Abstract
BACKGROUND
Neuraxial clonidine is utilized for peri-operative analgesia in children of all ages. Preclinical studies in the postnatal rat allow comparison of the relative toxicity and safety of spinal analgesics throughout postnatal development.
METHODS
Rat pups aged 3, 7 or 21 postnatal (P) days were briefly anesthetized for intrathecal injections of saline or clonidine. At each age, the maximum tolerated, anti-nociceptive (increased hindlimb mechanical withdrawal threshold) and anti-hyperalgesic (hindpaw carrageenan inflammation) doses were determined. Lumbar spinal cord sections were assessed for apoptosis and cell death (histology, activated caspase-3 immunohistochemistry, Fluoro-Jade C staining), histopathology (haematoxylin and eosin staining), and increased glial reactivity (microglial and astrocytic markers). P3 intrathecal ketamine sections served as positive controls. In additional groups, thermal latency and mechanical withdrawal threshold were measured at P35.
RESULTS
Intrathecal clonidine produces age- and dose-dependent analgesia in rat pups. Maximal doses of clonidine did not alter the degree or distribution of apoptosis or increase glial reactivity in the neonatal spinal cord. No spinal histopathology was seen 1 or 7 days following injection at any age. Intrathecal clonidine did not produce persistent changes in reflex sensitivity to mechanical or thermal stimuli at P35.
CONCLUSIONS
Intrathecal clonidine in the postnatal rat did not produce signs of spinal cord toxicity, even at doses much greater than required for analgesia. The therapeutic ratio (maximum tolerated dose/anti-hyperalgesic dose) was >300 at P3, >30 at P7, and >10 at P21. These data provide additional information to inform the clinical choice of spinal analgesic agent in early life.
Introduction
Preclinical work demonstrated that spinal delivery of clonidine produced potent analgesia (1,2) through an effect upon spinal alpha2-adrenergic receptors (3). Subsequent human studies with neuraxial clonidine demonstrated clinical efficacy in adults (4) for the management of acute post-operative (5,6), labor (7,8) and post-cesarean (9) pain; as well as for longer-term management of chronic neuropathic (10,11) and cancer pain (12). In pediatric practice, spinal clonidine has been used primarily for peri-operative pain management; either as an additive to prolong analgesia with single-shot caudal local anesthetic (13–15) or to improve analgesia and reduce local anaesthetic requirements in epidural infusions (16,17). Although the majority of controlled trials have been conducted in infants and children, neuraxial clonidine has also been used in neonates, via either the caudal (18–20) or intrathecal route (21,22).
Laboratory studies have confirmed that spinal alpha2 agonist analgesic mechanisms are functional from early development, and intrathecal clonidine (23) and epidural dexmedetomidine (24,25) have both anti-hyperalgesic and anti-nociceptive effects in rat pups from the third postnatal (P3) day. Epidural dexmedetomidine produces spinally-mediated dose-dependent analgesic effects at all postnatal ages, but the sensitivity to sedative (24) and cardiovascular (25) side-effects is also increased in early life.
The benefits of spinal analgesia must always be balanced by the potential risk of neurotoxicity when drugs are administered in relatively high concentrations close to the spinal cord, and adequate preclinical testing before routine clinical use has been advocated (26–28). In adult animals, repeated injections or continuous infusions of spinally administered clonidine for 14 days or longer, have not produced neurotoxic effects in rats (29,30) or dogs (31,32), and bolus doses did not reduce spinal cord blood flow in sheep (33) or pigs (34).
Responses to analgesics and anesthetics may differ in the developing nervous system (35). In particular, anesthetics with γ–amino-butyric-acid (GABA) agonist or n-methyl-D-aspartate (NMDA) antagonist action increase neuronal apoptosis or cell death in the primate (36–39) and rodent brain (40–42). Accordingly, it is important when evaluating novel neuraxial therapeutics for neonates and infants, to define the potential effects of the agent with respect to the developing spinal cord. We have recently developed a model for assessing spinal toxicity in the neonatal rat that includes evaluation of: i) dose-dependent analgesic effects; ii) histopathology, neuronal apoptosis and glial reactivity in the spinal cord at doses up to the maximum tolerated; and iii) functional outcomes (43,44). Calculation of a therapeutic ratio (toxic dose/analgesic dose) allows comparison of the effects of different drugs at different postnatal ages. In this model, morphine had a high therapeutic ratio, i.e. toxicity was not seen at 300 times the analgesic dose in neonatal rats (44). In contrast, intrathecal ketamine increased apoptosis and glial reactivity in the spinal cord and produced persistent changes in gait and mechanical threshold in the same dose range as analgesia, i.e. its therapeutic ratio was <1 (43). As clonidine is a potential alternative to ketamine for caudal analgesia in pediatric patients (15), we have now evaluated the analgesic effects and potential for spinal apoptosis and toxicity following single-dose intrathecal clonidine in the postnatal rat.
Methods
Experimental animals
The study protocol and experiments were performed with personal and project licenses approved by the UK Home Office, in a laboratory approved for regulated procedures, and in accordance with the requirements for the care of laboratory animals of the United Kingdom Animal (Scientific Procedures) Act 1986. Sprague-Dawley dams and litters were bred in-house in the Biological Services Unit University College London, and were maintained on a 12-h light/dark cycle at constant ambient temperature with free access to food and water. Postnatal (P) day 3, 7 or 21 male and female rat pups, with mean body weights of 10, 16 and 54 grams respectively, were assigned to treatment groups. Within each litter, pups were randomly selected, numbered, and assigned to treatment groups. Data from animals within the same litter were treated as independent values, and treatment groups and group comparisons comprised animals from more than one litter. Overall, 12 litters from each age group were utilized. An independent colleague coded drug solutions to ensure the experimenter was unaware of treatment allocation during testing. The degree of handling and duration of maternal separation was minimized, and was the same for saline control and clonidine treatment animals. Litters were restricted to a maximum of 12, and pups were weaned into same sex cages at P21.
Behavioral testing
In P3, P7 and P21 rats, mechanical withdrawal thresholds were measured with hand-held von Frey hairs (vFh) that apply a logarithmically increasing force (vFh 5 = 0.13g to vFh 13 = 7.8g)(45). Testing was performed on the dorsal hindpaw as it is difficult to maintain body temperature or reliably apply mechanical stimuli to the plantar surface through an elevated mesh platform in small pups. Rats were lightly restrained on a flat bench surface and von Frey hairs were applied 5 times at one-second intervals to the dorsal surface of the hindpaw, as previously described (24,43,45,46). The number of evoked flexion reflexes to increasing intensity stimuli was recorded, until a given stimulus evoked five withdrawal responses or a supra-threshold cut-off pressure was reached (7.8g at P3, 13 g at P7, or 60g at P21).
To evaluate any persistent changes in sensory function following intrathecal injection at P3, P7 or P21, sensory thresholds were measured at 5 weeks of age (P35). Following habituation on an elevated mesh platform, the mechanical stimulus (electronic von Frey device; Dynamic Plantar Aesthesiometer, Ugo Basile) was applied to the mid-plantar surface of the hindpaw. The mechanical threshold was averaged from 3 measures of the force (0 to 50 grams; ramp 20 grams/sec) required to produce hind-limb withdrawal. Thermal withdrawal latency was determined using a modified Hargreaves Box (University Anesthesia Research and Development Group, University of California San Diego, La Jolla, California)(47), consisting of a glass surface (maintained at 30 °C) on which the rats were placed in individual Plexiglas cubicles. The thermal nociceptive stimulus from a focused projection bulb positioned below the glass surface was directed to the mid-plantar hindpaw. Latency was defined as the time required for the paw to show a brisk withdrawal as detected by photodiode motion sensors that stopped the timer and terminated the stimulus. In the absence of a response within 20 seconds, the stimulus was terminated (cut-off time). Thermal latency was the average of three measures from each hindpaw.
Intrathecal injection technique
Pups were anesthetized with halothane (3–5%) in oxygen and air. Percutaneous intrathecal injections were made at the low lumbar level (intervertebral space L4–5 or L5–L6) with a 30-gauge needle perpendicular to the skin, connected to a micro-injector and 50 µl Hamilton syringe. Based on our previous work (44), an injectate volume of 0.5 µl per gram bodyweight was used as this provides a uniform spread across lumbar and low thoracic segments in rat pups of all current age (and corresponding weight) groups. Following preparation of different concentrations of clonidine, an independent colleague coded samples of drug and saline, to ensure the experimenter (SMW) was unaware of treatment group during behavioral testing.
Intrathecal clonidine dose
Determination of maximum tolerated dose
In pilot experiments, intrathecal clonidine hydrochloride (Sigma-Aldrich, St Louis, MO) dissolved in sterile saline were administered to P3, P7 and P21 pups in escalating doses of 0.1, 0.3, 1, 3, 10, and 30mg/kg. Dose escalation was limited by behavioral disturbance and weight loss, rather than by acute cardiovascular collapse or sedation and respiratory depression. Doses resulting in marked behavioral disturbance (reduced activity and freezing, alternating with agitation or excitation) were also associated with failure of normal daily weight gain or actual weight loss. Behavioral and motor changes were more apparent in the older pups, and occurred at lower doses. Following doses of 3mg/kg clonidine and above in P21 pups, piloerection, urinary voiding and shivering with significant hypothermia (temperature <31C) were also observed. As a result, temperature was monitored at intervals in all pups by holding a surface electrode between the upper limb and thorax in the ‘axilla’ of P3 pups or inserting a rectal probe in P10 or P21 pups. A warming protocol (thermostatically controlled heating blanket within open topped Perspex container) was established to ensure that body temperature was maintained after injection of saline or clonidine in subsequent experimental groups. The maximum tolerated dose of intrathecal clonidine was defined as the dose that did not produce weight loss in individual animals (when comparing values pre- and 24 hrs post-injection of clonidine) or significant group differences from saline controls. This provided a quantifiable outcome that could be standardized across the postnatal ages. In subsequent experimental groups, the maximum tolerated dose at each age produced mild acute behavioral changes, but body temperature and body weight were maintained.
