Abstract
The advent of more effective antiretroviral therapies has reduced the frequency of HIV dementia, however the prevalence of milder HIV associated neurocognitive disorders [HAND] is actually rising. Neurodegenerative mechanisms in HAND might include toxicity by secreted HIV-1 proteins such as Tat, gp120 and Nef that could activate neuro-inflammatory pathways, block autophagy, promote excitotoxicity, oxidative stress, mitochondrial dysfunction and dysregulation of signaling pathways. Recent studies have shown that Tat could interfere with several signal transduction mechanisms involved in cytoskeletal regulation, cell survival and cell cycle re-entry. Among them, Tat has been shown to hyper-activate cyclin-dependent kinase [CDK] 5, a member of the Ser/Thr CDKs involved in cell migration, angiogenesis, neurogenesis and synaptic plasticity. CDK5 is activated by binding to its regulatory subunit, p35 or p39. For this manuscript we review evidence showing that Tat, via calcium dysregulation, promotes calpain-1 cleavage of p35 to p25, which in turn hyper-activates CDK5 resulting in abnormal phosphorylation of downstream targets such as Tau, collapsin response mediator protein-2 [CRMP2], doublecortin [DCX] and MEF2. We also present new data showing that Tat interferes with the trafficking of CDK5 between the nucleus and cytoplasm. This results in prolonged presence of CDK5 in the cytoplasm leading to accumulation of aberrantly phosphorylated cytoplasmic targets [e.g.: Tau, CRMP2, DCX] that impair neuronal function and eventually lead to cell death. Novel therapeutic approaches with compounds that block Tat mediated hyper-activation of CDK5 might be of value in the management of HAND.
Keywords: CDK5, CRMP2, HIV-1 Tat, neurodegeneration, nuclear translocation, Tau
INTRODUCTION
The management and reduction of neurological complications associated with AIDS remains an important goal to lower levels of morbidity associated with HIV patients. In the CNS, microglial cells represent a potential a primary reservoir for HIV-1 infection [1–4], and in some cases infection is also detected in astrocytes [5]. Increased utilization of highly active antiretroviral therapies [HAART] has reduced the abundance of active HIV in the brain and overt dementia. Despite these advances in treatment, the increasing number of patients living with chronic HIV infection may be resulting in the rising prevalence of milder forms of HIV-associated neurocognitive disorders [HAND] [6–13].
Neurocognitive disorders are a major complication in HIV-infected patients [14], afflicting 15–50% of HIV patients [11, 15], and evidence suggests that the aging HIV population is particularly susceptible to cognitive alterations [12, 16, 17]. Thus, new targets capable of protecting the CNS from HIV neurotoxicity could represent important therapies for HAND patients. Clinico-pathological studies suggest that the development of HAND might be associated with the severity of HIV encephalitis [HIVE] [18, 19]. HIVE is an inflammatory condition [11, 20–22] involving the presence of HIV-infected macrophages/microglial cells forming microglial nodules and multi-nucleated giant cells [MNGC], astrogliosis, white matter damage and neurodegeneration [23]. While in the pre-HAART era severe HIVE was a common finding in 30–40% of the cases, after the institution of HAART this is a less common finding and burn-out or milder forms of HIVE are observed [11, 24, 25]. About half of the HAND cases display some degree of HIVE, however others do not show HIVE and might be associated with silent forms of HIV [26, 27] and other co-morbidities in HIV patients such as aging, HCV co-infection and drug abuse [11,28–30]. HIV proteins [e.g., Tat, gp120, nef] released from infected immune cells trafficking in the CNS might be responsible for the neurodegenerative phenotype in HAND. Among them, Tat plays a major role [11, 31, 32].
Viral proteins such as Tat, gp120 and Nef and cytokines produced by macrophages/microglia have been shown to induce neuronal dysfunction, neuron damage, and loss of nerve cells [33–42]. In HIV patients, early stage neurode-generation is characterized by dendritic and synaptic damage to pyramidal neurons in the neocortex and hippocampus [43], and non-spine neurons in the basal ganglia. Supporting an important role of Tat in neurodegeneration and HIV, Tat levels positively correlate with cognitive alterations in HAND patients [31].
The mechanisms leading to neurodegeneration in HAND might involve a variety of pathways including excitotoxicity [46, 47], oxidative stress [33, 48], calcium dysregulation [57, 58], autophagy [11, 49–52], proteasome abnormalities [53, 54], mitochondrial dysfunction [55, 56], damage to the blood-brain barrier [BBB] [44, 45], and signaling alterations [11, 32, 59–65]. Among the signaling pathways affected by HIV proteins, recent studies have shown that Tat and gp120 could abnormally activate c-Jun N-terminal kinase [JNK], double-stranded RNA-activated protein kinase [PKR] [66], glycogen synthase kinase-3β [GSK3β] [67–69] and receptor and non-receptor tyrosine kinases [RTK] pathways [70, 71]. Furthermore, Tat associates with growth factor receptor-bound protein 2 [Grb2], an adaptor protein containing two SH3 domains involved in RTK activity [72]. Chronic HIV and simian immunodeficiency virus [SIV] infection also dysregulate the vascular endothelial growth factor [VEGF] [74], RTKs recepteur d'orgine nantais [73], and platelet-derived growth factor [PDGF] signaling [75]. In addition to these signaling pathways, recent studies have shown that CDK5 is hyper-activated [67, 76, 77] in patients with HAND. Therefore, among other pathways through which Tat might lead to neurodegeneration is by aberrant activation of signaling pathways such as CDK5, which is an important pathway for cell survival. CDK5 is also of interest because it might be a target for therapeutical development to manage HAND.
CDK5 belongs to the Ser/Thr CDK family and is activated by binding to its regulatory subunit, p35 or p39 [78]. Most CDKs are activated in a manner dependent on the phase of the cell cycle in dividing cells and induce cell cycle progression. In contrast, CDK5 is mostly active in post-mitotic cells such as neurons, although CDK5 is expressed widely in many cells types and tissues [78]. CDK5 plays a role in neuronal survival and migration [79, 80], neurogenesis [81–83], neurite growth [84] and synaptic plasticity [85, 86].
The abnormal activation of CDK5 might play a role in Alzheimer’s Disease [AD] [87, 88], Parkinson’s Disease [PD] [89] and Huntington’s Disease [HD] [90]. Under pathological conditions, increased intracellular calcium levels activate calpain-I, which then abnormally cleaves p35 into p25 [88] (Fig. 1A). In contrast to p35, the p25 fragment is more stable and constitutively activates CDK5 [88], resulting in aberrantly phosphorylated neuronal substrates [91] and neuronal cell death that may be related to CDK5-mediated cell cycle reentry [92, 93]. In patients with AD, the active p25/CDK5 complex results in the hyperphosphorylation of the microtubule-associated protein Tau, which is abundantly present in the neurofibrillary tangles [87, 88, 91].
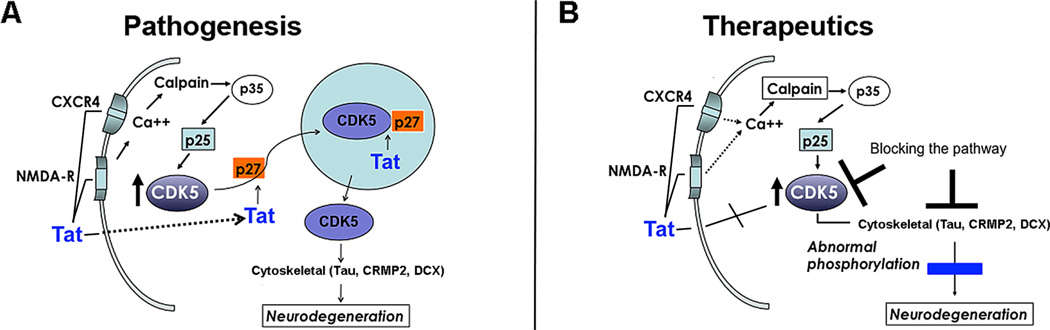
Fig. (1). Role of Tat and other HIV-1 proteins in neurodegeneration and treatment of HAND.
[A] Schematic representation of the mechanisms through which Tat might abnormally activate CDK5 in HAND. Tat could activate calpain 1, which in turn cleaves p35 to p25, this later fragment hyperactivates CDK5 leading to abnormal phosphorylation of Tau, CRMP2 and DCX and neurodegeneration. Alternatively, Tat could interact with p27, which is necessary to carry CDK5 to the nucleus. When Tat interferes with this role of p27, CDK5 is retained in the cytoplasm resulting in hyperactivation of CDK5 and neurodegeneration. (B) Potential targets for HAND therapeutics including blocking Tat, CDK5, p25 and pTau.
