Abstract
Background
Maternal diabetes causes neural tube defects (NTDs) in the embryos via activating protein kinase Cs (PKCs), which regulate programmed cell death (apoptosis). The aims of this study are to investigate the role of proapoptotic PKCδ in NTD formation and the underlying mechanisms.
Methods
PKCδ heterozygous (pkcδ+/−) female mice were diabetic (DM) induced by intravenous injection of streptozotocin. Occurrence of NTDs was evaluated at embryonic day 11.5 and compared between wild type (WT) and PKCδ homozygous (pkcδ−/−) embryos. Changes in oxidative and endoplasmic reticulum (ER) stress-associated factors and stress-response c-Jun N-terminal kinases (JNKs) were assessed using Western blot assay.
Results
Compared to DM/WT, the DM/PKCδ−/− embryos had significantly lower NTD rate and lower levels of oxidative and ER stress factors and JNK activation. These values were similar to those in the non-diabetic control group.
Conclusion
PKCδ plays a critical role in diabetes-induced NTDs, potentially through increasing oxidative and ER stress and JNK-associated stress-response pathways.
Keywords: diabetic embryopathy, endoplasmic reticulum stress, neural tube defects, oxidative stress, protein kinase Cδ
Introduction
Diabetes is a metabolic disease and causes neural tube defects (NTDs) in offspring of mothers with the disease [1–3]. Maternal hyperglycemia disturbs intracellular signaling leading to increases in stress conditions which, in turn, trigger programmed cell death (apoptosis) in the neural tube of the embryo [4,5].
Among the intracellular signaling factors, protein kinase C (PKC) isoforms have been implicated in NTD formation in diabetic embryopathy [6–9]. PKC family of serine/threonine kinases consists of 12 isoforms. Among them, PKCα, PKCβ, and PKCδ have been shown by our group to play important roles in neural tube malformations induced by hyperglycemic condition [10,11]. PKCδ has been demonstrated as a proapoptotic factor in many pathological conditions [12–16]. Unlike PKCα and PKCβ, PKCδ is a novel PKC isoform and activated via diacylglycerol-independent mechanism [17].
PKCδ is closely associated with increases in reactive oxygen species (ROS) and apoptosis in different models [18–20]. High levels of ROS in a low antioxidant condition results in oxidative stress. This stress condition is associated with NTDs in diabetic embryopathy [21–23].
In addition, hyperglycemia also perturbs the function of organelles, including the endoplasmic reticulum (ER). When ER function is disturbed by hyperglycemia, protein folding, maturation, and trafficking are impaired, leading to abnormal accumulation of unfolded or misfolded polypeptides in the ER [24]. Under such ER stress, cells activate so-called unfolded protein response, which is manifested by activation of a number of associated factors, including immunoglobulin heavy chain-binding protein (BiP), C/EBP homologous protein (CHOP), and eukaryotic translation initiation factor 2α (eIF2α) [24]. ER stress-induced apoptosis is associated with a variety of diseases, including diabetes and cardiovascular diseases [25]. Inhibition of ER stress protects cells from damage under different pathological conditions [26]. Although the regulation of ER stress is not fully understood, recent studies showed that PKCδ plays an important role in ER stress-induced apoptosis [27].
In response to stress conditions, a number of intracellular factors are activated. These include c-Jun N-terminal kinases (JNKs), which are members of the mitogen-activated protein kinase family [28]. Activation of JNKs via phosphorylation has been observed in the embryos of diabetic animals [29,30]. The role of JNK2 in NTD formation in diabetic embryopathy has been demonstrated using a jnk2 gene knockout model [30].
Previous observations suggest that PKCδ is a potential key factor in triggering molecular cascades leading to apoptosis in the embryonic neural tube in diabetic embryopathy [10]. The aims of this study are to address the role of PKCδ in diabetes-induced NTDs and delineate underlying mechanisms involving intracellular stress formation and responses, using a gene-specific pkcδ knockout animal model.
