Abstract
Background
The standard of care for patients with advanced renal cell carcinoma (RCC) has changed to favor targeted therapy over immunotherapy. Differences in patterns of progression between patients treated with these 2 modalities, and the impact of disease stabilization on outcome, were investigated.
Methods
Patients who progressed on first line antivascular therapy (AVT) or interferon were identified, and their medical records reviewed.
Results
A total of 162 patients met inclusion criteria for this analysis. Patients in the AVT group had better baseline performance status, fewer liver metastases, and more responses (CR + PR) compared with the interferon group. Both groups were equally likely to develop distant metastases; however, for patients in the AVT group, these new metastases were more likely to arise in the setting of controlled disease at baseline sites (18% vs 4%, P = .012). There was no difference in anatomic sites of progression between the 2 groups. Patients responding (CR + PR) to AVT trended toward longer progression-free survival (PFS) compared with patients with stable disease (SD) (P =.06). No difference between responders and SD was seen in the interferon group.
Conclusions
Patients with RCC treated with antivascular therapy were more likely to progress at new sites in the setting of stable disease at baseline sites, suggesting that AVT may be more effective at controlling existing sites of disease than it is at preventing new metastases. Patients with SD on AVT had shorter PFS compared with responders (CR + PR). Whether this relationship extends to overall survival requires further study.
Keywords: renal cell carcinoma, immunotherapy, survival, metastases, response criteria, sorafenib, bevacizumab, erlotinib, interferon
The treatment landscape for metastatic renal cell carcinoma (RCC) has changed dramatically in the past few years. Previously, the standard of care for patients with advanced disease was cytokine therapy, such as high dose interleukin-2 (IL-2) or interferon-alpha. Although a small percentage of durable complete responses (CRs) were seen with these therapies, overall response rates were low, with modest improvement in progression-free survival (PFS), and a small but consistent CR rate seen in patients who received high-dose IL-2.1,2
Recently, multiple new agents targeting the cell signaling pathways involved in angiogenesis have made their way into the clinic. Among these are sorafenib, sunitinib, bevacizumab, and erlotinib—agents which inhibit signaling by vascular endothelial growth factor, platelet-derived growth factor, or both. In randomized controlled trials, antiangiogenic agents have shown increased response rates and improved survival in patients with RCC when compared with interferon.3-5
Despite these significant advances, none of these new treatments are curative, and the vast majority of patients with RCC ultimately demonstrate progression of their disease while on treatment. Although anatomic patterns of metastatic spread have previously been described at first recurrence after resection, we are unaware of any published data describing patterns of disease progression in patients on systemic treatment.6
Controversy exists about how best to measure clinical benefit in patients on targeted agents. The Response Evaluation Criteria in Solid Tumors (RECIST) were published in 2000 to standardize response assessment in cancer patients and to align disease regression with clinical benefit.7 Unfortunately, not all diseases respond in the same manner to systemic therapy. With the advent of targeted agents, the validity of RECIST has been called into question. We sought to better understand the relationship between disease stabilization and progression-free survival (PFS), an endpoint that has begun to gain acceptance as a valid surrogate for treatment benefit in RCC patients treated with targeted therapy.
We report here the results of a retrospective evaluation of patients treated with interferon-alpha and antivascular agents at M. D. Anderson Cancer Center, and comparatively assess their patterns of relapse, and the relationship between commonly used outcome measures.
Materials and Methods
Patient Population
Data were collected on all eligible patients enrolled on 1 of 3 institutional review board approved clinical trials conducted at M. D. Anderson Cancer Center between March 2002 and October 2007. This retrospective investigation was approved by the M. D. Anderson Cancer Center internal review board and the requirement for informed consent was waived. Study treatments included 1 of the following: sorafenib, sorafenib plus interferon, erlotinib, bevacizumab plus erlotinib, or interferon alone at 1 of 2 candidate dose levels (0.5 million U twice daily or 5 million U daily). Inclusion and exclusion criteria for all 3 trials were similar. All trials required patients to have metastatic, measurable, histologically confirmed RCC. Patients who had received prior anticancer therapy or who had known brain metastases were excluded. The antivascular trials limited enrollment to patients with Eastern Cooperative Oncology Group performance status (PS) of 0 or 1. The interferon study included patients with PS of 0, 1, or 2. Patients with non–clear-cell RCC were included in the interferon trial but excluded from the antivascular trials. Patients on the bevacizumab alone or in combination with erlotinib study were required to have their primary kidney tumor in place and be candidates for cytoreductive nephrectomy. Patients on the other 2 studies were allowed regardless of prior nephrectomy status. There were no criteria in any of the studies to exclude or include patients on the basis of their metastatic sites, with the exception of brain metastases as described.
