Abstract
Background
Acute alcohol exposure produces cognitive deficits in adults but less is known about the acute cognitive effects of alcohol in adolescents. The cognitive impact of acute alcohol exposure includes deficits in discrimination and reversal learning, but traditional experimental approaches make it difficult to distinguish the effect of alcohol on discrimination learning from the effect of alcohol on reversal learning. Young rhesus macaques can be used to model some aspects of human adolescence because of their anatomical, neurophysiological and cognitive similarities with humans.
Methods
Adolescent male rhesus monkeys (N=10) were trained to respond to visual stimuli on touch-sensitive LCD panels controlled by the nonhuman primate version of CANTAB software. Discrimination and reversal learning tasks were subsequently assessed after monkeys were allowed to consume varying amounts of ethanol in a flavored vehicle (vehicle only, up to 0.5 g/kg ethanol, up to 1.0 g/kg ethanol and up to 1.5 g/kg ethanol).
Results
Acute exposure to ethanol reduced perseverance, increased response accuracy, and reduced errors during reversal learning when the task was completed within 90 minutes of ethanol consumption. No reduction in reversal errors was observed when ethanol was consumed 3 or 24 hrs prior to reversal learning. Ethanol only impaired discrimination learning when monkeys had very little previous ethanol exposure.
Conclusions
The temporal relationship between ethanol consumption and reversal learning was consistent with selective ethanol-induced impairment of retrieval, but not storage, processes. This was evidenced by diminished perseverance on the previously correct stimulus leading to decreased errors to criterion.
Keywords: ethanol, monkey, reversal learning, perseverance
Introduction
In adult humans, acute ethanol exposure can impair several cognitive domains thought to play an important role in learning and memory (Field M, 2008, Fillmore MT, 1999, Mulvihill et al., 1997), (Doughtery DM, 2000, Allen EJ, 2009). In keeping with these findings, MRI studies with adult humans indicate that acute alcohol exposure decreases activity in brain regions involved in error processing, behavioral regulation and cognitive control (Anderson BM, 2011, Marinkovic K, 2012, Van Horn JD, 2006). However, little is known about how acute exposure to ethanol alters cognitive function in adolescents, in large part because legal and ethical considerations prohibit alcohol-related experiments with human adolescents. Due to their anatomical and physiological similarities with humans, and wide behavioral repertoires, monkeys provide excellent models for studying the cognitive impact of ethanol (Grant and Bennett, 2003). Despite this, little is known about the relative cognitive impairment produced by acute ethanol exposure within various psychological dimensions in nonhuman primate laboratory models.
In nonhuman primate models, ethanol impairs match-to-sample memory tasks (Mello, 1971), discrimination learning tasks (Pieper and Skeen, 1973, Pieper and Skeen, 1975), a conditional object identification task (Melia KF, 1989) and the performance component of a repeated-acquisition learning task (Winsauer et al., 2002). It was also recently shown that adult rhesus macaques are impaired on a discrimination and reversal learning task under acute intoxication (Jedema et al., 2011). This latter task is intriguing because it can dissociate simple associative learning from the ability to inhibit pre-potent responses under changing reinforcement contingencies
In addition to data derived from experiments with humans and nonhuman primates, other animal models can be used to probe the impact of ethanol on cognitive function. For instance, there is evidence that acute ethanol exposure impairs spatial memory in rats (Popke EJ, 2000, Wright JW, 2003, Chin VS, 2011, Matthews DB, 1999). However, the memory deficits observed in these experiments were sometimes accompanied by significant response rate reductions, leaving open the possibility that observed changes were attributable to nonmnemonic effect of ethanol.
There have been a few direct attempts to determine age-related differences in cognitive effects of ethanol, but the results have been equivocal. Under some conditions, adult rats were shown to be more sensitive than adolescent rats to memory impairments produced by ethanol (Rajendran P, 2004). Other studies indicate the opposite, with adult rats (Markwiese BJ, 1998) and mice (Spanos M, 2012) being less sensitive to the memory-disrupting effects of ethanol than adolescents. Finally, there is evidence that the effect of ethanol on memory does not vary between adolescent and adult rats (Acheson SK, 2001, Chin VS, 2011). There is, however, consistent evidence from rats that early exposure to ethanol can produce long-term cognitive impairments (Tomlinson D, 1998, Pauli J, 1995, Girard TA, 2002).
So, while ethanol has been shown to impair discrimination and reversal learning in nonhuman primates, it is not clear if the effects of ethanol on discrimination learning are dissociable from effects on reversal learning. It is also unclear whether acute exposure to ethanol alters discrimination and reversal learning in adolescent and adult nonhuman primates differently. Accordingly, a series of experiments were undertaken to determine the temporal relationship between ethanol consumption and performance in discrimination and reversal learning tasks in adolescent rhesus monkeys.
Methods
Subjects
Experiments were conducted using 10 adolescent male rhesus macaques (Macaca mulatta; Primate Products, Inc., Miami, FL, USA). At the onset of these studies, the median age of the monkeys was 45 months (range = 36-47 months) and 70% of the monkeys were born within 60 days of each other. The mean weight was 6.0 kg (range = 4.8 – 6.9 kg). Prior work in this lab indicates that male rhesus macaques increase their rate of monthly bodyweight gain around 32 months of age and do not reach stable mature weight of 12-16 kg until about 8-9 years of age. Plasma testosterone levels in intact male monkeys increase between 36-48 month of age (Rose RM, 1978) and brain growth begins to taper off between 40-50 months of age (Knickmeyer, 2010). Thus, the age range of the monkeys in these experiments is consistent with a peri-pubertal time point stretching into late adolescence. Monkeys were fed a diet of standard nonhuman primate chow (Harlan Teklad 15% Monkey Diet #8714, Harlan Laboratories Inc., Madison, WI USA). Each monkey was fed approximately 37 grams of chow/kg bodyweight/day and their diet was supplemented with fresh fruit and a multi-vitamin tablet (Kirkland Signature Sugar-free Children's Chewable Vitamins, Seattle WA USA). Monkeys were fed approximately 20% of their daily chow at least 1 hour before the morning testing sessions. Water was available ad libitum unless otherwise noted. All of the monkeys were single-housed in the colony room.
