Abstract
Background
Chloral hydrate (CH), a sedative and metabolite of the environmental contaminant trichloroethylene, is metabolized to trichloroacetic acid, trichloroethanol, and possibly dichloroacetate (DCA). DCA is further metabolized by glutathione transferase zeta 1 (GSTZ1), which is identical to maleylacetoacetate isomerase (MAAI), the penultimate enzyme in tyrosine catabolism. DCA inhibits its own metabolism through depletion/inactivation of GSTZ1/ MAAI with repeated exposure, resulting in lower plasma clearance of the drug and the accumulation of the urinary biomarker maleylacetone (MA), a metabolite of tyrosine. It is unknown if GSTZ1/MAAI may participate in the metabolism of CH or any of its metabolites and, therefore, affect tyrosine catabolism. Stable isotopes were utilized to determine the biotransformation of CH, the kinetics of its major metabolites, and the influence, if any, of GSTZ1/MAAI.
Methods
Eight healthy volunteers (ages 21 – 40 years) received a dose of 1 g of CH (clinical dose) or 1.5 μg/kg (environmental) for five consecutive days. Plasma and urinary samples were analyzed by gas chromatography-mass spectrometry.
Results
Plasma DCA (1.2 – 2.4 μg/mL), metabolized from CH, was measured on the fifth day of the 1 g/day CH dosage but was undetectable in plasma at environmentally relevant doses. Pharmacokinetic measurements from CH metabolites did not differ between slow and fast GSTZ1 haplotypes. Urinary MA levels increased from undetectable to 0.2 – 0.7 μg/g creatinine with repeated CH clinical dose exposure. Kinetic modeling of a clinical dose of 25 mg/kg DCA administered after 5 days of 1 g/day CH closely resembled DCA kinetics obtained in previously naïve individuals.
Conclusions
These data indicate that the amount of DCA produced from clinically relevant doses of CH, although insufficient to alter DCA kinetics, is sufficient to inhibit MAAI and tyrosine catabolism, as evidenced by the accumulation of urinary MA.
Keywords: dichloroacetate, glutathione transferase zeta 1, maleylacetoacetate isomerase, maleylacetone, phenylalanine, trichloroacetic acid, trichloroethanol, trichloroethylene
Introduction
Chloral hydrate (CH) is one of the oldest medicinal sedatives [1] and is still used in pediatric medicine and dentistry to induce conscious sedation [2]. Standard doses for sedation vary from 50 mg/kg for infants to up to 1 g/day in adults. In addition to its clinical medications, CH remains a drug of abuse and is regulated as a controlled substance.
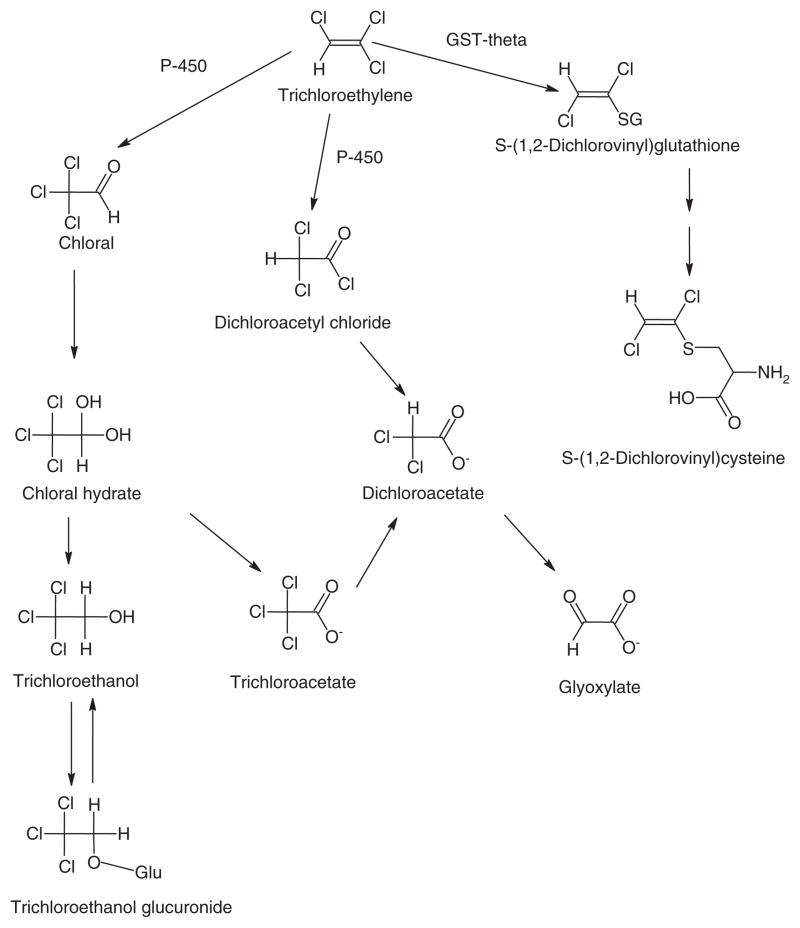
CH’s relevance to environmental science is derived from its formation through water chlorination and from its role as a metabolic intermediate of the Superfund chemical trichloroethylene (TCE) [3]. Human environmental exposure to CH is estimated to be about 1 – 2 μg/kg/day. CH is rapidly reduced to trichloroethanol (TCOH) in the presence of NADH and is oxidized to trichloroacetic acid (TCA) in the presence of NAD+ by alcohol dehydrogenase. TCOH can exist as the intact compound or its glucuronide conjugate (TCOH-Glu) through metabolism by UDP-glucuronosyltranferase [4] (Figure 1). In contrast to the rapid metabolism of CH, the elimination half-life for TCOH and TCA after a single dose of 1 g can be several hours. Repeated exposure to clinical levels of CH can increase the elimination half-life for TCOH and TCA from hours to several days [6].