For clarity, intrathecal doses are expressed in terms of mg/kg. However, as the concentration of drug is important for the direct effect upon local tissues after intrathecal delivery, the range of concentrations and mean total dose for each treatment group are also presented in Table 1.
Table 1.
Postnatal Age and Clonidine Dose
| INTRATHECAL INJECTATE | AVERAGE TOTAL DOSE PER ANIMAL | |||
|---|---|---|---|---|
| Dose (per kg body wt) |
Injectate concentration |
P3 (mean body weight 10g) |
P7 (mean body weight 16g) |
P21 (mean body weight 54g) |
| 0.03 mg/kg | 0.06 mg/ml | 0.3 mcg | 0.48 mcg | 1.62 mcg |
| 0.1 mg/kg | 0.2 mg/ml | 1 mcg | 1.6 mcg | 5.4 mcg |
| 0.3 mg/kg | 0.6 mg/ml | 3 mcg | 4.8 mcg | 16.2 mcg |
| 1 mg/kg | 2 mg/ml | 10 mcg | 16 mcg | 54 mcg |
| 3 mg/kg | 6 mg/ml | 30 mcg | 48 mcg | 162 mcg |
| 10 mg/kg | 20 mg/ml | 100 mcg | - | - |
Summary of intrathecal clonidine administration by dose/kg based on mean body weight, injectateconcentration, and average total dose per animal for three age groups.
Legend: g, grams; kg, kilogram; P=postnatal age; wt, weight
Evaluation of anti-nociceptive doses
Intrathecal clonidine doses up to the maximum tolerated were administered to evaluate acute anti-nociceptive effects. Individual pups received single doses of saline or clonidine. Mechanical withdrawal threshold was determined at baseline, correct intrathecal placement was confirmed by a significant increase in threshold 30 minutes after injection, and thresholds were again measured prior to sacrifice and spinal cord harvesting at 24 hours or 7 days post injection. Single injections of the maximum tolerated dose were administered for tissue analysis (44), as our model aims to deliver the highest possible concentration of drug to evaluate local tissue toxicity, and repeated administration is not feasible as intrathecal catheters can produce confounding injuries in small pups (23). To confirm that effects at these doses were not solely due to systemic absorption, mechanical thresholds 30 minutes following subcutaneous administration of clonidine in the midlumbar region (10mg/kg at P3 or 3mg/kg at P7 and P21 in 5 µl/g; n=6 per group) were also evaluated.
In separate groups of animals, the maximum tolerated dose of intrathecal clonidine was administered to P3 (10mg/kg), P7 (3mg/kg) or P21 (1mg/kg) pups (n=8 per group). Control animals received intrathecal saline (n=6–8 per group). Mechanical withdrawal thresholds were measured at baseline and 30 minutes after injection to confirm intrathecal placement. Animals were maintained and regularly reviewed in the UCL Biological Services Unit, weaned into same-sex cages at P21, and weight, mechanical withdrawal threshold and thermal withdrawal latency were measured at 5 weeks of age.
Evaluation of anti-hyperalgesic doses
Hindpaw inflammation was induced in P3, P7 and P21 pups by injection of 1 µl/g of 2% lambda carrageenan (Sigma-Aldrich) into the mid-plantar surface of the left hindpaw under brief halothane (2–4%) in oxygen anesthesia (24). Mechanical withdrawal thresholds were measured at baseline and 3 hours following carrageenan to quantify the degree of hyperalgesia. Intrathecal saline or clonidine (0.03 – 0.3 mg/kg; n=5–6 all groups) was administered as described above. Mechanical withdrawal thresholds were measured 15 and 30 minutes following injection, with the investigator (SMW) unaware of the treatment allocation.
Spinal cord preparation, staining and analysis
Spinal cord tissue was analyzed 24 hours and 7 days after injection as previously described (43,44) (n=4 per treatment group). In brief, animals were terminally anesthetized with 100mg/kg intraperitoneal pentobarbitone, and then transcardially perfused with heparinised saline followed by 4% paraformaldehyde. Spinal cords were dissected, postfixed in 4% paraformaldehyde, and then cryoprotected in 30% sucrose. Using a cryostat, 7 and 14 micron sections of lower lumbar spinal cord were taken and mounted on Fischer Superfrost Plus (Fischer Scientific, Houston, TX) slides and stored at −70°C.
For each analysis, sections were coded and the investigator was unaware of the treatment group. For quantitative analyses, at least 4 non-consecutive sections of lumbosacral cord from each animal were examined and the mean determined. Different sections were utilized for caspase-3 immunohistochemistry, Fluoro-Jade C staining or haematoxylin and eosin (H&E) staining at 24 hours, and for H&E staining and glial immunoreactivity at 7 days. Sections from P3 pups that had received 10mg/kg intrathecal preservative-free ketamine hydrochloride (Sigma-Aldrich, St Louis, MO) as previously described (43), were stained in parallel to act as a positive control for the staining protocol and to ensure the sample size was sufficient to detect treatment effects previously associated with both structural and functional changes (43). All statistical analysis was calculated with n representing the number of animals within each treatment group.
Fluoro-Jade C staining
Fluoro-Jade C (FJ-C; Millipore, Temecula, CA) staining was performed as previously described (48) on 14 micron spinal cord sections prepared from tissue collected 24 hours following intrathecal injection. In addition to 0.0001% FJ-C, slides were incubated with the DNA stain 4',6-diamidino-2-phenylindole (DAPI 0.0001%; MP Biomedicals, Solon, OH) to aid visualization and confirm tissue integrity of the sections. Slides were examined with the appropriate wavelength fluorescent microscopy, and the number of immunofluorescent cells and their distribution in the dorsal horn, ventral horn or adjacent to the central canal were recorded.
Activated caspase-3 immunohistochemistry
To further assess apoptosis, additional spinal cord sections obtained from P3 and P7 animals 24 hours following intrathecal injection were incubated with monoclonal activated caspase-3 antibody (1:100 Cell Signaling, Beverly, MA) for 72 hours at 4C. Biotinylated goat anti-rabbit secondary antibody (Vector, Burlingame, CA) was applied at 1:250 for 45 min at room temperature, followed by avidin-biotin-peroxidase complex (ABC reagent; Vector, Burlingame, CA) for 30 min. Staining was developed with DAB (Vector, Burlingame, CA) for 12 min. Sections were lightly counterstained with haematoxylin, dehydrated and coverslipped (Permount, Fischer SP15, Fair Lawn, NJ). Slides were coded and the number and location (dorsal horn, ventral horn or adjacent to central canal) of caspase-3 immunoreactive cells were counted under high power light microscopy.
Histology
A neuropathologist (MG) evaluated seven-micron spinal cord sections from all experimental groups, sacrificed at 1 or 7 days post-injection. Haematoxylin and eosin stained sections were examined for histopathological changes (including tissue necrosis, gliosis, and inflammation) and apoptotic cell numbers were counted under high power.
Glial fibrillary acidic protein (GFAP) and ionized calcium binding adapter molecule 1 (Iba1) immunohistochemistry
Tissue obtained 7 days following intrathecal injection was incubated with primary antibodies against astrocyte (1:1500 mouse anti-GFAP; Chemicon, Temecula, CA) and microglial (1:1000 rabbit anti-Iba-1; WAKO, Richmond, VA) markers for 48 hours at 4C. Flourescent secondary antibodies 1:250 Alexa 488 goat anti-mouse and 1:250 Alexa 594 goat anti-rabbit (Molecular Probes, Eugene, OR) were applied for 1 hour at room temperature. Slides were coverslipped with ProLong antifade reagent with DAPI (Molecular Probes, Eugene, OR). Spinal cord sections were imaged using standardized settings on a microscope (Olympus BX51 microscope with appropriate wavelength fluorescence illuminator; Olympus America Inc, Center Valley, PA) equipped with a digital camera and image-capture software. The mean intensity of immunofluorescence within a fixed area region of interest in the dorsal horn was quantified for each section (Image Pro Plus software, Media Cybernatics Inc, Silver Spring, MD).
Statistical Analysis
For evaluation of mechanical withdrawal threshold in pups, the number of withdrawal responses was plotted against the mechanical stimulus (force expressed as grams on log10 scale). A sigmoidal stimulus-response curve with non-variable slope was constructed using non-linear regression curve fit. The mid point of the curve (50% effective force; EF50) was determined and designated the threshold (24,46). In adults, mechanical withdrawal thresholds and thermal latencies were the mean of 3 values for each hindpaw. The degree of reversal of inflammatory hyperalgesia for each animal was calculated from the mechanical withdrawal thresholds at different time points: [(30 minutes post-clonidine - inflamed) / (baseline – inflamed) × 100]. Treatment groups were compared with one way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test for multiple comparisons or Dunnett’s multiple comparison, or Student's t-test if comparison was limited to 2 groups. P35 sensory threshold values were tested for normality (D’Agostino and Pearson normality test) and also assessed by two-way ANOVA with treatment (saline or different clonidine doses) and sex (male or female) as variables. Data was analyzed using Prism Version 5.0 (GraphPad, San Diego, CA). P<0.05 was considered statistically significant.
Results
Intrathecal clonidine produces dose-dependent analgesic effects at all postnatal ages
Effects of clonidine on mechanical withdrawal threshold
The maximum tolerated intrathecal clonidine dose was 10mg/kg at P3 and 3mg/kg at P7. These doses produced mild behavioral effects initially, that did not preclude sensory testing at 30 minutes, and body weight was maintained at 24 hours and 7 days. In P21 pups, 3mg/kg clonidine produced more marked behavioral effects (reduced movement but initial excitatory responses on handling), but minimal weight loss at 24 hours (56.7±1.1g at baseline vs 54.7±1.8g), and this dose was used for acute evaluations. However, as body weight was lower than saline controls by 3 days post-injection, the lower dose of 1mg/kg was designated the maximum tolerated dose in P21 pups for evaluation at 7 days post injection and at P35.