Testing environment
The colony room was maintained at 22° C - 25° C on a 12-hour light cycle (lights on at 6:00 am). Ethanol consumption and behavioral testing took place in the home cages between 9:00 am and 3:00 pm. These experiments followed guidelines adopted by the US National Institutes of Health (Clark et al., 1997) and took place in an AALAC-approved facility. The experimental protocol was approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Apparatus
Monkeys responded to compound visual stimuli presented on 23 cm × 30 cm touch-sensitive LCD panels controlled by a monkey version of the Cambridge Neurological Test Automated Battery (CANTAB, Lafayette Instruments, Lafayette, IN, USA)..
General Experimental Methodology
The overall approach utilized the 2-choice compound discrimination task that is part of the monkey CANTAB Intra-Dimensional/Extra-Dimensional Attentional Set Shift paradigm. This task has been previously described for use in macaque monkeys (Weed et al., 1999, Weed et al., 2008, Zurcher et al., 2010). Two compound visual stimuli, each approximately 5 cm × 8 cm in size, were used in these experiments. Each compound stimulus consisted of a white line superimposed over a purple shape set against a black background. During these sessions, shape elements were the salient stimuli and responses on line elements were never reinforced. The location of the lines and shapes were independent of each other and varied pseudorandomly; thus, reinforcer delivery was uniquely associated with one of the shape elements, but not with either line element. Each trial consisted of a single response and test sessions were limited to 240 trials or 30 minutes.
During discrimination learning, touching the correct visual stimulus ended the trial and resulted in a food reward (two 190 mg fruit-flavored nonhuman primate tablets, Product #5TUR, TestDiet, Richmond, IN USA). Touching the incorrect shape also ended the trial, but resulted only in 10 seconds of screen darkness. The learning criterion was met after the monkeys emitted at least 12 correct responses within 15 sequential trials. During reversal learning, the stimuli presented during a prior discrimination learning session were presented again, but the reinforcement contingencies were reversed (i.e., responses on the previously reinforced shape ended the trial, but did not result in a food reward). Reversal learning continued until monkeys again met the learning criterion (12/15 correct). Monkeys were presented with ethanol challenges no more than twice in any 7 day period. Each drinking session was separated by at least 48 hours to mitigate carry-over effects.
Ethanol Consumption
Ethanol was added to 300 mL of a 6% (w/v) solution of Tang ® orange-flavored drink mix (Kraft Foods, Glenville, IL, USA). It has been demonstrated that adding ethanol to a flavorant such as Tang ® produces controlled and behaviorally relevant levels of ethanol consumption in rhesus macaques (Katner et al., 2004, Katner et al., 2007). The concentration of ethanol (1.5% to 4.5% w/v) was adjusted for each monkey to achieve the desired maximum ethanol dose. Each monkey was fed 20% of their daily food ration 1 hour before their drinking session. The balance of the daily food ration was provided after all the monkeys had finished working. Ethanol solutions were added to 1L drinking bottles that bore visual indicators of remaining volume. Volume consumed was recorded 5, 10, 15, 20, 30, 45 and 60 minutes (where applicable) after the beginning of the drinking session. At the end of each drinking session, individual doses and mean group doses were calculated.
The maximum duration of the drinking session during Experiment 1 was 60 minutes. This was reduced to 30 minutes in Experiments 2 and 3 because, during Experiment 1, monkeys reliably consumed the majority of their ethanol dose within 15 minutes and drank very little after the first 30 minutes of the drinking session. These experiments were designed to explore the effects of acute ethanol consumption on discrimination and reversal learning. In order to limit total ethanol exposure and preserve the integrity of the design, the intermediate dose of ethanol (i.e., 1.0 g/kg) was omitted after Experiment 1.
Experiment 1: The influence of acute ethanol on discrimination and reversal learning
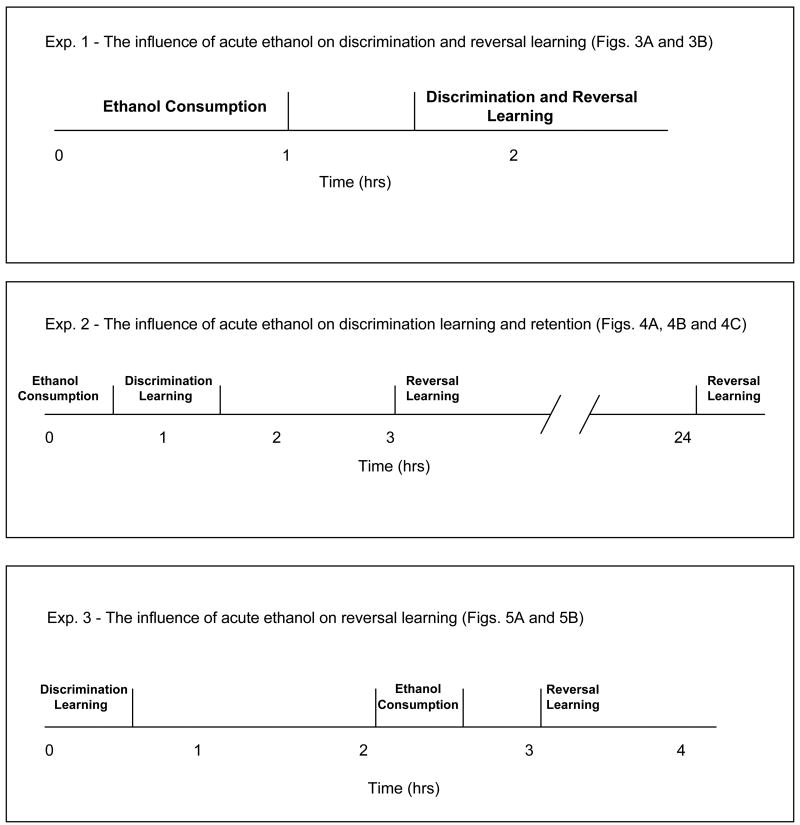
The purpose of Experiment 1 was to determine the effect of acute ethanol consumption on discrimination and reversal learning (Figure 1). Over a series of 4 test sessions, monkeys were given up to 1 hr to drink escalating doses of ethanol (0 g/kg, 0.5 g/kg, 1.0 g/kg and 1.5 g/kg). The escalating-dose design was used in this experiment to minimize total lifetime exposure to ethanol and because the focus was on identifying doses with specific cognitive effects that did not produce generalized behavioral disruption. Two of the 10 monkeys did not receive the 1.0 and 1.5 g/kg doses. Discrimination learning began 90 minutes after the beginning of the drinking session or 30 minutes after ethanol consumption, whichever came first. Reversal learning began immediately after the discrimination learning criterion (12/15 correct) was satisfied. Reversal learning continued until the learning criterion was again met.