Materials and methods
Diabetic mouse model
The use of animals was approved by Institutional Animal Care and Use Committee of the University of Maryland Baltimore. Female PKCδ heterozygous (pkcδ+/−) mice in C57BL/6J background(31) were injected with streptozotocin (60 mg/kg; in 0.1 M citrate buffer, pH 4.5) intravenously for two days. Blood glucose level was monitored until it reached 250 mg/dl and higher. An insulin pellet was implanted to restore the blood glucose level to normal range (80–150 mg/dl). The female mice were paired with normal male mice of the same genotype. The day when a vaginal plug was present was designated as embryonic (E) day 0.5. The insulin pellet was removed at E5.5 to make the mice hyperglycemic. Embryos were collected at E11.5, genotyped using polymerase chain reaction [31], and examined under a dissecting microscope. In each litter of diabetic mice (DM), embryos in three genotypes, wild type (WT), PKCδ heterozygous (PKCδ+/−) and PKCδ homozygous (PKCδ−/−), were exposed to the same condition of maternal diabetes. Comparisons were made between DM/WT and DM/PKCδ−/−. Wild type female mice injected with the citrate buffer alone served as non-diabetic control (CON).
Western blot
Neural tissues were isolated from the embryos using fine scissors under a dissecting microscope and homogenized in a lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA; Millipore Corporation, Bedford, MA) containing protease and phosphatase inhibitor cocktail (Thermo Scientific Rockford, IL). Samples were centrifuged at a speed of 14,000 rpm for 20 min to obtain supernatants.
Equal amounts of protein were electrophoretically separated in SDS-PAGE and electroblotted onto an Immobilon-P nylon membrane (Millipore Corporation Bedford, MA). The membranes were incubated with primary antibodies against BiP, CHOP, p-eIF2α, and p-JNK (Cell Signaling Technology Danvers, MA) overnight at 4°C after being blocked with non-fat milk. The membrane was, then, incubated with a secondary antibody that is conjugated with peroxidase (Santa Cruz Biotechnology Santa Cruz, CA) for two hours at room temperature. Detection of specific proteins was carried out with an ECL chemiluminescence system (Amersham Biosciences Piscataway, NJ). An antibody against β-actin (Sigma-Aldrich St. Louis, MO) was used to re-probe the membranes after being stripped to control sample loading. Images of fluorescent bands were captured using a UVP Bioimaging System (Upland, CA) and converted into grayscale format. Density of the bands was quantified using VisionWork software.
Statistical analysis
NTD rate is calculated as a percentage of embryos with NTDs in total embryos. NTD rates were analysed using two sided Fisher's exact test (StaXact) to compare between experimental groups. Student's t-test was used to analyse the data of Western blot assays. p < 0.05 was considered statistically significant in all tests.
Results
NTDs in diabetic PKCδ knockout model
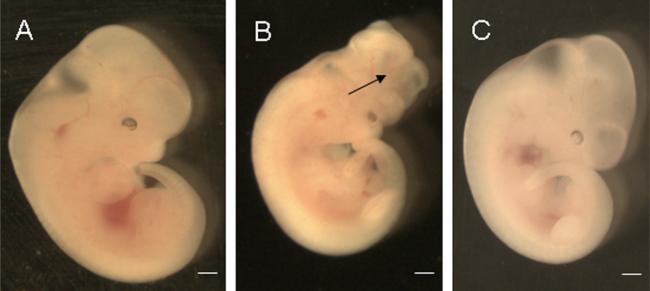
NTDs were characterized as open neural tube in the dorsal midline in any region along the neural axis (Figure 1). The NTD rates in the DM/PKCδ−/− (0%) and DM/PKCδ+/− (1.7%) groups were significantly lower than that in the DM/WT group (15.6%), but similar to that in the CON group (0%; Table I and Figure 1).
Figure 1.
Neural tube development in the embryos. E11.5 embryos of DM/WT (A), DM/PKCδ−/− (B), and CON (C). The arrow indicates opened neural tube in the brain region. Scale bars = 200 μm.
Table I.