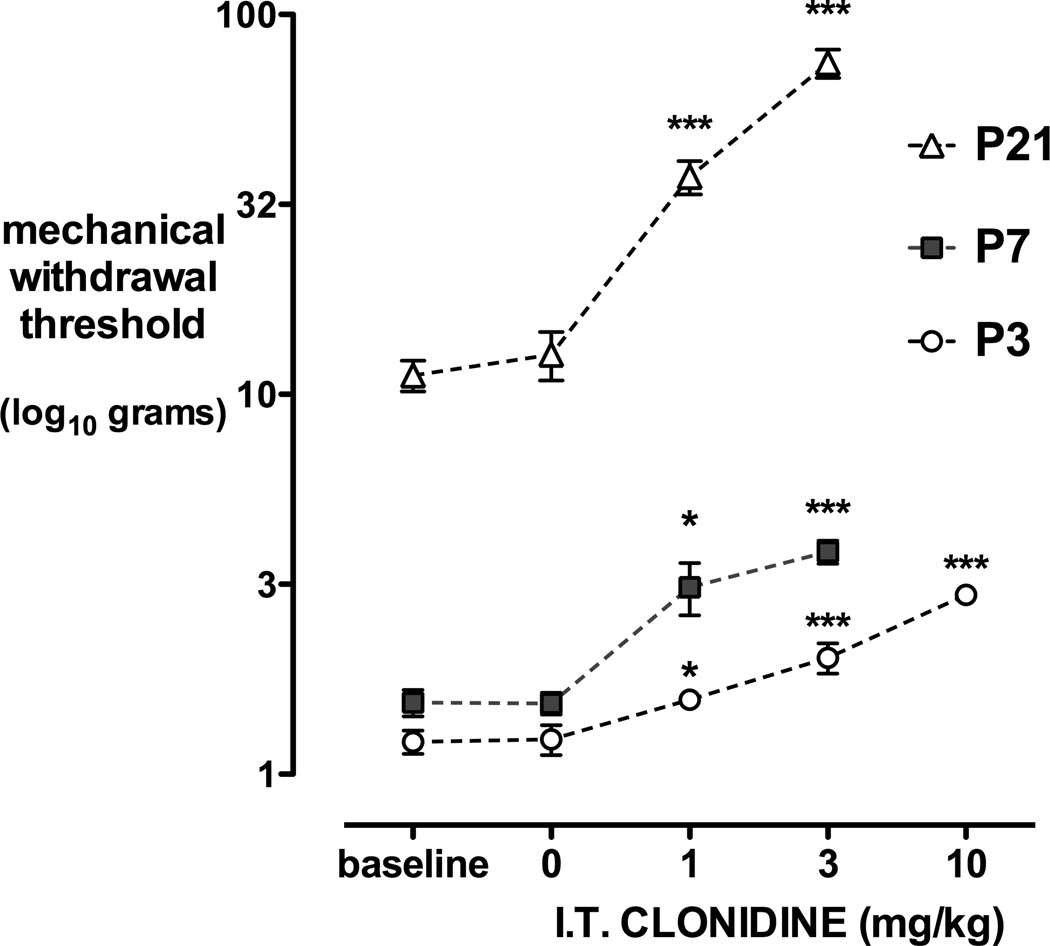
Mechanical withdrawal thresholds increase with postnatal age, from a mean of 1.3g at P3, 1.6g at P7 to 11.9g at P21 (Table 2). Intrathecal clonidine produces dose-dependent anti-nociceptive effects at all postnatal ages. Thirty minutes following intrathecal injection, mechanical withdrawal thresholds did not differ from baseline in the saline control groups, but were significantly increased by 1mg/kg clonidine at all postnatal ages (P<0.05 or P<0.01; one way ANOVA with Dunnett’s comparison to baseline; Fig. 1). Systemic administration of the highest clonidine dose also increased mechanical withdrawal threshold compared with baseline, but effects were significantly less than when the same dose was administered intrathecally at P3 (10mg/kg s.c. vs IT = 1.3±0.08g vs 3.0±0.14g; P<0.01), at P7 (3mg/kg s.c. vs IT = 2.8±0.19g vs 3.8 ± 0.25g; P<0.05), or at P21 (3mg/kg s.c. vs IT = 30±3g vs 74±6g; P<0.01 one way ANOVA with Bonferroni post hoc comparisons).
Table 2.
Sensory Thresholds Following Intrathecal Injection
| Treatment Group | Mechanical Withdrawal Threshold (g) | Thermal Latency (sec) |
|||
|---|---|---|---|---|---|
| baseline pre-IT |
24 hours post IT |
7 days post IT |
P35 (electronic vFh) |
P35 | |
| P3 saline | 1.3 ± 0.09 | 1.3 ± 0.15 | 5.5 ± 0.35 | 36 ± 1.8 | 11.1 ± 0.6 |
| P3 clonidine 10mg/kg | 1.3 ± 0.09 | 1.7 ± 0.06 | 5.9 ± 0.44 | 34 ± 0.6 | 10.8 ± 0.4 |
| P7 saline | 1.6 ± 0.14 | 1.6 ± 0.26 | 5.8 ± 0.31 | 33 ± 1.6 | 11.3 ± 0.5 |
| P7 clonidine 3mg/kg | 1.6 ± 0.06 | 2.0 ± 0.23 | 7.0 ± 0.62 | 36 ± 1.5 | 10.5 ± 0.5 |
| P21 saline | 11.7 ± 1.2 | 12.4 ± 0.8 | 30.0 ± 2.3 | 36 ± 2.0 | 10.4 ± 0.5 |
| P21 clonidine 1mg/kg | 12.1 ± 1.2 | 12.1 ± 1.4 | 40.7 ± 7.3 | 37 ± 1.5 | 10.3 ± 0.5 |
Legend: g=grams; sec=seconds; P = postnatal age; IT=intrathecal; vFh = von Frey hair. Values = mean±SEM; treatment groups at 24 hrsand 7 days n=4–6; at P35 n=6–8 saline groups, n=8 all clonidine groups.
Figure 1.
Dose-dependent anti-nociceptive effects of intrathecal clonidine. Mechanical withdrawal thresholds at baseline and 30 minutes following intrathecal (I.T.) injection of saline or clonidine (CL) 1, 3 or 10mg/kg in postnatal day (P) 3, 7 or 21 rat pups are shown. Data points = mean±SEM, n=6–8 per treatment group, *P<0.05 ***P<0.001 one way ANOVA with Dunnett’s comparison to baseline.
Inflammatory hyperalgesia
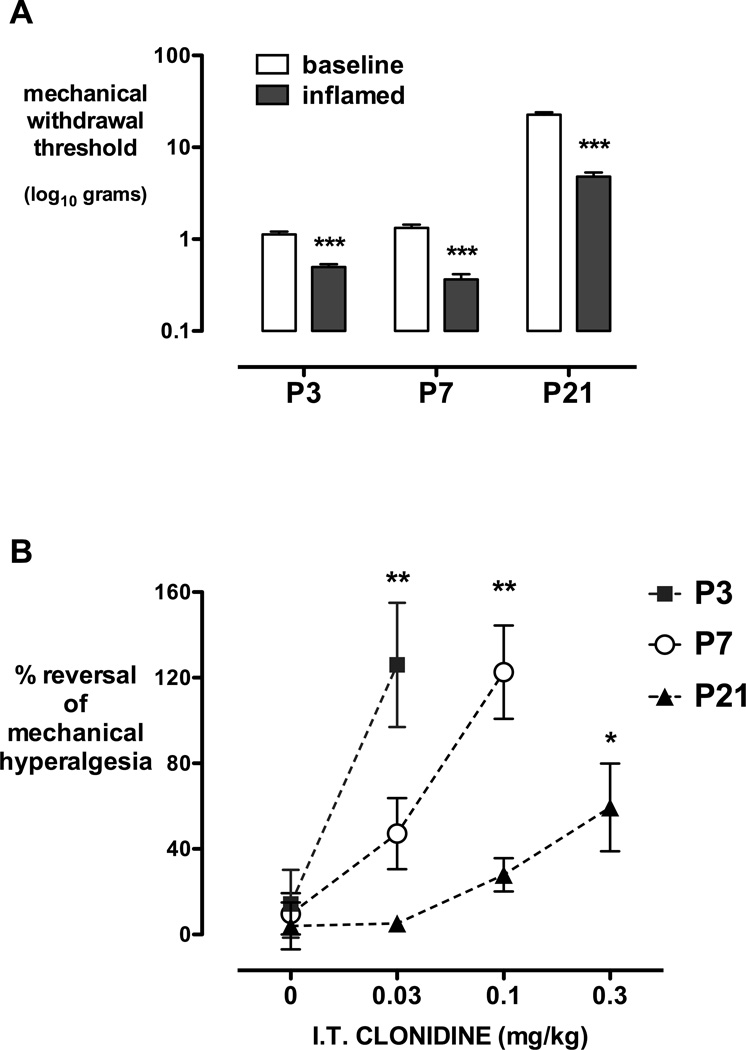
Three hours following hindpaw injection of carrageenan, mechanical withdrawal thresholds in the inflamed paw were significantly decreased at all postnatal ages (Fig. 2A; P<0.001 paired two-way Student’s t-test). The minimum dose of intrathecal clonidine that produced a significant reversal of inflammatory hyperalgesia was 0.03mg/kg at P3 and 0.1mg/kg at P7 (P<0.01), and 0.3mg/kg at P21 (P<0.05, one way ANOVA with Dunnett’s comparison to saline; Fig. 2B). Consistent with previous data following epidural dexmedetomidine (24), anti-hyperalgesic effects of intrathecal clonidine were seen at lower doses than anti-nociceptive effects at all ages. In addition, age-dependent analgesic effects were evident following inflammation, with lower dose requirements in the younger pups.
