Abstract
Biological barriers regulate the passage of cells, pathogens, fluids, nutrients, ions and signalling molecules between anatomical compartments during homeostasis and disease. Yet strategies that allow for reversible therapeutic modulation of these barriers are still in their infancy. The enhancement or protection of natural barriers is desirable in conditions such as acute respiratory distress syndrome or ischaemia–reperfusion injuries, whereas a temporary disruption could facilitate the penetration of drugs across such barriers. This Review discusses the role of sphingosine-1-phosphate receptors in the regulation and protection of biological barriers, and the potential of therapeutic strategies that target this receptor family.
Tight regulation of molecular and cellular exchanges between organs and the bloodstream is central to the maintenance of homeostasis in the body, and the dysregulation of this dynamic process is known to cause pathology. A greater understanding of the mechanisms involved in these exchanges during homeostasis and disease might allow the identification of novel strategies to control cellular and molecular trafficking, or delivery of therapeutic molecules to specific anatomical compartments. Of key importance to the control of these exchanges is the barrier constituted by the network of contiguous endothelial cells that line the lumen of blood vessels, which is known as the vascular endothelium. Endothelia can regulate the passage of cells by modifying the cell surface expression of adhesion molecules, such as integrins and selectins, and regulate molecular exchanges through paracellular gaps by acting on intercellular adhesion molecules from the cadherin, occludin and junctional adhesion molecule families.
Endothelia are found throughout the body and are highly heterogeneous. For example, the blood–brain barrier (BBB) is a stringent endothelial interface specialized in the protection of the central nervous system (CNS) from xenobiotics and toxins. The specificity of the BBB also hinders the delivery of pharmacological agents to the CNS, although the 600 km network of blood vessels within the CNS feeds virtually every neuron1. It is well established that almost 100% of large-molecule drugs and 98% of small-molecule drugs do not cross the BBB2. When the BBB is disrupted during meningitis or tumorigenesis, leakage of intravascular material to the extravascular tissue leads to increased pressure, vascular dysfunction and, in the case of meningitis, ionic imbalance and accumulation of inflammatory infiltrates, which leads to neuronal damage.
The integrity of the pulmonary endothelial barrier is crucial for the maintenance of gas exchange. When the pulmonary barrier is disrupted during pneumonia or sepsis, the accumulation of fluids and inflammatory cells in the extracellular space, and ultimately in the alveolar airspace, leads to impaired gas exchange. Various other organs are susceptible to barrier-mediated dysfunction, particularly during inflammatory conditions. During the reperfusion phase that occurs after a period of ischaemia — as in the case of myocardial infarction or after solid organ transplantation — the endothelium becomes activated and recruits inflammatory cells, which can irreversibly damage the organ. Consequently, enhancement, protection or even disruption of endothelial barriers could contribute to a range of useful therapeutic strategies for different pathologies.
Although the function of physiological barriers was the focus of scientific curiosity in the nineteenth century3, when the organ distribution of intravenously delivered dyes was studied, few currently used therapeutics elicit their effects through barrier modulation. The biosynthetic pathway of the lysophospholipid sphingosine-1-phosphate (S1P) has been extensively characterized4–8 (FIG. 1) and S1P has emerged as a potent modulator of barrier integrity owing to its ability to modulate a family of high-affinity G protein-coupled receptors (GPCrs)7. S1P receptors, previously called endothelial differentiation gene receptors, were discovered in the early 1990s, in parallel with the immunosuppressive myriocin analogue FTY720. Mandala et al.9 showed that FTY720 was a prodrug that required in vivo phosphorylation to induce immunosuppression, by acting as an agonist on receptors of the structurally similar endogenous ligand, S1P. Five high-affinity receptors have been cloned: S1P1, S1P2, S1P3, S1P4 and S1P5.
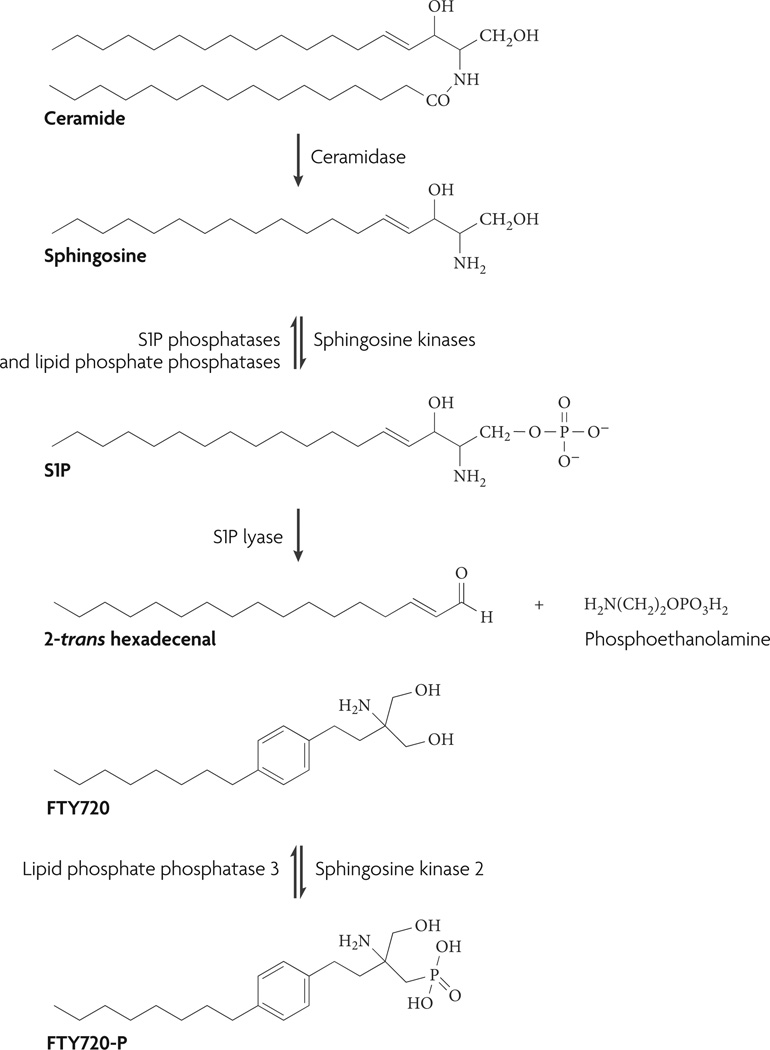
Figure 1. The sphingosine-1-phosphate (s1P) biosynthetic pathway.