NTD in embryos of diabetic PKCδ knockout mice.
| CON | DM/WT | DM/PKCδ–/– | DM/PKCδ+/– | |
|---|---|---|---|---|
| Total embryos | 42 | 32 | 35 | 59 |
| Embryos with NTD | 0 | 5 | 0 | 1 |
| NTD rate (%) | 0 | 15.6 | 0* | 1.7# |
p < 0.05 vs. DM/WT group.
p < 0.05 vs. DM/WT group
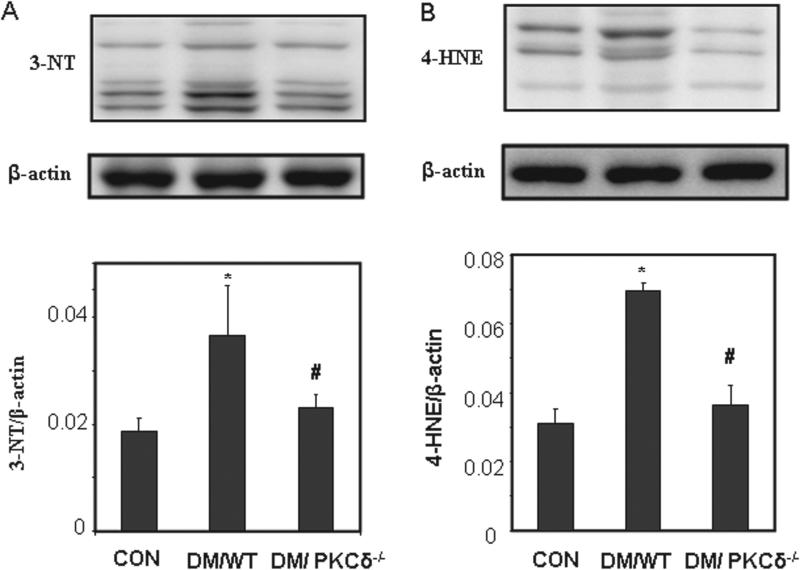
Oxidative stress in PKCδ knockout model
The levels of oxidative stress markers, 3-NT and 4-HNE, in the DM/WT group were higher than those in CON group (Figure 2). In the DM/PKCδ−/− embryos, the expression of these factors was significantly lower than that in DM/WT group, but at the similar level to that in the CON group (Figure 2).
Figure 2.
Western blot analysis of oxidative stress markers. (A) 3-NT; (B) 4-HNE. The bar charts represent band densities normalized to β-actin (Mean ± SEM; *p < 0.05 vs. CON, #p < 0.05 vs. DM/WT; n = 4).
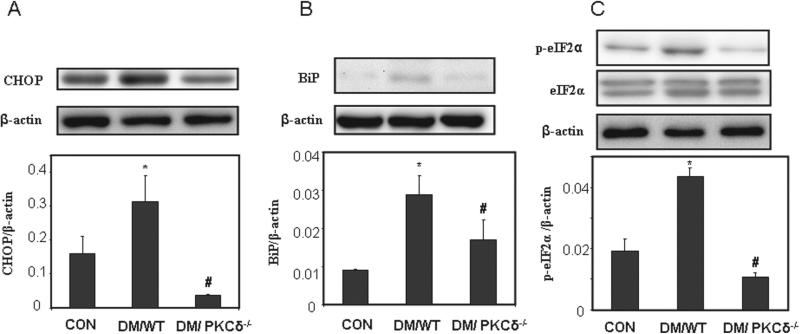
ER stress in PKCδ knockout model
Expression of ER stress-associated factors was assessed in the embryos. The levels of CHOP, BiP, and p-eIF2α were increased significantly in the DM/WT group, compared with the CON group (Figure 3). In the embryos of DM/PKCδ−/−, the levels were significantly lower than those in the DM group and similar to those in the CON group (Figure 3).
Figure 3.
Alteration of ER stress in PKCδ knockout embryos. ER stress markers (A) CHOP; (B) BiP; and (C) p-eIF2α and eIF2α. The bar charts represent band densities normalized to β-actin (Mean ± SEM; *p < 0.05 vs. CON, #p < 0.05 vs DM/WT; n = 4).