Figure 1.
Biotransformation of trichloroethylene. Adapted from [5].
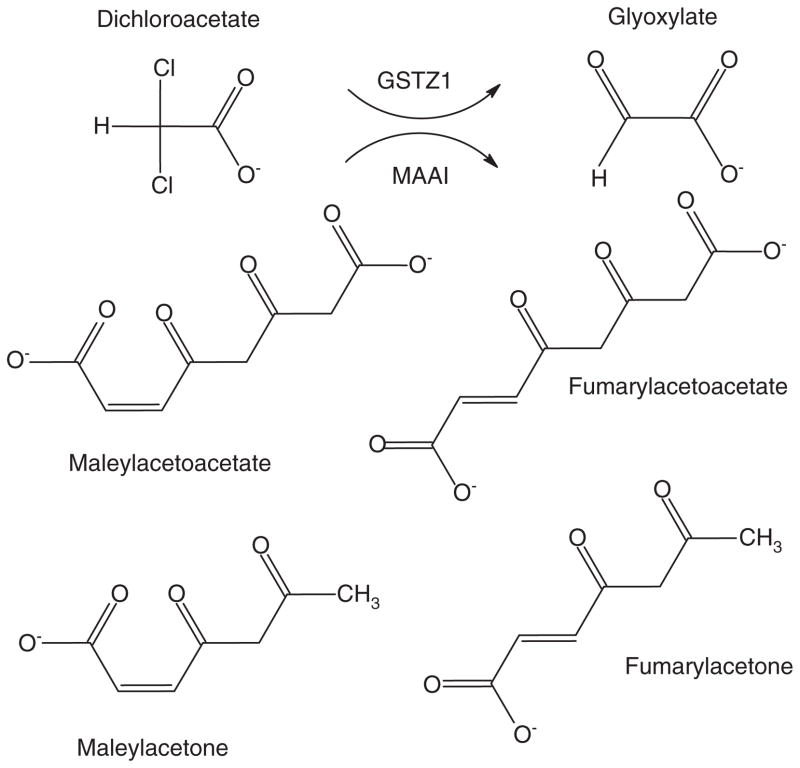
Dichloroacetate (DCA) has also been tentatively identified as CH metabolite, formed via several routes, including dechlorination of TCA (Figure 1). DCA is also a major by-product of water chlorination, as well as a metabolite of TCE and certain drugs. Human environmental exposure to DCA is approximately 2 – 4 μg/kg/day (reviewed in [7]). DCA is dehalogenated to glyoxylate by cytosolic (and, to a lesser degree, mitochondrial) glutathione transferase zeta 1 (GSTZ1), which is identical to maleylacetoacetate isomerase (MAAI), the penultimate enzyme in the phenylalanine/ tyrosine catabolic pathway [8] (Figure 2). Both subject age and genotype influence the kinetics of DCA. The GSTZ1 protein is a dimer consisting of two 24.2 kDa subunits [9], and DCA is a mechanism-based inhibitor of the enzyme, resulting in decreased plasma drug clearance with repeated exposure. GSTZ1/MAAI exhibits five major haplotypes: KRT (Z1A), KGT (X1B), EGT (Z1C), EGM (Z1D), and KGM (Z1F) [10]. Individuals possessing at least one EGT allele metabolize DCA more rapidly than do subjects lacking this allele [10]. Consequently, the plasma elimination half-life after 5 days of 25 mg/kg oral DCA in healthy adults can vary from 2 to as long as 100 h, based on GSTZ1 haplotype [10].
Figure 2. Bifunctionality of GSTZ1/MAAI.
GSTZ1 dehalogenates DCA to the naturally occurring molecule glyoxylate. MAAI isomerizes maleylacetoacetate and maleylacetone, respectively, to fumarylacetoacetate and fumarylacetone.