Degradation of ceramide to sphingosine by ceramidase and subsequent phosphorylation by sphingosine kinase 1 or sphingosine kinase 2 produces S1P. S1P can be reversibly degraded to sphingosine by S1P phosphatase and lipid phosphate phosphatases or irreversibly degraded by S1P lyase to 2-trans hexadecenal and phosphoethanolamine4,22. Notably, FTY720 and some of its analogues are preferentially phosphorylated by sphingosine kinase 2 (REF. 26) and were shown to be degraded by lipid phosphate phosphatase 3 in vitro105.
Synthetic S1P receptor modulators have shown therapeutic efficacy in clinical trials for multiple sclerosis21 and in a range of animal models, including acute respiratory distress syndrome (ARDS)10,11, disseminated intravascular coagulation12,13, ischaemia–reperfusion injury14–16, solid graft transplantation17,18 and experimental autoimmune encephalomyelitis (EAE)19,20. Here, we focus on the involvement of S1P receptors in the regulation and protection of the endothelial barrier in both physiological and pathophysiological conditions. On this basis, we elaborate on how the use of current investigative tools can help to refine our understanding of the involvement of S1P receptors in barrier modulation and protection, and how this may lead to the development of highly specific, barrier-oriented small-molecule-based therapeutics.
S1P receptor signalling
Sphingosine is a member of the sphingolipid family and consists of an aliphatic chain with 18 carbon atoms, with hydroxyl groups on carbon atoms 1 and 3, and an amine moiety on carbon atom 2. Sphingosine is generated from N-deacylation of ceramide by ceramidase, and can be phosphorylated by sphingosine kinases (SphKs) on its primary hydroxyl group to produce S1P (FIG. 1). Two major sphingosine kinase isoforms have been identified so far — SphK1 and SphK2 — and both possess a number of splice variants22. These two enzymes are differentially regulated and distributed within the cell and in different tissues. For example, in mice, SphK1 is mostly expressed in the lungs, spleen, kidneys and blood, whereas high expression of SphK2 occurs in the liver, kidneys, brain and heart23,24. SphK1 is present in the cytosol and translocates to the plasma membrane after phosphorylation, which is thought to account, at least in part, for the compartmentalization of S1P synthesis inside the cell (FIG. 2). moreover, SphK1 can be secreted and might contribute to plasma S1P levels, whereas SphK2 is only found intracellularly25, mostly in the cytosol and nucleus. Notably, the SphK2 isoform efficiently phosphorylates synthetic sphingosine analogues, such as FTY720 and AAL-R. This is in contrast to SphK1 (REF. 26) (FIG. 1), which has slow enzyme kinetics for phosphorylation of FTY720. After phosphorylation to S1P, sphingosine can activate any of its five differentially distributed, high-affinity GPCRs27. These receptors regulate a range of biological processes, including cell adhesion, migration and endocytosis. Physiologically, S1P receptors modulate smooth-muscle cell contraction, vascular tone, heart rate, lymphocyte trafficking and barrier integrity (FIG. 3). The role of S1P as a potential ligand for the orphan receptors GPR3, GPR6 and GPR12 (REF. 28) remains controversial, and no compelling experimental evidence has been found.
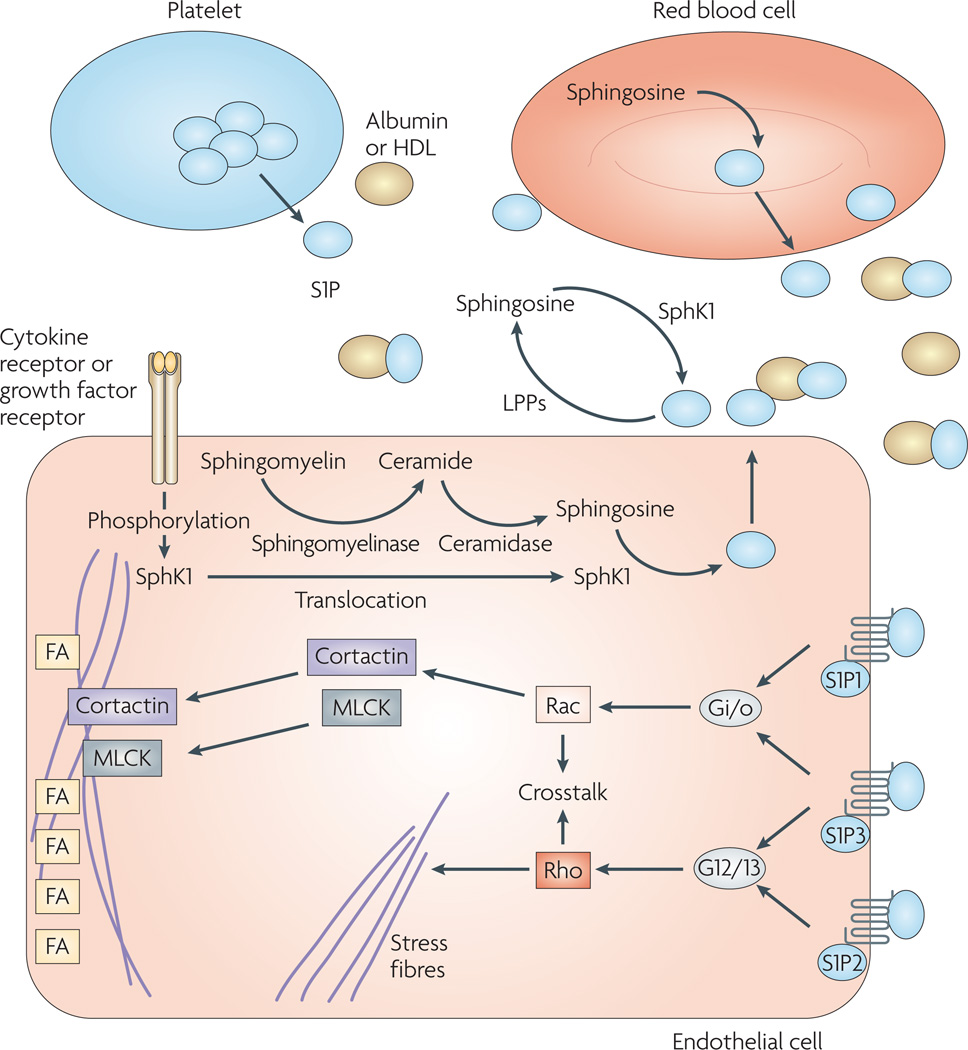
Figure 2. sphingosine-1-phosphate (s1P) receptor signalling.