Figure 2.
Anti-hyperalgesic effects of clonidine. A, Mechanical withdrawal thresholds are significantly reduced from baseline 3 hours following hindpaw carrageenan injection at postnatal day (P) 3, 7, and 21. Bars = mean±SEM, ***P<0.001 Student’s two-tailed paired t-test. B, The degree of reversal of carrageenan-induced hindpaw inflammatory hyperalgesia [30mins post dose – inflamed/baseline – inflamed) ×100] is shown following intrathecal (I.T.) saline (0) or increasing doses of intrathecal clonidine at postnatal (P) day 3, 7 and 21. Data=mean±SEM, n=5–7 per group, *P<0.05 **P<0.01 vs saline, one way ANOVA with Dunnett’s comparison to saline.
Intrathecal clonidine does not produce persistent changes in sensory thresholds
Following intrathecal injection of clonidine 10mg/kg at P3, 3mg/kg at P7 or 1mg/kg at P21, mechanical withdrawal thresholds (Table 2) and body weight (Table 3) did not differ from saline control groups 24 hours or 7 days following injection. In additional animals, more persistent effects on spinal reflex sensitivity were assessed at P35. There were no significant differences in mechanical withdrawal threshold or thermal withdrawal latency following intrathecal injection of clonidine or saline at P3, P7 or P21 (Table 2; n.s. one way ANOVA with Bonferroni multiple post-hoc comparisons). There was no main effect of treatment (F3,36 = 0.43, P=0.73) or sex (F1,36 = 0.71, P=0.55) on mechanical withdrawal threshold at P35. Similarly, there was no main effect of treatment (F3,36 = 0.23, P=0.87) or sex (F1,36 = 0.43, P=0.43) on thermal withdrawal latency at P35. Treatment accounted for 5.3% and 4%, and sex for <0.1% and 1.6% of total variance in mechanical threshold and thermal latency respectively (two-way ANOVA with treatment and gender as variables).
Table 3.
Body Weight and Intrathecal Treatment Group
| Treatment Group | baseline pre-IT |
24 hours post IT |
7 days post IT |
P35 |
|---|---|---|---|---|
| P3 saline | 8.8 ± 0.4 | 10.3 ± 0.6 | 26.5 ± 1.2 | 144 ± 10.1 |
| P3 clonidine 10mg/kg | 9.6 ± 0.4 | 9.6 ± 0.8 | 25.5 ± 1.3 | 146 ± 5.3 |
| P7 saline | 15.6 ± 0.5 | 18.8 ± 1.2 | 30.8 ± 1.8 | 122 ± 3.6 |
| P7 clonidine 3mg/kg | 16.2 ± 0.6 | 16.6 ± 1.2 | 28.3 ± 1.3 | 121 ± 3.3 |
| P21 saline | 50.1 ± 1.7 | 56.2 ± 1.0 | 82 ± 3.9 | 129 ± 4.7 |
| P21 clonidine 1mg/kg | 54.0 ± 1.7 | 56.5 ± 1.1 | 90.5 ± 2.1 | 132 ± 3.3 |
Legend: IT=intrathecal; P = postnatal age. Values = mean±SEM body weight in grams
Maximal tolerated doses of intrathecal clonidine do not increase apoptosis or produce histopathological changes in rat pups
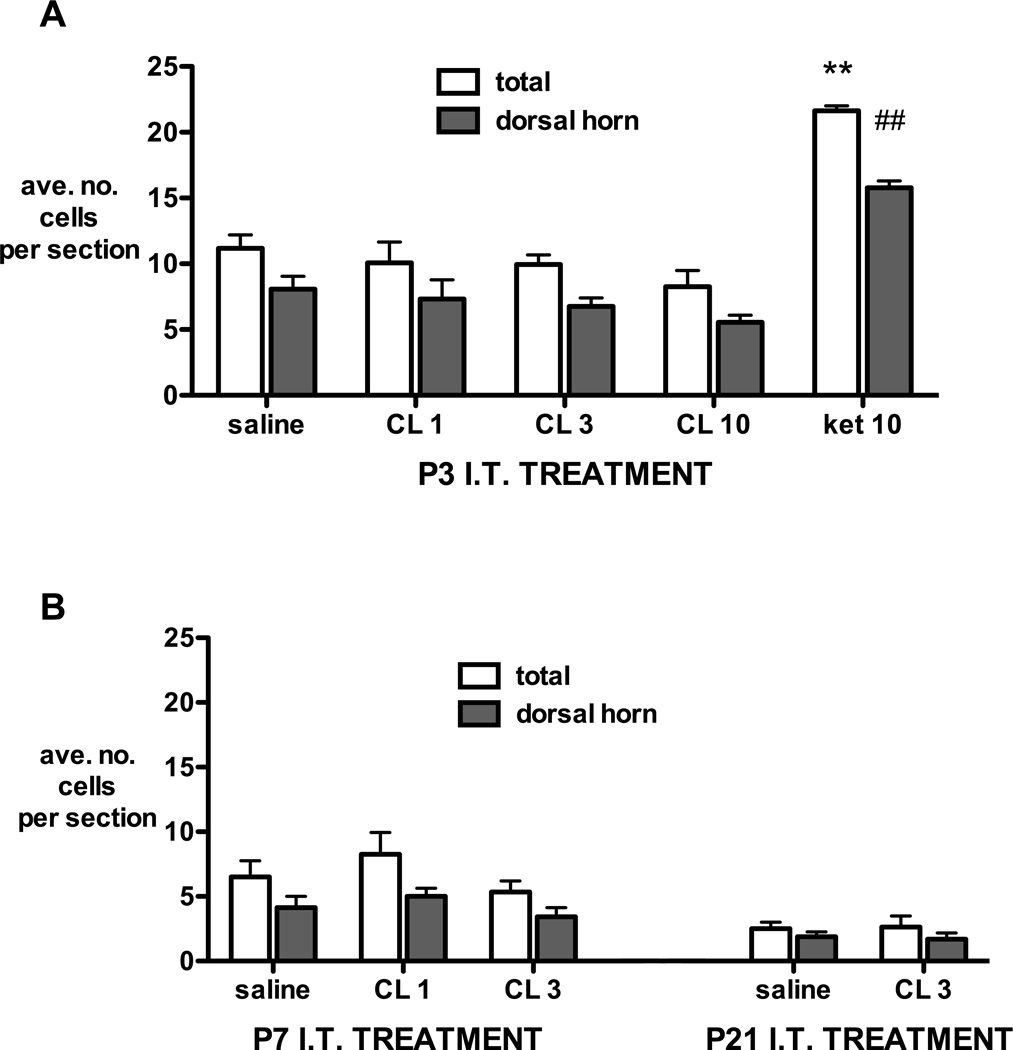
The number of Fluoro-Jade C positive neurons 24 hours following saline injection decreased with age (P4: 11.1±1.02; P8: 6.5±1.2; P22: 2.5±0.5; P<0.01 one way ANOVA linear trend) and the majority were found in the dorsal horn (Fig. 3A,B). Intrathecal clonidine had no effect on the number or distribution of Fluoro-Jade C positive cells at any age. By contrast, intrathecal ketamine 10mg/kg at P3 significantly increased the number of Fluoro-Jade C positive cells in the lumbar spinal cord, with the greatest increase in the dorsal horn (Fig. 3A).
Figure 3.
Fluoro-Jade C positive cell counts 24 hours following intrathecal injection at postnatal day 3 (A), 7 or 21 (B). The number of cells in the whole section (total) and the proportion distributed within the dorsal horn (dorsal horn) are averaged from at least 4 non-consecutive sections per animal. I.T.=intrathecal; P=postnatal day; CL=clonidine 1, 3 or 10mg/kg; ket 10 = ketamine 10mg/kg. Bars = mean±SEM, n= 4 animals per group. **P<0.01 ket 10 vs all groups in total section; ##P<0.01 ket 10 vs all groups in dorsal horn; one way ANOVA with Bonferroni post-hoc comparisons.
Similarly, maximum tolerated doses of intrathecal clonidine at P3 or P7 did not increase apoptotic cell counts as identified by activated caspase-3 immunohistochemistry (Fig. 4). Significant increases were seen in the P3 ketamine 10mg/kg positive control group.
Figure 4.