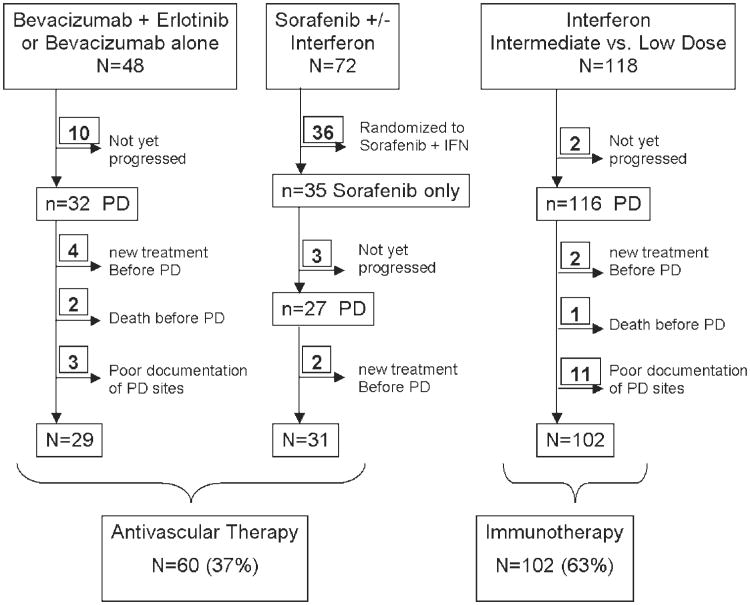
The following groups of patients were excluded from our analysis (see Fig. 1): patients who had not yet progressed on study therapy, patients who changed therapies or who died before disease progression, and patients in whom progression or sites of progression could not be verified by imaging. In addition, patients treated with combination sorafenib and interferon were excluded as they would fit in both categories of treatment used for comparison.
Figure 1.
Patient selection. PD indicates progressive disease.
Data were collected prospectively in the M. D. Anderson genitourinary research database describing baseline patient characteristics, toxicities, and response to therapy. Response to therapy was defined as either complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to RECIST in all cases.7 PFS was defined as time from date of enrollment to date of progression or last follow-up. Imaging reports contained in the patients' medical records were retrospectively reviewed to determine sites of metastases at baseline and at time of progression. New sites of disease were defined as new organ sites as distinct from new metastases in a previously involved organ site. Organ sites of metastases at baseline and at progression were classified into 8 categories: lymph nodes, lung, bone liver, brain, skin, contralateral kidney/renal fossa, and other. The primary tumor, when in place, was included in the assessment of RECIST response, but was not considered a metastatic site for the analysis of patterns of metastatic spread.
Statistical Methods
Patient characteristics were summarized by treatment group. The difference in continuous patient characteristics between the 2 treatment groups was assessed using Wilcoxon rank sum test and chi-square test or Fisher exact test was used to assess the association between patient categorical characteristics and treatment. Kaplan-Meier method was used to generate PFS curves by response and by treatment. Log-rank test was used to assess the difference in PFS among response groups and Bonferoni method was applied to adjust for the multiple pair-wise tests. All computations were carried out using SAS (SAS institute, Cary, NC).
Results
Patient Characteristics
From March 2002 to October 2007, 238 eligible patients were enrolled in the 3 clinical trials included in this analysis. Patients were separated into 2 groups: those enrolled on the antivascular therapy trials, and those enrolled on the interferon trial. After exclusion criteria were applied (Fig. 1), 162 patients, all of whom had progressed on study by April 1, 2008, were determined to be eligible for this retrospective review. Of these, 15 were treated with erlotinib and bevacizumab, 14 with bevacizumab alone, 31 with sorafenib, and 102 with interferon at either intermediate or low dose.