S1P is present at high nanomolar concentrations in plasma; 98.5% is bound to albumin and other transporters such as high density lipoproteins (HDLs)29. S1P is released to the blood from various sources. It was originally thought to be mainly produced by platelets106, but recent evidence suggests that red blood cells are also a source of S1P in mice30–32 and that secreted sphingosine kinase 1 (SphK1) could also help regulate plasma S1P levels25. S1P can act intracellularly (not shown) or be transported to the extracellular space by ATP-binding-cassette transporters107. Another S1P transporter was recently identified in the zebrafish108. Once in the extracellular space, S1P is susceptible to degradation to sphingosine by different classes of lipid phosphate phosphatases (LPPs). In turn, sphingosine might also be phosphorylated by secreted SphK1 in the extracellular space. S1P1 and S1P3 are known to couple to Gi/o, which is associated with endothelial barrier enhancement. Signalling through Gi/o leads to Rac activation, which is coupled to the translocation of cortactin to the periphery of the cell, where it co-localizes with myosin light chain kinase (MLCK) and leads to the formation of the cortical polymerized actin ring. Moreover, cortactin is also thought to contribute to the organization of tight junctions. The cortical actin ring is stabilized by the assembly of focal adhesions (FAs) at the periphery of the cell, leading to tightening of the endothelial barrier. In an apposite manner, S1P3 and S1P2 couple to G12 and G13. G12 and G13 activation leads to Rho activation, stress fibre formation and disruption of the endothelial barrier. This figure was inspired by various working models4,10,49,109.
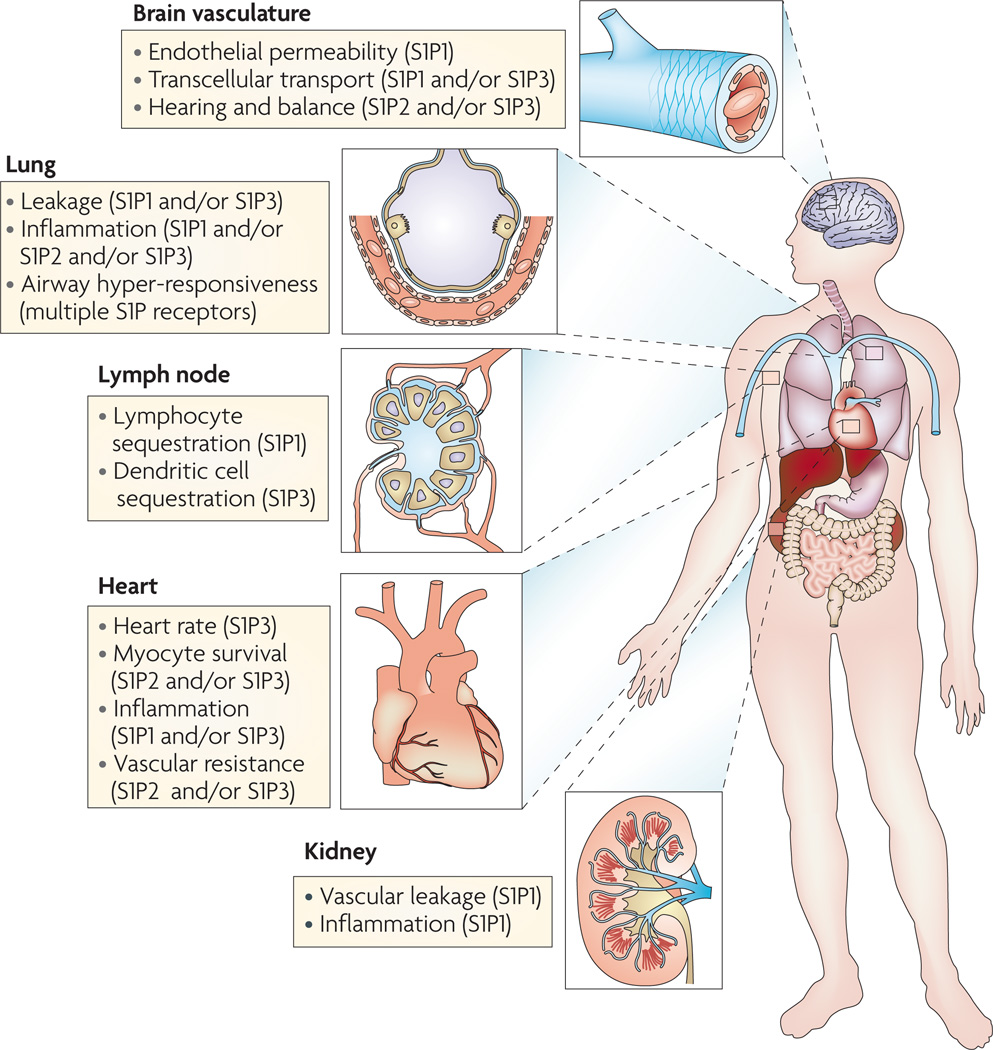
Figure 3. involvement of sphingosine-1-phosphate (s1P) receptors in the regulation of physiological and pathophysiological phenomena.
S1P receptors are involved in the regulation of various physiological and pathophysiological phenomena, including hearing36,37, vasodilation and vasoconstriction110, heart rate99, airway hyper-responsiveness111 and lymphoid tissue function9. Accumulating literature supports the use of small molecules that target the S1P immunoregulatory pathway to modulate barrier activity in different organs. For example, S1P receptor activation was shown to favour pulmonary barrier integrity in models of acute lung injury and acute respiratory distress syndrome10,11,48, and in the kidneys and myocardium after ischaemia–reperfusion stresses15,112, and to favour blood–brain barrier protection during experimental autoimmune encephalomyelitis19. Moreover, the S1P pathway was shown to enhance the lymphatic endothelial barrier integrity in lymph nodes, leading to sequestration of T cells in the lymph nodes9.
S1P is present at high nanomolar concentrations in the plasma, although only low nanomolar concentrations of free S1P are available for activity as 98.5% is bound to high density lipoprotein (HDL) and albumin29. Although the exact sources of plasma S1P are still a matter of debate, S1P is thought to be produced by endothelial cells, platelets and red blood cells30–32. Different subsets of S1P receptors (S1P1, S1P2 and S1P3, discussed below) have different and sometimes opposing effects on the regulation of cellular functions, thereby providing a teleological basis for the use of specific molecular probes directed towards a unique receptor to achieve unidirectional modulation of barrier function.