Figure 1.
Schematic Representation of Temporal Relationship between ethanol Consumption and Learning Tasks. Experiment 1 was designed to determine the effect of acute ethanol consumption on discrimination and reversal learning. Experiment 2 was designed to dissociate the effects of acute ethanol consumption on discrimination learning from the effects on reversal learning and retention. Experiment 3 was designed to determine the effect of ethanol on reversal learning directly.
Experiment 2: Temporal uncoupling of reversal learning from ethanol consumption
The purpose of Experiment 2 was to dissociate the effects of acute ethanol consumption on discrimination learning from the effects on reversal learning and retention. This was accomplished by evaluating discrimination learning immediately after ethanol consumption, but then waiting 3 hours to evaluate reversal learning (Figure 1). The effect of ethanol on retention of a previously acquired discrimination was evaluated when the monkey completed reversal learning 24 hours after ethanol consumption. Monkeys were given up to 30 minutes to drink 300 mL of the ethanol solution (0 g/kg, up to 0.5 g/kg and up to 1.5 g/kg). The dose order was randomized and each monkey consumed all doses. Discrimination learning started 60 minutes after the beginning of the drinking session or 30 minutes after consumption, whichever came first. Exp. 2 began with two discrimination learning sessions, presented sequentially, each containing different sets of stimuli. The second discrimination learning session began immediately after learning criterion was met for the first session (12/15 correct).
As stated above, the first reversal learning session, began 3 hours after ethanol consumption. The second reversal learning session, which utilized the remaining stimuli set from the previous discrimination session, was conducted 24 hours after ethanol consumption. The order in which each of the two previously-learned stimulus sets were presented during reversal learning was balanced across the group, with half being used for the 3 hour reversal test and half being used for the 24 hour reversal test.
Experiment 3: The influence of acute ethanol on reversal learning
The goal of Experiment 3 was to determine the effect of ethanol on reversal learning, per se. This was accomplished by conducting discrimination learning without ethanol pretreatment and then testing reversal learning 3 hours later and immediately after ethanol consumption (Figure 1). Two hours after completion of the original discrimination learning session, monkeys were given up to 30 minutes to drink 300 mL of an ethanol solution (0 g/kg, up to 0.5 g/kg and up to 1.5 g/kg). The dose order was randomized and each monkey again completed all doses. The reversal learning session began 60 minutes after the beginning of the drinking session or 30 minutes after ethanol consumption, whichever came first. Immediately after the satisfaction of the criterion for reversal learning was met (12/15 correct), a second discrimination learning session began. The purpose of the second discrimination learning session was to again quantify the effect of acute ethanol consumption on discrimination learning. The stimulus set used in the second discrimination session was different from the first, but the learning criterion was the same (12/15 correct).
Experiment 4: Determination of blood-ethanol concentrations (BEC) after oral consumption
After the acute effects of ethanol on discrimination and reversal learning were determined, a separate experiment was conducted to determine BEC after ethanol consumption. Monkeys were allowed 30 minutes to consume ethanol doses up to 3.0 g/kg. The maximum dose was increased in this study to produce a wider dose range and improve BEC analysis. Monkeys were then immobilized with ketamine (10 mg/kg, i.m.) immediately after the drinking session and blood samples were taken 30 minutes after ethanol consumption. Blood samples were centrifuged at 20,000g for 10 minutes. Plasma samples were drawn off, transferred to separate vials and stored at -80C until analysis. Blood-ethanol concentrations were determined using an Analox AM1 ethanol analyzer (Analox Instruments USA, Lunenburg, MA) and expressed as mg% (i.e., mg/dl).
Data Collection and Analysis
The primary dependent variables in these experiments were errors-to-criterion (ETC) and nearly-consecutive errors (NCE). Errors-to-criterion were defined as the total number of errors committed before the performance criterion was met (12/15 correct). Generally, the ETC data were not distributed normally. Distributions were normalized with a square-root transformation in order to better comply with the assumptions of parametric analysis (Roberts et al., 1988). A logarithmic transformation of these data was not appropriate because it was possible for a monkey to satisfy the learning criterion without making any errors and log(0) is undefined.
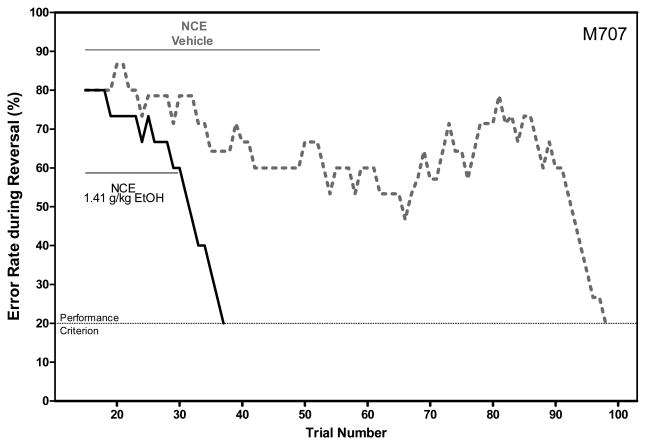
NCE was used as an index of perseverance and was defined as the number of successive incorrect responses made without emitting more than one consecutive correct response. Although it was not uncommon for a series of successive incorrect responses to be interrupted by occasional correct response, these were typically followed by another series of successive incorrect responses (Figure 6). Conversely, two sequential correct responses frequently presaged continued correct responses and rapid achievement of the criterion. Therefore defining the index of perseverance to include nearly-consecutive errors (NCE), instead of consecutive errors only, provides a more comprehensive portrayal of behavior during reversal learning.