Recent work indicates that CDK5 might also be involved in the mechanisms of neurotoxicity in patients with HAND [67, 76, 77]. For example, a previous gene array study showed that expression levels of CDK5 and related family members are abnormally expressed in patients with HIVE [94]. In the brains of patients infected with HIV-1, calpain-1 is activated by Tat resulting in breakdown of p35 to p25, leading to hyper-activation of CDK5 [64] (Fig. 1A). This study showed that increased p25/CDK5 activity in the nuclear compartment could lead to phosphorylation of MEF2, a nuclear neuronal pro-survival transcription factor, and result in neurotoxicity (Fig. 1A) [64]. Therefore, Tat might promote neurodegeneration by activating CDK5 via aberrant calpain-1 cleavage of p35 to p25. This results in hyperphosphorylation of downstream substrates, and as such this pathway may be a potential target for therapeutic purposes (Fig. 1B). For this manuscript we review evidence supporting a role of Tat in neurodegeneration in HAND via dysregulation of CDK5. We also present new data showing that Tat interferes with the trafficking of CDK5 between the nucleus and cytoplasm resulting in prolonged presence of CDK5 in the cytoplasm. This leads to accumulation of aberrantly phosphorylated targets such as Tau, collapsin response mediator protein-2 [CRMP2], and doublecortin [DCX].
RESULTS
Tat Hyperactivates CDK5 by Interfering with it’s Nucleo-Cytoplasmic Translocation
Although there is consensus in the field that Tat mediated CDK5 hyper-activation leads to neurodegeneration in HAND, it is now important to investigate in greater detail how HIV proteins promote this effect. CDK5 is the predominant CDK [95] in the CNS, and is a Ser-Thr protein kinase with post-mitotic activity that phosphorylates cytoskeletal proteins [e.g., Tau, collapsin response mediator protein-2 [CRMP2]], synaptic proteins [e.g., PSD95], and transcription factors [e.g., MEF2] [96–98]. CDK5 is normally localized in the nucleus and cytoplasm of mature neurons [99]. New evidence indicates that CDK5 nuclear localization is key in the maintenance of the post-mitotic state of mature neurons [100–102] (Fig. 1A). Some studies suggest that interactions with cell cycle regulators such as p27 are responsible for nuclear localization [103, 104] (Fig. 1A). Tat has been shown to interact with p27, but also with other cell cycle proteins such as cyclins, CDK’s, and proliferating cell nuclear antigen [PCNA] [105]. Since Tat and CDK5 have been shown to interact with similar cell cycle regulators [105], then it is possible that in the presence of Tat protein, physiological interactions between CDK5 and cell cycle regulators might be disrupted resulting in alterations of CDK5 localization to the nucleus. Under stress conditions in post-mitotic neurons, loss of CDK5 interactions with cell cycle regulators in the nucleus may result in nuclear export of CDK5 to the cytoplasm, triggering abnormal cell cycle re-entry [105]. Prolonged elevation of levels of CDK5 in the cytoplasm may lead to an accumulation of aberrantly phosphorylated cytoplasmic targets that impair neuronal function and eventually lead to cell death (Fig. 1A).
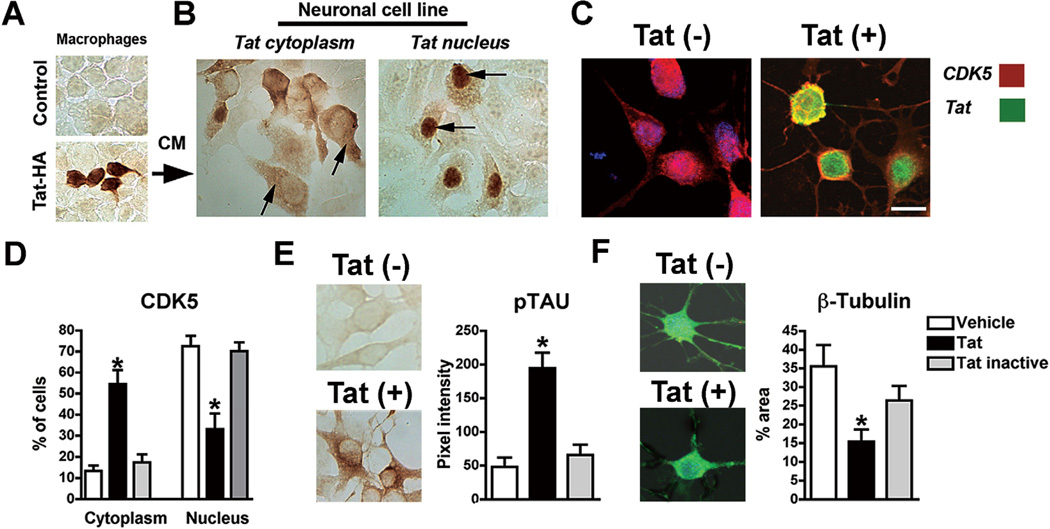
To investigate the possibility that Tat abnormally activates CDK5 by interfering with its cellular localization, recombinant HA-tagged Tat was produced in human monocyte/macrophage cells transfected with a pcDNA plasmid expressing Tat tagged with HA [Tat-HA] [Addgene plasmid 14654] [106] (Fig. 2A). Then neuronal cells [B103 rat neuroblastoma] [107] were exposed to the conditioned media [CM] from transfected cells as well as to a number of control conditions including heat inactivation of the CM, CM spiked with recombinant Tat, or CM obtained from cells transfected with control pcDNA-GFP alone. Immunoblot of Tat protein in cyotosolic and nuclear fractions produced inconclusive results in these studies. Therefore, immunocytochemistry [ICC] analysis with antibodies against HA or Tat showed that after 24 hrs, Tat was detected in 30–40% of the neuronal cells (Fig. 2B). In most cases Tat was found in the cytoplasm, however 45% of the neurons displaying Tat in the cytoplasm also displayed nuclear labeling (Fig. 2B). Double-labeling showed that in neuronal cells that contained Tat in the nucleus, CDK5 labeling was predominantly detected in the cytoplasm, while in the control conditions without Tat, CDK5 was localized to the nucleus (Fig. 2C, D). Neuronal cells displaying nuclear Tat localization also showed higher levels of pTau (Fig. 2E), and analysis with antibodies against β-tubulin showed that neuritic processes were reduced (Fig. 2F) and the microtubule cytoskeleton was simplified. These results support the notion that Tat can traffic into the neuronal cells including the nucleus where it could potentially interfere with the localization of CDK5.
Fig. (2). In vitro studies showing that Tat reduces the translocation of CDK5 from the cytoplasm to the nucleus.
(A) Tat expression in macrophages following 5 days; (B) immunocytochemical analysis of neuronal cells treated with conditioned media [CM] showing that Tat traffics into neuronal cells and localizes in the cytoplasm and nucleus; (C, D) double immunolabeling with antibodies against CDK5 [red] and Tat [green] showing that Tat re-distributes CDK5 from the nucleus to the cytoplasm of neuronal cells [p<0.0001; df=5, F=75.82 by one-way ANOVA]. (E, F) Tat-mediated translocation of CDK5 leads to increased pTau [p<0.0001; df=2, F=320.7 by one-way ANOVA] and reduced neurite outgrowth [β-tubulin] [p<0.0001; df=2, F=17.57 by one-way ANOVA]. Bar= 10 um.
Tat Mediates CDK5 Mis-Localization in HIVE and GFAP-Tat tg Mice
Secreted HIV proteins such as gp120 and Tat have been shown to be neurotoxic. Tat penetrates the cell membrane [108], is released from infected macrophages [109, 110], triggers abnormal signaling in CNS cell populations [111], accumulates in neurons [112], and is axonally transported [113], leading to neuronal damage [33, 110, 112, 114, 115]. Exogenous Tat might be up-taken into cells, facilitating interactions with cell cycle-related proteins such as p27 (Fig. 1) in neuronal models, either preventing CDK5 trafficking to the nucleus or promoting CDK5 trafficking from the nucleus to the cytoplasm. Given the non-cycling state of neurons, favoring cytoplasmic localization of CDK5 [due to potential interactions between Tat and p27, for example] in combination with the induction of p25, results in CDK5 activation and hyperphosphorylation of substrates such as CRMP2 [116] and Tau [76], leading to impaired neuronal function and eventual cell death (Fig. 1A). Although we have focused on the effects of Tat as an example of how HIV proteins might dysregulate cell cycle activation and trigger neurotoxicity, we recognize that other HIV proteins such as nef, vif, and vpr might also dysregulate CDKs [117, 118].