It has been difficult to unequivocally determine whether DCA is a metabolite of CH from studies in humans or animals [11, 12]. As recently reviewed [7], DCA is not only an environmentally important xenobiotic but also an investigational drug in the treatment of several congenital and acquired diseases, the latter at exposure levels of 10 – 50 mg/kg/day. In one study of adults who received 1 g CH, the measured DCA plasma levels were so low as to be considered analytical artifacts of the method [6]. However, in a separate study, clinically significant levels of approximately 20 μg/mL of DCA were found in the plasma of children given a single oral dose of 50 mg/kg CH [13]. This amount of CH-derived DCA was sufficient to increase the drug’s elimination half-life, as compared with DCA naïve subjects, when 1,2-13C-DCA pharmacokinetic modeling was used.
Repeated exposure to clinically relevant DCA doses also inhibits tyrosine catabolism and leads to the urinary accumulation of the reactive tyrosine metabolite, maleylacetone (MA) [10]. Urinary MA is nondetectable in healthy adults, regardless of their GSTZ1 haplotype [10]. However, repeated mg/kg doses of DCA result in measurable levels of urinary MA that are highest in those individuals who lack the EGT allele and, thus, possess GSTZ1/MAAI isoforms conferring slowest metabolism of DCA [10]. Nevertheless, urinary MA has been monitored in individuals exposed to clinical doses of DCA from several months to years but does not accumulate over time and elicits no apparent toxicity [14]. This suggests that urinary MA can reach a steady state, reflecting a balance between DCA-induced depletion of the enzyme and new enzyme synthesis [14]. In children who received 25 mg/kg/day for up to 30 months, a strong correlation (r = 0.90) was found between urinary MA and DCA plasma trough concentrations [15].
We undertook the present study to determine whether DCA is a metabolite of CH when administered to healthy adults at clinical and environmental exposure levels and, if so, to determine whether the quantity of DCA generated from CH can, through the inactivation of GSTZ1/MAAI, alter plasma DCA plasma kinetics and the urinary accumulation of MA. We also tested the hypothesis that TCA or some other CH metabolite could inhibit GSTZ1/MAAI, as evidenced by differences in plasma clearance based on GSTZ1/MAAI haplotype.
Materials and methods
Chemicals
Pure CH standard, TCE, TCA, and TCOH were obtained from Sigma Chemical Co (St. Louis, MO, USA), and 10% syrup used for clinical administration was from Pharmaceutical Associates, Inc. (Greenville, SC, USA). [13C1] CH (chemical purity > 98%, isotopic purity > 99%) and [13C1,2] sodium DCA (chemical purity > 98%, isotopic purity > 99%) were custom synthesized by Cambridge Isotopes (Andover, MD, USA). [12C1,2] DCA (chemical purity > 99%, clinical grade) was from TCI America (Portland, OR, USA). TCA, TCE, and TCOH were analyzed for DCA as an impurity by gas chromatography-mass spectrometry (GC-MS). DCA was found < 0.02%. MA was synthesized in-house according to the procedure described by [10], and its structure was verified by mass spectrometry and nuclear magnetic resonance analyses. All other chemicals were reagent grade or higher.
Clinical studies
This research was approved by the University of Florida Institutional Review Board and the Scientific Advisory Committee of the Clinical Research Center (CRC). Eight male and nine female healthy adults (aged 21 – 40 years) were studied in the CRC inpatient ward. Subjects were randomized into two separate groups: Group 1 to measure plasma CH kinetics and Group 2 to measure the effect of CH-derived DCA on DCA kinetics. Subjects in the first group received 13C-CH on day 1, 12C-CH from days 2 to 4, and another dose of 13C-CH on day 5. Each subject was studied sequentially at two different CH exposure levels, first at an environmentally relevant dose of 1.5 μg/kg CH and, subsequently at a clinically relevant dose of 1 g by mouth, with a washout period of at least 30 days. Plasma and urine samples were collected and analyzed by mass spectrometry for both 12C and 13C compounds to determine the kinetics of the metabolites of CH, whether 13C-DCA was present as a metabolite of 13C-CH, and whether urinary MA accumulates (as a result of inactivation of MAAI). Subjects in Group 2 received 5 days of 12C-CH at the doses described above but were given an additional 25 mg/kg oral dose of 13C-DCA on the morning of day 6 to determine the kinetics of 13C-DCA and to determine whether the DCA formed from CH influenced 13C-DCA kinetics. Urine for MA was collected daily. There was no dietary control for tyrosine/phenylalanine intake.