Figure 6.
Error Rate During Reversal Learning. Graph depicts error rates for a single monkey after consumption of vehicle and 1.41 g/kg Ethanol. The error rate patterns displayed by this monkey were typical of those observed during reversal learning. In these trials, the error rate is the percent incorrect across the previous 15 trials. Error rates commonly increased or remained unchanged while monkeys perseverated on unreinforced stimuli. Monkeys satisfied the performance criterion after error rates fell to 20% across at least 15 trials. Error rates were typically near 100% during the first 15 trials of reversal learning, but these data were excluded by this analysis. Periods of perseverance are indicated by solid lines and labeled as “NCE”.
Behavioral data were analyzed with one-factor or two-factor (as appropriate) repeated measures analyses of variance (SigmaStat, ver. 3.5, Systat Software, Inc, Richmond, California, USA). Post-hoc analyses were conducted using the Holm-Sidak method with all possible comparisons. The criterion for significance was p < 0.05 for all tests. The mathematical relationship between ethanol dose and blood-ethanol concentration was determined by linear regression analysis.
Results
Experiment 1: The influence of acute ethanol on discrimination and reversal learning when tested within 90 minutes after consumption (Figures 2A and 2B)
Figure 2.
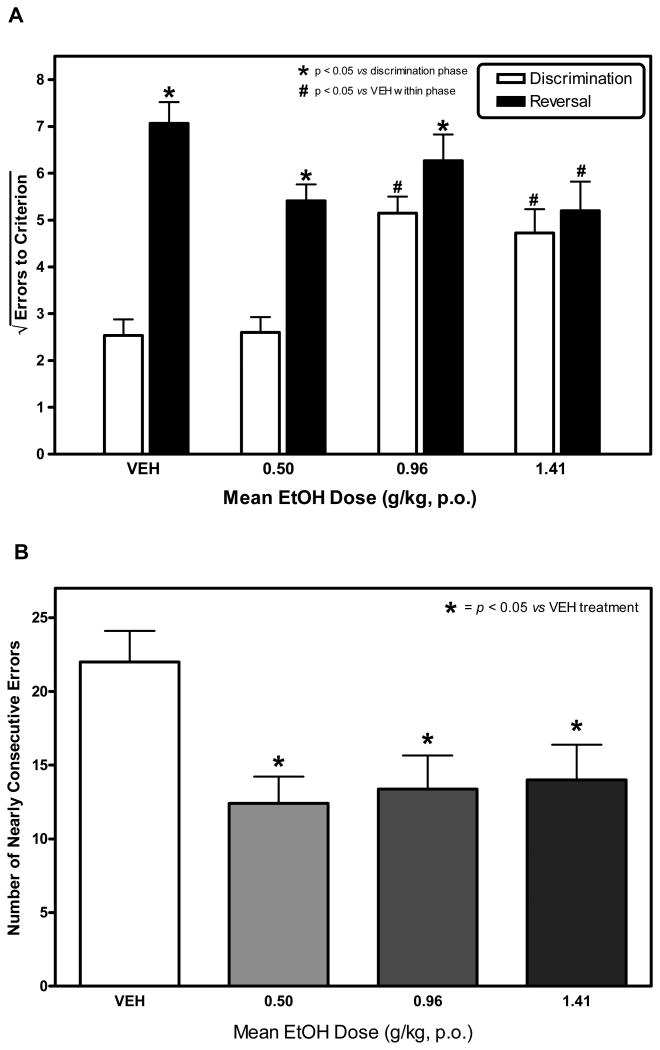
A and B. The Influence of Acute Ethanol on Discrimination and Reversal Learning. In these trials, both discrimination and reversal learning took place within 90 minutes of ethanol consumption. Under these conditions, the effect of ethanol on errors-to-criterion (ETC) was dependent upon whether the monkey was engaged in discrimination or reversal learning. Ethanol increased ETC during discrimination learning and reduced ETC in reversal learning in a dose-related fashion (Fig 2A). As such, ethanol abolished the reversal effect commonly observed during reversal learning. At each dose tested, ethanol also reliably reduced perseverance as indexed by NCE (Figure 2B).
Monkeys reliably consumed the majority of their ethanol dose within 15 minutes of presentation as previously reported (Katner et al., 2004). Mean ethanol doses were 0.50 g/kg (+/- 0.00 SEM), 0.96 g/kg (+/- 0.03 SEM), and 1.41 g/kg (+/- 0.05 SEM). In this experiment, ethanol increased the number of errors committed before reaching criterion during discrimination learning and reduced the number of errors committed before reaching criterion during reversal learning (Figure 2A.). Statistical analysis confirmed that the effect of ethanol on errors-to-criterion (ETC) depended upon whether the monkey was engaged in discrimination or reversal learning. A two-factor repeated measures ANOVA confirmed a significant interaction of ethanol dose and type of learning task on ETC, F3, 21 = 11.889, p < 0.001. Post-hoc analyses confirmed that ETC committed during discrimination learning were significantly higher after consuming 0.96 g/kg (p < 0.001) and 1.41 g/kg (p < 0.001) of ethanol than after consuming vehicle (6% Tang). The analysis also confirmed an increase in ETC when compared to errors during discrimination learning (i.e., a reversal effect) under vehicle conditions and after treatment with 0.50 g/kg and 0.96 g/kg ethanol (p < 0.05 for each); this effect was eliminated by the 1.41 g/kg ethanol dose. ETC during discrimination learning were indistinguishable from ETC during reversal learning after the 1.41 g/kg ethanol dose. Similarly, ETC during reversal learning were significantly lower after consumption of a 1.41 g/kg dose of ethanol (p < 0.01) when compared to vehicle.