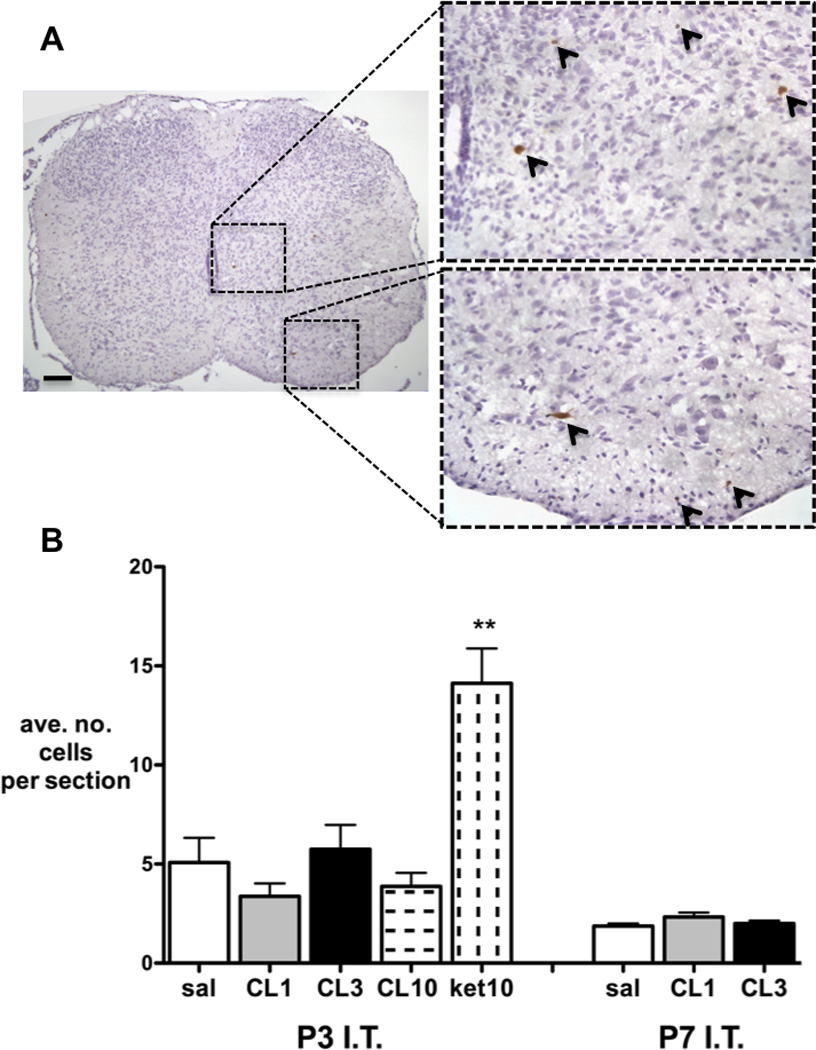
A, Example of lumbar spinal cord section following intrathecal ketamine at P3 with activated-caspase 3 immunopositive cells stained brown and highlighted with arrows. B, Activated-caspase 3 immunopositive cell counts 24 hours following intrathecal injection at postnatal day 3 or 7. Numbers are averaged from at least 4 non-consecutive sections per animal. I.T=intrathecal; P=postnatal day; CL=clonidine 1, 3 or 10mg/kg; ket=ketamine 10mg/kg. Bars = mean±SEM, n= 4 animals per group. **P<0.01 ket 10 vs all other P3 groups; one way ANOVA with Bonferroni post-hoc comparisons.
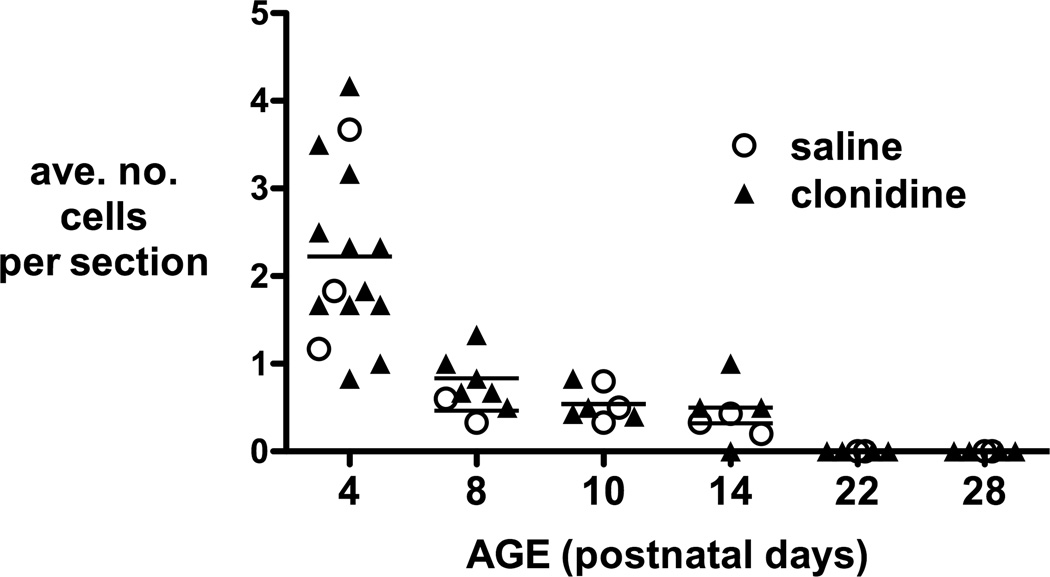
Spinal cord sections stained with haematoxylin and eosin were evaluated for histopathological changes after intrathecal injection. The age related decrease in apoptosis is again demonstrated in data from animals 24 hours post injection (P4, P8, P22) and 7 days post injection (P10, P14, P28). Within each age group, the number of apoptotic profiles following any dose of intrathecal clonidine did not differ from the saline control group (Fig. 5). None of the spinal cords showed histopathological changes such as necrosis, inflammation, or microglial nodules.
Figure 5.
Apoptotic cell counts from haematoxylin and eosin stained sections. Numbers are averaged from at least 5 sections per animal 24 hours or 7 days following intrathecal injection on postnatal day 3, 7 and 21. Counts following intrathecal saline or any dose of intrathecal clonidine did not differ at any time point (n.s. one way ANOVA).
Intrathecal clonidine does not increase glial reactivity in the neonatal spinal cord
Glial reactivity 7 days following intrathecal injection was assessed by immunohistochemistry for microglial (Iba1; Fig 6A,B) and astrocytic (GFAP; Fig.6C) markers. The mean density of Iba-1 or GFAP immunofluorescence in the dorsal horn was not altered by maximum doses of intrathecal clonidine at P3, P7 or P21. However, significant increases were seen in the P3 ketamine group (Fig. 6).
Figure 6.
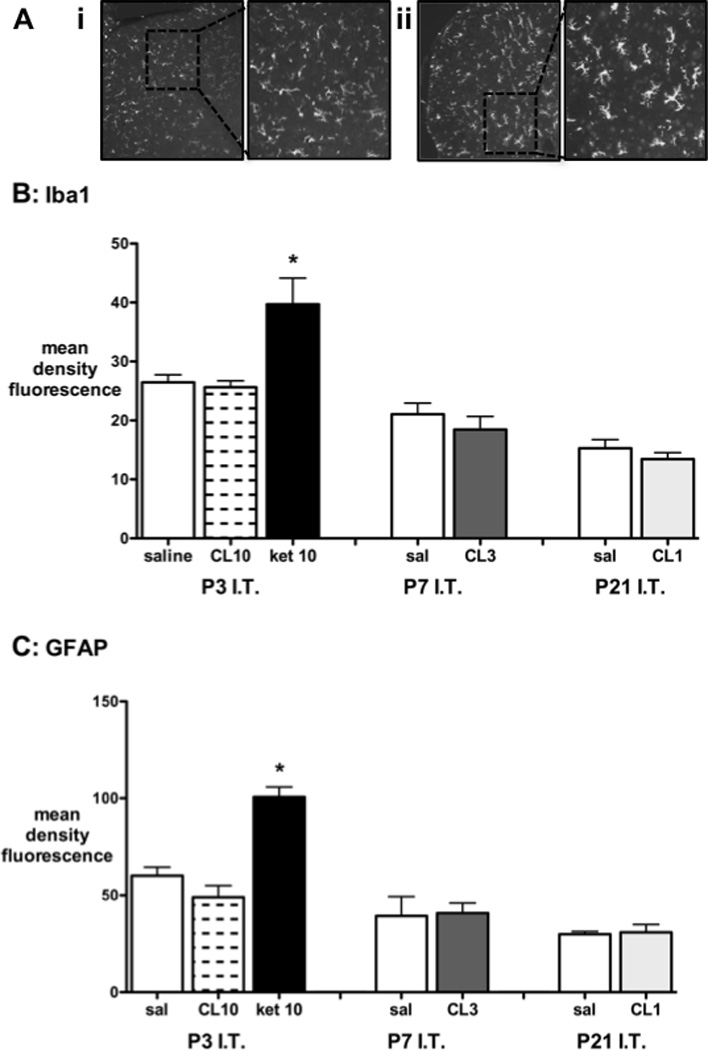
A, Representative spinal cord sections demonstrating Iba1 immunofluorescent staining 7 days following injection of i) intrathecal clonidine 10mg/kg or ii) intrathecal ketamine 10mg/kg. B, Quantification of Iba1 immunofluoresence in the dorsal horn 7 days following intrathecal (I.T.) saline, clondine 1, 3 or 10mg/kg (CL 1, 3, 10) or ketamine 10mg/kg (ket 10) at postnatal day (P)3, 7 or 21. C, Quantification of GFAP immunofluoresence in the dorsal horn in the same experimental groups as above. Bars=mean±SEM, n=3–4 animals per group. *P<0.05 ketamine 10mg/kg vs saline or clonidine 10mg/kg at P3; one way ANOVA with Bonferroni post-hoc comparisons.
Postnatal age and therapeutic ratio of intrathecal clonidine
Based on the above results, the therapeutic ratio calculated as the quotient of the maximum tolerated dose and the minimum dose that significantly increased mechanical threshold in the uninjured paw was calculated as >10 at P3 (10/1) and >3 at P7 and P21 (3/1). Using the anti-hyperalgesic dose as the measure of analgesic activity, the therapeutic ratio at P3 was >300 (10/0.03), at P7 was >30 (3/0.1), and at P21 was >10 for acute apoptosis (3/0.3) and >3 for persistent sensory effects (1/0.3). As the maximum dose was limited by systemic side-effects and there were no signs of local spinal toxicity, the ratio for spinal effects is designated as greater than the given ratio rather than an absolute value.