GC-MS analysis
All compounds were analyzed by an Agilent 5975C Inert GC-MS after derivatization in plasma and urine to form their methyl esters with 12% boron trifluoride in methanol and subsequent extraction with methylene chloride [16]. Several samples were analyzed by an alternative methylation method using diazomethane with similar results. DCA was not measured as an analytical artifact from either analytical method. Specific ions were used to monitor both 13C-and 12C-molecular fragments independently. The limit of quantitation for DCA, TCA, and MA was 0.1 μg/mL, and TCOH was 2.0 μg/mL. Plasma samples from the environmental CH dose were analyzed for DCA and TCA [17] and MA [18], using gas chromatography negative chemical ionization mass spectrometry. Limits of quantitation for the environmental dosage samples are DCA (50 ng/mL), TCA (250 ng/mL), and MA (50 ng/mL).
DNA isolation, genotyping and haplotype analysis
The coding region of the GSTZ1/MAAI gene contains three functionally important nonsynonymous single-nucleotide polymorphisms (SNPs) that give rise to five major GSTZ1 haplotypes: KRT (Z 1 A), KGT (Z 1 B), EGT (Z 1 C), EGM (Z 1 D) and KGM (Z 1 F). EGT is the most frequent haplotype among the major racial and ethnic groups [7]. Two other very rare SNPs in GSTZ1 have also been reported [19, 20]. Kinetics investigations indicate that subjects who harbor at least one EGT allele metabolize DCA more rapidly than non-EGT carriers [19].
Accordingly, DNA from blood samples was genotyped for three nonsynonymous SNPs: G94 > A (rs7975) Glu → Lys at amino acid position 32 (E32K), G124 > A (rs7972) Gly → Arg at position 42 (G42R), and C245 > T(rs1046428) Thr → Met at position 82 (T82M) in the GSTZ1/ MAAI gene by pyrosequencing [21]. Haplotypes were inferred by computational methods using the Bayesian haplotype reconstruction program, PHASE version 2.1.39 [22].
Pharmacokinetic analysis
The plasma-concentration time curve for all measurements was fitted to a noncompartmental pharmacokinetic model for each patient using WinNonlin, version 5.01 software (Pharsight, Mountain View, CA, USA) obtained through the academic license program. We calculated the area under the plasma concentration-time curve (AUC) from time 0 to 1440 min (24 h) for 13C-TCA, using the linear-trapezoidal method. At least three sampling points were used by the modeling software to estimate the first-order elimination rate constant (λz) for each time-concentration curve.
Statistical analyses
The mean, standard deviation, and statistical significance of the data were determined using Excel software (Microsoft, Redmond, WA, USA). A two-sided Student’s t-test was used to analyze kinetic and metabolic data between groups; and in all cases, a p value of ≤ 0.05 was considered statistically significant.
Results
CH is rapidly metabolized into TCA and TCOH, and no effort was made to measure CH directly in plasma. Repeated doses of CH lead to accumulation of TCA and TCOH in plasma [6]. 13C-TCA (detection limit 250 ng/mL) and 13C-DCA (detection limit 50 ng/mL) were undetected in plasma taken from subjects receiving the environmentally relevant dose of 1.5 μg/kg 13C-CH. Similarly, MA was not detected (detection limit 50 ng/mL) in the urinary samples of the same subjects. 13C-TCA plasma clearance, half-life, Cmax, and AUC changed significantly from day 1 to day 5 of 1 g/day CH administration. The average half-life of 13C-TCA after five doses of CH was approximately 100 h, in agreement with previous studies [6]. Repeated dosing of CH over 5 days approximately doubled the TCA half-life. However, in contrast to kinetic studies of DCA, no statistical difference was found in the clearance of 13C-TCA and 13C-TCOH between “fast” (EGT carriers) and “slow” (EGT noncarriers) metabolizers of DCA after five consecutive days of a 1 g dose of CH per day (Table 1). Therefore, it is doubtful that GSTZ1/MAAI influences the elimination of either TCA or TCOH. 13C-and 12C-DCA were not found in the plasma after the first CH dose, but 12C-DCA and trace levels of 13C-DCA were found in samples obtained on the fifth day of CH exposure, suggesting that trace accumulation of DCA occurred from repeated doses of CH (Table 2). Plasma 12C-DCA concentrations were markedly less (0.9 – 2.1 μg/mL) than the levels of 12C-TCA (151 – 234 μg/mL). Moreover, plasma accumulation of DCA did not correlate with GSTZ1/MAAI haplotype, and there was no significant difference in plasma DCA concentrations between fast and slow metabolizers of the compound. This is not surprising because reduced DCA plasma clearance and urinary MA accumulation in individuals who are EGT noncarriers become most pronounced only after repeated clinical (mg/kg/day) DCA dosages [14]. It is possible that the amount of DCA formed from CH is insufficient to show differences in the accumulation of MA between EGT carriers and noncarriers. In subjects who received 1 g of 12C-CH for 5 consecutive days, followed by 13C-DCA on day 6, 12C-DCA was found in the plasma (1.2 – 2.4 μg/mL) of all subjects after 6 days (Table 3). However, this amount of DCA was insufficient to alter the kinetics of the 25 mg/kg of 13C-DCA administered on day 6, in contrast to an earlier study of CH kinetics conducted in children [13]. Furthermore, no significant difference was found in DCA plasma half-life, Cmax, AUC, or clearance between EGT carriers and noncarriers. Very low levels of plasma 12C-DCA (mean ± SD, 1.6 ± 0.4 μg/mL) were measured in comparison with the levels of 12C-TCA (280 ± 58 μg/mL) found in the same samples.
