Abstract
Introduction
There is a paucity of research about the impact of palliative care (PC) on perceived control (i.e. one’s perceived influence over outcomes or events in the environment) and activation (i.e. ability to self-manage) in patients with symptomatic heart failure (HF). Likewise, little is known about the association between perceived control, activation, and symptom distress in this patient population. We hypothesized that patients with advanced HF who received ongoing PC services (i.e. ≥2 PC consultations) vs no access or a single PC consultation would have greater improvements in perceived control and activation and greater reductions in symptom distress three months post-discharge for HF exacerbation.
Methods
Forty-two patients (average age 53.9±8.0 years; predominantly male (72%), White (61%) and married (69%)) participated in the study. However, only 36 (85.7%) patients completed an outpatient PC consultation of which 29 (69%) patients returned for additional follow-up visits with the PC team. Data on perceived control, activation, and symptom distress were collected at baseline and three months. Parametric statistical models were applied to draw conclusions.
Results
Findings showed that the patients who received ≥2 PC consultations had greater improvements in perceived control and activation than their counterparts; these increases were associated with greater reductions in symptom distress.
Conclusion
Our findings suggest that on-going PC interventions enhance perceived control and activation in patients with advanced HF and open up the possibility of planning larger studies to assess the effect of PC on these variables as possible mediators to improvements in self-management and clinical outcomes.
Keywords: Heart failure, palliative care, perceived control, activation, self-care
Introduction
Chronic heart failure (HF) is a health care epidemic characterized by progressive decline of cardiac performance and functional status with frequent decompensation of the chronic state resulting in recurrent hospitalizations.1 Despite tremendous advances achieved in medical management, HF continues to present patients with challenges that lead to marked physical, psychological, social, and extreme distress; furthermore, amongst these patients diverse symptoms are common and result in feelings of loss of control over their own health outcomes (i.e. perceived control) or inadequate self-management knowledge, skills, and self-efficacy, which are expressed by the composite construct of patient activation (i.e. activation).2 Self-management is the ability of the patient to deal with symptoms, treatment, complications, and lifestyle changes; it goes beyond traditional knowledge-based patient education to include processes that enhance self-advocacy, improve self-efficacy, and support application of knowledge to maintain a satisfactory quality of life. Since patient self-management is so critical to health outcomes, greater attention to symptom management earlier in the HF trajectory may potentially reduce suffering from both physical and psychological symptoms and lessen the distress associated with this incurable condition.3
One approach to addressing the needs of patients living with HF is the integration of palliative care (PC) with standard HF care.4–6 Likewise, guidelines advocate for PC, referral to hospice, and end of life support for patients suffering with terminal illness.7 Palliative care is ‘an interdisciplinary team approach to optimizing QOL (quality of life) and symptom management that does not necessarily exclude any medical therapy and takes into account physical, psychosocial, and spiritual needs and patient/family preferences.’8 A substantial literature calls for PC in older adults and caregivers suffering with HF. Moreover, recent position statements and health care delivery models emphasize several critical needs to enhance a new protocol for PC in this vulnerable group of chronically ill patients that includes: (a) Interdisciplinary team evaluation and symptom management with the integration of psychosocial, functional, and behavioral support; (b) Multidimensional assessment to identify, prevent, and alleviate suffering; and (c) Early integration of PC with updates based on changes in clinical status.9–11 However, research that focuses on the impact of PC on symptom control in HF is still in its infancy. Likewise, although there is increasing advocacy for timely symptom control in patients with HF, there is limited research examining the efficacy of PC services on perceived control and activation.12
The primary objective of the current descriptive correlational study was to obtain preliminary data on the efficacy of PC services on enhancing perceived control and activation in patients with symptomatic HF. The specific aims of the study were to: (a) assess levels of perceived control and activation immediately after discharge with acute HF decompensation and three months thereafter; (b) compare the impact of no access or limited access to PC services (i.e. single PC consultation) vs access to on-going PC services (i.e. ≥ 2 PC consultations) on perceived control and activation in a sample of patients with symptomatic HF; and (c) determine the association between perceived control, activation, and symptom distress in patients immediately after and three months post-discharge for HF exacerbation. We hypothesized that patients with advanced HF who received on-going PC services would have greater improvements in perceived control and activation and consequently, greater reductions in symptom distress three months post-discharge for HF exacerbation than their counterparts.