In vitro model systems involving receptor overexpression or gene silencing were employed to dissect the function of S1P1, which was shown to be involved in cytoskeletal rearrangement and the formation of intercellular adherens junctions33,34. Genetic deletion in mice subsequently proved that S1P1 is essential for complete vascular maturation during embryogenesis35, and resulted in embryonic lethality. Genetic deletion of S1P2 leads to deafness36,37, whereas S1P3 knockout mice show no obvious phenotypic abnormalities38. Perinatal mortality is observed in S1P2–S1P3 double-knockout mice38. Furthermore, a number of small-molecule chemical probes have been generated (TABLE 1) to study the S1P receptor system. For example, probes that are selective for S1P1 revealed that expression of this receptor was sufficient to induce lymphocyte arrest in the lymph nodes in rodents, and that tonic activation of S1P1, caused by constant exposure of the endothelial S1P1 receptor to blood-borne S1P, was required for the maintenance of blood-barrier integrity in specific vascular beds (FIG. 4), including the lungs and kidneys, in the adult mouse39–41.
Table 1.
Synthetic chemical modulators of sphingosine-1-phosphate (S1P) receptors
| Compound | Activity and usage | Structure | Ref. |
|---|---|---|---|
| FTY720 (prodrug; phosphorylated to FTY720-P in vivo) | Agonist for S1P1, S1P3, S1P4,and S1P5; in Phase III clinical trials for multiple sclerosis | 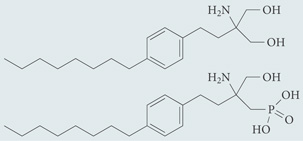 |
9 |
| AAL-R (prodrug; phosphorylated to AFD-R in vivo) | Agonist for S1P1, S1P3, S1P4 and, S1P5; chemical probe* | 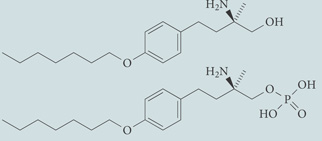 |
89 |
| AAL-S (chiral enantiomer of AAL-R that cannot be phosphorylated) | Inactive on S1P receptors; chemical probe* | 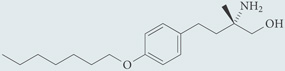 |
89 |
| KRP-203 (prodrug) | Agonist with greater affinity for S1P1 than S1P3 or S1P4; in Phase I clinical trials for multiple sclerosis | 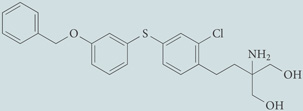 |
114 |
| AUY954 | Agonist for S1P1; chemical probe* | 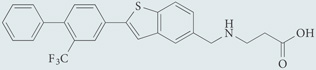 |
17 |
| CYM-5442 | Agonist for S1P1; chemical probe* | 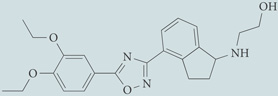 |
101 |
| SEW2871 | Agonist for S1P1; chemical probe* | 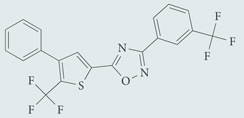 |
99 |
| W146 | Antagonist for S1P1; chemical probe* | 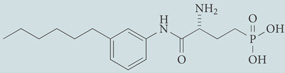 |
40 |
| W140 | Control enantiomer for W146; chemical probe* | 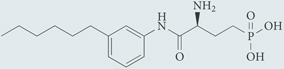 |
40 |
| VPC44116 | Antagonist for S1P1 and S1P3; chemical probe* | 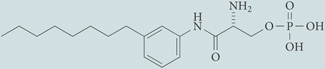 |
41 |
| VPC23019 | Antagonist for S1P1 and S1P3; chemical probe* | 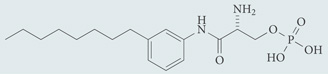 |
115 |
| JTE-013 | Antagonist for S1P2; chemical probe* | 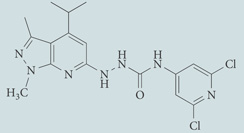 |
116 |
The term chemical probe describes compounds with usefulness for fundamental research that may not have potential for use in humans.
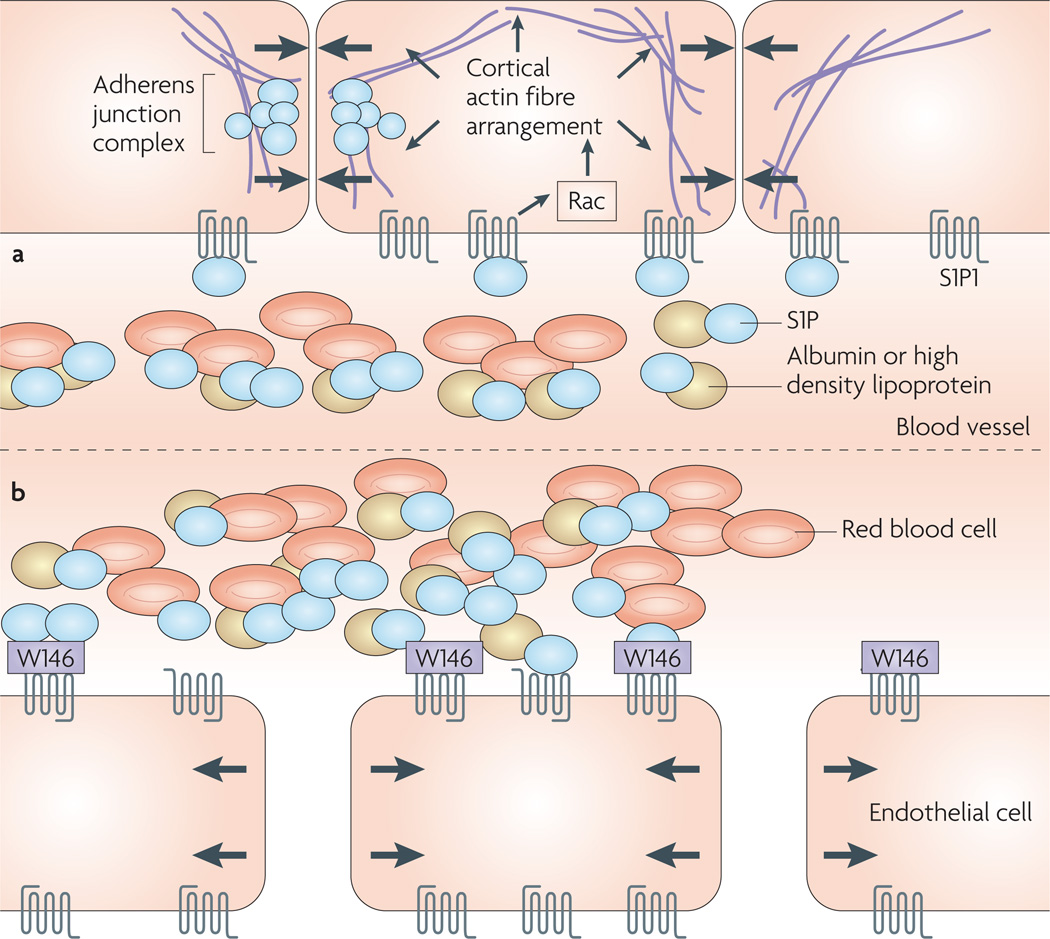
Figure 4. effects of sphingosine-1-phosphate (s1P)–s1P receptor 1 (s1P1) tone on the endothelium of blood vessels.
a | During homeostasis, blood-borne S1P can activate S1P1, which induces Rac activation. This leads to the arrangement of the cortical actin ring and tightening of adherens junctions, favouring the maintenance of barrier integrity. b | The essential requirement of tonic S1P1 activation for the maintenance of endothelial barrier integrity can be shown by in vivo blockade of S1P1 by a chemical probe, such as W146, which leads to disruption of the endothelial barrier and plasma leakage into the interstitium.
