6.03 (−12.62, 0.56) | 2,202 | −2.69 (−6.16, 0.78) | 3,461 | −1.02 (−9.32, 7.28) |
| Model 3 | 10,122 | 3 (−6, 12) | 10,233 | −5.7 (−12.3, 0.91) | 2,180 | −2.67 (−6.15, 0.8) | 3,436 | −0.58 (−8.9, 7.74) |
| NOx | ||||||||
| Model 1 | 10,197 | 0 (−7, 6) | 10,310 | 2.37 (−2.19, 6.93) | 2,202 | 1.85 (−0.21, 3.92) | 3,461 | 3.88 (−1.97, 9.73) |
| Model 2 | 10,197 | −4 (−11, 2) | 10,310 | −0.54 (−5.24, 4.15) | 2,202 | 0.49 (−1.61, 2.58) | 3,461 | −0.13 (−5.67, 5.41) |
| Model 3 | 10,122 | −4 (−11, 2) | 10,233 | −0.54 (−5.24, 4.16) | 2,180 | 0.3 (−1.8, 2.41) | 3,436 | 0.07 (−5.49, 5.62) |
| NO2 | ||||||||
| Model 1 | 10,197 | −5 (−15, 4) | 10,310 | −12.55 (−19.06, −6.03) | 2,202 | 1.07 (−1.94, 4.08) | 3,461 | −0.93 (−9.54, 7.68) |
| Model 2 | 10,197 | −7 (−17, 2) | 10,310 | −13.1 (−19.82, −6.37) | 2,202 | −0.57 (−3.72, 2.59) | 3,461 | −0.54 (−8.78, 7.7) |
| Model 3 | 10,122 | −7 (−17, 3) | 10,233 | −13.01 (−19.75, −6.28) | 2,180 | −0.53 (−3.7, 2.64) | 3,436 | −0.09 (−8.37, 8.18) |
Air pollution metrics are annual average concentrations.
Model 1 is adjusted for age, race/ethnicity, gender, exam, and site.
Model 2 is adjusted for the covariates in Model 1 as well as education, employment, income, neighborhood SES, second-hand smoke exposure, smoking status, alcohol consumption, physical activity, BMI, waist–hip ratio, recent infection, and for calendar time splines. CRP, IL-6, and fibrinogen models include 48 df calendar time splines (4 df * 12 years), D-dimer models include 40 df splines (4df * 10 years) and E-selectin and sICAM-1 models includes 20 df splines (4df * 5 years). sICAM-1 models were also adjusted for the gene RS4591 (no T versus at least one T).
Model 3 is adjusted for the covariates in Model 2 as well as for hypertension, diabetes, and anti-inflammatory medications.
A 5 µg/ml increase in PM2.5 on the day of blood draw was associated with 0.60 ng/ml increase in E-selectin (95% CI = 0.06, 1.14) and suggestive of a positive association with CRP (1% difference, 95% CI = 0, 3) and fibrinogen (1.16 mg/dl, 95% CI = −0.28, 2.61; model 2, Table 7). However, the association between D-dimer and sICAM-1 and the 3-, 4-, and 5-day lagged PM2.5 were suggestive of an inverse relation. Associations between other blood markers and short-term PM2.5 were null. Finally, including short-term PM2.5 exposures in the long-term associations between PM2.5 and IL-6, and between NOx and D-dimer, did not substantially alter point estimates (eFigure 2; http://links.lww.com/EDE/A886).
TABLE 7.