Discussion
These are the first systematic studies evaluating the potential spinal toxicity of intrathecal clonidine in a neonatal model. Intrathecal clonidine dose-dependently reverses inflammatory hyperalgesia at all postnatal ages, and higher doses increase sensory thresholds in uninjured paws (i.e. have anti-nociceptive effects). Single maximum tolerated doses/concentrations of intrathecal clonidine did not produce signs of local tissue toxicity at any postnatal age as measured by several criteria. Firstly, intrathecal clonidine did not increase apoptotic cell counts identified by activated caspase-3 immunohistochemistry or histological examination, or alter the number or distribution of Fluoro-Jade C positive neurons 24 hours following injection. Intrathecal clonidine did not produce histopathological changes in the spinal cord or alter markers of microglial or astrocytic reactivity. Finally, intrathecal clonidine had no persistent effects on spinal cord reflex sensitivity to mechanical or thermal stimuli at P35. The absence of effects of clonidine were similar to those following morphine (44), but in contrast to the effects following intrathecal ketamine in P3 animals as reported previously (43) and confirmed in parallel sections in the current study.
Clonidine is an alpha-adrenergic agonist with an approximate 160-fold selectivity for the alpha2 vs alpha1 receptor (49). Early preclinical work pointed to the potent spinal action of alpha2 agonists such as clonidine in regulating the processing of nociceptive information following both tissue and nerve injury in adult animals (1,2,50). Spinal alpha2 agonists have age- and dose-dependent analgesic effects throughout postnatal development (23–25). The current study confirms reversal of hyperalgesia by intrathecal clonidine, with lower dose requirements in the youngest pups. Higher intrathecal doses are anti-nociceptive, and although maximum tolerated intrathecal doses produce additional systemic side-effects, analgesic effects are still more marked than when the same dose is administered systemically.
Safety evaluation of spinal clonidine has been undertaken in adult dogs after repeated bolus (32) or extended continuous epidural (31) and intrathecal (51) delivery. No evidence of spinal pathology was observed. Similarly, intrathecal administration for 14 days did not result in toxicity in the adult rat (29,30). These findings are based on histological evaluation performed by a neuropathologist, and similar methodology here has also failed to demonstrate histopathology in the cord of neonatal and young rats following single dose intrathecal clonidine. Of particular interest, clonidine has been reported to a have anti-inflammatory actions (52–55), and co-administration reduces the local inflammatory effects evoked by chronic low dose intrathecal morphine in the canine model (51). Alpha2 agonists may also have specific advantages in combination therapy in early development. Systemic dexmedetomidine reduced general anesthetic-related apoptosis in P7 rats, although efficacy varied in different brain regions (56,57), and effects in the spinal cord have not been evaluated. The effect of combinations of different intrathecal analgesics and local anesthetics throughout postnatal development warrant future evaluation.
Given the concern regarding general anesthesia in neonates and increased interest in neuraxial anesthesia (58), we have emphasized the need to develop a safety database to define the potential toxicity of agents for spinal delivery at different developmental ages (59). The present work with clonidine, and previous work with morphine (44), ketamine (43), and bupivacaine (60) represent the only systematic preclinical spinal safety data in postnatal rats. Several points should be emphasized. Assessment of developmental safety must take cognizance of the fact that there are age-dependent changes in connectivity in the developing spinal cord (35,61), and treatments that alter afferent traffic, such as NMDA antagonists (43) and general anesthetics (60,62) can increase apoptosis in the spinal cord, and persistently modify spinal cord structure and function (43,63,64). Accordingly, as in our previous studies (43,44), we specifically examined treatment effects at P3, P7 and P21; a developmental time span that extends from the critical neonatal period through to childhood in humans. Consistent with our current and previous reports, spontaneous apoptosis in the spinal cord of postnatal rats occurs largely in the dorsal horn from P2–P5, decreasing by P8–10 (65,66). The brief anesthesia used for our intrathecal injection protocol has no additional impact, as the number of apoptotic cells did not differ between naïve and intrathecal saline animals (44). Here, maximum tolerated doses of clonidine did not alter the number or distribution of apoptotic cell profiles as assessed by histological examination, activated-caspase 3 immunohistochemistry or Fluoro-Jade C staining. In addition, there was no evidence of a delayed injury response as there was no histopathological change or increase in glial reactivity 7 days following injection. Following general anesthetic exposures that increase cortical and hippocampal apotosis in rodents, changes in higher cognitive functions such as learning and memory have been reported at intervals from 4 weeks to 8 months (67). We evaluated sensory thresholds at P35, when both sensory (61) and motor (68,69) components of reflex function are fully mature, and descending modulation from the rostroventral medulla has reached adult patterns by P25 (70). Whereas intrathecal ketamine at P3 increased apoptosis and altered mechanical withdrawal thresholds and gait (43), single maximal doses of intrathecal clonidine did not alter reflex sensitivity at P35.
Clonidine has an established role as a spinal adjuvant analgesic in pediatric practice, and clonidine via the intrathecal (71) or caudal/epidural (72) route has a greater effect than the same dose intravenously. Epidural clonidine 0.08–0.12 µg/kg/hr produces dose-dependent analgesia when added to local anesthetic infusions (17), and higher doses of clonidine alone (0.2µg/kg/hr preceded by bolus of 2µg/kg) provide analgesia at rest following abdominal surgery (73). Clonidine 1–2µg/kg prolongs analgesia when added to caudal local anesthetic, with cardiovascular and sedative side-effects at doses of 5µg/kg in children (15), but sensitivity to side-effects (apnoea, oxygen desaturation, and bradycardia) is greater in neonates (18–20). Similarly, when added to intrathecal local anesthetic, clonidine prolonged analgesia in neonates (21), but an increased proportion were sedated and developed self-limiting apnea postoperatively (22). Caudal dexmedetomidine has analgesic properties (74) similar to clonidine (75) in infants and children, but there has been limited preclinical evaluation of toxicity (59,76).
The merits of different spinal analgesics have been discussed in several clinical reviews, but concerns about the relative safety of different drugs and preparations have also been expressed (15,77–79). Current practice differs across centers and countries as the availability of preparations varies and there is limited clinical data relating to comparative efficacy or long-term outcomes (16,80). Within study comparisons of caudal clonidine and ketamine have variably reported more prolonged analgesia with ketamine (81,82) or no difference (83). Addition of clonidine to ketamine further prolonged analgesia in one study (84), but provided no benefit in another (85). Between 2002 and 2009, the proportion of pediatric anesthetists adding clonidine to caudal local anesthetic in the UK increased, and use of caudal ketamine remained relatively stable (13,14). However, decreased use of ketamine in Europe (86) has been attributed to guidelines reporting laboratory toxicity with preservative-free ketamine in adult animals (87). Our previous study (43) and the direct comparisons utilized here, suggest that ketamine has specific developmental effects that reduce the safety margin for spinal administration in early development.
One of the conundrums facing the interpretation of preclinical safety data is the translation of dosing from the animal model to the human condition. We have suggested that one useful strategy is to compare the therapeutic ratio (i.e. the minimum spinal dose at which toxicity is observed divided by the maximally therapeutic dose), as this would provide a conservative estimate for comparison of different agents. The definition of the therapeutic dose clearly depends upon the end point selected. In previous work, we evaluated anti-nociceptive effects of morphine to a mechanical stimulus (44). As ketamine has minimal effects on baseline thresholds, we evaluated anti-hyperalgesic effects following hindpaw inflammation (43). Here we evaluated both anti-nociceptive and anti-hyperalgesic effects of clonidine, and as shown previously in rat pups following epidural morphine (88,89) or epidural dexmedetomidine (24), lower doses of intrathecal clonidine were required to reverse inflammatory hyperalgesia than to produce anti-nociceptive effects. Analgesic dose requirements for morphine, ketamine and clonidine were age-dependent and lowest at P3. As analgesic requirements vary with the type of drug and with age (e.g. carrageenan-induced hyperalgesia was reversed by clonidine 30mcg/kg and ketamine 3mg/kg at P3, and by clonidine 300mcg/kg and ketamine 15mg/kg at P21), equi-analgesic effects rather than absolute doses are used to calculate the relative therapeutic ratio. Using anti-hyperalgesic doses, therapeutic ratios for intrathecal clonidine were >300 at P3, >30 at P7, and >10 at P21. Similarly, maximal doses of morphine did not produce toxicity, and the therapeutic ratio was high (44). By contrast, intrathecal ketamine increased apoptosis in the same dose range as analgesia, and the calculated therapeutic ratio did not exceed 1 (43). This approach has potential weaknesses. From the perspective of the spinal agent, the principal variable defining local tissue effects are the local concentration of the drug in the spinal canal and the duration of that exposure (26). As repeated dosing via intrathecal catheters is not practical in small pups, single doses were given and the maximum testable dose of clonidine, morphine and ketamine was limited by systemic side effects. While systemic side effects are of concern, our principal focus for toxicity is the local effect upon spinal tissues. In the event that the maximum tolerable dose after intrathecal delivery is not associated with toxicity, we can only conclude that the therapeutic ratio is at least, or greater than, a certain value. This value will be low if systemic side-effects preclude assessment of higher doses/concentrations. Conversely, if local spinal toxicity occurs at a dose lower than the therapeutic analgesic effect, one can assert that the therapeutic ratio is 1 or less. We would argue that despite these limitations on achieving absolute values, this methodology allows us to compare the relative safety margin of different drugs at given stages of development. A relatively wide safety margin has been demonstrated with morphine (44) and now with clonidine. The comparisons with ketamine presented here and previously (43), confirm that spinal clonidine has a wider safety margin than spinal ketamine in the neonatal rat. These data provide additional information to inform the clinical choice of spinal analgesic in early life.
Acknowledgments
Funding: Supported by: British Journal of Anaesthesia/Royal College of Anaesthetists Project Grant and Great Ormond Street Hospital Children’s Charity (SMW) and NIDA 15353 (TLY)
Information for LWW regarding depositing manuscript into PubMed Central: This research was funded by National Institutes of Health grant number NIDA 15353.