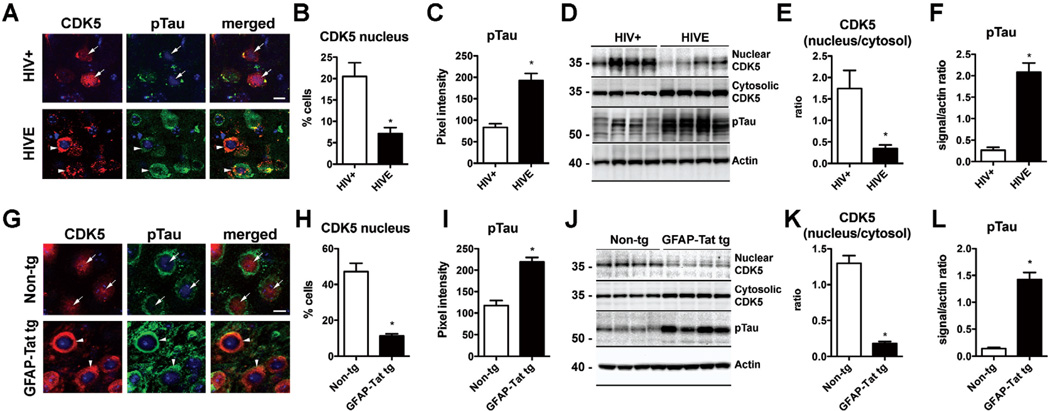
To determine if HIV-1 promotes CDK5 mis-localization with the resulting cytoplasmic accumulation and Tau hyperphosphorylation we analyzed the distribution and levels of CDK5 and pTau in HIV+ and HIVE donor brain tissue. Analysis by double immunolabeling and confocal microscopy in the frontal cortex neurons from HIV+ control patients showed CDK5 was preferentially localized in the nucleus and to a lesser extent in the cytoplasm (Fig. 3A, B). In contrast, in the neocortex from patients with HIVE the levels of CDK5 were reduced while greater CDK5 immunoreactivity was detected in the cytoplasm of neurons (Fig. 3A, B). Consistent with these findings, and levels of pTau were low in HIV+ cases while in HIVE pTau immunoreactivty in the neuronal cytoplasm was elevated (Fig. 3A, C). To further verify these findings by an independent method, immunoblot analysis was performed with the nuclear and cytosolic fractions from the frontal cortex of HIV cases. In agreement with the immunocytochemical analysis, immunoblot showed that in HIV+ control cases CDK5 was more abundant in the nuclear than cytosolic fractions, while in the HIVE cases CDK5 levels were reduced in the nuclear fraction and increased in the cytosolic fraction (Fig. 3D, E). Likewise, levels of pTau were increased by immunoblot in HIVE cases compared to HIV+ controls (Fig. 3D, F). To further investigate the involvement of Tat in the CDK5 mislocalization, the brains of non-tg and GFAP-Tat tg mouse brains were analyzed. By double immunolabeling and confocal microscopy, in the non-tg mice CDK5 was localized in the nucleus and to a lesser extent in the cytoplasm (Fig. 3G, H) while in the GFAP-Tat tg mouse CDK5 was more abundant in the neuronal cytoplasm than in the nucleus (Fig. 3G, H). Moreover, levels of pTau immunoreactivity were elevated in the GFAP-Tat tg mouse (Fig. 3G, I). By immunoblot analysis, CDK5 was more abundant in the nuclear than cytosolic fractions in the non-tg mice, whereas in the GFAP-Tat tg mouse CDK5 immunoreactivity was reduced in the nuclear fraction and increased in the cytosolic fraction (Fig. 3J, K). Similarly, levels of pTau were increased by immunoblot in GFAP-Tat tg versus non-tg controls (Fig. 3J, L).
Fig. (3). Nucleo-cytoplasmic translocation of CDK5 and Tau hyperphosphorylation in HIVE and GFAP-Tat tg mice.
(A–C) Double immunolabeling and confocal microscopy for CDK5 [red] and pTau [green] in the frontal cortex of HIV+ control and HIVE patients showing increased localization of CDK5 immunostaining to the cytoplasm of HIVE cases as well as increased pTau. n=6 HIV+ and n=6 HIVE; B: p=0.0033, df=10, F=5.111; C: p=0.0002, df=10, F=3.709 by two-tailed, unpaired T-test. (D–F) Immunoblot analysis with nuclear and cytosolic fractions showing decreased CDK5 in the nuclear fraction and increased in the cytosolic fraction of HIVE cases. Levels of pTau were also increased in HIVE cases by immunoblot. n=6 HIV+ and n=6 HIVE. E: p=0.0085, df=10, F=26.69; F: p<0.0001, df=10, F=9.501 by two-tailed, unpaired T-test. Bar= 10 um. (G–I) Double immunolabeling and confocal microscopy for CDK5 [red] and pTau [green] in the cortex of non-tg and GFAP-Tat tg mice showing increased localization of CDK5 immunoreactivity to the cytoplasm of HIVE cases as well as increased pTau. n=6 non-tg and n=6 tg mice; H: p<0.0001, df=10, F=14.02; I: p<0.0001, df=10, F=1.298 (J–L) Immunoblot analysis with nuclear and cytosolic fractions showing decreased CDK5 in the nuclear fraction and increased in the cytosolic fraction of GFAP-Tat tg mice compared to non-tg mice. Levels of pTau were also increased in GFAP-Tat tg mice by immunoblot. n=6 non-tg and n=6 tg mice; K: p<0.0001, df=10, F=19.06; L: p<0.0001, df=10, F=30.13 by two-tailed, unpaired T-test. Bar= 10 um.
These results bolster the notion that Tat might mediate the mislocalization of CDK5. Accumulation of CDK5 in the cytoplasm of neurons may cause Tau hyperphosphorylation, and thereby lead to neuronal cytoskeleton alterations and neurodegeneration.
Alterations in p27 Might Mediate Tat Effects on CDK5 Mis-Localization in HIVE
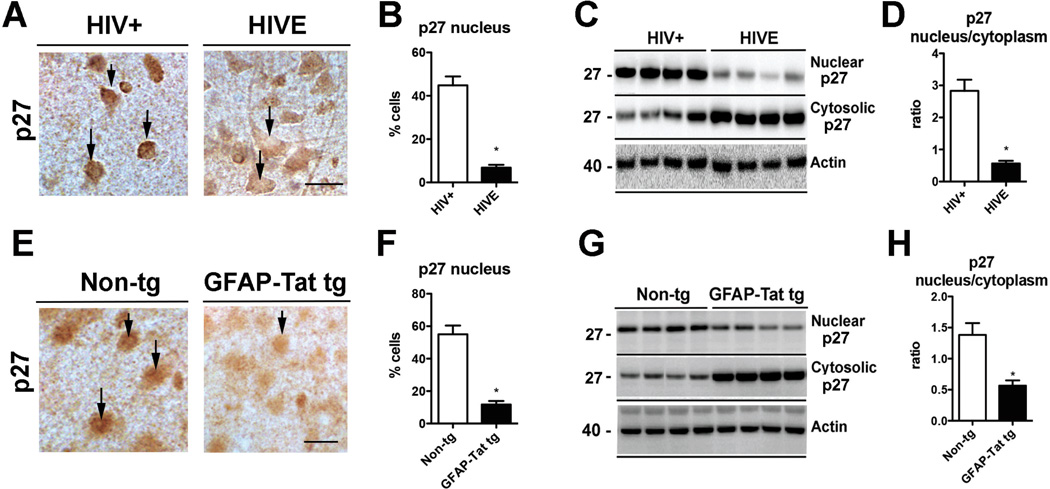
Since CDK5 has no intrinsic functional nuclear localization sequence [NLS], the nuclear localization of CDK5 relies on its binding to p27, whereas the cytoplasmic re-distribution of CDK5 is achieved through its nuclear export sequence [NES] interacting with the chromosome region maintenance [CRM1] nuclear export mechanism [103] (Fig. 1A). Tat has been shown to interact with p27, but also with other cell cycle proteins such as cyclins, CDK’s, and proliferating cell nuclear antigen [PCNA] [105] and Tat has been shown to traffic to the cell nucleus (Fig. 2). Hence, it is possible that Tat interaction with p27 may mediate the cytoplasmic mis-localization of CDK5 in HIVE (Fig. 1A). To begin to investigate this, the distribution and levels of p27 in the nucleus and cytoplasm were analyzed. By immunocytochemistry and digital bright field microscopy, p27 was preferentially localized in the nucleus and less so in the cytoplasm in the frontal cortex neurons from HIV+ control patients (Fig. 4A, B). In contrast, CDK5 immunoreactivity was reduced in the nucleus and increased in cytoplasm of neurons in HIVE cases (Fig. 4A, B). By immunoblot analysis in HIV+ control cases p27 was more abundant the nuclear than in the cytoplasmic fraction, whereas in HIVE cases p27 levels were reduced in the nuclear compartment and increased in the cytosolic compartment (Fig. 4C, D). Similar analysis was performed in the brains of non-tg and GFAP-Tat tg mice. In the non-tg mice p27 was localized to the nucleus and to a lesser extent in the cytoplasm (Fig. 4E, F) while in the GFAP-Tat tg mouse p27 was reduced in the nucleus (Fig. 4E, F). By immunoblot analysis, p27 was more abundant in the nuclear than in the cytoplasmic fractions in the non-tg mice, whereas in the GFAP-Tat tg mouse p27 immunoreactivity was reduced in the nuclear fraction and increased in the cytosolic fraction (Fig. 4G, H).