All patients were assessed for sites of metastases at baseline and at progression as described. Summary statistics of patient characteristics by treatment group are shown in Table 1. Patients treated on the interferon trial were more likely to have hepatic metastases, poor performance status, and lower response rates compared with patients treated with antivascular therapy. Patients treated on the antivascular studies were less likely to have lung metastases. Otherwise there was no significant difference between the 2 groups. Response rates at measurable sites as defined by RECIST criteria were higher in the antivascular therapy group compared with interferon. The most common sites of baseline metastases in both groups were lung, lymph node, and renal fossa/contralateral kidney. In addition to the common anatomic sites, 11 patients had baseline sites of metastases categorized as “other”. Baseline sites coded as “other” were mainly visceral sites of disease including pancreas and bowel.
Table 1. Patients' Characteristics by Treatment Group.
| Patient Characteristics | Treatment | |||
|---|---|---|---|---|
| Interferon n=102 (63%) | Antivascular Therapy n=60 (37%) | P | ||
| Age (y) | Mean | 59.1 | 59.6 | .63 |
| Median | 59 | 61 | ||
| Race | Nonwhite | 17 (17) | 7 (14) | .67 |
| White | 85 (83) | 43 (86) | ||
| Sex | Women | 22 (22) | 12 (24) | .74 |
| Men | 80 (78) | 38 (76) | ||
| PS | 0 | 46 (45) | 24 (48) | .04 |
| 1 | 45 (44) | 26 (52) | ||
| 2 | 11 (11) | 0 (0) | ||
| Nephrectomy | Yes | 91 (89) | 45 (90) | NS |
| No | 11 (11) | 5 (10) | ||
| Best response | PD | 53 (52) | 15 (31) | .002 |
| SD | 44 (43) | 23 (47) | ||
| CR or PR | 5 (5) | 11 (22) | ||
| Original sites | Contralateral kidney/renal fossa | 42 (41) | 16 (32) | .27 |
| Lymph Node | 50 (49) | 29 (58) | .30 | |
| Lung | 71 (70) | 43 (86) | .03 | |
| Osseous | 20 (20) | 9 (18) | .81 | |
| Hepatic | 35 (34) | 4 (8) | <.0001 | |
| Brain | 0 (0) | 1 (2) | .33 | |
| Others | 7 (7) | 4 (8) | .75 | |
| Skin | 11 (11) | 4 (8) | .77 | |
PS indicates performance status; PD, progressive disease; SD, stable disease; CR, complete response; PR, partial response.
Sites of Disease Progression
Patients in both groups were equally likely to develop metastases at new sites. However, patients treated with antivascular therapy who progressed with metastases at new sites were more likely to do so in the setting of controlled disease at existing sites, compared with patients treated with interferon (P = .012) (Table 2). There was no significant difference between the treatment groups when comparing rates of progression at old sites only, or progression at both old and new sites concurrently.
Table 2. Association Between Treatment Group and Progression Pattern.
| Sites of Progression | Treatment | P | |
|---|---|---|---|
| Interferon n=102 (63%) | Antivascular Therapy n=60 (37%) | ||
| Old sites only | 77 (75) | 43 (72) | 1.0 |
| New sites only | 4 (4) | 11 (18) | .012 |
| Both old and new sites | 21 (21) | 6 (10) | .39 |
| Solitary sites | 49 (48) | 31 (52) | .656 |
| Multiple sites | 53 (52) | 29 (48) | |
The single most common anatomic site of disease progression in both groups was the lung, with 50% of the patients in the interferon group and 66% of patients in the antivascular therapy group showing either new lung metastases or growth of existing lesions. This finding is not surprising, as the lung was the most prominent site of disease at baseline.
Patients treated with antivascular therapy were as likely to progress at any given anatomic site as those treated with interferon (see Table 3). The most common sites of new disease were the same for both groups: bone and lymph node. There was no difference in the incidence of clinically significant brain metastases between the 2 treatment groups. However, although patients with known brain metastases were excluded from all 3 clinical trials, patients on the antivascular therapy trials were screened for brain metastases before study entry with baseline brain imaging, whereas patients on the interferon trial were not.