In vitro model systems proved that S1P can rescue endothelial cell monolayers from thrombin or lipopolysaccharide (LPS)-induced disruption42,43, and that S1P, FTY720-phosphate (FTY720-P) and AFD-R, which are nonspecific agonists of S1P receptors7, can inhibit vascular endothelial growth factor (VEGF)-induced permeability in embryonic endothelial cells in vitro44. Enhancement of endothelial barriers proceeds through a pathway that has been shown to include S1P1 receptor engagement, activation of Rac1, recruitment to membrane rafts of anchoring substrates for polymerized actin45, an increase in the amount of phosphorylated myosin light chain kinase and cortactin at the cell periphery, cortical rearrangement of polymerized actin46 and cortical relocalization of focal adhesions47 (FIG. 2). S1P2 and S1P3 have opposite effects to S1P1 regarding the regulation of endothelial barrier permeability (FIG. 2). Whereas S1P1 couples solely to Gi/o, S1P2 and S1P3 couple to Gi/o, Gq, and G12 and G13. Activation of G12 and G13 leads to activation of the small GTPase, Rho, which induces destabilization of cortical actin, favours stress-fibre formation and induces endothelial barrier disruption48,49. Sanchez et al.50 observed that overexpression of S1P2 in human umbilical vein endothelial cell (HUVEC) monolayers increased both basal and S1P-triggered permeability. In accordance with these results, the S1P2 antagonist JTE-013 diminishes basal permeability of HUVEC monolayers and synergizes with the barrier-enhancing effect of S1P. S1P3 gene silencing inhibited thrombin- and low-molecular-weight hyaluronan-induced disruption of endothelial monolayers51,52, suggesting that some stimuli need S1P3 transactivation to induce barrier disruption. After exposure of endothelial cells to various barrier-disrupting agents, the S1P3 receptor is phosphorylated on threonine residues, which seems to depend on the activity of rhoA and of the serine–threonine kinases rOCK1 and rOCK2 (REF. 52). The importance of S1P3 phosphorylation remains to be determined.
The combination of in vitro and in vivo data suggests that the tonic activation of the different S1P receptor subtypes at the surface of endothelial cells favours barrier maintenance under basal conditions, and that this equilibrium is changed in favour of barrier disruption in pathological states. This change might be due to modification of the relative expression of S1P1 and S1P3 and/ or S1P2 combined with variable G protein coupling of S1P3 (in contrast to the uniform Gi/o coupling of S1P1). Adding to the complexity of S1P receptor signalling, cognate S1P receptors homodimerize and heterodimerize, not only with each other53,54 but also with members of other receptor families, such as chemo kine receptors55. Furthermore, S1P receptor expression varies among different endothelia34, cell types and pathophysiological conditions50,56,57. The involvement of the S1P receptors in the dysregulated steps that lead to barrier dysfunction has also been addressed in several animal models10,11,15,16,58, and acute lung injury is perhaps the model that has provided the largest amount of information regarding the barrier-modulating capacity of the S1P system.
Pulmonary barrier regulation by S1P receptors
Activation and disruption of the pulmonary endothelium reaches its apotheosis in ARDS, in which increased barrier permeability results in leakage of protein-rich fluid from the vascular compartment to the interstitium and the alveolar airspaces, leading to a life-threatening impairment of gas exchange59. Supportive strategies, such as extracorporeal membrane oxygenation, adoption of the prone position, ventilator-assisted strategies and pharmacological interventions (such as corticosteroid administration and intravenous infusion of activated protein C) were shown to be ineffective, to have a modest therapeutic effect or produced inconclusive outcomes60–63. Pharmacological treatment remains an important unmet need for ARDS.
The involvement of S1P receptors in the regulation of pulmonary barrier integrity was addressed in various models, including intradermal VEGF injection and intratracheal instillation of barrier-disrupting agents, such as bacterial LPS. The sphingosine analogues FTY720 and AAL-R, but not the enantiomer AAL-S, which cannot be phosphorylated, reduced VEGF-induced leakage in mouse ears44. S1P infusion protected the lungs from excessive protein-rich fluid accumulation in the pulmonary parenchyma and/or alveolar airspace in ARDS models; for example in dogs10 and rodents10,11. Furthermore, intraperitoneal injection of FTY720 alleviated features of ARDS 24 hours post LPS instillation in mice; however, this might not only result from pulmonary barrier modulation, but also reflect alterations in leukocyte trafficking64. So far, little or no documentation is available regarding the respective contribution of S1P receptors to ARDS in vivo, which could speculatively be explained, in the case of genetic knockdown models, by adaptive mechanisms, receptor redundancy and the opposing effects of S1P receptors on different effector cells involved in the exacerbation of pulmonary leakage. The lack of publications on positive effects of S1P1 mono-selective agonists in models of ARDS, in spite of their availability for more than 3 years, suggests that multiple receptors are involved in pulmonary endothelial barrier protection in pathological conditions. The exact function of the different S1P receptors during the course of ARDS in vivo needs to be further investigated.
S1P signalling in inflammatory conditions
An increasing body of evidence suggests that S1P could function as a modulator of the innate immune response. Activation of the endothelium is characterized by increased permeability and stimulation of cell surface expression of adhesion molecules, leading to recruitment and accumulation of inflammatory cells that can exacerbate disease by further promoting barrier damage. This paradigm is not only relevant to ARDS, but also to other pathologies, such as myocardial infarction, transplantation-associated ischaemia and multiple sclerosis. Essentially, in response to an insult, sentinel cells, such as fibroblasts65, macrophages66, epithelial cells67 and dendritic cells68, release a plethora of proinflammatory and chemotactic mediators (FIG. 5a). The activated endothelium expresses adhesion molecules, including selectins and integrins, allowing for rolling and adhesion of leukocytes to the vascular wall69. Adherent and infiltrating leukocytes release proteases and toxic molecules for migration and defence, which induce host cell death and tissue damage. This exacerbates endothelial dysfunction and tissue injury, with dramatic consequences for highly differentiated cells and organs that have limited regenerative capacity70. For these clinical phenomena, in which activation of sentinel cells and endothelial cells leads to excessive leukocyte accumulation and immunopathological damage, animal models have highlighted the potential usefulness of a range of molecules. These include anti-inflammatory molecules, pro-survival factors, neutralizers of pro-inflammatory mediators and adhesion molecule blockers71,72. All have resulted in a disappointing translation to human diseases73,74, increasing the need for the identification of new therapeutic targets.