Fig. (4). p27 localization and levels in HIVE and GFAP-Tat tg mice.
(A–B) Immunocytochemistry and bright field microscopy in the frontal cortex of HIV+ control and HIVE patients showing increased localization of p27 to the nucleus of HIV+ and increased cytoplasmic p27 in HIVE cases. n=6 HIV+ and n=6 HIVE; p=0.0246, df=10, F=9.97 by two-tailed, unpaired T-test. (C–D) Immunoblot analysis with nuclear and cytosolic fractions showing decreased p27 in the nuclear fraction and increased in the cytosolic fraction of HIVE cases. n=6 HIV+ and n=6 HIVE; p=0.0061, df=10, F=18.59 by two-tailed, unpaired T-test. Bar=10 um. (E–F) Immunocytochemistry and bright field microscopy in the cortex of non-tg control and GFAP-Tat tg mice showing nuclear localization of p27 in non-tg and reduced nuclear localization in GFAP-Tat tg mice n=6 Non-tg and n=6 tg mice; p<0.0001, df=10, F=5.971 by two-tailed, unpaired T-test. (G–H) Immunoblot analysis with nuclear and cytosolic fractions showing decreased p27 in the nuclear fraction and increased in the cytosolic fraction of GFAPTat tg mice compared to non-tg mice. n=6 non-tg and n=6 tg mice. p=0.0027, df=10, F=5.008 by two-tailed, unpaired T-test. Bar=10 um.
These results suggest that retention of p27 in the cytoplasm might play a role in the Tat mediated mislocalization of CDK5 in HIVE.
CRMP2 and DCX Hyperphosphorylation in HIVE and Tat tg Mice
Downstream targets might be involved in mediating the neurotoxic effects of Tat upon abnormal CDK5 activation. Among them, studies in AD patients suggest that phosphorylation of Tau might be important [87, 88]. Similarly in HAND, CDK5 hyperactivation leads to neurodegeneration via abnormal phosphorylation of Tau and other substrates such as CRMP2 [119] and DCX. Hyperphosphorylation of other substrates such as postsynaptic density protein 95 [PSD95] and MEF2 [96–98] might also play a critical role. This is consistent with studies in the SIV encephalitis [SIVE] [120, 121] and gp120 [122] models that have shown that CDKs are dysregulated [123, 124], and with previous studies by Maggirwar et al. showing that abnormal activation of GSK3® and CDK5 play an important role in HIV neurotoxicity [62–64]. Aberrant modifications of Tau [125, 126] and CRMP2 [127, 128] by GSK3β and CDK5 play a key role in AD and frontotemporal dementia [FTD] [129]. In the CSF of HIV patients with cognitive impairment, pTau [130–132] is elevated, while total Tau is not changed [133, 134]. A previous study found an association between Tau phosphorylation and HIV infection [135].
Similar to the alterations that were detected for Tau phosphorylation in HIV brains, in vitro studies of CDK5 identified a novel substrate of CDK5 in the pathogenesis of HIVE, namely CRMP2. CRMP2 is a growth cone associated protein that binds to tubulin and regulates microtubule assembly and axon development in neurons [136]. Specifically, CRMP2 may act as an inhibitor of CRMP-associated molecule [CRAM] interaction with mitochondria [137] to regulate neuronal differentiation. Abnormal activation of CDK5 impairs neurogenesis by hyperphosphorylation of CRMP2, which in turn results in de-stabilization of the cytoskeleton and defective neuronal maturation [116, 119].
Further studies, using 2-dimensional differential in-gel electrophoresis, have identified CRMP2 as a protein increased in the frontal cortex of HIV-associated dementia patients, compared to non-demented HIV patients [138]. Interestingly, CRMP2 is also implicated in AD, and may promote AD like neuropathology in HIV patients. Tau-mediated phosphorylation may activate CRMP2 and thereby contribute to neurodegeneration through aberrant regulation of mitochondrial function.
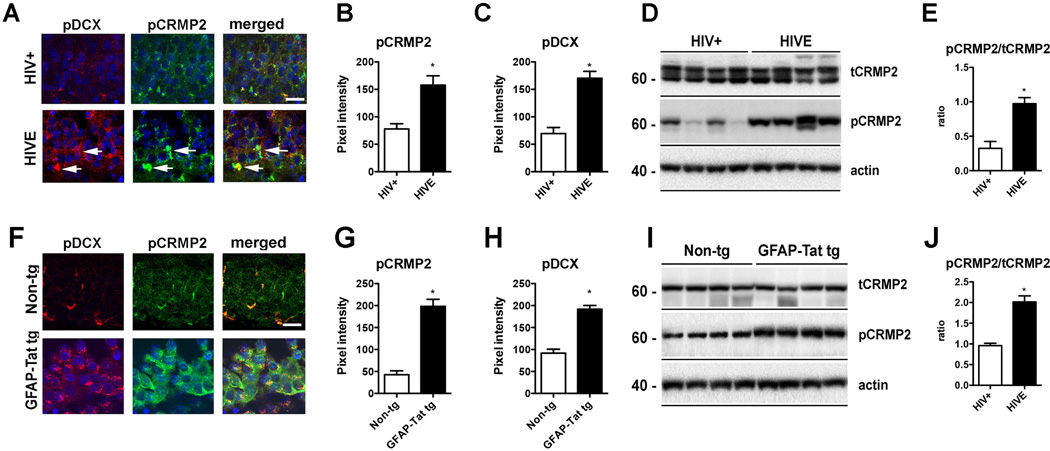
Here we investigate the state of phosphorylation of CRMP2 and DCX in this new set of HIVE cases where the nucleo-cytoplasmic localization of CDK5 and p27 localization was analyzed, and then immunochemical analysis was performed. By double immunolabeling and confocal microscopy, levels of pCRMP2 and pDCX were elevated in the hippocampus of HIVE cases versus HIV+ control patients (Fig. 5A–C). By immunoblot analysis, levels of tCRMP2 were similar in HIV+ control and HIVE cases, whereas levels of pCRMP2 were increased in HIVE compared to HIV+ control (Fig. 5D, E). Similar analysis was performed in the brains of non-tg and GFAP-Tat tg mice. By double-immunolabeling, levels of pCRMP2 and pDCX were elevated in the GFAP-Tat tg mice compared to non-tg controls (Fig. 5F–H). By immunoblot analysis, tCRMP2 levels were similar in the non-tg mice and GFAP-Tat tg mice, while levels of pCRMP2 were increased in GFAP-Tat tg mice (Fig. 5I, J).
Fig. (5).
CRMP2 and DCX hyperphosphorylation in HIVE and GFAP-Tat tg mice
(A–C) Double immunolabeling and confocal microscopy for pDCX [red] and pCRMP2 [green] in the hippocampus of HIV+ control and HIVE patients showing increased pCRMP2 and pDCX in HIVE cases. n=6 HIV+ and n=6 HIVE; B: p=0.0022, df=10, F=3.19; C: p=0.0001, df=10, F=1.277 by two-tailed, unpaired T-test. (D-E) Levels of pCRMP2 were also increased in HIVE cases by immunoblot. n=6 HIV+ and n=6 HIVE; E: p=0.0006, df=10, F=1.295; by two-tailed, unpaired T-test. (F–H) Double immunolabeling and confocal microscopy for pDCX [red] and pCRMP2 [green] in the hippocampus of non-tg and tg mice showing increased pCRMP2 and pDCX in GFAP-Tat tg mice. n=6 non-tg and n=6 tg mice. G: p<0.0001, df=10, F=3.179; H: p<0.0001, df=10, F=1.133 by two-tailed, unpaired T-test. (I–J) Levels of pCRMP2 were also increased in GFAP-Tat tg mice by immunoblot. n=6 non-tg and n=6 tg mice. J: p<0.0001, df=10, F=6.875. Bar=10 um.