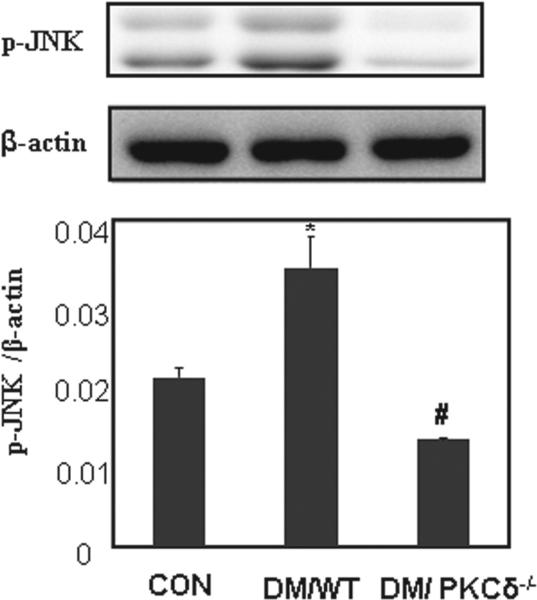
Stress-response factors in PKCδ knockout model
Stress-response JNKs in the embryo are activated by maternal diabetes [29,30]. To investigate if PKCδ also influences JNK activation, the levels of p-JNK were quantified using immunoblot assay. Significant increases in p-JNK were seen in the embryos of the DM/WT group, compared with those in the CON group (Figure 4). In the DM/PKCδ−/− embryos, the level of p-JNK was significantly reduced in comparison with that in the DM/WT group, and in the similar level in the CON group (Figure 4).
Figure 4.
Expression of phosphorylated JNK (p-JNK). The bar charts represent band density normalized to β-actin (Mean ± SEM; *p < 0.05 vs CON, #p < 0.05 vs. DM/WT; n = 4).
Discussion
In humans, NTDs occur in about 6–10% in infants of diabetic pregnancies, which is higher than that in the general population [1,3,32]. Although the underlying mechanism is not very clear, we and others have shown that NTDs are closely related to increased apoptosis in the neural epithelium [4,11,21,33]. It has been known that PKCδ is critical in cell apoptosis [34–36], and deletion of pkcδ significantly reduced the apoptosis in different tissues and models. For example, smooth muscle cell death was decreased in pkcδ null mice [37]; and deletion of pkcδ protects against β cell death [38]. These observations imply that PKCδ may be also involved in diabetic embryopathy. Our previous work has revealed that some PKC isoforms including PKCδ are activated in the embryos of diabetic rats [10]. Furthermore, inhibition of PKCδ in the embryos cultured in high glucose reduces NTD rate [10]. Here, using a gene knockout model, we provide strong evidence that PKCδ plays a critical in NTD formation in diabetic embryopathy.
PKCδ plays an important role in response to various pathological conditions. Our results clearly demonstrate that PKCδ responses to hyperglycemia by exacerbating oxidative and ER stress. Deleting one of the pkcδ alleles appears sufficiently to ameliorate the adverse effects of hyperglycemia on embryonic development. The question of whether the effects of pkcδ+/− and pkcδ−/− are in a gene dosage-dependent fashion remains to be addressed.
It has been speculated that PKCs respond to the early events of aberrant glucose metabolism by being activated [6,7]. Activated PKCs trigger phospholipid peroxidation to produce isoprostanes, and subsequently increase ROS production [39]. It has been hypothesized that PKCs activate cytosolic phopholipase A2 to trigger lipoperoxidation in the embryonic neural cells under hyperglycemic conditions, and further exacerbate oxidative stress [10]. Although the mechanisms need to be further delineated, our data show that PKCδ is responsible for the manifestation of oxidative stress in the embryos of diabetic mice.
ER stress has been shown to be responsible for initiation of apoptosis, subsequently leading to a variety of diseases including cardiovascular diseases, neural diseases, and diabetes [24]. Although the underlying mechanism of ER stress is not clear, recent studies have demonstrated that PKCδ is critical for ER stress-induced apoptosis [27,40,41]. Our data have provided strong evidence that deletion of the pkcδ gene significantly reduces the levels of ER stress-associated factors, indicating that PKCδ mediates the effects of maternal hyperglycemia on ER stress elevation. Interaction between oxidative stress and ER stress has also been implicated in many systems [24,42]. Therefore, further studies are needed to delineate the pathway involving PKCδ, oxidative stress, and ER stress in diabetic embryopathy.
JNK is well noted as a mediator of cell stress responses and is involved in mediating apoptosis [43]. Previous studies by our group on malformed neural tubes of diabetic mice showed that JNK activation is the critical pathway involved [29,30]. Consistent with these findings, we further show in this report that JNK activation is increased in neural tube of wild type diabetic mice, however, deletion of PKCδ restored a level similar to that observed in control mice. However, the mechanism by which PKCδ regulates JNKs in diabetic embryopathy remains to be addressed.