Methods
Study design and setting
This prospective, single-cohort, study was conducted at a single, tertiary care medical center with both a specialized HF disease management program led by seven heart failure specialist and four nurse practitioners with expertise in HF disease management and a PC clinic comprised of two board certified PC physicians, a nurse practitioner with expertise in PC, and PC support staff (e.g. pharmacist, psychiatrist, social worker, physical, occupational, and speech therapist, and chaplain).13 The appropriate Institutional Review Board reviewed and approved the research protocol; all participants gave written informed consent.
Study participants
Participants were recruited from the inpatient setting during an episode of acute HF exacerbation through HF provider referrals. Eligible participants were at least 18 years old, able to read, write, and speak English or Spanish; and were willing to be referred for a PC consultation. Patients were precluded from study participation if they had: (a) cognitive decline (e.g. dementia); (b) other co-morbid terminal illness (e.g. malignancy); (c) surgically implanted left ventricular assist device; and (d) currently receiving PC services for symptom management.
Procedures
Prior to hospital discharge, a member of the research team provided the patient with a packet containing: (a) a PC program brochure; (b) a cover letter explaining the purpose of the PC consultation with a date and time of their PC appointment; the letter encouraged participants to bring their spouse, partner, or other family member to the initial visit; and (c) an information sheet to instruct the study participant to schedule a telephone interview with a member of the research team; the purpose of the 20–30 min interview was to obtain baseline information from participants prior to the their initial PC consultation (~7–10 days after discharge). After completion of the baseline telephone interview, the research staff conducted chart reviews to extract data about participants’ medical history and current clinical status and treatment regimen. A follow-up telephone interview was scheduled three months after the initial PC consultation and was conducted by the same research staff.
The initial PC consultation was scheduled a week following hospital discharge, and in conjunction with participants’ follow-up visit with their HF provider. During this initial consultation, the PC specialist (e.g. physician or advance practice nurse) completed a standardized intake summary (e.g. current health status, treatment regimen), assessed physical and psychological symptoms, determined illness understanding, established goals of care with the patient and family, and assisted with treatment decision making and coordination of care. The intervention is described in greater depth in another paper.13 All patients were encouraged to avail themselves of on-going PC services based on their identified goals of care; they were given the number for the 24-hour on-call service staffed by the PC team and were encouraged to call for additional PC support (e.g. worsening of symptoms, support for care coordination, etc.).
Measures
During the baseline interviews, participants were asked to provide information related to their sociodemographic (i.e. personal characteristics—age, gender, race/ethnicity, marital status, level of education, employment status, occupation) and clinical status (i.e. medical history and current treatment regimen). Participants were also asked to complete a series of surveys at baseline and three-months later.
To measure perceived control, participants completed the revised Control Attitude Scale (CAS-R),14 an eight-item tool designed to measure a person’s belief that he or she has the resources to cope with the negative events associated with cardiac illness; sample items are ‘I can do a lot of things myself to cope with my heart condition’ and ‘Regarding my heart problems, I feel lots of control.’15 The total score is obtained by reversing the ratings on negatively phrased items and adding the item scores – each item is rated on a scale of 1 (totally disagree) to 5 (totally agree); scores range from 8–40 with higher scores indicating greater perceived control.14 Cronbach’s alpha values for the CAS-R in patients with coronary heart disease, acute myocardial infarction, and HF were all greater than 0.70.14