In summary, Tat mediated hyperactivation of CDK5 might lead to neurodegeneration by aberrant phosphorylation of substrates such as Tau, CRMP2 and DCX.
DISCUSSION
The present study showed that Tat enters neuronal cells, increases CDK5 levels in the cytoplasm of neurons and thereby increases phosphorylation of CDK5 targets and neurodegeneration. We found that CM from macrophages expressing Tat caused an increase in cytoplasmic CDK5 with a concomitant reduction in nuclear CDK5 levels, an increase in pTau and a decrease in β-tubulin in neuronal cells. Furthermore, in HIVE cases and in GFAP-Tat tg mice, CDK5 and p27 nuclear localization was decreased resulting in increased CDK5 levels in the cytoplasm with concomitant hyperphosphorylation of Tau, CRMP2 and DCX. Under pathological [e.g., HIV infection] or stress conditions [e.g., excitotoxicity, oxidative stress] the combination of two distinct events may contribute to aberrant activation of CDK5 in neurons: A] as has been previously shown, neurotoxins and HIV proteins such as Tat might interact with cell surface receptors and raise intracellular calcium with activation of calpain I and atypical cleavage of p35 to p25, further feeding into the pathological activation of CDK5, and B] HIV-1 Tat might interfere with interactions between CDK5 and cell cycle regulators [e.g., p27] which might result in translocation of CDK5 from nucleus to cytoplasm (Fig. 1A). In patients with HIV, both of these mechanisms might contribute to promote aberrant CDK5 signaling [76, 94, 119], which in turn results in aberrant CRMP2 phosphorylation [119] and Tau [76], neurodegenerative alterations, and behavioral deficits [76, 139] (Fig. 1).
Once Tat and other HIV-1 proteins engage signaling pathways resulting in hyperphosphorylation of substrates such as Tau and CRMP2, downstream mechanisms might be at play as key and final mediators of the neurodegenerative process. Among them, recent studies have identified alterations of mitochondrial biogenesis as an important pathway. Mitochondrial alterations and dysfunction are hallmarks of several neurodegenerative disorders, possibly, in part, as a consequence of Tau hyperphosphorylation. HIV proteins Gp120, vpr and Tat have been shown to cause mitochondrial membrane permeablization [MMP] and cell death [140, 141]. Tat, specifically, is linked to alterations in expression of apoptosis-regulating proteins, Bcl-2 and Bax [142], and down regulation of super oxide dismutase [143]. Interestingly, recent studies have shown that Tau and CRMP2 are associated with mitochondrial and neuronal function [137, 144], both of which are targets of CDK5 phosphorylation that may be influenced by Tat in neurons [76, 116]. Another recent study showed that CDK5 directly phosphorylates a mitochondrial fission protein, dynamin-related protein [DRP]1, increasing DRP1 localization to microtubules and causing mitochondrial elongation [145]. Mitochondrial biogenesis is regulated through a balance of mitochondrial fission and fusion, and subsequent mitophagy or transport of mitochondria throughout the cell [146]. These processes are important during neurogenesis, synapse formation and maintenance of neuronal connections [147, 148]. DRP1 is a GTPase responsible for mitochondrial fission [148]. Neuronal homeostasis, and consequently CNS function, is highly dependent upon a balance between mitochondrial fission and fusion [144, 149, 150]. Mitochondrial fission is important for removal of damaged mitochondria by mitophagy [151] and elongated mitochondria have been associated with altered mitochondrial bioenergetics and increased reactive oxygen species [152]. A recent study showed that Tau-mediated stabilization of actin reduces DRP1 localization to the mitochondrial membrane in drosophila and mouse hippocampal neurons [144]. Interestingly, mitochondrial length and neurotoxicity were increased; however, inhibition of actin stabilization reversed this effect [144]. These results support the assertion that Tat-mediated translocation of CDK5 to the cytoplasm may have downstream effects on Tau function and ultimately mitochondrial biogenesis via DRP1 phosphorylation. Hence, Tat-induced cytoplasmic CDK5 translocation and activity may reduce DRP1 function and promote an imbalance in fission/fusion processes and mitochondrial biogenesis. Future studies will focus on elucidating the effects of Tat on CDK5 and DRP1 in HAND.
Tat promotes nucleo-cytoplasmic translocation and pathological activation of CDK5, which suggests that Tau reduction or CDK5 inhibition with a new class of compounds, such as roscovitine, may be neuroprotective in preclinical models of HIV neurotoxicity [76]. Thus, strategies directed at reducing Tau might represent an alternative approach for the treatment of HAND and might help deepen our understanding of the pathogenesis of the neurodegenerative process. Remarkably, recent studies have shown that Tau reduction is neuroprotective in APP tg models of AD [153, 154] and models of excitotoxicity. Since in HIV and in models of HIV neurotoxicity there is evidence of excitotoxicity [110], CDK5 is abnormally activated [64, 76] and Tau and CRMP2 are abnormally phosphorylated [76, 116, 135], it is possible that reducing Tau or down-modulating CDK5 activity might rescue the neurodegenerative phenotype in models of HIV neurotoxicity (Fig. 1B).
In addition to reducing Tau, genetic knockdown of CDK5 in GFAP-gp120 tg mice, or intracerebral infusion [osmotic minipumps] of the CDK5 inhibitor roscovitine ameliorates the behavioral and neurodegenerative alterations and reduces levels of pTau [76]. Therefore, inhibitors of CDK5 might represent an alternative strategy for managing HAND. Although roscovitine is a potent CDK5 inhibitor, critical problems with this drug are the poor capacity to cross the BBB [155], and the lack of specificity. In recent years, our laboratory has used structure-based design and pharmacophore modeling techniques to discover novel and more selective inhibitors of the CDK5 active site [156] that exhibit improved parameters predicting BBB permeability. Further development of such CDK5 modulators in combination therapies might represent an important step toward establishing new neuroprotective treatments for HAND (Fig. 1B).
In conclusion, with the advent of HAART, clinicians are challenged with the task of diagnosing and treating an aging HIV-infected population for an array of neurocognitive impairments. Identifying the mechanisms leading to these impairments in aged HIV patients, and subsequently developing therapies to combat these molecular insults is paramount to securing a healthy life for these individuals. It seems clear that HIV proteins are expressed in the CNS despite low-level viral load in the periphery. This report describes recent, and new, data that suggest Tat interferes with function of CDK5 in neurons, and possibly leads to neurodegeneration and HAND. The parallel pathways of Tau and CRMP2 present an innovative possible molecular mechanism that could underlie the neurodegenerative and neurogenic alterations in HIVE. Abnormal CDK5 activation is involved in the neurodegenerative process in HIV via abnormal Tau-CRMP2 phosphorylation, supporting the notion that reducing CDK5 or Tau might ameliorate impairments associated with HAND. The mechanisms outlined here may serve as promising therapeutic targets for maintaining CNS health in aging HIV patients.
MATERIALS AND METHODS
Study Population
Briefly, as previously described [119], for this study we included a total of n=6 HIV+ cases and n=6 HIVE cases acquired from the HIV Neurobehavioral Research Center and California Neuro acquired immunodeficiency syndrome [AIDS] Tissue Network at the University of California, San Diego. Cases had neuromedical and neuropsychological examinations within a median of 12 months before death. The diagnosis of HIVE was based on the presence of microglial nodules, astrogliosis, HIV p24–positive cells, and myelin pallor.
Generation of Inducible Tat Transgenic Mice and Doxycycline Infusion
Briefly, as previously described [157], inducible Tat transgenic mouse colonies [GT-tg] were obtained by generation of two separate transgenic lines Teton-GFAP mice [G-tg] and TRE-Tat86 mice [T-tg], and then crossbreeding of these two lines of transgenic mice. Founder animals and progeny carrying the transgenes were identified by PCR analysis of genomic DNA, which was extracted from mouse tail clippings [0.5 to 1 cm long] using the Wizard genomic DNA isolation kit [Promega]. With this construct, mice express Tat upon DOX treatment. For these experiments a total of n=8 non-tg mice and n=8 GFAP-Tat tg mice were utilized [7–8 months old]. The GFAP-Tat tg mice were treated with DOX at 80 mg/kg [daily IP] for two weeks and then sacrificed immediately after [week 2], the other groups were sacrificed at 2 week intervals after cessation of DOX.