In combination with previous findings on diabetic embryopathy, this study provides new insight into the mechanism of hyperglycemia-induced NTDs. It further confirms that ER stress is the downstream event of PKCδ action because deletion of PKCδ restored the ER function and thus significantly reduced NTDs in diabetic mice. The present data clearly show a relationship between ER stress and PKCδ. These data also implicate a potential therapeutic target in treatment or prevention of NTDs.
Acknowledgements
The authors thank Dr. Keiichi I. Nakayama (Fukuoka, Japan) for providing the PKCδ knockout mice. We are grateful for Dr. Ming Tan for statistical analyses and Hua Li for technical assistance.
Footnotes
Declaration of Interest: This study was supported by NIH grant R01DK083770.
References
- 1.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e1–237.e9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills JL. Malformations in infants of diabetic mothers. Teratology. 1982;25:385–394. doi: 10.1002/tera.1420250316. [DOI] [PubMed] [Google Scholar]
- 3.Reece EA. Obesity, diabetes, and links to congenital defects: a review of the evidence and recommendations for intervention. J Matern Fetal Neonatal Med. 2008;21:173–180. doi: 10.1080/14767050801929885. [DOI] [PubMed] [Google Scholar]
- 4.Chappell JH, Jr, Wang XD, Loeken MR. Diabetes and apoptosis: neural crest cells and neural tube. Apoptosis. 2009;14:1472–1483. doi: 10.1007/s10495-009-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Z, Reece EA. Experimental mechanisms of diabetic embryopathy and strategies for developing therapeutic interventions. J Soc Gynecol Investig. 2005;12:549–557. doi: 10.1016/j.jsgi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu Y, Sekiguchi N, Hayashi M, Isshiki K, Yokota T, King GL, Loeken MR. Diacylglycerol production and protein kinase C activity are increased in a mouse model of diabetic embryopathy. Diabetes. 2002;51:2804–2810. doi: 10.2337/diabetes.51.9.2804. [DOI] [PubMed] [Google Scholar]
- 7.Wentzel P, Wentzel CR, Gäreskog MB, Eriksson UJ. Induction of embryonic dysmorphogenesis by high glucose concentration, disturbed inositol metabolism, and inhibited protein kinase C activity. Teratology. 2001;63:193–201. doi: 10.1002/tera.1034. [DOI] [PubMed] [Google Scholar]
- 8.Gäreskog M, Wentzel P. Altered protein kinase C activation associated with rat embryonic dysmorphogenesis. Pediatr Res. 2004;56:849–857. doi: 10.1203/01.PDR.0000145295.88601.B9. [DOI] [PubMed] [Google Scholar]
- 9.Gäreskog M, Wentzel P. N-Acetylcysteine and α-cyano-4-hydroxycinnamic acid alter protein kinase C (PKC)-δ and PKC-zeta and diminish dysmorphogenesis in rat embryos cultured with high glucose in vitro. J Endocrinol. 2007;192:207–214. doi: 10.1677/joe.1.06966. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Wu YK, Reece EA. Demonstration of the essential role of protein kinase C isoforms in hyperglycemia-induced embryonic malformations. Reprod Sci. 2008;15:349–356. doi: 10.1177/1933719108316986. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Zhao Z, Eckert RL, Reece EA. Protein kinase Cβ2 inhibition reduces hyperglycemia-induced neural tube defects through suppression of a caspase 8-triggered apoptotic pathway. Am J Obstet Gynecol. 2011;204:226.e1–226.e5. doi: 10.1016/j.ajog.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villalba M. A possible role for PKC δ in cerebellar granule cells apoptosis. Neuroreport. 1998;9:2381–2385. doi: 10.1097/00001756-199807130-00042. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cδ targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C δ-dependent. Am J Respir Cell Mol Biol. 2003;29:198–205. doi: 10.1165/rcmb.2002-0248OC. [DOI] [PubMed] [Google Scholar]
- 15.Pongracz J, Webb P, Wang K, Deacon E, Lunn OJ, Lord JM. Spontaneous neutrophil apoptosis involves caspase 3-mediated activation of protein kinase C-δ. J Biol Chem. 1999;274:37329–37334. doi: 10.1074/jbc.274.52.37329. [DOI] [PubMed] [Google Scholar]