Table 3. Association Between Anatomic Site of Progression and Treatment Group.
| Treatment | |||||
|---|---|---|---|---|---|
| Site | Involved at Baseline? | Involved at PD? | Interferon n=102 (63%) | Antivascular Therapy n=60 (37%) | P |
| Contralateral kidney/renal fossa | No | No | 56 (93) | 36 (97) | .65 |
| Yes | 4 (7) | 1 (3) | |||
| Yes | No | 18 (43) | 9 (39) | .77 | |
| Yes | 24 (57) | 14 (61) | |||
| Lymph node | No | No | 47 (90) | 24 (92) | 1.0 |
| Yes | 5 (10) | 2 (8) | |||
| Yes | No | 20 (40) | 17 (50) | .38 | |
| Yes | 30 (60) | 17 (50) | |||
| Lung | No | No | 29 (94) | 10 (91) | 1.0 |
| Yes | 2 (6) | 1 (9) | |||
| Yes | No | 23 (32) | 15 (31) | .84 | |
| Yes | 48 (68) | 34 (69) | |||
| Bone | No | No | 74 (90) | 40 (85) | |
| Yes | 8 (10) | 7 (15) | .38 | ||
| Yes | No | 4 (20) | 3 (23) | 1.0 | |
| Yes | 16 (80) | 10 (77) | |||
| Liver | No | No | 64 (96) | 52 (94) | 1.0 |
| Yes | 3 (4) | 3 (6) | |||
| Yes | No | 9 (26) | 1 (20) | 1.0 | |
| Yes | 26 (74) | 4 (80) | |||
| Brain* | No | No | 97 (95) | 57 (97) | 1.0 |
| Yes | 5 (5) | 2 (3) | |||
| Skin | No | No | 90 (99) | 55 (98) | 1.0 |
| Yes | 1 (1) | 1 (2) | |||
| Yes | No | 6 (55) | 1 (25) | .57 | |
| Yes | 5 (45) | 3 (75) | |||
| Others | No | No | 95 (100) | 52 (98) | .36 |
| Yes | 0 (0) | 1 (2) | |||
| Yes | No | 5 (71) | 5 (50) | 1.0 | |
| Yes | 2 (29) | 2 (50) | |||
PD indicates progressive disease.
Patients with known brain metastases at baseline were excluded from all 3 clinical trials. However, patients on the antivascular therapy trials were screened for brain metastases prior to study entry with baseline brain imaging. Patients on the interferon trial were not.
Analysis of RECIST Endpoints
In the past 8 years, the response rate measured using RECIST has been used as a primary endpoint in clinical trials.7 Because antivascular therapy is presumably working at the level of the endothelial cell compartment, and is less likely to directly affect the epithelial compartment, SD has been considered an indicator of treatment benefit. To assess the significance of SD in the context of antivascular therapy, we compared the PFS rates of patients responding to therapy (CR + PR) with those with SD or PD. Patients with CR or PR to antivascular therapy trended toward longer PFS compared with patients with SD (P = .06) (Fig. 2). No significant difference in PFS was observed between responders (CR + PR) and patients with SD in the interferon group (Fig. 3). By definition, a statistically significant difference in PFS existed between patients (with CR, PR,) or SD and patients with PD in both groups.
Figure 2.
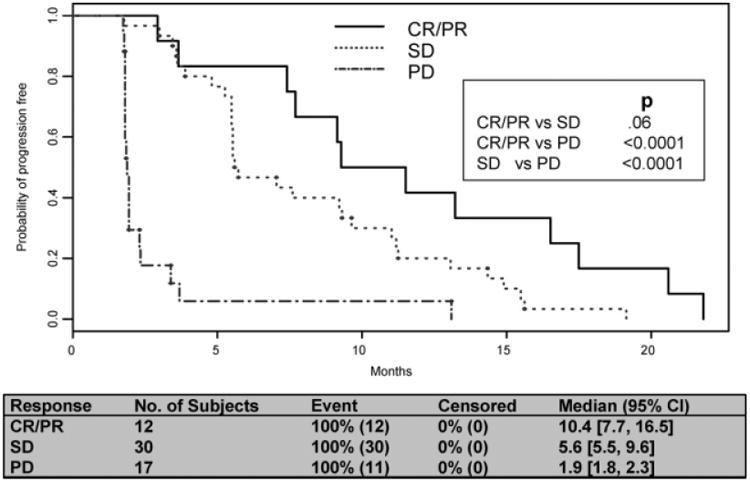
Progression-free survival by response: antivascular therapy. CR indicates complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival.