Furthermore, the effect of ethanol on response accuracy depended on whether the monkey was engaged in discrimination or reversal learning, F3, 21 = 11.324, p < 0.001 (data not shown). Ethanol reliably reduced response accuracy during discrimination learning at 0.96 and 1.41 g/kg. In contrast, ethanol reliably increased response accuracy during reversal learning at 0.50 g/kg and 1.41 g/kg.
A separate one-factor repeated measures ANOVA confirmed a significant main effect of ethanol on the number of nearly consecutive errors (NCE) during reversal learning (F 3, 23 = 6.373, p < 0.01; Figure 2B). Post-hoc analysis confirmed that ethanol reliably reduced NCE at each dose tested (p < 0.05).
Experiment 2: The influence of acute ethanol on discrimination learning and retention
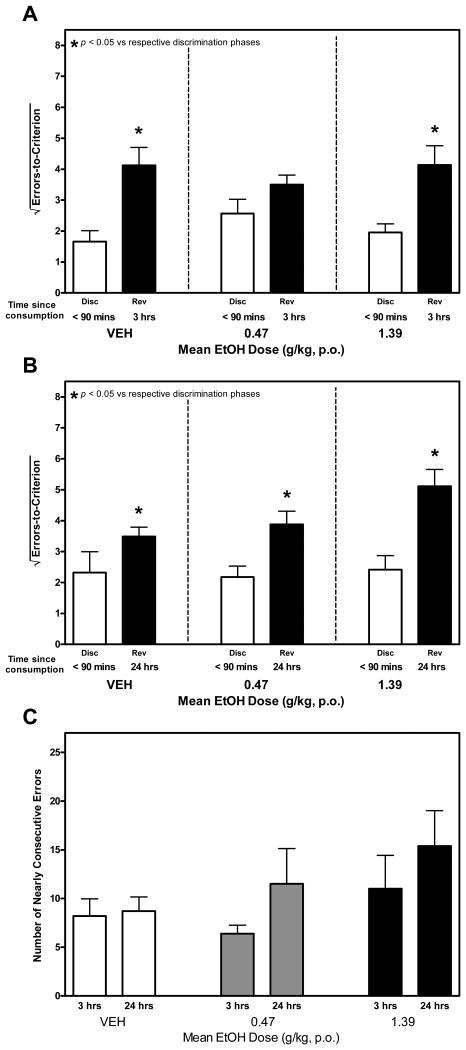
A two-factor repeated measures ANOVA confirmed a significant main effect of type of learning task on ETC, F3, 27 = 30.435, p < 0.001. An increase in ETC during the reversal learning (i.e., the reversal effect) was observed at even the highest ethanol dose tested when reversal learning took place 3 (Figure 3A) and 24 (Figure 3B) hours after consumption of ethanol. With a single exception, post-hoc comparisons confirmed a reliable difference in ETC during reversal and discrimination learning. This contrasts with the elimination of the reversal effect which reversal learning took place less than 90 minutes after ethanol consumption in Exp. 1.
Figure 3.
A, B and C. Temporal Uncoupling of Ethanol Consumption from Reversal Learning. In these trials, discrimination learning (Disc) was conducted within 90 minutes of ethanol consumption. Reversal learning (Rev) was conducted 3 hrs (Fig 3A) and 24 hrs (Fig 3B) later. Unlike Exp 1, ethanol did not alter ETC during discrimination learning or reversal learning. Similar to the results observed under vehicle conditions during Exp 1, a reliable difference in ETC committed during reversal and discrimination learning was generally observed. These data indicate that ethanol did not impair the reversal effect when reversal learning took place 3 or 24 hours after ethanol consumption. Additionally, ethanol did not affect retention at these time points. Perseverance during reversal learning, as indexed by NCE, was not reliably affected ethanol when tested 3 and 34 hours after (Fig 3C).
A separate two-factor repeated measures ANOVA failed to confirm a main effect of ethanol on NCE during reversal learning however, a main effect of time since ethanol consumption on NCE was confirmed, F1, 18 = 5.945, p < 0.05 (Figure 3C). When collapsed across all doses, NCE was higher 24 hours after ethanol consumption than 3 hours after ethanol consumption.
Experiment 3: The influence of acute ethanol on reversal learning
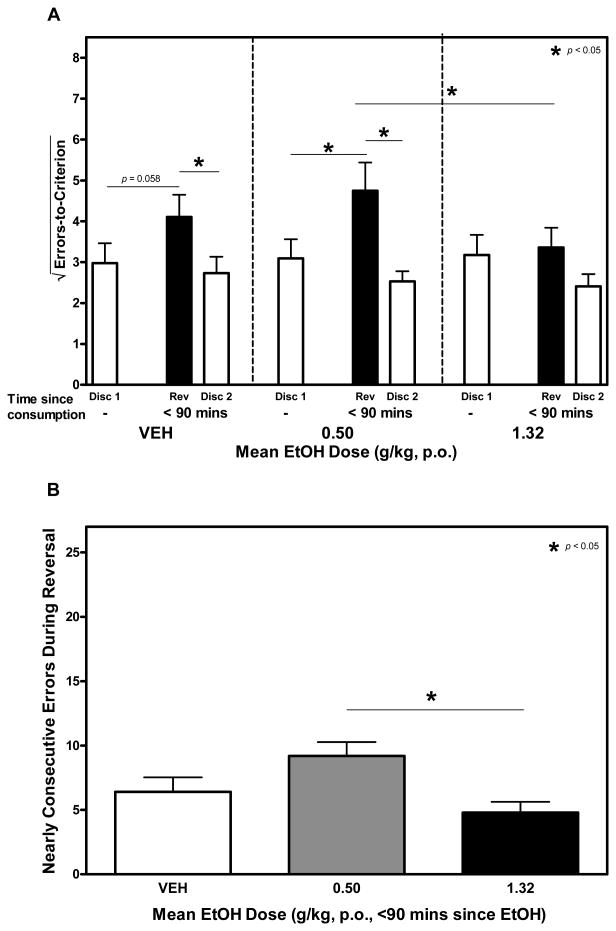
Two-factor repeated measures ANOVA confirmed a significant main effect of learning type on ETC, F2, 18 = 7.800, p < 0.01 (Figure 4A). Post-hoc analyses confirmed that more errors were committed during reversal learning than during discrimination learning after treatment with 0.50 g/kg ethanol. A similar pattern was noted after vehicle, though the observed differences in ETC were not statistically significant (p = 0.058). After treatment with the highest dose of ethanol, however, the number of errors committed during discrimination and reversal learning were very similar (means = 10.09 & 11.28, respectively).