The participants also completed the Patient Activation Measure (PAM), a 13-item, interval-level, unidimensional tool, developed by Hibbard and colleagues16 to assess patient’s self-rated ability to take preventive actions, manage symptoms, access medical care, and work with health care providers to make decisions about care.2 A four-point Likert scale that ranges from 1 (strongly agree) to 4 (strongly disagree) is used for each item and these scores are added to derive a single score previously shown to be reliable and valid.17 Hibbard & colleagues describe four levels of activation, viewed as sequential across a hierarchical continuum: 1 (low level of activation)—believing the patient role is important (score≤47); 2 (also a low level)—having the knowledge and confidence to take action (score=47.1–55.1); 3 (medium)—taking action to maintain or improve health (score=55.2–67); and 4 (high)—maintaining healthy lifestyle changes under stress (score ≥67.1).18 A study conducted in the target population of HF patients suggest that the PAM is highly reliable at the individual patient level and a valid instrument for assessing activation and individualizing care in HF patients with a Cronbach’s α of 0.88.19
Finally, to measure symptom distress, participants completed the Edmonton Symptom Assessment Scale (ESAS), a self-reported visual analog scale developed for use in assessing the symptoms of patients receiving palliative care.20 It includes nine common symptoms of advanced cancer (pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, shortness of breath); scores range from 9–90 with higher scores indicating greater symptom distress.21 It is designed to enable repeated quantitative measurements of multidimensional symptom intensity with minimal patient burden, numerically rated from zero (no symptom at all) to 10 (worse possible symptom).22 Since its original inception by Bruera et al. in 1991,20 the ESAS has been adopted in diverse palliative and cancer care programs and countries.21 The reliability of the modified ESAS was previously established in patients with chronic illness with a Cronbach’s alpha coefficient of 0.86.20 In a group of patients with HF, the ESAS was highly correlated with quality of life measures.23
Data analysis
Data analysis was performed using SPSS for Windows Statistical Program (version 18, 1.0, SPSS, Inc., Chicago, Illinois, USA). Descriptive statistics including means, ranges, standard deviations and chi-square statistics were used to characterize the study population and summarize distribution of perceived control, activation scores, and symptom distress. Data were analyzed using both parametric and non-parametric statistics; data were fairly similar so only parametric data are presented in the paper. Differences in sociodemographic and clinical variables and variables of interest were examined using independent sample t-tests or chi-squared tests depending on levels of measurement. Bivariate analyses examined correlations between sociodemographic characteristics, PC group, perceived control, activation, and symptom distress scores. Reported p-values are two-sided and adjusted for multiple comparisons.
Results
Patient characteristics
A total of 42 patients provided informed consent for participation in the study at the time of hospital discharge; 85.7% came for the initial PC consultation. Of the 36 patients who came for the PC consultation, 29 (69%) received additional PC services beyond the initial consultation (Figure 1). The median number of follow-up visits for each participant over three months was two days (mean, 2.21± 0.27, range 1–4 days). The number of follow-up visits and telephone calls for the 29 patients who availed themselves of ≥2 PC consultations totaled 64 and 45, respectively.13 Table 1 shows the sociodemographic and clinical characteristics of participants who reported ≤1 PC consultation vs ≥2 PC consultations; no significant differences between the two groups were observed.
Figure 1.
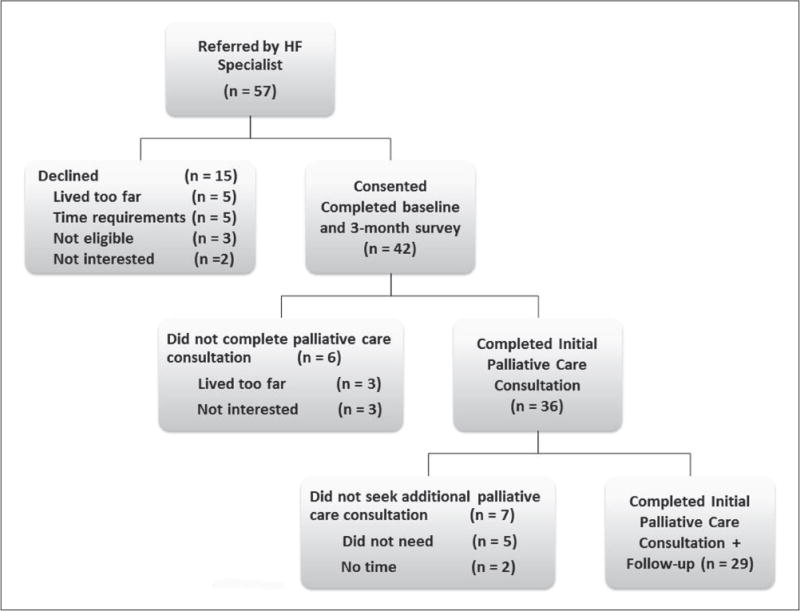
Flow diagram of study process. HF: heart failure.
Table 1.