Table 1.
Pharmacokinetic parameters of 13C-TCA and 13C-TCOH after 4 days of 1 g CH followed by 1 g 13C-CH on day 5.
| Parameter | Units | GSTZ1 EGT fast metabolizer
|
GSTZ1:non-EGT slow metabolizer
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 EGT/EGM |
PT2 EGT/EGT |
PT3 EGT/EGM |
PT4 EGT/EGT |
Avg. | St. dev. | Pt 5 KRT/EGM |
PT 6 KGT/EGM |
Pt 7 KGT/EGM |
PT 8 KGT/EGM |
Avg. | St. dev. | ||
| 13C-TCA | |||||||||||||
| t1/2 | min | 5300 | 6500 | 5600 | 4100 | 5400 | 1000 | 4300 | 6700 | 7200 | 6100 | 6100 | 1300 |
| Cmax | μg/mL | 84 | 69 | 63 | 66 | 70.5 | 9.3 | 65 | 68 | 71 | 60 | 66 | 4.7 |
| AUC | min* μg/mL | 700,000 | 640,000 | 610,000 | 360,000 | 580,000 | 150,000 | 370,000 | 530,000 | 600,000 | 460,000 | 490,000 | 99,000 |
| Clearance | mL/min | 1.4 | 1.6 | 2.0 | 2.8 | 1.9 | 0.5 | 2.7 | 1.9 | 1.7 | 2.2 | 2.1 | 0.4 |
| 13C-TCOH | |||||||||||||
| t1/2 | min | 570 | 700 | 490 | 640 | 600 | 100 | 510 | 620 | 690 | 550 | 620 | 80 |
| Cmax | μg/mL | 11.5 | 12.0 | 8.0 | 13 | 11.1 | 2.2 | 10.0 | 11.4 | 12.6 | 8.3 | 10.6 | 1.9 |
| AUC | min* μg/mL | 60,000 | 73,000 | 52,000 | 70,000 | 64,000 | 9600 | 51,000 | 65,000 | 72,000 | 48,000 | 59,000 | 11,400 |
| Clearance | mL/min | 12.1 | 9.5 | 15.9 | 10.6 | 12 | 2.8 | 15.1 | 12.9 | 9.8 | 16.4 | 12.1 | 2.4 |
t1/2, elimination half-life; AUC: area under curve; Cmax, maximum concentration.
Table 2.
Plasma 13C-DCA and Urinary MA prior to treatment and after 4 days of 1 g CH followed by 1 g 13C-CH on day 5.
| Parameter | Units | GSTZ1 EGT carrier (fast metabolizer)
|
GSTZ1 non-EGT carrier (slow metabolizer)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 EGT/EGM |
PT2 EGT/EGT |
PT3 EGT/EGM |
PT4 EGT/EGT |
Avg. | St. dev. | Pt 5 KRT/EGM |
PT 6 KGT/EGM |
Pt 7 KGT/EGM |
PT 8 KGT/EGM |
Avg. | St. dev. | ||
| 13C-DCA | μg/mL | 0.9 | 1.1 | 0.7 | 0.8 | 0.9 | 0.2 | 1.0 | 0.9 | 0.6 | 1.2 | 0.9 | 0.3 |
| 12C-DCA | μg/mL | 1.3 | 1.7 | 1.2 | 2.1 | 1.6 | 0.4 | 1.5 | 1.6 | 0.9 | 1.9 | 1.5 | 0.4 |
| 13C-TCA | μg/mL | 175 | 215 | 187 | 162 | 184 | 22 | 234 | 198 | 151 | 174 | 204 | 40 |
| MA | μg/g C | 0.3 | 0.4 | 0.5 | 0.2 | 0.4 | 0.1 | 0.4 | 0.7 | 0.2 | 0.4 | 0.4 | 0.2 |
MA, maleylacetone; C, creatinine.
Table 3.