Cell Culture
B103 cells [rat neuroblastoma] and blood derived mononuclear cells were cultured at 37° C and 5% CO2. B103 rat neuroblastoma cells were utilized here for the cholinergic and GABAergic phenotypes [158]; both of which are implicated in frontal cortex and basal ganglia function [159, 160] and relevant to HAND [23, 161]. B103 were grown in dubelco’s modified essential medium [DMEM] with 5% fetal bovine serum [FBS]. Cells were treated with CM from macrophages transfected pTat-HA for 24 hours and then fixed in 4% PFA for immunocytochemistry.
Antibodies
For immunohistochemical and immunoblot antibodies were attained for Tat [NIH AIDS Reagents Program cat# 705], CDK5 [Santa Cruz Biotechnology, cat# sc-173], p27 [Cell signaling], pDCX [S28, Abcam], CRMP2 [Kinasource, cat# AB-042], pCRMP2 [pSer522, Kinasource, cat# PB-042], pTau [PHF1, courtesy of Dr. Peter Davies], MAP2 [Millipore, cat# MAP378] and actin [Sigma, cat# A5441] were obtained.
Immunoblot Analysis
Immunoblot analysis was performed as previously described [50].
Immunohistochemistry, Image Analysis and Laser Scanning Confocal Microscopy
Immunohistochemistry, Image Analysis and Laser Scanning Confocal Microscopy was performed as previously described [50].
Analysis of CDK5 and pTau and pCRMP2 and pDCX was performed as previously described [119] by double labeling. For this purpose, coverslips or brain sections were incubated with a rabbit polyclonal primary antibody against p-Tau or p-CRMP2 detected with FITC-conjugated secondary antibodies [1:75, Vector Laboratories] and CDK5 or pDCX detected with Tyramide Red. All sections were processed under the same standardized conditions. The immunolabeled blind-coded sections were serially imaged with a laser scanning confocal microscope [MRC-1024; Bio-Rad] and analyzed with Image J v1.43 software [NIH, Bethesda, MD], as previously described [162]. For each case, a total of three sections were analyzed and four fields were examined. Results were expressed as percent of cells displaying CDK5 in the nucleus or levels of pixel intensity.
Statistical Analysis
Values in the figures are expressed as means ± SEM. To determine the statistical significance, values were compared by one-way ANOVA with post-hoc Tukey-Kramer test for comparisons made among groups (Fig. 2) and T-test when only comparing two groups (Figs. 3, 4). The differences were considered to be significant if p values were less than 0.05.
ACKNOWLEDGEMENTS
This work was supported by NIH grants AG043384, MH062962, MH5974 and MH83506, and NS083426 [to JF]. NIH AIDS Reagents Program for recombinant Tat [Cat# 2222].
Role of the Funding Source
The funding source had no role in the study design, data collection, data analysis, data interpretation or writing of this report. The corresponding author had full access to all the data and had final responsibility for the decision to submit the paper for publication.
Biography

Jerel A. Fields
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 2.Gendelman HE, Persidsky Y, Ghorpade A, et al. The neuropathogenesis of the AIDS dementia complex. Aids. 1997;11(Suppl A):S35–S45. [PubMed] [Google Scholar]
- 3.Haas DW, Clough LA, Johnson BW, et al. Evidence of a source of HIV type 1 within the central nervous system by ultraintensive sampling of cerebrospinal fluid and plasma. AIDS Res Hum Retroviruses. 2000;16(15):1491–1502. doi: 10.1089/088922200750006010. [DOI] [PubMed] [Google Scholar]
- 4.Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10(8):843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Carroll-Anzinger D, Al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol. 2006;80(1):541–544. doi: 10.1128/JVI.80.1.541-544.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003;62(5):429–440. doi: 10.1093/jnen/62.5.429. [DOI] [PubMed] [Google Scholar]
- 7.McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9(2):205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 8.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 9.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 10.Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69(3):376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valcour V, Paul R, Neuhaus J, Shikuma C. The Effects of Age and HIV on Neuropsychological Performance. J Int Neuropsychol Soc. 2011;17(1):190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24(9):1367–1370. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- 15.McArthur J, Hoover D, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 16.Valcour V, Paul R. HIV infection and dementia in older adults. Clin Infect Dis. 2006;42(10):1449–1454. doi: 10.1086/503565. [DOI] [PubMed] [Google Scholar]
- 17.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 18.Gelman BB, Chen T, Lisinicchia JG, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7(9):e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherner M, Cysique L, Heaton RK, et al. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol. 2007;13(1):23–28. doi: 10.1080/13550280601089175. [DOI] [PubMed] [Google Scholar]
- 20.Wiley C, Achim C. HIV encephalitis is the pathologic correlate of dementia in AIDS. AnnNeurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- 21.Budka H, Costanzi G, Cristina S, et al. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol(Berl) 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- 22.Budka H, Wiley C, Kleihues P, et al. HIV-associated disease of the nervous system: Review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 23.Masliah E, Achim C, DeTeresa R, Ge N, Wiley C. Cellular neuropathology in HIV encephalitis. In: Price R, editor. AIDS and the brain. New York: Raven Press; 1994. pp. 119–131. [Google Scholar]
- 24.Langford T, Letendre SL, Larrea GJ, ande Masliah E. Changing Patterns in the Neuropathogenesis of HIV During the HAART Era. Brain Pathol. 2002;11:306–312. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masliah E, DeTeresa R, Mallory M, Hansen L. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000;14:69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- 26.Desplats P, Dumaop W, Patrick C, et al. Latent and chronic HIV infection in the CNS; impact on epigenetic regulation. AIDS. 2009 Submitted. [Google Scholar]
- 27.Desplats P, Dumaop W, Smith D, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80(15):1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 29.Bharti AR, Letendre SL, Wolfson T, et al. Clinical variables identify seronegative HCV co-infection in HIV-infected individuals. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011;52(4):328–332. doi: 10.1016/j.jcv.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Iudicello JE, Shi C, et al. Absence of neurocognitive impairment in a large Chinese sample of HCV-infected injection drug users receiving methadone treatment. Drug Alcohol Depend. 2014;137:29–35. doi: 10.1016/j.drugalcdep.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath A, Steiner J. Synaptodendritic injury with HIV-Tat protein: What is the therapeutic target? Exp Neurol. 2014;251:112–114. doi: 10.1016/j.expneurol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehn-Hall K, Guendel I, Carpio L, et al. Inhibition of Tat-mediated HIV-1 replication and neurotoxicity by novel GSK3-beta inhibitors. Virology. 2011;415(1):56–68. doi: 10.1016/j.virol.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 34.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. PNAS. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meucci O, Fatatis A, Simen AA, et al. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95(24):14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders VJ, Pittman CA, White MG, et al. Chemokines and receptors in HIV encephalitis. Aids. 1998;12(9):1021–1026. [PubMed] [Google Scholar]
- 37.Giulian D, Vaca K, Noonan C. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 38.Pulliam L, Zhou M, Stubblebine M, Bitler CM. Differential modulation of cell death proteins in human brain cells by tumor necrosis factor alpha and platelet activating factor. J Neurosci Res. 1998;54(4):530–538. doi: 10.1002/(SICI)1097-4547(19981115)54:4<530::AID-JNR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Pulliam L, Clarke JA, McGuire D, McGrath MS. Investigation of HIV-infected macrophage neurotoxin production from patients with AIDS dementia. Adv Neuroimmunol. 1994;4(3):195–198. doi: 10.1016/s0960-5428(06)80257-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Trillo-Pazos G, Kim SY, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- 41.Brandimarti R, Khan MZ, Fatatis A, Meucci O. Regulation of cell cycle proteins by chemokine receptors: A novel pathway in human immunodeficiency virus neuropathogenesis? J Neurovirol. 2004;10(Suppl 1):108–112. doi: 10.1080/753312761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Garcia J, Kolson DL, Gonzalez-Scarano F. Chemokine receptors in the brain: their role in HIV infection and pathogenesis. Aids. 2002;16(13):1709–1730. doi: 10.1097/00002030-200209060-00003. [DOI] [PubMed] [Google Scholar]