Baseline sociodemographic and clinical characteristics (n=42).
| All participants (n=42) |
Participants receiving≤1 palliative care consultation (n=13) | Participants receiving > 2 palliative care consultations (n=29) | Sig. | |
|---|---|---|---|---|
| Age, years (mean±SD) | 53.7±7.9 | 52.5±7.6 | 53.3±7.3 | 0.930 |
| Male, n (%) | 30 (71.4) | 8 (61.5) | 22 (84.6) | 0.383 |
| Race, n (%) | 0.168 | |||
| Hispanic | 7 (16.7) | 3 (23.1) | 4 (13.8) | |
| White | 24 (57.1) | 5 (38.5) | 19 (55.5) | |
| Black | 11 (26.2) | 5 (38.5) | 6 (20.7) | |
| Married, n (%) | 29 (69.0) | 10 (76.9) | 19 (65.5) | 0.578 |
| Education, n (%) | 0.664 | |||
| <High school graduate | 18 (42.9) | 4 (30.8) | 14 (48.3) | |
| Some college | 12 (28.6) | 4 (30.8) | 8 (27.6) | |
| >College graduate | 12 (28.6) | 5 (38.5) | 7 (24.1) | |
| Ejection fraction, % (mean±SD) | 26.1±6.2 | 30.5.14±9.7 | 23.1±4.3 | 0.094 |
| Charlson Comorbidity Index | 3.7±1.5 | 3.5±1.0 | 2.7±1.4 | 0.134 |
| NYHA class, n (%) | 0.983 | |||
| Class II | 29 (69.0) | 9 (69.2) | 20 (69.0) | |
| Class III | 13 (31.0) | 4 (30.8) | 9 (31.0) | |
| Comorbidities | ||||
| Hypertension, n (%) | 26 (61.9) | 7 (53.8) | 19 (65.5) | 0.823 |
| Coronary artery disease, n (%) | 23 (54.8) | 5 (38.5) | 18 (62.1) | 0.365 |
| Diabetes mellitus, type 2, n (%) | 16 (38.1) | 7 (53.8) | 9 (31.0) | 0.246 |
| Overweight or obese, n (%) | 29 (69.0) | 8 (61.5) | 21 (72.4) | 0.875 |
| History of smoking (previous), n (%) | 15 (35.7) | 4 (30.8) | 11 (37.9) | 0.842 |
| Medications use, n (%) | ||||
| ACE inhibitors | 31 (73.8) | 10 (76.9) | 21 (72.4) | 0.747 |
| Angiotensin receptor blockers | 8 (19.0) | 3 (23.1) | 5 (17.2) | 0.649 |
| Beta-blockers | 31 (73.8) | 10 (76.9) | 21 (72.4) | 0.847 |
| Diuretics | 28 (66.7) | 10 (76.9) | 18 (62.1) | 0.178 |
| Pain medications | 14 (33.3) | 6 (46.1) | 8 (27.6) | 0.335 |
| Antidepressants | 11 (41.0) | 5 (38.5) | 6 (20.6) | 0.430 |
p<0.05; ACE: Angiotensin Converting Enzyme; NYHA: New York heart Association; SD: standard deviation.
Perceived control, activation, and symptom distress
Data showed that perceived control and activation levels were fairly low at baseline in both groups (Table 2). Using a repeated measures general linear model, and assessing the total study population, time was a statistically significant predictor of perceived control (F=65.1, p<0.001), activation (F=27.0, p<0.001), and symptom distress (F=68.3, p<0.001). No differences were observed in in perceived control, activation, and symptom distress at baseline between the two groups; however, participants who received on-going PC care reported significantly greater improvements in perceived control (F=26.5, p<0.001) and activation scores (F=14.4, p<0.001) and greater reductions in symptom distress (F=4.5, p=0.040) compared to their counterparts.
Table 2.
Comparison of perceived control and activation levels at baseline and three months for participants receiving≤1 palliative consultation (n=13) and those receiving >2 palliative care consultations (n=29).
| Participants
|
≤1 Palliative consultation
|
>2 Palliative care consultations
|
p value (time) | p value (G×T) | ||
|---|---|---|---|---|---|---|
| Variable | Baseline | 3-month | Baseline | 3-month | ||
| Perceived control (CAS-R) |
26.5±5.9 | 29.2±5.0 | 24.9±7.4 | 37.4±9.1 | <0.001 | <0.001 |
| Patient activation (PAM) |
39.3±6.4.3 | 41.4±7.5 | 37.3±7.3 | 50.2±10.4 | <0.001 | <0.001 |
| Symptom distress (ESAS) |
27.6±11.4 | 23.1±10.2 | 35.7±7.1 | 28.2±5.5 | <0.001 | 0.040 |
CAS-R: Control Attitude Scale-Revised; PAM: Patient Activation Measure; ESAS: Edmonton Symptom Assessment Score.