Figure 4.
A and B. The Influence of Acute Ethanol on Reversal Learning. In these trials, the first discrimination learning task (Disc 1) took place without drug treatment. Reversal learning (Rev) and the second discrimination learning task (Disc 2) took place within 90 minutes of ethanol consumption. Under these conditions, a 1.32g/kg dose of ethanol abolished the reversal effect observed after treatment with vehicle or a 0.50 g/kg dose of ethanol (Figure 4A). The 1.32 g/kg dose of ethanol also reduced ETC during reversal learning when compared to the 0.50 g/kg dose. (Figure. 4B).
A separate one-factor repeated measures ANOVA confirmed a significant main effect of ethanol on NCE committed during reversal learning, F2, 18 = 4,583, p < 0.05 (Figure 4B). Post-hoc analyses confirmed that NCE after consumption of a 0.50 g/kg dose of ethanol were reliably higher than after consumption of a 1.32 g/kg dose.
Experiment 4: Determination of blood-ethanol concentrations after oral consumption
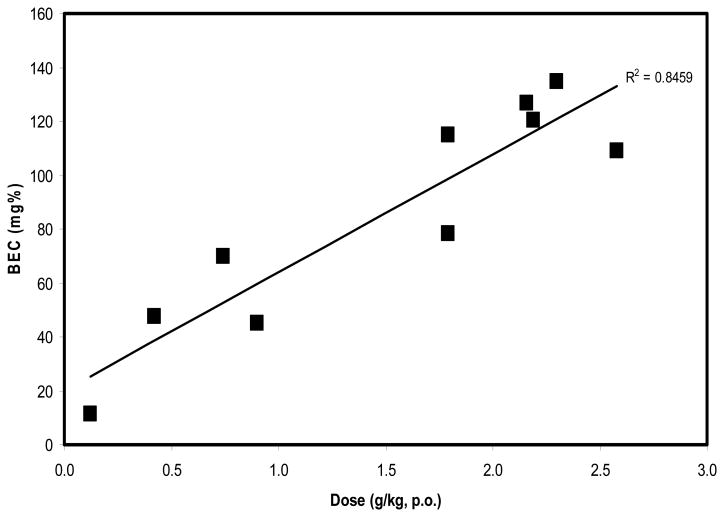
Mean ethanol dose consumed was 1.50 g/kg (+/- 0.94 SEM) and mean BEC was 85.8 mg% (+/- 6.5). Linear regression analysis confirmed that blood-ethanol concentrations were significantly related to does of ethanol consumed, F1,8 = 43.909, p < 0.001 (Figure 5). The coefficient of determination (R2) for these data was 0.846 and standard error of the estimate was 17.368. These observations are consistent with other studies that used similar dosing protocols (Katner et al., 2004, Katner et al., 2007, Green et al., 1999, Vivian et al., 2001); any differences in absolute levels would depend on the precise timing of blood collection after consumption in various models.
Figure 5.
Blood-Ethanol Concentrations (BEC) after Ethanol Consumption. BEC were reliably affected by the dose of ethanol consumed and were consistent with other studies that used similar drinking protocols.
Discussion
The central finding of these experiments is that, in adolescent monkeys, ethanol reliably attenuated the increase in errors committed during reversal learning (i.e., reversal effect) (Figures 2A, 4A). Ethanol attenuated this reversal effect in a dose-related fashion and the reversal effect was completely eliminated by doses of ethanol of 1.32 g/kg equal or greater. Although prior investigations have studied reversal learning under acute ethanol treatment in adult monkeys (Jedema et al., 2011), those procedures conflated effects on the original discrimination, the detection of changing reinforcement contingencies and the establishment of new response patterns. By temporally dissociating ethanol consumption from the discrimination and reversal learning tasks, it was demonstrated that effects of ethanol were specific to reversal learning and did not depend on altering the acquisition of the original discrimination.
One potential explanation for the outcome of the first experiment is that ethanol weakened the associations formed during discrimination learning. In subsequent trials, however, ethanol reduced the reversal cost when reversal learning occurred less than 90 minutes after ethanol consumption (Figures 2A, 4A), but not when reversal learning was tested 3 hours and 24 hours after ethanol consumption (Figures 3A and 3B). If ethanol affected the associations formed during discrimination learning, then the reversal effect should have been attentuated whether reversal learning took place 90 minutes, 3 hours or 24 hours later. These data suggest that the effects of ethanol are on the reversal learning process specifically.