Pharmacokinetic parameters of 25 mg/kg 1,2- 13C-DCA and urinary MA after 5 days of 1 g of 12C-CH.
| Parameter | Units | DCA fast metabolizers day 6
|
DCA slow metabolizers day 6
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 2 EGT/EGT |
Pt 3 EGT/EGM |
Pt 4 EGT/EGT |
Pt 9 EGT/EGT |
Avg. | St. dev. | Pt 5 KRT/EGM |
Pt 8 KGT/EGM |
Pt 10 KGT/KGT |
Pt 11 KRT/EGM |
Avg. | St. dev. | ||
| Elimination half life | min | 75.1 | 119.8 | 135.5 | 49.9 | 95.1 | 39.5 | 80.7 | 52.0 | 67.0 | 97.8 | 74.3 | 19.5 |
| Cmax | μg/mL | 52 | 50 | 62 | 49.8 | 53.5 | 5.8 | 65 | 54 | 69.3 | 31.4 | 54.9 | 17.0 |
| AUC | min* μg/mL | 9460 | 10,156 | 11,276 | 6654 | 9387 | 1969 | 6716 | 8068 | 10,058 | 3834 | 7169 | 2613 |
| Clearance | mL/min | 2.6 | 2.5 | 2.2 | 3.8 | 2.8 | 0.7 | 3.7 | 3.1 | 2.5 | 6.5 | 4.0 | 1.8 |
| 12C-DCA | μg/mL | 1.7 | 1.2 | 2.4 | 1.5 | 1.7 | 0.5 | 2 | 1.6 | 1.3 | 1.4 | 1.6 | 0.3 |
| MA | μg/g C | 0.2 | 0.3 | 0.5 | 0.4 | 0.4 | 0.1 | 0.2 | 0.4 | 0.3 | 0.7 | 0.4 | 0.2 |
AUC, area under curve; Cmax, maximum concentration; MA, maleylacetone.
Urinary MA was undetectable (< 0.1 μg/g creatinine) in the CH naïve individuals but was present after 5 days of CH (range 0.2 – 0.7 μg/g creatinine) in all of the subjects. The non-EGT carrier group did not have higher urinary MA values than the EGT carrier group. However, accumulation of urinary MA in non-EGT carriers were found only after chronic DCA treatment at clinically relevant doses.
Discussion
In an earlier study of one child with a primary mitochondrial disease, but with normal hepatic and renal function, in whom CH was used to induce conscious sedation, DCA was reported to exist at much higher plasma concentrations (approximately 20 μg/mL) after a single dose of 50 mg/kg CH [13]. On a weight basis, a 50 mg/kg dose represents approximately five times the single daily dose used in this study of adults (mean 15.1 ± 4 mg/kg). In addition, much less TCA (approximately 10 μg/mL) was found in the plasma samples of that child than was found in this study or by others [6] investigating the biotransformation of CH. It is unknown if age or dosage plays a major causative role in these differences. Moreover, no urinary samples were collected from the child mentioned above, and the relationships among DCA, MA, and GSTZ1/MAAI were unknown at the time of that report.
DCA and MA were not found in the plasma or urine of subjects who received environmentally relevant doses (1.5 μg/kg/day) of either 13C or 12C-CH, despite using a more sensitive analytical method [17, 18] than applied to clinical samples [16]. Thus, it is unknown what impact, if any, DCA formation from environmental levels of CH may have on the environmental risk-assessment of these compounds.
On a molar basis, DCA constitutes a relatively small fraction of clinically relevant CH doses and represents < 1% of the peak plasma concentrations of TCA and TCOH. In addition, there is no evidence that CH, TCA, or TCOH influences GSTZ1/MAAI activity. No statistical differences were found in the clearance of these compounds between the fastest and slowest metabolizers of DCA. This suggests that the DCA, derived from the biotransformation of a clinically relevant dose of CH administered to adults, is responsible for the inhibition of the enzyme, resulting in accumulation of urinary MA.
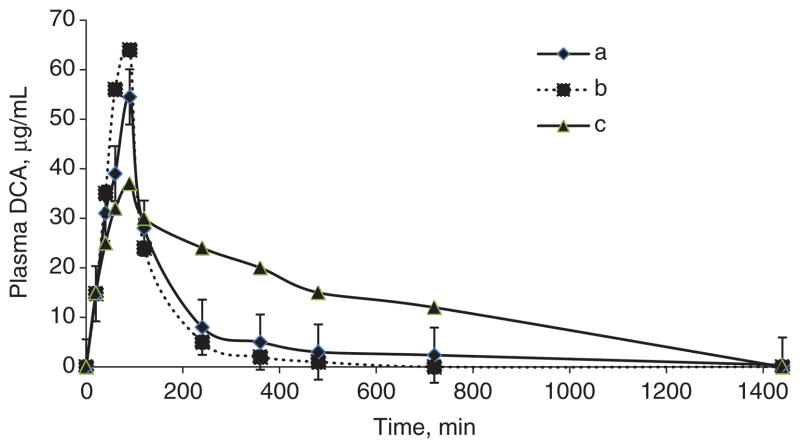
DCA clearance and urinary MA levels are dependent on chronological age, with younger rats or humans metabolizing the drug most rapidly and accumulating less urinary MA than older individuals [23]. The rate of DCA biotransformation varies widely across species, with an approximate order of rat (t1/2 ~ 3 h) > human (t1/2 ~ 5 h) > dog (t1/2 ~ 9 h) after 5 days of clinical relevant doses [11, 24]. Pharmacokinetic modeling of plasma samples from 25 mg/kg doses of 13C-DCA after 5 days of 1 g of CH did not vary among GSTZ1 haplotypes, and there was no statistical difference between individuals possessing the EGT and non-EGT alleles. In addition, the kinetics parameters at this dose closely resemble those of DCA naïve subjects found in a previous study [10] (Figure 3). This suggests that the amount of DCA by CH metabolism did not inhibit GSTZ1 activity to a degree sufficient to affect DCA kinetics. In contrast, MAAI activity in this bifunctional enzyme appeared to be inactivated, as evidenced by accumulation of MA during CH exposure. Nevertheless, urinary MA concentrations found in this study are quite small in comparison with the levels found in MAAI-knockout mice [25], in patients with hereditary tyrosinemia type I [16], or in subjects chronically treated with DCA.