Table 3 shows the proportion of participants who reported increased activation levels at three months and the corresponding average increase at three months according to the baseline activation level and number of PC services accessed. A significantly greater proportion of participants who attended two or more PC consults showed increases in their activation levels (p<0.001) following the intervention compared to their counterpart.
Table 3.
Participants with improvements in activation levels at three-month follow-up participants receiving< 1 palliative participants receiving > 2 palliative care consultation (n= 13) care consultations (n=29).
| 3-Month activation levela n (%) | 3-Month activation levela n (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Baseline activation levela n (%) | 1 | 2 | 3 | 4 | Change in activation %
|
Change in activationb mean±SD | Baseline activation levela n (%) | 1 | 2 | 3 | 4 | Change in activation %
|
Change in activationb mean±SD | ||||
| Increased | Decreased | Increased | Decreased | ||||||||||||||
| 1 | 10 (77) | 6 (60) | 4 (40) | 0 (0) | 0 (0) | 40% | 0% | −1.8±6.1 | 1 | 24 (83) | 8 (33) | 13 (54) | 3 (13) | 0 (0) | 67% | 0% | 22.6±4.9 |
| 2 | 3 (23) | 2 (67) | 1 (33) | 0 (0) | 0 (0) | 0% | 66.7% | −8.1±5.5 | 2 | 5 (17) | 0 (0) | 0 (0) | 4 (80) | 1 (20) | 100% | 0% | 16.2±6.4 |
Patient activation scores were derived from the Patient Activation Measure (range, 0–100) and are divided into four levels. Level 1 (score <47.0) is associated with not believing that one has a role to play in self-management of their condition. Level 2 (score 47.1–55.1) is associated with a lack of knowledge and confidence to take action in self-management of their condition. Level 3 (score 55.2–67.0) is associated with beginning to take action in self-management. Level 4 (scores >67.1) is associated with maintaining behavior change, although individuals may still experience difficulties overcoming obstacles; for the current study, none of the participants had scores in levels 3 and 4.
Any increases or decreases in patient activation scores have taken into account the baseline activation levels.
Bivariate analyses
Table 4 illustrates the correlation between the key variables. In the entire sample, race was associated with increased perceived control at baseline (r=0.430, p<0.001); Hispanics had the lowest perceived control scores followed by Blacks. On-going PC was related to perceived control (p<0.001); activation levels (p=0.006); and symptom distress (p=0.038) at three-month follow-up. Perceived control at baseline was associated with perceived control at three-month follow-up and activation scores at baseline and three-month follow-up (all p’s<0.001), but not symptom distress at either time points. Activation scores at baseline were related to activation scores (p=0.001), perceived control (p=0.007), and symptom distress (p=0.026) at three-month follow-up. Intuitively, symptom distress at baseline and symptom distress at the three-month follow-up and perceived control and activation at three months were highly correlated (both p<0.001). There was a moderate correlation between perceived control and symptom distress and activation and symptom distress at three months (both p’s<0.05).
Table 4.
Correlational matrix for the key variables (n=42).
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Race | 1.000 | |||||||
| 2. | Palliative care | −0.222 | 1.000 | ||||||
| 3. | Perceived control (baseline) | −0.430a | −0.107 | 1.000 | |||||
| 4. | Patient activation (baseline) | −0.259 | −0.069 | 0.618a | 1.000 | ||||
| 5. | Symptom distress (baseline) | 0.285 | 0.203 | −0.057 | −0.219 | 1.000 | |||
| 6. | Perceived control (3-months) | 0.157 | 0.432a | 0.604a | 0.487a | 0.089 | 1.000 | ||
| 7. | Patient activation (3-months) | 0.087 | 0.383b | 0.417a | 0.412a | −0.057 | 0.838a | 1.000 | |
| 8. | Symptom distress (3-months) | 0.221 | 0.352b | −0.298 | −0.371a | 0.915a | 0.344b | 0348b | 1.000 |
p<0.001,
p <0.05.