Percent Differences and Difference from Mean Blood Markers per 5 µg/m3 Increase in Short-term PM2.5 Concentrations from Fixed Effects Models
| CRP (% Difference) |
IL-6 (% Difference) |
D-Dimer (% Difference) |
Fibrinogen (mg/dl) |
Fibrinogen (mg/dl) |
sICAM-1 (ng/ml) |
|
|---|---|---|---|---|---|---|
| Day of blood draw | ||||||
| Model 1a | 2 (0, 3) | 0 (−1, 1) | 1 (−1, 3) | 1.09 (−0.36, 2.54) | 0.61 (0.05, 1.16) | 0.43 (−1.73, 2.59) |
| Model 2b | 1 (0, 3) | 0 (−1, 1) | 1 (−1, 3) | 1.16 (−0.28, 2.61) | 0.6 (0.06, 1.14) | 0.43 (−1.7, 2.55) |
| Model 3c | 1 (0, 3) | 0 (−1, 1) | 1 (−1, 3) | 1.19 (−0.28, 2.66) | 0.6 (0.05, 1.14) | 0.42 (−1.74, 2.58) |
| Day prior | ||||||
| Model 1 | 0 (−1, 2) | 0 (−1, 1) | 0 (−1, 2) | −0.37 (−1.75, 1) | 0.27 (−0.26, 0.81) | −1.03 (−3.01, 0.96) |
| Model 2 | 0 (−1, 2) | 0 (−1, 1) | 0 (−1, 2) | −0.33 (−1.7, 1.04) | 0.39 (−0.13, 0.91) | −0.6 (−2.57, 1.36) |
| Model 3 | 1 (−1, 2) | 0 (−1, 1) | 0 (−1, 2) | −0.27 (−1.66, 1.12) | 0.35 (−0.18, 0.89) | −0.88 (−2.89, 1.13) |
| Average 2 day | ||||||
| Model 1 | −1 (−3, 1) | 0 (−1, 1) | 0 (−2, 2) | −0.08 (−1.73, 1.57) | 0.3 (−0.36, 0.96) | −1.43 (−3.86, 1) |
| Model 2 | −1 (−3, 1) | 0 (−1, 1) | 0 (−2, 2) | −0.04 (−1.69, 1.6) | 0.44 (−0.2, 1.08) | −0.93 (−3.33, 1.47) |
| Model 3 | −1 (−3, 2) | 0 (−1, 1) | 0 (−2, 2) | 0.05 (−1.63, 1.73) | 0.27 (−0.39, 0.93) | −1.29 (−3.75, 1.17) |
| Average 3 day | ||||||
| Model 1 | −2 (−4, 1) | 0 (−1, 2) | −2 (−4, 0) | 0.16 (−1.73, 2.05) | −0.23 (−0.96, 0.51) | −3.99 (−6.75, −1.23) |
| Model 2 | −1 (−4, 1) | 0 (−1, 2) | −2 (−4, 0) | 0.12 (−1.76, 2) | −0.02 (−0.74, 0.69) | −3.21 (−5.93, −0.48) |
| Model 3 | −1 (−4, 1) | 0 (−1, 2) | −2 (−4, 0) | 0.26 (−1.66, 2.18) | −0.22 (−0.95, 0.51) | −3.58 (−6.36, −0.79) |
| Average 4 day | ||||||
| Model 1 | −2 (−4, 1) | 1 (−1, 2) | −2 (−5, 0) | −0.56 (−2.64, 1.52) | −0.61 (−1.42, 0.2) | −5.19 (−8.24, −2.13) |
| Model 2 | −1 (−4, 1) | 1 (−1, 2) | −2 (−5, 0) | −0.51 (−2.58, 1.56) | −0.37 (−1.16, 0.42) | −4.22 (−7.24, −1.21) |
| Model 3 | −1 (−4, 1) | 1 (−1, 2) | −2 (−5, 0) | −0.32 (−2.43, 1.79) | −0.59 (−1.39, 0.22) | −4.5 (−7.57, −1.42) |
| Average 5 day | ||||||
| Model 1 | −2 (−5, 1) | 1 (0, 3) | −3 (−5, 0) | −1.59 (−3.89, 0.71) | −0.57 (−1.42, 0.28) | −4.17 (−7.47, −0.88) |
| Model 2 | −2 (−4, 1) | 1 (0, 3) | −3 (−5, 0) | −1.4 (−3.68, 0.89) | −0.33 (−1.16, 0.5) | −3.13 (−6.38, 0.13) |
| Model 3 | −2 (−4, 1) | 1 (0, 3) | −3 (−5, 0) | −1.22 (−3.55, 1.1) | −0.5 (−1.35, 0.34) | −3.23 (−6.53, 0.08) |
Model 1 is adjusted for site, age, race/ethnicity, gender, and exam.
Model 2 is adjusted for all variables in Model 1 as well as education, employment, income, neighborhood SES, recent infection, second-hand smoke exposure, smoking status, alcohol consumption, BMI, exercise, waist–hip ratio. sICAM-1 models also adjusted for the gene RS4591 (no T versus at least one T).
Model 3 is adjusted for all covariates in Model 2 as well as hypertension, diabetes, and anti-inflammatory medications.
DISCUSSION
We found evidence of a positive association between long-term PM2.5 and a marker of inflammation (IL-6), and between NOx (a TRAP) and a marker of fibrinolysis (D-dimer). Based on previous research, the magnitude of effects is likely in the range of what is to be expected for a typical long-term ambient exposure.22 Furthermore, the findings were not consistent across all the available markers of inflammation and coagulation or across all TRAPs, thus we approach our findings with some caution. The research on short-term air pollution exposure and blood markers has commonly found inconsistencies across categories of blood markers (eg, findings were robust for sICAM-1 but null for e-Selectin, two markers of endothelial activation).10 There was no association among CRP, fibrinogen, e-Selectin, and sICAM-1 and long-term concentrations of air pollution.