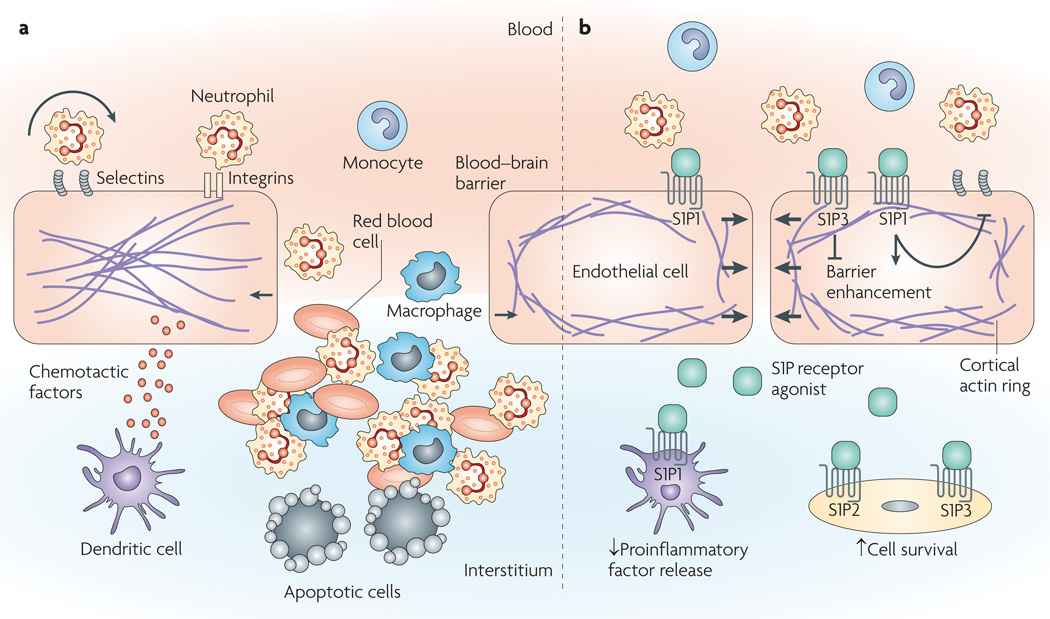
Figure 5. Targets for endothelial barrier protection during inflammation.
a | After tissue insult, tissue-homing cells, such as macrophages or dendritic cells, release proinflammatory and chemotactic factors, which leads to activation of the endothelium. The endothelium expresses adhesion molecules, such as selectins and integrins, at its surface, thereby inducing tethering, rolling, firm adhesion and infiltration of leukocytes. Pro-inflammatory mediators, vasodilatory agents and infiltrating cells induce barrier disruption and injury, resulting in leakage of plasma into the interstitium and infiltration of inflammatory cells that can release toxic molecules and induce cell death. b | Sphingosine-1-phosphate (S1P) and sphingosine analogues were shown to impair the release of pro-inflammatory factors in various models, such as ischaemia–reperfusion injuries or viral infections16,58,102. Although S1P3 activation can disrupt barrier integrity, it seems that broad S1P receptor agonists, such as FTY720, favour barrier integrity. S1P1 activation was also shown to reduce expression of adhesion molecules, to impair interactions between leukocytes and the endothelium and to reduce inflammatory-cell infiltration15,82–84,113. Moreover, S1P2 and S1P3 activation might favour cell survival by activating the pro-survival Akt (also known as protein kinase B) pathway14.
The S1P–S1P receptor regulatory axis has also emerged as a modulator of leukocyte infiltration. Neutrophils isolated from healthy humans express S1P1, S1P4 and S1P5 (REF. 55), whereas monocytes express S1P1, S1P2 and S1P3 (REF. 75). S1P modulates key cellular events involved in leukocyte migration, such as store-operated calcium entry76, cytoskeletal organization42,77 and chemokine receptor expression78. moreover, S1P receptor expression is modulated by receptor stimulation. For example, S1P3 expression increases in the neutrophils of patients suffering from pneumonia55 and S1P3 heterodimerizes with the interleukin-8 (IL-8) receptor IL-8RA (also known as CXCR1), although the functional corollary of this association remains to be determined. As tumour necrosis factor-α (TNFα) has been shown to induce SphK1 expression and S1P release from human monocytes79, and pharmacological inhibition of SphK1 impairs neutrophil migration in vitro by decreasing surface expression of the adhesion molecule CD11b80, S1P biosynthetic enzymes also seem to influence leukocyte migration. The exact mechanism of SphK1-mediated regulation of leukocyte migration remains to be determined, but seems to occur through autocrine S1P receptor activation. Paradoxically, the inflammatory response seems to be intact in SphK1-deficient mice81, which might result from adaptive genetic compensation. S1P1 expression was also increased in the kidneys of mice after ischaemia–reperfusion injury15. FTY720 diminished the extent of ischaemia-induced injury by downregulating neutrophil accumulation, which was reversed by the S1P1 antagonist VPC-44116 (REF. 15). This suggests that S1P1 plays a crucial part in the mediation of leukocyte infiltration to the injured site. Accordingly, SEW2871, a specific agonist of S1P1, decreased expression of intracellular adhesion molecule 1 (ICAM1), P selectin, vascular cell adhesion molecule 1 (VCAM1) and TNFα after renal ischaemia16. These effects might be due to the upstream downregulation of pro-inflammatory TNFα16 or due to S1P1 activation interfering with signalling cascades downstream of TNFα that would normally lead to upregulation of adhesion molecules82. moreover, S1P and SEW2871 impaired TNFα-induced monocyte– endothelial cell interactions, which was reversed by the inhibition of S1P1 and/or S1P3 with the dual antagonist VPC23019 (REF. 82). In a similar model system, S1P1 but not S1P3 modulation downregulated endothelial activation, as measured by monocyte adhesion and VCAM1 expression83. Aoki et al.84 showed that dual blockade of S1P1 and S1P3 reversed S1P-mediated inhibition of basal U937 monocyte adhesion to endothelial cells in vitro, not by causing VCAM1 down-modulation, but rather by affecting α5β1 and αvβ3 integrin relocalization. Surprisingly, in vitro stimulation of HUVECs with S1P induced IL-8 and monocyte chemoattractant protein 1 release in an IL-1-dependent manner85. Although the data described above are scarce and sometimes contradictory, they suggest that S1P receptors, especially S1P1, might constitute feasible targets for therapeutic chemical modulation by interfering at multiple steps. For example, S1P receptor activation could lead to alleviation of endothelial barrier injury by decreasing the release of pro-inflammatory mediators and reducing activation of the endothelium (FIG. 5).