Discussion
Our study shows that HF patients have a significant need for control and care activation. We compared the impact of no access or limited access to PC services versus on-going receipt of PC interventions on perceived control, activation, and symptom distress in a cohort of patients recently hospitalized with HF decompensation immediately after and three months post discharge. The study’s results suggest that co-management of a PC outpatient clinic alongside or as part of a specialty HF clinic is effective and can make an impact on HF patients’ perception of control and care activation. The importance of a longitudinal outpatient relationship between the PC professional and the HF patient is demonstrated by the difference in outcomes with on-going receipt of PC. This study adds to the growing literature about the effectiveness of outpatient PC for HF patients and the need for early PC interventions in HF management.
Our findings also showed very low levels of perceived control and activation in our patient sample at baseline; a majority of the patients presented with low levels of activation. Furthermore, none of the patients reported activation levels beyond stage 2 which reflects patients’ low confidence in their ability to take control of their health. Seeking out additional PC support beyond the initial PC consultation helped patients achieve greater perceived control and enhanced their confidence to become increasingly active in self-managing their health as reflected in patients achieving higher levels of activation during the three month follow-up. Studies have shown that as an individual progresses through the second and third levels of activation, they develop the knowledge and skills to become actively involved in self-managing their condition.2,19,24 Likewise, patients who believe that they can impact their own health are more likely to play a role in making decisions about their health and are more likely to adhere to behaviors that promote symptom control.18 We speculate that this argument explains why patients who received on-going PC had greater reductions in symptom distress and supports the premise that referring patients for PC services early in the disease trajectory can potentially enhance problem solving skills that enable the individual to confidently engage in decision-making and actions to effectively manage their chronic health condition.25
Our findings related to race are consistent with a previous study examining perceived control and activation in patients with chronic illness; Hispanics and Blacks are less likely to have higher levels of perceived control and activation compared to Whites.26 Future research examining relationships between sociodemographic variables, perceived control, and activation in a larger sample are warranted to better explicate the impact of personal characteristics on self-management.
As the concept of patient-centered care gains momentum, health care providers need to be proactive in providing patients with the tools necessary to make informed decisions about their health care and to solve problems encountered daily from living with a chronic condition. Palliative care interventions have been seen as a means for reducing disability and promoting quality of life through more proactive measures to enhancing access to care, increasing patient involvement in managing their health, and promoting better symptom control.27 The current study provides researchers and clinicians with a better understanding of the potential role of PC interventions in enhancing perceived control and care activation and promoting patient’s readiness, willingness, and ability to manage their own care. We also demonstrate the potential benefits of initiating PC earlier in the HF trajectory as reflected in the data that a large proportion of our sample (69%) was New York Heart Association functional class II.
Study limitations and future work
There are several important limitations to our findings. First, we had a small, heterogeneous sample which limits the strength and generalizability of our conclusions. For example, we observed a trend for higher comorbidity scores in patients with no or limited PC access; however, the sample size was too small to detect any significant differences. Second, enrollment in the study was based on a convenience sample of patients willing to participate in the study and be referred for PC services resulting in a sample that was probably skewed toward patients with a more favorable view toward PC even before their participation. Likewise, it is possible that patients who had higher symptom distress were less likely to agree to participate in the study, thus leading to the underestimation of the prevalence and severity of symptoms. Third, because of the lack of a true control group and the possibility of selection bias, our findings should be viewed as hypothesis- generating and in need of testing in a long-term, randomized, controlled trial. Additionally, future work designed to draw conclusions about perceived control and activation should incorporate a larger sample. Investigating other relevant outcomes, such as social support, may also be useful for future research.
Conclusion
Our findings suggest that on-going PC interventions show promise in being able to enhance perceived control and activation in patients with advanced HF and open up the possibility of planning larger studies to assess the effect of PC on these variables as possible mediators to improvements in self-management and clinical outcomes. Measuring perceived control and activation and using the information to improve PC programs and processes that support patient self-management could be an important key to reducing symptom distress and improving outcomes of care in patients with symptomatic HF.3–4 Thus, developing PC programs to promote active self-management and determining the mechanisms by which they influence outcomes warrant additional investigation.
Implications for Practice.
On-going palliative care interventions may potentially enhance perceived control and activation and warrant further investigation in a larger clinical trial.
Integrating measures to increase perceived control and activation to support self-management may be key to implmenting effective palliative care programs in heart failure patients.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, National Institutes of Health, and the National Institute on Aging.