- 43.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42(6):963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 44.Kanmogne GD, Schall K, Leibhart J, et al. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77(2):212–219. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 47.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78(3):457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 48.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004;4(2–3):119–129. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4(5):704–706. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fields J, Dumaop W, Rockenstein E, et al. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol. 2013;19(1):89–101. doi: 10.1007/s13365-012-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008;4(7):963–966. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui L, Chen X, Haughey NJ, Geiger JD. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN neuro. 2012;4(4):243–252. doi: 10.1042/AN20120017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5(1):92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. Am J Pathol. 2010;176(2):893–902. doi: 10.2353/ajpath.2010.090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maragos WF, Young KL, Turchan JT, et al. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83(4):955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- 56.Turchan J, Pocernich CB, Gairola C, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60(2):307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 57.Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- 58.Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer's disease: bad genes and bad habits. J Mol Neurosci. 2001;17(2):205–224. doi: 10.1385/JMN:17:2:205. [DOI] [PubMed] [Google Scholar]
- 59.New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem. 1998;273(28):17852–17858. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- 60.Sui Z, Fan S, Sniderhan L, et al. Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. J Immunol. 2006;177(1):702–711. doi: 10.4049/jimmunol.177.1.702. [DOI] [PubMed] [Google Scholar]
- 61.Sui Z, Sniderhan LF, Fan S, et al. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3beta to antagonize nuclear factor-kappaB survival pathway in neurons. Eur J Neurosci. 2006;23(10):2623–2634. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- 62.Dewhurst S, Maggirwar SB, Schifitto G, Gendelman HE, Gelbard HA. Glycogen synthase kinase 3 beta (GSK-3 beta) as a therapeutic target in neuroAIDS. J Neuroimmune Pharmacol. 2007;2(1):93–96. doi: 10.1007/s11481-006-9051-1. [DOI] [PubMed] [Google Scholar]
- 63.Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73(2):578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, White MG, Akay C, et al. Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. J Neurochem. 2007;103(2):439–455. doi: 10.1111/j.1471-4159.2007.04746.x. [DOI] [PubMed] [Google Scholar]
- 65.Rappaport J, Joseph J, Croul S, et al. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol. 1999;65(4):458–465. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- 66.Alirezaei M, Watry DD, Flynn CF, et al. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci. 2007;27(41):11047–11055. doi: 10.1523/JNEUROSCI.2733-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crews L, Patrick C, Achim CL, Everall IP, Masliah E. Molecular Pathology of Neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10(3):1045–63. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dewhurst S, Maggirwar SB, Schifitto G, Gendelman HE, Gelbard HA. Glycogen synthase kinase 3 beta (GSK-3 beta) as a therapeutic target in neuroAIDS. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2007;2(1):93–96. doi: 10.1007/s11481-006-9051-1. [DOI] [PubMed] [Google Scholar]
- 69.Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. JNeurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 70.Krogh KA, Wydeven N, Wickman K, Thayer SA. HIV-1 protein Tat produces biphasic changes in NMDA-evoked increases in intracellular Ca2+ concentration via activation of Src kinase and nitric oxide signaling pathways. J Neurochem. 2014;130(5):642–656. doi: 10.1111/jnc.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra R, Singh SK. HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neurosci. 2014;15:80. doi: 10.1186/1471-2202-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rom S, Pacifici M, Passiatore G, et al. HIV-1 Tat binds to SH3 domains: cellular and viral outcome of Tat/Grb2 interaction. Biochimica et biophysica acta. 2011;1813(10):1836–1844. doi: 10.1016/j.bbamcr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cary DC, Clements JE, Henderson AJ. RON receptor tyrosine kinase, a negative regulator of inflammation, is decreased during simian immunodeficiency virus-associated central nervous system disease. J Immunol. 2013;191(8):4280–4287. doi: 10.4049/jimmunol.1300797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasheed S, Yan JS, Hussain A, Lai B. Proteomic characterization of HIV-modulated membrane receptors, kinases and signaling proteins involved in novel angiogenic pathways. Journal of translational medicine. 2009;7:75. doi: 10.1186/1479-5876-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bethel-Brown C, Yao H, Hu G, Buch S. Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation. Journal of neuroinflammation. 2012;9:262. doi: 10.1186/1742-2094-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patrick C, Crews L, Desplats P, et al. Increased CDK5 Expression in HIV Encephalitis Contributes to Neurodegeneration via Tau Phosphorylation and Is Reversed with Roscovitine. Am J Pathol. 2011;178(4):1646–1661. doi: 10.1016/j.ajpath.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, White MG, Akay C, et al. Activation of cyclin-dependent kinase 5 by calpains contributes to human immunodeficiency virus-induced neurotoxicity. Journal of neurochemistry. 2007;103(2):439–455. doi: 10.1111/j.1471-4159.2007.04746.x. [DOI] [PubMed] [Google Scholar]
- 78.Kimura T, Ishiguro K, Hisanaga S. Physiological and pathological phosphorylation of tau by Cdk5. Front Mol Neurosci. 2014;7:65. doi: 10.3389/fnmol.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohshima T, Kozak CA, Nagle JW, et al. Molecular cloning and chromosomal mapping of the mouse gene encoding cyclin-dependent kinase 5 regulatory subunit p35. Genomics. 1996;35(2):372–375. doi: 10.1006/geno.1996.0370. [DOI] [PubMed] [Google Scholar]
- 80.Hirota Y, Ohshima T, Kaneko N, et al. Cyclin-dependent kinase 5 is required for control of neuroblast migration in the postnatal subventricular zone. J Neurosci. 2007;27(47):12829–12838. doi: 10.1523/JNEUROSCI.1014-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jessberger S, Aigner S, Clemenson GD, Jr, et al. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6(11):e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jessberger S, Gage FH, Eisch AJ, Lagace DC. Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends Neurosci. 2009;32(11):575–582. doi: 10.1016/j.tins.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lagace DC, Benavides DR, Kansy JW, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105(47):18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10(7):816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 85.Molnar E. Motor learning and long-term plasticity of parallel fibre- Purkinje cell synapses require post-synaptic Cdk5/p35. J Neurochem. 2014;131(1):1–3. doi: 10.1111/jnc.12788. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Zhang C, Zi X, Tu Q, Guo K. Epigenetic modulation of Cdk5 contributes to memory deficiency induced by amyloid fibrils. Exp Brain Res. 2014 doi: 10.1007/s00221-014-4100-0. [DOI] [PubMed] [Google Scholar]
- 87.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336(3):417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 88.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405(6784):360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 89.Qu D, Rashidian J, Mount MP, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55(1):37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 90.Anne SL, Saudou F, Humbert S. Phosphorylation of huntingtin by cyclin-dependent kinase 5 is induced by DNA damage and regulates wild-type and mutant huntingtin toxicity in neurons. J Neurosci. 2007;27(27):7318–7328. doi: 10.1523/JNEUROSCI.1831-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahlijanian MK, Barrezueta NX, Williams RD, et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97(6):2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25(42):9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopes JP, Oliveira CR, Agostinho P. Neurodegeneration in an Abeta-induced model of Alzheimer's disease: the role of Cdk5. Aging Cell. 2010;9(1):64–77. doi: 10.1111/j.1474-9726.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- 94.Masliah E, Roberts ES, Langford D, et al. Patterns of gene dysregulation in the frontal cortex of patients with HIV encephalitis (Erratum appeared in J Neuroimmunol 2005, 162: 197) J Neuroimmunol. 2004;157(1-2):163–175. doi: 10.1016/j.jneuroim.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 95.Fischer A, Sananbenesi F, Spiess J, Radulovic J. Cdk5 in the adult non-demented brain. Curr Drug Targets CNS Neurol Disord. 2003;2(6):375–381. doi: 10.2174/1568007033482706. [DOI] [PubMed] [Google Scholar]