Figure 3. Plasma DCA kinetics.
(a) Composite plasma time-concentration curve of eight subjects after a single dose of 12.5 mg/kg 1,2- 13C-DCA, preceded by 5 consecutive days of 1 g 12C-CH. (b) Typical plasma time-concentration curve of naïve subject (EGT/EGT) after a single dose of 12.5 mg/kg 1,2- 13C-DCA. Adapted from [10]. (c) Typical plasma time-concentration curve of subject (EGT/EGT) after five consecutive daily doses of 12.5 mg/ kg DCA, demonstrating delay in plasma clearance after repeated dosing. Adapted from [10].
Conclusions
We conclude that DCA is a minor metabolite of CH in humans. DCA cannot be detected in adults receiving repeated environmentally relevant doses of CH. Furthermore, the amount of DCA metabolized from milligrams per kilogram doses of CH is insufficient to affect its own kinetics. However, at these same doses, sufficient DCA is formed from CH to perturb tyrosine catabolism and increase MA formation. Plasma DCA and urinary MA are undetectable after a single CH dose of 1 g. Therefore, the toxicological risk, if any, applies only to chronic CH exposure at clinically relevant doses.
Acknowledgments
We thank Ms. Candy Caputo for editorial assistance. This work was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Award to the University of Florida UL1 TR000064 and National Institutes of Health National Environmental Health Sciences TCE/Health Effects of Chlorinated Compounds grant P42 ES07375.
Research funding: None declared.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Albert L. Shroads, Division of Endocrinology and Metabolism, Department of Medicine, College of Medicine, University of Florida, Gainesville, FL, USA
Bonnie S. Coats, Division of Endocrinology and Metabolism, Department of Medicine, College of Medicine, University of Florida, Gainesville, FL, USA
Taimour Langaee, Center for Pharmacogenomics, Department of Pharmacotherapy and Translational Research, College of Pharmacy, University of Florida, Gainesville, FL, USA.
Jonathan J. Shuster, Department of Health Outcomes and Policy, College of Medicine, University of Florida, Gainesville, FL, USA
Peter W. Stacpoole, Professor of Medicine and Biochemistry and Molecular Biology, University of Florida College of Medicine, PO Box 100226, Gainesville, FL 32610, USA, and Division of Endocrinology and Metabolism, Department of Medicine, College of Medicine, University of Florida, Gainesville, FL, USA.
References
- 1.Leibreich O. Das Chloralhydrat, ein neues Hyponoticum und Anaestheticum. 3. Berlin: Otto Mullers Verlag; 1869. [Google Scholar]
- 2.Bracken J, Hesslip I, Ryan S. Chloral hydrate sedation in radiology: retrospective audit of reduced dose. Pediatr Radiol. 2013;42:349–54. doi: 10.1007/s00247-011-2279-9. [DOI] [PubMed] [Google Scholar]
- 3.Lash LH, Fisher JW, Lipscomb JC, Parker JC. Metabolism of trichloroethylene. Environ Health Perspect. 2000;108:177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbas RR, Seckel CS, Kidney JK, Fisher JW. Pharmacokinetic analysis of chloral hydrate and its metabolism in B6C3F1 mice. Drug Metab Dispos. 1996;24:1340–6. [PubMed] [Google Scholar]
- 5.Chiu W, Okino M, Lipscomb J, Evans M. Issues in the pharmacokinetics of trichloroethylene and its metabolites. Environ Health Perspect. 2006;114:1450–6. doi: 10.1289/ehp.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merdink JL, Robinson LM, Stevens DK, Hu M, Parker JC, Bull RJ. Kinetics of chloral hydrate and its metabolites in male human volunteers. Toxicology. 2008;245:130–40. doi: 10.1016/j.tox.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Stacpoole PW. The dichloroacetate dilemma: environmental hazard versus therapeutic goldmine – both or neither ? Environ Health Perspect. 2011;119:155–8. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornett R, James MO, Henderson GN, Cheung J, Shroads AL, Stacpoole PW. Inhibition of glutathione S-tranferase zeta and tyrosine metabolism by dichloracetate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochem Biophys Res Commun. 1999;262:752–6. doi: 10.1006/bbrc.1999.1287. [DOI] [PubMed] [Google Scholar]