S1P and the modulation of the BBB
The BBB is the most stringent endothelial interface of the human body, and its dysregulation is associated with a range of devastating diseases with no satisfactory therapies. For example, the aetiology of multiple sclerosis is still incompletely understood but is known to involve BBB endothelium activation and disruption, as well as accumulation of autoreactive lymphocytes and macrophages in the CNS86,87. So far, there is no direct evidence that S1P receptors can regulate the BBB. recently, FTY720 was shown to ameliorate clinical scores during the relapsing phase in the EAE rodent model of multiple sclerosis20,88, as well as in human clinical trials19. The effect of FTY720 is likely to result, at least in part, from protection of the BBB. Indeed, FTY720 was recently shown to reduce the expression of pro-inflammatory genes and metalloproteases, and to favour the expression of tissue inhibitors of metallo proteases19 in the brain of EAE rats. The design of the study did not allow any direct modulation of S1P receptors to be determined. However, other studies have shown that, although FTY720 was protective in an EAE model in rats, an analogue that cannot be phosphorylated did not confer any protection89, suggesting that phosphorylation of sphingosine analogues and subsequent activation of S1P receptors are required. moreover, FTY720 and FTY720-P levels are increased by more than tenfold in the CNS when compared with blood content90, and both species were present in blood at an approximate 1:2 FTY720 to FTY720-P ratio. S1P receptors are widely and differentially expressed on various cells of the CNS (reviewed in REF. 90), and were shown to contribute to astrocyte, oligodendrocyte and neuronal fate and functions (reviewed in REFS. 6, 86). S1P1 and S1P3 are expressed on BBB endothelial cells and astrocytes86,91 that work cooperatively to coordinate exchanges between the blood and brain. Nonetheless, little is known regarding the regulation of the BBB by S1P receptors during EAE or in other models of CNS inflammation. By contrast, modulation of S1P receptors is better characterized in the periphery and could explain, at least partially, the beneficial effect of FTY720 in EAE models.
Administration of S1P1 receptor agonists can disrupt the chemokine–chemokine receptor system92 and enhance inter-endothelial junctions in the lymphatic sinus of lymph nodes93, leading to an accumulation of lymphocytes on the abluminal side of the sinus-lining endothelial cells and ultimately to impaired egress of lymphocytes to the efferent lymphatic vessels, causing blood lymphopaenia9,39. FTY720-P was shown to prevent the development of EAE in naive mice adoptively transferred with congenic T cells autoreactive for myelin94 and to decrease activated lymphocyte accumulation in the CNS, which is likely to result from S1P1-dependent impairment of T-lymphocyte recirculation94,95 (FIG. 6).
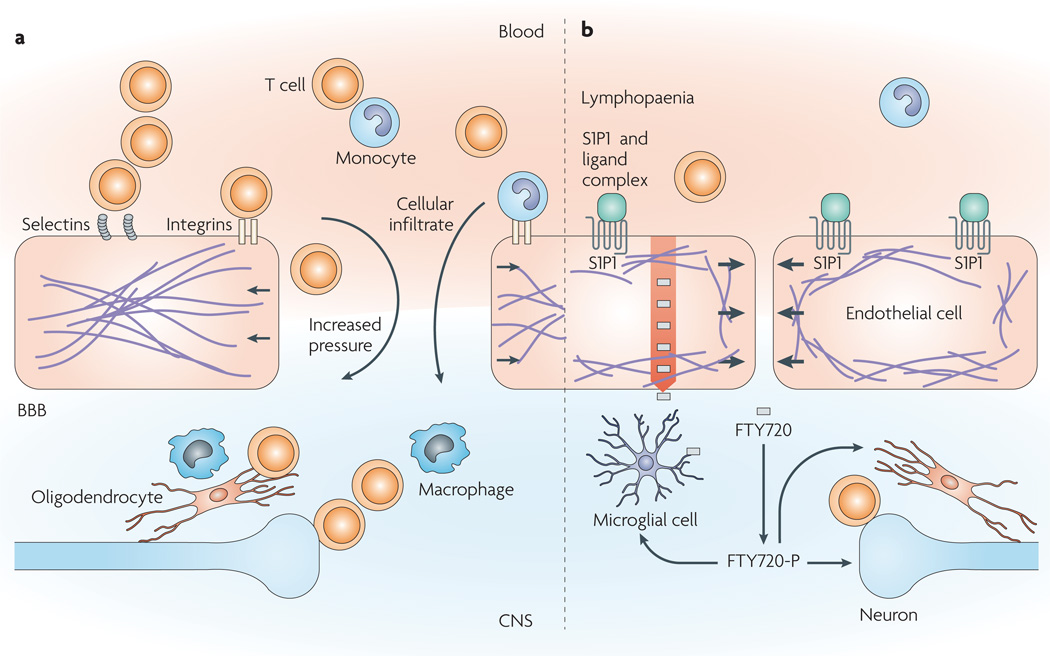
Figure 6. Putative strategies for modulating the immune response in the central nervous system (cNs).
a | The blood–brain barrier (BBB) is central to the pathogenesis of various CNS diseases, as it controls the passage of molecules and cells, and can be damaged in the course of disease. During autoimmune disease, autoreactive lymphocytes access the brain through the bloodstream. Like the endothelium of acutely injured tissues, the BBB expresses adhesion molecules and becomes disrupted, leading to accumulation of fluids, macrophages and autoreactive T cells in the CNS. b | The sphingosine-1-phosphate (S1P) analogue FTY720 (grey rectangles) and S1P receptor 1-specific agonists (shown in green) induce sequestration of lymphocytes in secondary lymphoid organs owing to lymphatic barrier tightening, which might decrease the capacity of these lymphocytes to reach the CNS9. In addition to reducing leukocyte infiltration, BBB enhancement with S1P1-specific agonists could lead to decreased fluid accumulation in the CNS, which is an aggravating factor in brain cancer and might interfere with drug delivery. Decreased accumulation of fluids and activated lymphocytes in the CNS could translate into alleviation of tissue damage. Blood-borne small, lipophilic molecules, such as FTY720, can accumulate in the CNS (indicated by a red arrow)90. FTY720 is then phosphorylated (FTY720-P) in the CNS, where it can act on widely distributed S1P receptors.