- 96.Tang X, Wang X, Gong X, et al. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2005;25(19):4823–4834. doi: 10.1523/JNEUROSCI.1331-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2(10):749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 98.Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem. 2004;88(6):1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- 99.Ino H, Chiba T. Intracellular localization of cyclin-dependent kinase 5 (CDK5) in mouse neuron: CDK5 is located in both nucleus and cytoplasm. Brain Res. 1996;732(1–2):179–185. doi: 10.1016/0006-8993(96)00523-9. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Cicero SA, Wang L, Romito-Digiacomo RR, Yang Y, Herrup K. Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc Natl Acad Sci USA. 2008;105(25):8772–8777. doi: 10.1073/pnas.0711355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7(22):3487–3490. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, Herrup K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle. 2011;10(8):1208–1214. doi: 10.4161/cc.10.8.15328. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 2010;285(18):14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Li H, Yabut O, Fitzpatrick H, D'Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1- DP1 complex. J Neurosci. 2010;30(15):5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou BY, He JJ. Proliferation inhibition of astrocytes, neurons, and non-glial cells by intracellularly expressed human immunodeficiency virus type 1 (HIV-1) Tat protein. Neurosci Lett. 2004;359(3):155–158. doi: 10.1016/j.neulet.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Cujec TP, Okamoto H, Fujinaga K, et al. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11(20):2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17(4):1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71(3):2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8(1–2):119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 110.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12 Suppl 1:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 111.Conant K, Ma M, Nath A, Major EO. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70(3):1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bruce-Keller AJ, Chauhan A, Dimayuga FO, et al. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23(23):8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chauhan A, Turchan J, Pocernich C, et al. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278(15):13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- 114.Nath A, Psooy K, Martin C, et al. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 116.Crews L, Ruf R, Patrick C, et al. Phosphorylation of collapsin response mediator protein-2 disrupts neuronal maturation in a model of adult neurogenesis: Implications for neurodegenerative disorders. Mol Neurodegener. 2011;6:67. doi: 10.1186/1750-1326-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakai K, Barnitz RA, Chaigne-Delalande B, Bidere N, Lenardo MJ. Human Immunodeficiency Virus Type 1 Vif causes dysfunction of Cdk1 and CyclinB1: implications for cell cycle arrest. Virol J. 2011;8(1):219. doi: 10.1186/1743-422X-8-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao RY, Liang D, Li G, Larrimore CW, Mirkin BL. Anti-cancer effect of HIV-1 viral protein R on doxorubicin resistant neuroblastoma. PLoS One. 2010;5(7):e11466. doi: 10.1371/journal.pone.0011466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crews L, Patrick C, Adame A, Rockenstein E, Masliah E. Modulation of aberrant CDK5 signaling rescues impaired neurogenesis in models of Alzheimer's disease. Cell Death Dis. 2011;2:e120. doi: 10.1038/cddis.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci. 2002;22(6):2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jordan-Sciutto KL, Wang G, Murphy-Corb M, Wiley CA. Induction of cell-cycle regulators in simian immunodeficiency virus encephalitis. Am J Pathol. 2000;157(2):497–507. doi: 10.1016/S0002-9440(10)64561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khan MZ, Brandimarti R, Musser BJ, Resue DM, Fatatis A, Meucci O. The chemokine receptor CXCR4 regulates cell-cycle proteins in neurons. J Neurovirol. 2003;9(3):300–314. doi: 10.1080/13550280390201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okamoto S, Kang Y-J, Brechtel CW, et al. HIV/gp120 Decreases Adult Neural Progenitor Cell Proliferation via Checkpoint Kinase- Mediated Cell-Cycle Withdrawal and G1 Arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 124.Chang KH, de Pablo Y, Lee HP, et al. Cdk5 is a major regulator of p38 cascade: relevance to neurotoxicity in Alzheimer's disease. J Neurochem. 2010;113(5):1221–1229. doi: 10.1111/j.1471-4159.2010.06687.x. [DOI] [PubMed] [Google Scholar]
- 125.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. In: Roses A, Weisgraber K, Christen Y, editors. Apoliprotein E and Alzheimer's disease. Berlin: Springer- Verlag; 1996. pp. 103–125. [Google Scholar]
- 126.Mandelkow EM, Mandelkow E. Tau in Alzheimer's disease. Trends Cell Biol. 1998;8(11):425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 127.Soutar MP, Thornhill P, Cole AR, Sutherland C. Increased CRMP2 phosphorylation is observed in Alzheimer's disease; does this tell us anything about disease development? Curr Alzheimer Res. 2009;6(3):269–278. doi: 10.2174/156720509788486572. [DOI] [PubMed] [Google Scholar]
- 128.Cole AR, Noble W, van Aalten L, et al. Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer's disease progression. J Neurochem. 2007;103(3):1132–1144. doi: 10.1111/j.1471-4159.2007.04829.x. [DOI] [PubMed] [Google Scholar]
- 129.Spillantini M, Murrell J, Goedert M, et al. Mutation in the tau gene in familiar mutliple system tauopathy with presenile dementia. PNAS. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73(23):1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65(9):1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 132.Andersson L, Blennow K, Fuchs D, Svennerholm B, Gisslen M. Increased cerebrospinal fluid protein tau concentration in neuro- AIDS. J Neurol Sci. 1999;171(2):92–96. doi: 10.1016/s0022-510x(99)00253-1. [DOI] [PubMed] [Google Scholar]
- 133.Gisslen M, Krut J, Andreasson U, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Green AJ, Giovannoni G, Hall-Craggs MA, Thompson EJ, Miller RF. Cerebrospinal fluid tau concentrations in HIV infected patients with suspected neurological disease. Sex Transm Infect. 2000;76(6):443–446. doi: 10.1136/sti.76.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111(6):529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 136.Fukata Y, Itoh TJ, Kimura T, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4(8):583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- 137.Takahashi S, Inatome R, Yamamura H, Yanagi S. Isolation and expression of a novel mitochondrial septin that interacts with CRMP/CRAM in the developing neurones. Genes Cells. 2003;8(2):81–93. doi: 10.1046/j.1365-2443.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 138.Zhou L, Diefenbach E, Crossett B, et al. First evidence of overlaps between HIV-Associated Dementia (HAD) and non-viral neurodegenerative diseases: proteomic analysis of the frontal cortex from HIV+ patients with and without dementia. Mol Neurodegener. 2010;5:27. doi: 10.1186/1750-1326-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang YJ, Digicaylioglu M, Russo R, et al. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 2010;68(3):342–352. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ferri KF, Jacotot E, Blanco J, Este JA, Kroemer G. Mitochondrial control of cell death induced by HIV-1-encoded proteins. Ann N Y Acad Sci. 2000;926:149–164. doi: 10.1111/j.1749-6632.2000.tb05609.x. [DOI] [PubMed] [Google Scholar]
- 141.Yusim A, Franklin L, Brooke S, Ajilore O, Sapolsky R. Glucocorticoids exacerbate the deleterious effects of gp120 in hippocampal and cortical explants. J Neurochem. 2000;74(3):1000–1007. doi: 10.1046/j.1471-4159.2000.0741000.x. [DOI] [PubMed] [Google Scholar]
- 142.Sastry KJ, Marin MC, Nehete PN, McConnell K, el-Naggar AK, McDonnell TJ. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene. 1996;13(3):487–493. [PubMed] [Google Scholar]
- 143.Prakash O, Teng S, Ali M, et al. The human immunodeficiency virus type 1 Tat protein potentiates zidovudine-induced cellular toxicity in transgenic mice. Arch Biochem Biophys. 1997;343(2):173–180. doi: 10.1006/abbi.1997.0168. [DOI] [PubMed] [Google Scholar]
- 144.DuBoff B, Gotz J, Feany MB. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75(4):618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Strack S, Wilson TJ, Cribbs JT. Cyclin-dependent kinases regulate splice-specific targeting of dynamin-related protein 1 to microtubules. J Cell Biol. 2013;201(7):1037–1051. doi: 10.1083/jcb.201210045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14(2):R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 147.Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011;149(3):241–251. doi: 10.1093/jb/mvr002. [DOI] [PubMed] [Google Scholar]
- 148.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 149.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36(5):449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 150.Delettre C, Lenaers G, Griffoin JM, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26(2):207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 151.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parone PA, Da Cruz S, Tondera D, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One. 2008;3(9):e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 154.Vossel KA, Zhang K, Brodbeck J, et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6(6):464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 156.Wrasidlo W, Crews LA, Tsigelny IF, et al. Neuroprotective effects of the anti-cancer drug Sunitinib in models of HIV- neurotoxicity: potential for drug repositioning for the treatment of neurodegenerative disorders. Br J Pharmacol. 2014 doi: 10.1111/bph.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162(5):1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schubert D, Heinemann S, Carlisle W, et al. Clonal cell lines from the rat central nervous system. Nature. 1974;249:224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- 159.Nguyen DP, Lin SC. A frontal cortex event-related potential driven by the basal forebrain. eLife. 2014;3:e02148. doi: 10.7554/eLife.02148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stocco A, Lebiere C, Anderson JR. Conditional routing of information to the cortex: a model of the basal ganglia's role in cognitive coordination. Psychological review. 2010;117(2):541–574. doi: 10.1037/a0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hesselgesser J, Horuk R. Chemokine and chemokine receptor expression in the central nervous system. J Neurovirol. 1999;5(1):13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- 162.Crews L, Spencer B, Desplats P, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5(2):e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]