Figure 3.
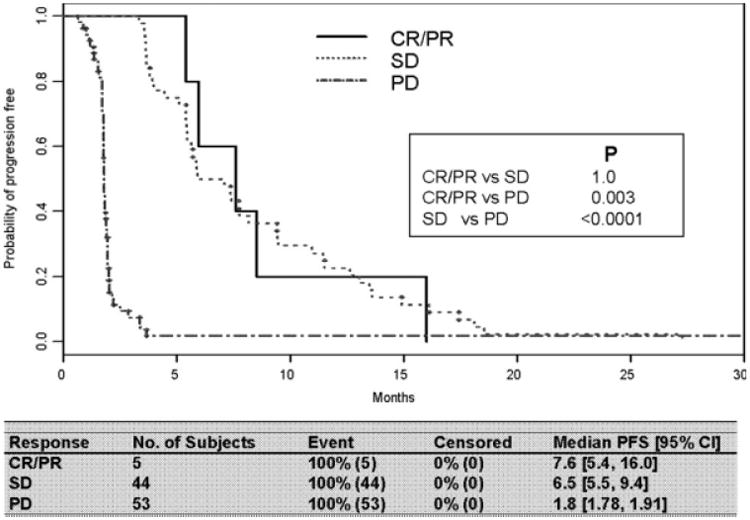
Progression-free survival (PFS) by response: interferon. CR indicates complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Discussion
Targeted therapies have revolutionized the treatment of RCC over the past 5 years. Now that these agents are widely used, there is a need to re-evaluate what we know about the behavior of RCC in this new treatment setting. The current study was performed to compare progression patterns of patients treated with antivascular targeted therapies with patients treated with interferon, and to assess the impact of disease stabilization on patient outcomes.
We found that anatomic sites of progression were similar in both treatment groups and were consistent with previously reported patterns of relapse in high risk localized disease after nephrectomy.6,8 Of greater significance is the finding that patients treated with antivascular therapy were significantly more likely to progress with new metastases in previously uninvolved anatomic sites in the setting of controlled disease at existing sites. This pattern was not seen in patients treated with interferon. Overall, we found the patterns of progression in the interferon group similar to those previously reported for cytokine therapy.9 Both groups were equally likely to progress at baseline sites alone, or to progress at baseline sites with concurrent development of metastases in a new anatomic site. The mechanism of action of the antivascular therapeutic agents investigated here—sorafenib, erlotinib, and bevacizumab—involves targeting of multiple pathways with a variety of proposed downstream effects. However, central to the mechanism of each is the effect on decreasing and disrupting tumor vasculature.10,11 These results strengthen the theory that antivascular therapy may be most effective at controlling established, vascularized tumors, whereas micrometastases or infiltrative disease may be less responsive to the effects of these targeted therapies.
This finding has significant implications in terms of how we conceptualize treatment for RCC and how we combine these targeted agents therapeutically. Typically, we consider systemic therapy as a means of “cleaning up” micrometastatic disease, while concurrently treating macroscopic disease, a theory that underlies the concept of adjuvant therapy in solid tumors. Our data suggest that targeted antivascular therapy may be better thought of as extended local therapy, targeting established primary tumors and metastases. It would be of interest to explore the combination of targeted therapies with treatments aimed at controlling micrometastatic disease such as cytotoxics or experimental therapies with an antimetastatic scientific rationale. These data also suggest caution in assuming that the efficacy of these agents is ensured in patients with high-risk, resected, RCC. The results of ongoing adjuvant studies are extremely important to define the scope of antivascular therapy.12 Neoadjuvant targeted therapy has also been considered for RCC, and recent reports show that in select patients treatment can downstage primary kidney tumors, making them more amenable to surgery.13 In light of data presented here, neoadjuvant therapy for the purposes of downstaging would be reasonable. However, use of such therapies in the neoadjuvant setting with the hopes of decreasing the micrometastatic burden is open to debate.