Funding: This study received funding from the National Heart, Lung, and Blood Institute (1R01HL093466-01) and University of California, Los Angeles, Resource Centers for Minority Aging Research/Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under National Institute in Aging (P30-AG02-1684, Principal investigator: C Mangione).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Lloyd-Jones D, Adam RJ, Brown TM. Heart disease and stroke statistics – 2010 update a report from the American Heart Association. Circulation. 2010;121:e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hibbard JH, Mahoney E. Toward a theory of patient and consumer activation. Patient Educ Couns. 2010;78:377–381. doi: 10.1016/j.pec.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Blinderman CD, Homel P, Billings JA, et al. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35:594–603. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Love R, Sewertsky J. Management of end-stage heart failure: Improving palliative care. Can J Cardiovasc Nurs. 2007;17:13–18. [PubMed] [Google Scholar]
- 5.Bekelman DB, Nowels C, Allen L, et al. Outpatient palliative care for chronic heart failure: A case series. J Palliat Med. 2011;14:814–821. doi: 10.1089/jpm.2010.0508. [DOI] [PubMed] [Google Scholar]
- 6.Hauptman PJ, Havranek EP. Integrating palliative care into heart failure care. Arch Inten Med. 2005;165:374–378. doi: 10.1001/archinte.165.4.374. [DOI] [PubMed] [Google Scholar]
- 7.Heart Failure Society Of America. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 9.Goodlin SJ, Hauptman PJ, Arnold R, et al. Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–209. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Clinical practice guidelines for quality palliative care. www.nationalconsensusproject.org [serial online] (2009, accessed 6 June, 2012)
- 11.Hupcey JE, Penrod J, Fenstermacher K. Review article: A model of palliative care for heart failure. Am J Hosp Palliat Care. 2009;26:399–404. doi: 10.1177/1049909109333935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14:449–464. doi: 10.1089/jpm.2010.0382. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista LS, Liao S, Motie M, et al. Does the type and frequency of palliative care services received by patients with advanced heart failure impact symptom burden? J Palliat Med. 2014;17:1–5. doi: 10.1089/jpm.2013.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser D, Riegel B, Mckinley S, et al. The Control Attitudes Scale-Revised: Psychometric evaluation in three groups of patients with cardiac illness. Nurs Res. 2009;58:42–51. doi: 10.1097/NNR.0b013e3181900ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinley S, Fien M, Riegel B, et al. Complications after acute coronary syndrome are reduced by perceived control of cardiac illness. J Adv Nurs. 2012;68:2320–2330. doi: 10.1111/j.1365-2648.2011.05933.x. [DOI] [PubMed] [Google Scholar]
- 16.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbard JH, Mahoney ER, Stockard J, et al. Development and testing of a short form of the Patient Activation Measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shively MJ, Gardetto NJ, Kodiath MF, et al. Effect of patient activation on self-management in patients with heart failure. J Cardiovasc Nurs. 2013;28:20–34. doi: 10.1097/JCN.0b013e318239f9f9. [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, Kuehn N, Miller M, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 21.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: Development and refinement. Psycho-Oncology. 2012;21:977–985. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- 22.Stromgren AS, Sjogren P, Goldschmidt D, et al. A longitudinal study of palliative care. Cancer. 2005;103:1747–1755. doi: 10.1002/cncr.20958. [DOI] [PubMed] [Google Scholar]
- 23.Opasich C, Gualco A, De Feo S, et al. Physical and emotional symptom burden of patients with end-stage heart failure: What to measure, how and why. J Cardiovasc Med. 2008;9:1104–1108. doi: 10.2459/JCM.0b013e32830c1b45. [DOI] [PubMed] [Google Scholar]
- 24.Stepleman L, Rutter MC, Hibbard J, et al. Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil Rehabil. 2010;32:1558–1567. doi: 10.3109/09638280903567885. [DOI] [PubMed] [Google Scholar]
- 25.Solomon M, Wagner S, Goes J. Effects of a web-based intervention for adults with chronic conditions on patient activation: Online randomized controlled trial. J Med Internet Res. 2012;14:e32. doi: 10.2196/jmir.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degner L, Kristjanson L, Bowman D, et al. Informational needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–1492. [PubMed] [Google Scholar]
- 27.Zimmermann C, Riechelmann R, Krzyzanowska M, et al. Effectiveness of specialized palliative care. JAMA. 2008;299:1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]