- 9.Board PG, Baker RT, Chelvanayagam G, Jermiin LS. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem J. 1997;328:929–35. doi: 10.1042/bj3280929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shroads AL, Langaee T, Coats BS, Kurtz TL, Bullock JR, Weithorn D, et al. Human polymorphisms in the glutathione transferase zeta1/ maleylacetoacetate isomerase gene influence the toxicokinetics of dichloracetate. J Clin Pharmacol. 2012;52:837–49. doi: 10.1177/0091270011405664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stacpoole PW, Henderson GN, Yan Z, Cornett R, James MO. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev. 1998;30:499–539. doi: 10.3109/03602539808996323. [DOI] [PubMed] [Google Scholar]
- 12.Merdink JL, Stenner RD, Stevens DK, Parker JC, Bull RJ. Effect of enterohepatic circulation on the pharmacokinetics of chloral hydrate and its metabolites in F344 rats. J Toxicol Environ Health A. 1999;57:357–68. doi: 10.1080/009841099157665. [DOI] [PubMed] [Google Scholar]
- 13.Henderson GN, Yan Z, James MO, Davydova N, Stacpoole PW. Kinetics and metabolism of chloral hydrate in children: identification of dichloroacetate as a metabolite. Biochem Biophys Res Commun. 1997;235:695–8. doi: 10.1006/bbrc.1997.6868. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmalak M, Lew A, Ramezani R, Shroads AL, Coats BS, Langaee T, et al. Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab. 2013;109:139–43. doi: 10.1016/j.ymgme.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shroads AL, Coats BS, McDonough CW, Langaee T, Stacpoole PW. Haplotype variations in glutathione transferase zeta 1 influence the kinetics and dynamics of chronic dichloroacetate in children. J Clin Pharmacol. doi: 10.1002/jcph.3712014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shroads AL, Henderson GN, Cheung J, James MO, Stacpoole PW. Unified gas chromatographic-mass spectrometric method for quantitating tyrosine metabolites in urine and plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;808:153–61. doi: 10.1016/j.jchromb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Jia M, Wu W, Yost RA, Chadik P, Stacpoole PW, Henderson GN. Simultaneous determination of trace levels of nine haloacetic acids in biological samples as their pentafluorobenzyl derivatives by gas chromatography/tandem mass spectrometry in electron capture negative Ion chemical ionization mode. Anal Chem. 2003;75:4065–80. doi: 10.1021/ac034036w. [DOI] [PubMed] [Google Scholar]
- 18.Zolodz MD, Jia M, Liu H, Henderson GN, Stacpoole PW. A GC-MS/ MS method for the quantitative analysis of low levels of the tyrosine metabolites maleylacetone, succinylacetone, and the tyrosine metabolism inhibitor dichloroacetate in biological fluids and tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;837:125–32. doi: 10.1016/j.jchromb.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Langlois C, Jorquera R, Finegold M, Shroads AL, Stacpoole PW, Tanguay RM. Evaluation of dichloracetate treatment in a murine model of hereditary tyrosinemia type 1. Biochem Parmacol. 2006;71:1648–61. doi: 10.1016/j.bcp.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Blackburn AC, Tzeng HF, Anders MW, Board PG. Discovery of a functional polymorphism in human glutathione transferase zeta by expressed sequence tag database analysis. Pharmacogenetics. 2000;10:49–57. doi: 10.1097/00008571-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Langaee TY, Ronaghi M. Genetic variation analyses by pyrosequencing. Mutat Res. 2005;573:96–102. doi: 10.1016/j.mrfmmm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shroads Al, Guo X, Dixit V, Liu HP, James MO, Stacpoole PW. Age-dependent kinetics and metabolism of dichloracetate: possible relevance to toxicity. J Pharmacol Exp Ther. 2008;808:1163–71. doi: 10.1124/jpet.107.134593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maisenbacher HW, Shroads AL, Zhong G, Daigle AD, Abdelmalak MM, Samper IS, et al. Pharmacokinetics or oral dichloroacetate in dogs [rapid communication] J Biochem Mol Toxicol. 2013;27:522–5. doi: 10.1002/jbt.21518. [DOI] [PubMed] [Google Scholar]
- 25.Ammini CV, Fernandez-Canon J, Shroads AL, Cornett R, Cheung J, James MO, et al. Pharmacologic or genetic ablation of maleylacetoacetate isomerase increases levels of toxic tyrosine catabolites in rodents. Biochem Pharmacol. 2003;66:2029–38. doi: 10.1016/j.bcp.2003.07.002. [DOI] [PubMed] [Google Scholar]