On the basis of the evolving experience in treating a variety of solid tumors with newer targeted therapies, many have called into question the entrenched practice of relying on RECIST endpoints to describe disease response to these agents.14-16 Here we sought to evaluate whether response, complete or partial as defined by RECIST, predicted superior PFS compared with SD as similarly defined. We found a strong trend toward prolonged PFS in patients with CR or PR as their best response to antivascular therapy, compared with those whose best response was disease stabilization (Fig. 2). However, in patients treated with interferon, response compared with SD did not affect PFS (Fig. 3). Further evaluation with larger numbers of patients is needed to confirm these results, and to investigate the appropriateness of using RECIST response as a surrogate for overall survival. In addition, investigation is ongoing into the use of radiographic calculations other than tumor measurements to describe disease response in solid tumors.17
There are some specific caveats that need to be raised about our analysis. The treatment groups were somewhat unbalanced at baseline, with patients with poor performance status and hepatic metastases clustered in the interferon group. This was due to the fact that poor performance status patients were excluded from the antivascular trials. Patients in the interferon group had lower response rates and higher rates of progressive disease compared with patients treated with antivascular therapy. These results are expected based on what we have learned from randomized clinical trials comparing interferon with various antivascular therapies, but may be a result of the poorer baseline patient characteristics in the interferon group. It is also not known whether the poorer baseline characteristics of the interferon patient group biased our progression analysis. Future analysis with prognostically matched groups may aid in answering these questions. Finally, this is a retrospective review subject to the limitations of such an analysis. These results are hypothesis generating and should be further investigated in a prospective manner before conclusions can be drawn.
In summary, we show that patients on antivascular agents will progress at new sites of disease alone more frequently than patients on immunotherapy, while achieving superior control at existing sites of disease. To improve therapy for RCC, we need to define and abrogate the biological processes that promote the generation of new sites of disease, while continuing to improve on the efficacy of existing tumoristatic treatments.
Footnotes
Conflict of Interest Disclosures: The authors made no disclosures.
References
- 1.Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet. 1999;353:14–17. [PubMed] [Google Scholar]
- 2.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–411. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Sivaramakrishna B, Gupta NP, Wadhwa P, et al. Pattern of metastases in renal cell carcinoma: a single institution study. Indian J Cancer. 2005;42:173–177. [PubMed] [Google Scholar]
- 9.Lee DS, White DE, Hurst R, Rosenberg SA, Yang JC. Patterns of relapse and response to retreatment in patients with metastatic melanoma or renal cell carcinoma who responded to interleukin-2 -based immunotherapy. Cancer J Sci Am. 1998;4:86–93. [PubMed] [Google Scholar]
- 10.Haas NB, Uzzo RG. Tyrosine kinase inhibitors and anti-angiogenic therapies in kidney cancer. Curr Treat Options Oncol. 2007;8:211–226. doi: 10.1007/s11864-007-0031-3. [DOI] [PubMed] [Google Scholar]
- 11.Hahn O, Stadler W. Sorafenib. Curr Opin Oncol. 2006;18:615–621. doi: 10.1097/01.cco.0000245316.82391.52. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsohn KM, Wood CG. Adjuvant therapy for renal cell carcinoma. Semin Oncol. 2006;33:576–582. doi: 10.1053/j.seminoncol.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Shuch B, Riggs SB, Larochelle JC, et al. Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int. 2008;102:692–696. doi: 10.1111/j.1464-410X.2008.07660.x. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 15.Gollob JA, Bonomi P. Historic evidence and future directions in clinical trial therapy of solid tumors. Oncology (Williston Park) 2006;20:10–18. [PubMed] [Google Scholar]
- 16.Tuma RS. Sometimes size doesn't matter: reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98:1272–1274. doi: 10.1093/jnci/djj403. [DOI] [PubMed] [Google Scholar]
- 17.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]