In assessing effect modification, we found suggestive evidence that older individuals, smokers, and participants with hypertension experienced larger increases in IL-6 and D-dimer compared with younger, non-smoking, and normotensive participants. This finding is consistent with some studies on short-term air pollution and inflammation and coagulation9,42,43 and with our general understanding of which populations are more susceptible to the health effects of air pollution.1
To put our results in context, it is useful to compare the magnitude of effects we observed for air pollutants with those observed for other common risk factors, such as smoking and gender. A 5 µg/m3 increase in PM2.5 corresponds to about 0.081 pg/ml higher IL-6 and a 40 ppb increase in NOx to about 0.018 µg/ml higher D-dimer. In our study, current smokers had about 0.32 pg/ml increase in IL-6 (22%) compared with those who never smoked and women had about 0.044 µg /ml increase in D-dimer (17%) compared with men. These are about four and two times higher, respectively, than the estimated effects of long-term concentrations of air pollution.
Two previous studies found a positive association between long-term air pollution and IL-6 and NO2 (a marker of TRAP).20,23 In our study, however, IL-6 was positively associated with PM2.5. IL-6 is an acute phase reactant elaborated by T-cells and macrophages, so our result suggests an effect of air pollution early in the inflammatory process. Similar to Forbes et al,21 we did not find an association with the other inflammatory marker, CRP; a German study did find a positive association between CRP and PM2.5, but only among men.22
Fibrinogen, an acute phase reactant and coagulation factor, is to our knowledge the only marker involved in the coagulation cascade that has been previously studied with respect to long-term air pollution. A positive association between fibrinogen and long-term PM2.5 was found in German men but not in an English or a Swedish cohort21–23; our study replicates the null findings of these two studies. We also found a null association between long-term air pollutants and E-selectin and sICAM-1, neither of which has been evaluated in existing research.
The association between long-term air pollution and D-dimer has not been previously evaluated, thus our findings of a positive association between NOx and D-dimer are, we believe, novel. D-dimer is a fibrin degradation product and is produced as a result of the breakdown of fibrin clots. Epidemiologic and controlled exposure studies of short-term D-dimer and air pollutants have been largely null,6,7,10,44 thus our results suggesting long-term air pollution has a role to play in fibrinolysis require additional confirmation.
We found some evidence of positive associations between day of blood draw and CRP, fibrinogen, and E-selectin. The evidence in this area is mixed: some studies have found similar positive associations for acute air pollution and CRP,10,45 fibrinogen,44,46 and E-selectin,47 whereas several others have found null associations.2 A study using the MESA cohort at baseline examined PM2.5 1 day, 2 days, and 7 days before blood draw and found null effects for both CRP and IL-6.48 It is unclear why associations between 3-, 4-, and 5-day averages and D-dimer and sICAM-1 were negative. The use of fixed effects models for the short-term exposures leveraged the within-person variability in blood markers and controlled for unmeasured confounders better than a random-effects model. The random effects model (eTable 7; http://links.lww.com/EDE/A886) gave similar results, except there was no association between day of blood draw and fibrinogen and there was a suggestion of a positive association between E-selectin and day before blood draw. The associations between 3-, 4-, and 5-day averages and D-dimer and sICAM-1 were negative in both models.
Consistent with Hoffmann et al,22 our study that found positive long-term associations between PM2.5 and IL-6 and between NOx and D-dimer were not greatly impacted by inclusion of acute PM2.5. We cannot, however, be certain that this association is free from confounding by short-term air pollution because we did not assess acute NOx exposures in the NOx–D-dimer association and our acute exposures are not spatially resolved.
Our approach to air pollution exposure assessment advances the field in two ways. First, individual PM2.5 prediction incorporates participant- and season-specific information about time participants spent indoors versus outdoors and residential infiltration efficiencies.34 To the best of our knowledge, this is the first study to use predictions that more realistically represent concentrations of ambient-origin PM2.5. Comparing the results of ambient to individual PM2.5, we see point estimates closer to the null and slight improvements in precision that may represent a reduction in one dimension of measurement error. Second, our use of pre-adjusted acute exposure is a novel approach which can improve efficiency while effectively minimizing concerns over statistical power.31 It should be noted that black carbon predictions relied solely on study-specific monitors (central monitoring data is unavailable), and in turn parameter estimates for BC from health effects models were less precise than those for other air pollutants (Figures 1 and 2, Table 6).