- 16.Zrachia A, Dobroslav M, Blass M, Kazimirsky G, Kronfeld I, Blumberg PM, Kobiler D, et al. Infection of glioma cells with Sindbis virus induces selective activation and tyrosine phosphorylation of protein kinase C δ. Implications for Sindbis virus-induced apoptosis. J Biol Chem. 2002;277:23693–23701. doi: 10.1074/jbc.M111658200. [DOI] [PubMed] [Google Scholar]
- 17.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase C δ. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 18.Cai W, Torreggiani M, Zhu L, Chen X, He JC, Striker GE, Vlassara H. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-δ: implications for vascular disease. Am J Physiol, Cell Physiol. 2010;298:C624–C634. doi: 10.1152/ajpcell.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato K, Yamanouchi D, Esbona K, Kamiya K, Zhang F, Kent KC, Liu B. Caspase-mediated protein kinase C-δ cleavage is necessary for apoptosis of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H2253–H2261. doi: 10.1152/ajpheart.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thamilselvan V, Menon M, Thamilselvan S. Oxalate-induced activation of PKC-α and -δ regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2009;297:F1399–F1410. doi: 10.1152/ajprenal.00051.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeken MR. Free radicals and birth defects. J Matern Fetal Neonatal Med. 2004;15:6–14. doi: 10.1080/14767050310001650662. [DOI] [PubMed] [Google Scholar]
- 22.Chang TI, Horal M, Jain SK, Wang F, Patel R, Loeken MR. Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia. 2003;46:538–545. doi: 10.1007/s00125-003-1063-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130, 131–137. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi X, Mochly-Rosen D. The PKCδ -Abl complex communicates ER stress to the mitochondria - an essential step in subsequent apoptosis. J Cell Sci. 2008;121:804–813. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- 28.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reece EA, Ma XD, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy. I. Membrane signalling. J Matern Fetal Neonatal Med. 2002;11:249–253. doi: 10.1080/jmf.11.4.249.253. [DOI] [PubMed] [Google Scholar]
- 30.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun. 2007;357:749–754. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 32.Reece EA, Homko CJ. Prepregnancy care and the prevention of fetal malformations in the pregnancy complicated by diabetes. Clin Obstet Gynecol. 2007;50:990–997. doi: 10.1097/GRF.0b013e31815a634b. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Z, Yang P, Eckert RL, Reece EA. Caspase-8: a key role in the pathogenesis of diabetic embryopathy. Birth Defects Res B Dev Reprod Toxicol. 2009;86:72–77. doi: 10.1002/bdrb.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Craven PA, Studer RK, Negrete H, DeRubertis FR. Protein kinase C in diabetic nephropathy. J Diabetes Complicat. 1995;9:241–245. doi: 10.1016/1056-8727(95)80012-4. [DOI] [PubMed] [Google Scholar]
- 36.Craven PA, DeRubertis FR. Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest. 1989;83:1667–1675. doi: 10.1172/JCI114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, et al. Exacerbated vein graft arteriosclerosis in protein kinase C δ-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantley J, Boslem E, Laybutt DR, Cordery DV, Pearson G, Carpenter L, Leitges M, Biden TJ. Deletion of protein kinase Cd in mice modulates stability of inflammatory genes and protects against cytokine-stimulated β cell death in vitro and in vivo. Diabetologia. 2011;54:380–389. doi: 10.1007/s00125-010-1962-y. [DOI] [PubMed] [Google Scholar]
- 39.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 40.Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C δ is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- 41.Santiago-Walker AE, Fikaris AJ, Kao GD, Brown EJ, Kazanietz MG, Meinkoth JL. Protein kinase C δ stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J Biol Chem. 2005;280:32107–32114. doi: 10.1074/jbc.M504432200. [DOI] [PubMed] [Google Scholar]
- 42.Csala M, Margittai E, Bánhegyi G. Redox control of endoplasmic reticulum function. Antioxid Redox Signal. 2010;13:77–108. doi: 10.1089/ars.2009.2529. [DOI] [PubMed] [Google Scholar]
- 43.Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]