These data bear some similarity with a prior finding with adult monkeys in which discrimination learning was not reliably impaired by intravenous ethanol (Jedema et al., 2011). In those studies, ethanol elevated the number of trials required to reach the discrimination learning criterion but a reliable effect on response accuracy could not be confirmed. In the experiments reported here, ethanol reliably increased errors and reduced response accuracy during discrimination learning, but only when the monkeys had very little previous exposure to ethanol. During subsequent trials (Figures 3A, 4A), ethanol did not alter errors during discrimination learning. It is therefore possible that these data illustrate a distinct cognitive vulnerability present only in monkeys with a limited history of ethanol exposure or, alternatively, that repeated exposure to the discrimination learning test obscures the drug effect. The latter is somewhat unlikely given that these monkeys had successfully completed 8 separate discrimination learning tasks before experiment 1 was undertaken and prior studies show that general task-familiarization and performance improvement is observed in the first few sessions (Weed et al., 2008, Weed et al., 1999, Zurcher et al., 2010)
In contrast with the findings of Jedema et al., however, the data presented here show that ethanol did not produce a reliable reduction in performance during reversal learning. In fact, ethanol increased response accuracy and produced fewer errors during reversal learning. These contrasting results may be attributable, at least in part, to nonspecific behavioral effects, possibly associated with the differences in the route of drug administration. While few overt behavioral alterations were noted during these experiments, Jedema et al. reported that intravenous treatment with a 1.0 g/kg dose of ethanol left the monkeys “highly impaired” and reduced the number that reached criterion during reversal learning dramatically.
It should also be noted that adolescent monkeys (∼4 years of age) were used in these experiments, and Jedema et al. used adult monkeys (7-8 years of age). These age differences take on additional relevance given data from rodents suggest that there are age-related differences in alcohol sensitivity (Silveri MM, 1998). Strong conclusions about the age difference would of course require additional direct study of age groups within a single behavioral model.
In addition to altering ETC, ethanol consumption also reliably decreased perseveration, as indexed by NCE, during reversal learning (Figures 2B and 4B). Under control conditions, monkeys exhibited a significant tendency to perseverate on previously reinforced stimuli during reversal learning. This observation can best be understood in the context of the disinhibitory properties of ethanol (Fillmore MT, 1999, Dougherty DM, 1999). After the discrimination is acquired, the discriminated stimulus becomes pre-potent and subsequent responses tend to be made on that stimulus. Perseveration is affected by the tendency to emit unreinforced responses on pre-potent stimuli. In order to satisfy the learning criterion during reversal learning, however, the tendency to respond on pre-potent stimuli must be inhibited. In these studies, ethanol appeared to impair this inhibitory process. These findings are consistent with other observations of ethanol-related impairments of inhibitory control (Fillmore MT, 1999, Mulvihill et al., 1997, Adams S, 2012, Weafer J, 2012). Interestingly, Jedema et al., reported that post-error slowing was reduced by ethanol, which may be consistent with decreased registration and integration of errors, an increase in compulsive behavior or a reduction in inhibitory control.
It is important to note that changes in perseverance and the reversal effect are not correlated perfectly. Monkeys that had very little experience with ethanol continued to make significantly more errors during reversal learning than during discrimination learning after treatment with both 0.50 g/kg and 0.96 g/kg doses of ethanol (Figure 2A). In contrast, all of the ethanol doses tested in experiment 1 were equally efficacious in reducing NCE (Figure 2B). This disparity demonstrates that diminished perseverance is not the only factor driving the attenuation of the reversal effect.
Finally, these studies validate the use of the monkey CANTAB learning tasks contained in the ID/ED Attentional Set Shift procedure for repeated-measures investigations of treatment effects. Prior studies have demonstrated efficacy for longitudinal analysis of permanent manipulations, typically using between-groups designs (Rodriguez et al., 2011, Dias et al., 1996a, Dias et al., 1996b, Muggleton et al., 2005, Pearce et al., 1999, Weed et al., 1999). Here it is shown that the tasks can be used for multiple acute treatment repeated-measures designs within a single group of subject. The observed stability in the ETC measures for original discrimination learning tasks (using random stimulus assignment) and in the reversal effect under vehicle conditions are necessary conditions for validating the task for the evaluation of the acute effect of multiple doses of a psychoactive agents.
In summary, these data indicate that, in adolescent monkeys, acute exposure to ethanol selectively attenuates the reversal effect that is commonly seen during reversal learning, but only when reversal learning occurred less than 90 minutes after ethanol consumption. These data are distinct from data collected in adult rhesus monkeys (Jedema et al., 2011) and suggest that the cognitive effects of ethanol are different in adolescent and adult monkeys. These data distinguish effects of alcohol on reversal learning from those on the learning of the original discrimination. Perseverance was also reduced after ethanol consumption, but changes in perseverance and the reversal effect were not perfectly correlated. Additionally, discrimination learning was impaired after ethanol treatment, but only monkeys that had very little previous ethanol exposure. Taken together, these data suggest that, in adolescent monkeys, ethanol consumption faciltates reversal learning, at least in part, by impairing inhibitory control. Unfortunately, it is not possible to contrast these results with data from adult monkeys because little is known about how acute ethanol consumption alters perseverance in adult monkeys. In contrast, ethanol produced only a modest effect on incremental associative learning in these studies.
Acknowledgments
This work was supported by USPHS grants AA016807 and AA007456. The authors would also like to acknowledge the support of the TSRI Alcohol Research Center as well as Sophia Vandewater and Maury Cole for their technical assistance. This is publication #21694 from The Scripps Research Institute.