Sensitivity analysis using nearest-monitor PM2.5 and year 2000 estimates did not change the conclusions of our study (eTable 6; http://links.lww.com/EDE/A886). A few point estimates changed signs, but given the wide confidence intervals containing the null, the study conclusions remain the same.
The strengths of our study included improvements in air pollution exposure assessment, the large number of measurements, the use of repeat blood measures performed at a central laboratory, and a well-characterized cohort that allowed good control for confounding. The study of some biological markers can be challenging because of inadequate reproducibility of assays. Each one of the biomarkers used in this study is highly reproducible and if large effects had existed, we likely would have detected them.
Our study had a few limitations. Given the relative inconsistency of our results and the multiple comparisons made, statistically significant results may be due to chance alone. However, our findings were in line with previous research. In addition, because NO2 is not measured directly (it is the difference between concentrations of NO and NOx) and because there are fewer AQS sites for NO compared with NOx, precision of our NO2 estimates is slightly worse than PM2.5 and NOx. Also, the MESA population is healthier than the general public: all participants were free of cardiovascular disease at baseline and those who remained in the cohort over time reflect a healthy participant bias. Several studies have shown stronger effects of air pollution on blood markers in populations with diabetes, hypertension, or heart disease.9,10,49 Finally, generalizability of our study is limited to older adults residing in mostly urban areas.
Overall, we found evidence that long-term exposure to air pollution was associated with some markers of inflammation and fibrinolysis, and short-term exposure was associated with some markers of inflammation, coagulation, and endothelial activation.
Supplementary Material
Acknowledgments
The main MESA study was funded by the National Institutes of Health NHLBI (N01-HC-95159 through N01-HC-95165, N01-HC-95169). The MESA Air study was funded by the US Environmental Protection Agency (EPA) under a STAR research assistance agreement (R831697). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication. The MESA abdominal body composition study was funded by R01-HL-088451 and the MESA Stress was funded by R01-HL-076831, R01 HL10161-01A1, and R21-DA-024273. AH was also funded by K99-ES-023498, and JDK was also funded by NIEHS (P30ES07033 and K24-ES-013195).
Footnotes
Disclosure: The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the authors.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, III, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Rittenhouse-Olson K, Scheider WL, Mu L. Effect of particulate matter air pollution on C-reactive protein: a review of epidemiologic studies. Rev Environ Health. 2012;27:133–149. doi: 10.1515/reveh-2012-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaptoge S, White IR, Thompson SG, et al. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol. 2007;166:867–879. doi: 10.1093/aje/kwm191. [DOI] [PubMed] [Google Scholar]
- 4.Kleinegris MC, ten Cate H, ten Cate-Hoek AJ. D-dimer as a marker for cardiovascular and arterial thrombotic events in patients with peripheral arterial disease. A systematic review. Thromb Haemost. 2013;110:233–243. doi: 10.1160/TH13-01-0032. [DOI] [PubMed] [Google Scholar]
- 5.Demetz G, Ott I. The interface between inflammation and coagulation in cardiovascular disease. Int J Inflam. 2012;2012:860301. doi: 10.1155/2012/860301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomberg A, Törnqvist H, Desmyter L, Deneys V, Hermans C. Exposure to diesel exhaust nanoparticles does not induce blood hypercoagulability in an at-risk population. J Thromb Haemost. 2005;3:2103–2105. doi: 10.1111/j.1538-7836.2005.01559.x. [DOI] [PubMed] [Google Scholar]
- 7.Carlsten C, Kaufman JD, Peretz A, Trenga CA, Sheppard L, Sullivan JH. Coagulation markers in healthy human subjects exposed to diesel exhaust. Thromb Res. 2007;120:849–855. doi: 10.1016/j.thromres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 9.Rückerl R, Greven S, Ljungman P, et al. AIRGENE Study Group. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rückerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 11.Cannon RO., III Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44(8 Pt 2):1809–1819. [PubMed] [Google Scholar]
- 12.Noll G, Tschudi M, Nava E, Lüscher TF. Endothelium and high blood pressure. Int J Microcirc Clin Exp. 1997;17:273–279. doi: 10.1159/000179239. [DOI] [PubMed] [Google Scholar]
- 13.Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003;45:443–458. doi: 10.1053/pcad.2003.YPCAD13. [DOI] [PubMed] [Google Scholar]