Footnotes
Suellen M. Walker, This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Suellen M. Walker has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Marjorie Grafe, This author helped analyze the data, write the manuscript, and histopathology
Attestation: Marjorie Grafe has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Tony L. Yaksh, This author helped design the study and write the manuscript
Attestation: Tony L Yaksh has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Conflicts: Suellen M. Walker reported no conflicts of interest
Conflicts: Marjorie Grafe reported no conflicts of interest
Conflicts: Tony L Yaksh reported no conflicts of interest
- Portex Unit: Pain Research, UCL Institute of Child Health and Great Ormond Street Hospital NHS Trust, London, United Kingdom
- Department of Pathology, Oregon Health and Science University, Portland, Oregon, USA
- Department of Anesthesiology, University of California San Diego, La Jolla, California, USA
This report was previously presented, in part, as a poster in the Late Breaking Abstracts Pediatric Anesthesia Neurotoxicity Panel at the IARS Annual Meeting in May 2011.
Contributor Information
Suellen M. Walker, Portex Unit: Pain Research, UCL Institute of Child Health and Great Ormond St Hospital NHS Trust, suellen.walker@ucl.ac.uk.
Marjorie Grafe, Oregon Health and Science University, Portland, OR, USA, grafem@ohsu.edu.
Tony L. Yaksh, Department of Anesthesiology, University of California San Diego, La Jolla, California, USA, tyaksh@ucsd.edu.
References
- 1.Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- 2.Yaksh TL, Reddy SV. Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha-adrenergic agonists and baclofen. Anesthesiology. 1981;54:451–467. doi: 10.1097/00000542-198106000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 4.Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–674. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Eisenach JC, Lysak SZ, Viscomi CM. Epidural clonidine analgesia following surgery: phase I. Anesthesiology. 1989;71:640–646. doi: 10.1097/00000542-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Paech MJ, Pavy TJ, Orlikowski CE, Lim W, Evans SF. Postoperative epidural infusion: a randomized, double-blind, dose-finding trial of clonidine in combination with bupivacaine and fentanyl. Anesth Analg. 1997;84:1323–1328. doi: 10.1097/00000539-199706000-00027. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelo R, Evans E, Dean LA, Gaver R, Eisenach JC. Spinal clonidine prolongs labor analgesia from spinal sufentanil and bupivacaine. Anesth Analg. 1999;88:573–576. doi: 10.1097/00000539-199903000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Paech MJ, Pavy TJ, Orlikowski CE, Evans SF. Patient-controlled epidural analgesia in labor: the addition of clonidine to bupivacaine-fentanyl. Reg Anesth Pain Med. 2000;25:34–40. doi: 10.1016/s1098-7339(00)80008-5. [DOI] [PubMed] [Google Scholar]
- 9.Paech MJ, Pavy TJ, Orlikowski CE, Yeo ST, Banks SL, Evans SF, Henderson J. Postcesarean analgesia with spinal morphine, clonidine, or their combination. Anesth Analg. 2004;98:1460–1466. doi: 10.1213/01.ane.0000111208.08867.3c. [DOI] [PubMed] [Google Scholar]
- 10.Rauck RL, Eisenach JC, Jackson K, Young LD, Southern J. Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology. 1993;79:1163–1169. [PubMed] [Google Scholar]
- 11.Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000;91:1493–1498. doi: 10.1097/00000539-200012000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- 13.Sanders JC. Paediatric regional anaesthesia, a survey of practice in the United Kingdom. Br J Anaesth. 2002;89:707–710. [PubMed] [Google Scholar]
- 14.Menzies R, Congreve K, Herodes V, Berg S, Mason DG. A survey of pediatric caudal extradural anesthesia practice. Paediatr Anaesth. 2009;19:829–836. doi: 10.1111/j.1460-9592.2009.03116.x. [DOI] [PubMed] [Google Scholar]
- 15.Ansermino M, Basu R, Vandebeek C, Montgomery C. Nonopioid additives to local anaesthetics for caudal blockade in children: a systematic review. Paediatr Anaesth. 2003;13:561–573. doi: 10.1046/j.1460-9592.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams DG, Howard RF. Epidural analgesia in children. A survey of current opinions and practices amongst UK paediatric anaesthetists. Paediatr Anaesth. 2003;13:769–776. doi: 10.1046/j.1460-9592.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 17.De Negri P, Ivani G, Visconti C, De Vivo P, Lonnqvist PA. The dose-response relationship for clonidine added to a postoperative continuous epidural infusion of ropivacaine in children. Anesth Analg. 2001;93:71–76. doi: 10.1097/00000539-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Fellmann C, Gerber AC, Weiss M. Apnoea in a former preterm infant after caudal bupivacaine with clonidine for inguinal herniorrhaphy. Paediatr Anaesth. 2002;12:637–640. doi: 10.1046/j.1460-9592.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 19.Bouchut JC, Dubois R, Godard J. Clonidine in preterm-infant caudal anesthesia may be responsible for postoperative apnea. Reg Anesth Pain Med. 2001;26:83–85. doi: 10.1053/rapm.2001.20455. [DOI] [PubMed] [Google Scholar]
- 20.Breschan C, Krumpholz R, Likar R, Kraschl R, Schalk HV. Can a dose of 2 microg.kg(−1) caudal clonidine cause respiratory depression in neonates? Paediatr Anaesth. 1999;9:81–83. [PubMed] [Google Scholar]
- 21.Rochette A, Raux O, Troncin R, Dadure C, Verdier R, Capdevila X. Clonidine prolongs spinal anesthesia in newborns: a prospective dose-ranging study. Anesth Analg. 2004;98:56–59. doi: 10.1213/01.ANE.0000093229.17729.6C. [DOI] [PubMed] [Google Scholar]
- 22.Rochette A, Troncin R, Raux O, Dadure C, Lubrano JF, Barbotte E, Capdevila X. Clonidine added to bupivacaine in neonatal spinal anesthesia: a prospective comparison in 124 preterm and term infants. Paediatr Anaesth. 2005;15:1072–1077. doi: 10.1111/j.1460-9592.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- 23.Hughes HE, Barr GA. Analgesic effects of intrathecally applied noradrenergic compounds in the developing rat: differences due to thermal vs mechanical nociception. Brain Res. 1988;469:109–120. doi: 10.1016/0165-3806(88)90174-5. [DOI] [PubMed] [Google Scholar]
- 24.Walker SM, Howard RF, Keay KA, Fitzgerald M. Developmental age influences the effect of epidural dexmedetomidine on inflammatory hyperalgesia in rat pups. Anesthesiology. 2005;102:1226–1234. doi: 10.1097/00000542-200506000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Walker SM, Fitzgerald M. Characterization of spinal alpha-adrenergic modulation of nociceptive transmission and hyperalgesia throughout postnatal development in rats. Br J Pharmacol. 2007;151:1334–1342. doi: 10.1038/sj.bjp.0707290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaksh TL, Rathbun ML, Provencher JC. Preclinical safety evaluation for spinal drugs. In: Yaksh TL, editor. Spinal Drug Delivery. Amsterdam: Elsevier Science B.V.; 1999. pp. 417–437. [Google Scholar]
- 27.Eisenach JC, Yaksh TL. Safety in numbers: how do we study toxicity of spinal analgesics? Anesthesiology. 2002;97:1047–1049. doi: 10.1097/00000542-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Eisenach JC, Shafer SL, Yaksh T. The need for a journal policy on intrathecal, epidural, and perineural administration of non-approved drugs. Pain. 2010;149:417–419. doi: 10.1016/j.pain.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Guevara-Lopez U, Aldrete JA, Covarrubias-Gomez A, Hernandez-Pando RE, Lopez-Munoz FJ. Absence of histological changes after the administration of a continuous intrathecal clonidine in Wistar rats. Pain Pract. 2009;9:122–129. doi: 10.1111/j.1533-2500.2008.00251.x. [DOI] [PubMed] [Google Scholar]
- 30.Gordh T, Jr, Post C, Olsson Y. Evaluation of the toxicity of subarachnoid clonidine, guanfacine, and a substance P-antagonist on rat spinal cord and nerve roots: light and electron microscopic observations after chronic intrathecal administration. Anesth Analg. 1986;65:1303–1311. [PubMed] [Google Scholar]
- 31.Yaksh TL, Rathbun M, Jage J, Mirzai T, Grafe M, Hiles RA. Pharmacology and toxicology of chronically infused epidural clonidine. HCl in dogs. Fundam Appl Toxicol. 1994;23:319–335. doi: 10.1006/faat.1994.1112. [DOI] [PubMed] [Google Scholar]
- 32.Gordh TE, Ekman S, Lagerstedt AS. Evaluation of possible spinal neurotoxicity of clonidine. Ups J Med Sci. 1984;89:266–273. doi: 10.3109/03009738409179507. [DOI] [PubMed] [Google Scholar]
- 33.Hood DD, Eisenach JC, Tong C, Tommasi E, Yaksh TL. Cardiorespiratory and spinal cord blood flow effects of intrathecal neostigmine methylsulfate, clonidine, and their combination in sheep. Anesthesiology. 1995;82:428–435. doi: 10.1097/00000542-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Gordh T, Jr, Feuk U, Norlen K. Effect of epidural clonidine on spinal cord blood flow and regional and central hemodynamics in pigs. Anesth Analg. 1986;65:1312–1318. [PubMed] [Google Scholar]
- 35.Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- 36.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 38.Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creeley CE, Olney JW. The young: neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–448. doi: 10.1213/ANE.0b013e3181c6b9ca. [DOI] [PubMed] [Google Scholar]
- 41.Loepke AW. Developmental neurotoxicity of sedatives and anesthetics: a concern for neonatal and pediatric critical care medicine? Pediatr Crit Care Med. 2010;11:217–226. doi: 10.1097/PCC.0b013e3181b80383. [DOI] [PubMed] [Google Scholar]