Footnotes
Conflicts of Interest: None
References
- Acheson SK, R E, Swartzwelder HS. Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol. 2001;23:167–175. doi: 10.1016/s0741-8329(01)00127-6. [DOI] [PubMed] [Google Scholar]
- Adams S, A A, Attwood AS, Munafo MR. Effects of alcohol on disinhibition towards alcohol-related cues. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Allen EJ, M S, Skudlarski P, Calhoun VD, Astur R, Ruopp KC, Pearlson GD. Effects of alcohol on performance on a distraction task during simulated driving. Alcohol Clin Exp Res. 2009;33:617–325. doi: 10.1111/j.1530-0277.2008.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BM, S M, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin VS, VS C, Berry RB, Kirk RE, Diaz-Granados J, Matthews DB. Effect of acute ethanol and acute allopregnanolone on spatial memory in adolescent and adult rats. Alcohol. 2011;45:473–483. doi: 10.1016/j.alcohol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. Ilar J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, M F, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- Doughtery DM, M D, Moeller FG, Chokshi RV, Rosen VC. Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in immediate and delayed memory. Alcohol Clin Exp Res. 2000;24:1702–1711. [PubMed] [Google Scholar]
- Field M, S T, Wiers RW. Cognitive processes in alcohol binges: a review and research agenda. Curr Drug Abuse Rev. 2008;24:1702–1711. doi: 10.2174/1874473710801030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, VS An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Girard TA, W P. Testing the spatial- versus object-learning distinction: water-maze performance of male rats exposed to ethanol during the brain growth spurt. Behav Brain Res. 2002;134:493–503. doi: 10.1016/s0166-4328(02)00091-8. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–55. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis) Alcohol Clin Exp Res. 1999;23:611–6. [PubMed] [Google Scholar]
- Jedema HP, Carter MD, Dugan BP, Gurnsey K, Olsen AS, Bradberry CW. The acute impact of ethanol on cognitive performance in rhesus macaques. Cereb Cortex. 2011;21:1783–91. doi: 10.1093/cercor/bhq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, Taffe MA. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004;28:873–83. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Von Huben SN, Davis SA, Lay CC, Crean RD, Roberts AJ, Fox HS, Taffe MA. Robust and stable drinking behavior following long-term oral alcohol intake in rhesus macaques. Drug Alcohol Depend. 2007;91:236–43. doi: 10.1016/j.drugalcdep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, et al. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. 2010;20:5. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, R E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Human Brain Mapp. 2012;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, A S, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Matthews DB, I M, White AM, Best PJ. Acute ethanol administration impairs spatial performance while facilitating nonspatial performance in rats. Neurobiol Learn Mem. 1999;72:169–179. doi: 10.1006/nlme.1998.3900. [DOI] [PubMed] [Google Scholar]
- Melia KF, E C. Signal detection analysis of ethanol effects on a complex conditional discrimination. Pharmacol Biochem Behav. 1989;33:581–584. doi: 10.1016/0091-3057(89)90391-2. [DOI] [PubMed] [Google Scholar]
- Mello NK. Alcohol effects on delayed matching to sample performance by rhesus monkey. Physiol Behav. 1971;7:77–101. doi: 10.1016/0031-9384(71)90239-3. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Smith AJ, Scott EA, Wilson SJ, Pearce PC. A long-term study of the effects of diazinon on sleep, the electrocorticogram and cognitive behaviour in common marmosets. J Psychopharmacol. 2005;19:455–66. doi: 10.1177/0269881105056521. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–5. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Pauli J, W P, Bedi KS. Spatial learning ability of rats following acute exposure to alcohol during early postnatal life. Physiol Behav. 1995;58:1013–1020. doi: 10.1016/0031-9384(95)00120-8. [DOI] [PubMed] [Google Scholar]
- Pearce PC, Crofts HS, Muggleton NG, Ridout D, Scott EA. The effects of acutely administered low dose sarin on cognitive behaviour and the electroencephalogram in the common marmoset. J Psychopharmacol. 1999;13:128–35. doi: 10.1177/026988119901300203. [DOI] [PubMed] [Google Scholar]
- Pieper WA, Skeen MJ. Development of functional tolerance to ethanol in rhesus monkeys (Macaca mulatta) Pharmacol Biochem Behav. 1973;1:289–94. doi: 10.1016/0091-3057(73)90119-6. [DOI] [PubMed] [Google Scholar]
- Pieper WA, Skeen MJ. Retention of functional tolerance to ethanol in rhesus monkeys (Macaca mulatta) Pharmacol Biochem Behav. 1975;3:909–13. doi: 10.1016/0091-3057(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Popke EJ, A S, Paule MG. Effects of acute ethanol on indices of cognitive-behavioral performance in rats. Alcohol. 2000;20:187–192. doi: 10.1016/s0741-8329(99)00081-6. [DOI] [PubMed] [Google Scholar]
- Rajendran P, S L. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci. 2004;1021:441–444. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Quart J Exp Psychol. 1988;40B:321–341. [PubMed] [Google Scholar]
- Rodriguez JS, Zurcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204:545 e1–10. doi: 10.1016/j.ajog.2011.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RM, B I, Gordon TP, Lindsley JG. Changes in testosterone and behavior during adolescence in the male rhesus monkey. Psychosom Med. 1978;40:60–70. doi: 10.1097/00006842-197802000-00007. [DOI] [PubMed] [Google Scholar]
- Silveri MM, S L. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spanos M, B J, Hodge CW. Increased sensitivity to alcohol induced changes in ERK Map kinase phosphorylation and memory disruption in adolescent as compared to adult C57BL/6J mice. Behav Brain Res. 2012;230:158–166. doi: 10.1016/j.bbr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D, W P, Bedi KS. Spatial learning ability of rats following differing levels of exposure to alcohol during early postnatal life. Physiol Behav. 1998;63:205–211. doi: 10.1016/s0031-9384(97)00424-1. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, Y M, Schmitt PJ, Grafton ST. Alcohol-induced suppression of BOLD activity during goal-directed visuomotor performance. Neuroimage. 2006;31:1209–1221. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–97. [PubMed] [Google Scholar]
- Weafer J, F M. Comparison of alcohol impairment of behavioral and attentional inhibition. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Bryant R, Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157:22–8. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Brauner IN, Purcell JE, Lancaster JR, Bagby JR, G J, Nelson S. Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcohol Clin Exp Res. 2002;26:1846–57. doi: 10.1097/01.ALC.0000042171.80435.F1. [DOI] [PubMed] [Google Scholar]
- Wright JW, M A, Reichert JR, Turner GD, Meighan SE, Meighan PC, Harding JW. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003;963:252–261. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- Zurcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, Mcdonald TJ, Nathanielsz PW, Nijland MJ. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Methods. 2010;188:219–25. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]