- 14.Nahser PJ, Jr, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–640. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 15.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 16.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 17.Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- 18.Toborek M, Kaiser S. Endothelial cell functions. Relationship to atherogenesis. Basic Res Cardiol. 1999;94:295–314. doi: 10.1007/s003950050156. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan RM, Adar SD, Szpiro AA, et al. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60:2158–2166. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med. 2011;68:64–68. doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- 21.Forbes LJ, Patel MD, Rudnicka AR, et al. Chronic exposure to outdoor air pollution and markers of systemic inflammation. Epidemiology. 2009;20:245–253. doi: 10.1097/EDE.0b013e318190ea3f. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann B, Moebus S, Dragano N, et al. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect. 2009;117:1302–1308. doi: 10.1289/ehp.0800362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panasevich S, Leander K, Rosenlund M, et al. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med. 2009;66:747–753. doi: 10.1136/oem.2008.043471. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman JD, Adar SD, Allen RW, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnett DK, McClelland RL, Bank A, et al. Biomarkers of inflammation and hemostasis associated with left ventricular mass: the Multiethnic Study of Atherosclerosis (MESA) Int J Mol Epidemiol Genet. 2011;2:391–400. [PMC free article] [PubMed] [Google Scholar]
- 27.Cava F, González C, Pascual MJ, Navajo JA, González-Buitrago JM. Biological variation of interleukin 6 (IL-6) and soluble interleukin 2 receptor (sIL2R) in serum of healthy individuals. Cytokine. 2000;12:1423–1425. doi: 10.1006/cyto.2000.0714. [DOI] [PubMed] [Google Scholar]
- 28.Sakkinen PA, Macy EM, Callas PW, et al. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: assessment and implications for epidemiology. Am J Epidemiol. 1999;149:261–267. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
- 29.Van Hoydonck PG, Schouten EG, Temme EH. Reproducibility of blood markers of oxidative status and endothelial function in healthy individuals. Clin Chem. 2003;49(6 Pt 1):963–965. doi: 10.1373/49.6.963. [DOI] [PubMed] [Google Scholar]
- 30.Keller JP, Olives C, Kim SY, et al. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect. 2014 doi: 10.1289/ehp.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szpiro AA, Sheppard L, Adar SD, Kaufman JD. Estimating acute air pollution health effects from cohort study data. Biometrics. 2014;70:164–174. doi: 10.1111/biom.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43:4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MESA Air Pollution Study. Exposure Monitoring Quality Assurance/Quality Control Report (QA/QC Report) [Accessed October 21, 2014]; Available at http://www.uwchscc.org/MESAAP/Data.aspx. [Google Scholar]
- 34.Allen RW, Adar SD, Avol E, et al. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Health Perspect. 2012;120:824–830. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajat A, Diez-Roux AV, Adar SD, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2013;121:1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A merican Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–S48. [PubMed] [Google Scholar]
- 37.Joint National Committee. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 38.Bielinski SJ, Pankow JS, Li N, et al. ICAM1 and VCAM1 polymorphisms, coronary artery calcium, and circulating levels of soluble ICAM-1: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;201:339–344. doi: 10.1016/j.atherosclerosis.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ Health. 2010;9:42. doi: 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampel R, Breitner S, Rückerl R, et al. Air temperature and inflammatory and coagulation responses in men with coronary or pulmonary disease during the winter season. Occup Environ Med. 2010;67:408–416. doi: 10.1136/oem.2009.048660. [DOI] [PubMed] [Google Scholar]
- 41.Schneider A, Panagiotakos D, Picciotto S, et al. AIRGENE Study Group. Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology. 2008;19:391–400. doi: 10.1097/EDE.0b013e31816a4325. [DOI] [PubMed] [Google Scholar]
- 42.Delfino RJ, Staimer N, Tjoa T, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhal Toxicol. 2003;15:1465–1478. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- 45.Pope CA, 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong J, Zhu T, Kipen H, et al. Comparisons of ultrafine and fine particles in their associations with biomarkers reflecting physiological pathways. Environ Sci Technol. 2014;48:5264–5273. doi: 10.1021/es5006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hildebrandt K, Rückerl R, Koenig W, et al. Short-term effects of air pollution: a panel study of blood markers in patients with chronic pulmonary disease. Part Fibre Toxicol. 2009;6:25. doi: 10.1186/1743-8977-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diez Roux AV, Auchincloss AH, Astor B, et al. Recent exposure to particulate matter and C-reactive protein concentration in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;164:437–448. doi: 10.1093/aje/kwj186. [DOI] [PubMed] [Google Scholar]
- 49.Alexeeff SE, Coull BA, Gryparis A, et al. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect. 2011;119:481–486. doi: 10.1289/ehp.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.