- 42.Stratmann G. Review article: neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 43.Walker SM, Westin BD, Deumens R, Grafe M, Yaksh TL. Effects of Intrathecal Ketamine in the Neonatal Rat: Evaluation of Apoptosis and Long-term Functional Outcome. Anesthesiology. 2010;113:147–159. doi: 10.1097/ALN.0b013e3181dcd71c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westin BD, Walker SM, Deumens R, Grafe M, Yaksh TL. Validation of a Preclinical Spinal Safety Model: Effects of Intrathecal Morphine in the Neonatal Rat. Anesthesiology. 2010;113:183–199. doi: 10.1097/ALN.0b013e3181dcd6ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker SM, Tochiki KK, Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain. 2009;147:99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Nandi R, Beacham D, Middleton J, Koltzenburg M, Howard RF, Fitzgerald M. The functional expression of mu opioid receptors on sensory neurons is developmentally regulated; morphine analgesia is less selective in the neonate. Pain. 2004;111:38–50. doi: 10.1016/j.pain.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- 48.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 49.Van Zwieten PA. The role of adrenoceptors in circulatory and metabolic regulation. Am Heart J. 1988;116:1384–1392. doi: 10.1016/0002-8703(88)90128-7. [DOI] [PubMed] [Google Scholar]
- 50.Yaksh TL, Pogrel JW, Lee YW, Chaplan SR. Reversal of nerve ligation-induced allodynia by spinal alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther. 1995;272:207–214. [PubMed] [Google Scholar]
- 51.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, Sommer C, Meschter C, Richter PJ, Hildebrand KR. Chronically infused intrathecal morphine in dogs. Anesthesiology. 2003;99:174–187. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Maes M, Lin A, Kenis G, Egyed B, Bosmans E. The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res. 2000;96:245–253. doi: 10.1016/s0165-1781(00)00216-x. [DOI] [PubMed] [Google Scholar]
- 53.Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, Niwa Y. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesth Analg. 1999;88:452–458. doi: 10.1097/00000539-199902000-00042. [DOI] [PubMed] [Google Scholar]
- 54.Lindgren BR, Grundstrom N, Andersson RG. Comparison of the effects of clonidine and guanfacine on the histamine liberation from human mast cells and basophils and on the human bronchial smooth muscle activity. Arzneimittelforschung. 1987;37:551–553. [PubMed] [Google Scholar]
- 55.Ruffolo RR., Jr Peripheral alpha-adrenergic receptors. Introduction. Fed Proc. 1984;43:2908–2909. [PubMed] [Google Scholar]
- 56.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 57.Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54:710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 58.McGowan FX, Jr, Davis PJ. Anesthetic-related neurotoxicity in the developing infant: of mice, rats, monkeys and, possibly, humans. Anesth Analg. 2008;106:1599–1602. doi: 10.1213/ane.0b013e31817330cf. [DOI] [PubMed] [Google Scholar]
- 59.Walker SM, Yaksh TL. New caudal additives in children: benefit vs. risk? Acta Anaesthesiol Scand. 2009;53:1097–1098. doi: 10.1111/j.1399-6576.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- 60.Yahalom B, Athiraman U, Soriano SG, Zurakowski D, Carpino EA, Corfas G, Berde CB. Spinal anesthesia in infant rats: development of a model and assessment of neurologic outcomes. Anesthesiology. 2011;114:1325–1335. doi: 10.1097/ALN.0b013e31821b5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 62.Sanders RD, Xu J, Shu Y, Fidalgo A, Ma D, Maze M. General anesthetics induce apoptotic neurodegeneration in the neonatal rat spinal cord. Anesth Analg. 2008;106:1708–1711. doi: 10.1213/ane.0b013e3181733fdb. [DOI] [PubMed] [Google Scholar]
- 63.Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- 64.Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci. 2008;28:5494–5503. doi: 10.1523/JNEUROSCI.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Louw AJ, de Vente J, Steinbusch HP, Gavilanes AW, Steinbusch HW, Blanco CE, Troost J, Vles JS. Apoptosis in the rat spinal cord during postnatal development; the effect of perinatal asphyxia on programmed cell death. Neuroscience. 2002;112:751–758. doi: 10.1016/s0306-4522(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 66.Lawson SJ, Davies HJ, Bennett JP, Lowrie MB. Evidence that spinal interneurons undergo programmed cell death postnatally in the rat. Eur J Neurosci. 1997;9:794–799. doi: 10.1111/j.1460-9568.1997.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 67.Stratmann G, Sall JW, May LD, Loepke AW, Lee MT. Beyond Anesthetic Properties: The Effects of Isoflurane on Brain Cell Death, Neurogenesis, and Long-Term Neurocognitive Function. Anesth Analg. 2010;110:431–437. doi: 10.1213/ANE.0b013e3181af8015. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonzalez-Forero D, Alvarez FJ. Differential postnatal maturation of GABAA, glycine receptor, and mixed synaptic currents in Renshaw cells and ventral spinal interneurons. J Neurosci. 2005;25:2010–2023. doi: 10.1523/JNEUROSCI.2383-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587:2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao JP, Miao XY, Liu J, Shi XY. An evaluation of intrathecal bupivacaine combined with intrathecal or intravenous clonidine in children undergoing orthopedic surgery: a randomized double-blinded study. Paediatr Anaesth. 2011;21:399–405. doi: 10.1111/j.1460-9592.2011.03543.x. [DOI] [PubMed] [Google Scholar]
- 72.Akin A, Ocalan S, Esmaoglu A, Boyaci A. The effects of caudal or intravenous clonidine on postoperative analgesia produced by caudal levobupivacaine in children. Paediatr Anaesth. 2010;20:350–355. doi: 10.1111/j.1460-9592.2010.03259.x. [DOI] [PubMed] [Google Scholar]
- 73.Klamt JG, Garcia LV, Stocche RM, Meinberg AC. Epidural infusion of clonidine or clonidine plus ropivacaine for postoperative analgesia in children undergoing major abdominal surgery. J Clin Anesth. 2003;15:510–514. doi: 10.1016/j.jclinane.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, Afifi W. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–256. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 75.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–274. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 76.Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25:403–409. doi: 10.1017/S0265021507003079. [DOI] [PubMed] [Google Scholar]
- 77.Cook B, Doyle E. The use of additives to local anaesthetic solutions for caudal epidural blockade. Paediatr Anaesth. 1996;6:353–359. doi: 10.1046/j.1460-9592.1996.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 78.de Beer DA, Thomas ML. Caudal additives in children--solutions or problems? Br J Anaesth. 2003;90:487–498. doi: 10.1093/bja/aeg064. [DOI] [PubMed] [Google Scholar]
- 79.Tsui BC, Berde CB. Caudal analgesia and anesthesia techniques in children. Curr Opin Anaesthesiol. 2005;18:283–288. doi: 10.1097/01.aco.0000169236.91185.5b. [DOI] [PubMed] [Google Scholar]
- 80.Howard RF, Carter B, Curry J, Morton N, Rivett K, Rose M, Tyrrell J, Walker SM, Williams DG. Association of Paediatric Anaesthetists: Good Practice in Postoperative and Procedural Pain. Pediatric Anesthesia. 2008;18(Suppl. 1):1–81. doi: 10.1111/j.1155-5645.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 81.De Negri P, Ivani G, Visconti C, De Vivo P. How to prolong postoperative analgesia after caudal anaesthesia with ropivacaine in children: S-ketamine versus clonidine. Paediatr Anaesth. 2001;11:679–683. doi: 10.1046/j.1460-9592.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 82.Cook B, Grubb DJ, Aldridge LA, Doyle E. Comparison of the effects of adrenaline, clonidine and ketamine on the duration of caudal analgesia produced by bupivacaine in children. Br J Anaesth. 1995;75:698–701. doi: 10.1093/bja/75.6.698. [DOI] [PubMed] [Google Scholar]
- 83.Akbas M, Akbas H, Yegin A, Sahin N, Titiz TA. Comparison of the effects of clonidine and ketamine added to ropivacaine on stress hormone levels and the duration of caudal analgesia. Paediatr Anaesth. 2005;15:580–585. doi: 10.1111/j.1460-9592.2005.01506.x. [DOI] [PubMed] [Google Scholar]
- 84.Hager H, Marhofer P, Sitzwohl C, Adler L, Kettner S, Semsroth M. Caudal clonidine prolongs analgesia from caudal S(+)-ketamine in children. Anesth Analg. 2002;94:1169–1172. doi: 10.1097/00000539-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Passariello M, Almenrader N, Canneti A, Rubeo L, Haiberger R, Pietropaoli P. Caudal analgesia in children: S(+)-ketamine vs S(+)-ketamine plus clonidine. Paediatr Anaesth. 2004;14:851–855. doi: 10.1111/j.1460-9592.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 86.Eich C, Strauss J. Prompt and powerful effect of a practice guideline on caudal additives. Paediatr Anaesth. 2009;19:271–272. doi: 10.1111/j.1460-9592.2009.02926.x. [DOI] [PubMed] [Google Scholar]
- 87.Vranken JH, Troost D, Wegener JT, Kruis MR, van der Vegt MH. Neuropathological findings after continuous intrathecal administration of S(+)-ketamine for the management of neuropathic cancer pain. Pain. 2005;117:231–235. doi: 10.1016/j.pain.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 88.Marsh D, Dickenson A, Hatch D, Fitzgerald M. Epidural opioid analgesia in infant rats II: responses to carrageenan and capsaicin. Pain. 1999;82:33–38. doi: 10.1016/S0304-3959(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 89.Marsh D, Dickenson A, Hatch D, Fitzgerald M. Epidural opioid analgesia in infant rats I: mechanical and heat responses. Pain. 1999;82:23–32. doi: 10.1016/S0304-3959(99)00028-7. [DOI] [PubMed] [Google Scholar]