In addition to protecting the BBB, modulating cell fate in the CNS and influencing lymphocyte recirculation, some evidence suggests that modulating the S1P pathway might allow direct manipulation of the BBB, extending therapeutic possibilities to other non-inflammatory diseases, such as brain cancer. For example, it was recently suggested that the disease-associated increase in hydrostatic pressure in the CNS might reduce the efficacy of drug delivery strategies that involve BBB disruption96, which could explain the unsuccessful delivery of chemotherapeutic agents to the CNS in several such trials97,98. A combination of barrier-enhancing agents and small lipophilic molecules that readily cross the BBB or strategies involving active drug transport might constitute a better approach to deliver drugs to the CNS1,2,78. For example, FTY720 accumulates in the CNS, where it is phosphorylated and can act on multiple S1P receptors and cellular targets. results obtained using immortalized rat brain endothelial cells showed that S1P functioned through S1P1 and S1P3 to upregulate the expression of ABCB1, a glycoprotein that is highly expressed in brain capillaries and can mediate efflux of xenobiotics from endothelial cells, ultimately protecting the CNS91 (FIG. 6). Hypothetically, antagonism of S1P3 might favour not only an enhancement of the BBB, but also the accumulation of therapeutic compounds in the CNS. Clearly, sphingosine analogues and the S1P regulatory axis show therapeutic potential for diseases of the CNS, such as multiple sclerosis and even brain cancer. However, the regulation and regulatory activities of the S1P receptors in the specialized BBB endothelium remain poorly understood. moreover, adverse effects that are related to S1P receptor activation, such as bradycardia99, arrhythmia100 and dyspnoea, as well as non-mechanistic adverse effects, such as upper respiratory tract infections and liver enzyme elevations21, have been associated with FTY720. Refining our knowledge of the minimal signalling requirement for each pathological process modulated by FTY720 is the next step towards the generation of drugs with higher specificity and fewer side effects.
Outlook
The discovery of FTY720 has facilitated new insights into the immunoregulatory system, and FTY720 can be viewed as a prototypic compound for further drug development. FTY720 has already led to the identification of other broad S1P receptor modulators, such as KRP-203 and AAL-R, and to the generation of structurally related and unrelated chemical probes, including monoselective S1P1 agonists, such as SEW2871, CYM-5442 and AUY954, and antagonists, such as W146 (REFS. 40,101–103) (TABLE 1).
In the quest to develop monoselective receptor modulators, numerous compounds are discarded because of their non-selective activities. Based on the opposing effects of the different S1P receptors in endothelial barrier regulation, these compounds — especially antagonists of similar potencies for different S1P receptors — would in fact be important proof-of-principle tools to address the contribution of S1P receptor interactions during disease. Indeed, S1P is present at high nanomolar concentrations in the plasma, which makes it difficult to determine whether the effect on outcome measures obtained after inhibition of a single receptor isoform results from intrinsic receptor inhibition or from relative alteration of S1P signalling tone between this receptor isoform and its opposing homologues. Availability of an array of monoselective antagonists, or of single molecules with multiple antagonistic activities on different S1P receptor subsets, could therefore be key to the study of this tonic receptor balance in different organs during homeostasis and disease.
In addition to the opposing activities of the various S1P receptors, the diversity of the receptor partners also has to beconsidered to develop clinically useful drugs. G protein-coupled receptors act as large signalling units and trigger numerous distinct signalling cascades. An increasing body of evidence suggests that different synthetic ligands can differentially affect receptor activity, for example by influencing their pairing with other receptors101,104, and S1P receptors seem to form distinct ‘signalplexes’ that vary according to the biological system studied. Genetic models need to be used to identify the involvement of specific receptors during disease and to determine cell-specific receptor repertoires and interactions, as exemplified by the recent identification of S1P3 on dendritic cells as a major exacerbating factor for mortality during sepsis12. After the identification of a disease-specific cellular target, mouse models with functional tagged receptors knocked in would allow cell-based proteomic analysis of physically interacting receptor partners during experimental disease. A similar approach has successfully been used to identify the content of lipid rafts after cell stimulation with S1P45. Using these findings to construct fluorescence–bioluminescence resonance energy transfer experiments or reporter-based assays, for example, along with the availability of public high-throughput screening facilities, would generate proof-of-concept molecules for the dissection of disease-specific receptor interactions and functions. Therefore, the design of assays for high-throughput screening should not be only based on feasibility, but should also be dictated by disease-specific signalling pathways of a single receptor isoform.
So far, the combined use of nonspecific and specific chemical probes supports the potential of S1P receptors as therapeutic modulators of endothelial barriers. A deeper understanding of the specific activities of S1P receptors in a cell- and disease-specific manner could facilitate the design of therapeutics that target S1P signalling for a range of conditions. Our experience suggests that specific-tool generation in both the pharmaceutical industry and academia, in combination with genetic approaches, is leading to the identification and validation of new points of chemical intervention that can modulate these complex pathophysiological interactions and ultimately benefit human health.
Acknowledgements
Work in the laboratory of H.R. is supported by the National Institutes of Health (AI-055509, MH-074404 and AI-074564) and by Specific Funding Proposal-1599 from Kyorin Pharmaceutical Company D.M. is supported by the Fonds de la Recherche en Santé du Québec. We thank our colleagues for useful discussions.
Footnotes
Competing interests statement
The authors declare competing financial interests: see web version for details.
DATABASES
UniProtKB: http://www.uniprot.org
S1P1 | S1P2 | S1P3 | S1P4 | S1P5
FURTHER INFORMATION
Hugh Rosen’s homepage: http://www.scripps.edu/chemphys/rosen/
All LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.de Boer AG, Gaillard PJ. Blood-brain barrier dysfunction and recovery. J. Neural. Transm. 2006;113:455–462. doi: 10.1007/s00702-005-0375-4. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. Blood-brain barrier delivery. Drug Discov. Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D, Nico B, Crivellato E, Artico M. Development of the blood-brain barrier: a historical point of view. Anat. Rec. B New Anat. 2006;289:3–8. doi: 10.1002/ar.b.20087. [DOI] [PubMed] [Google Scholar]
- 4.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 2002;16:1596–1602. doi: 10.1038/sj.leu.2402611. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nature Rev. Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 9.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 10.McVerry BJ, et al. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 2004;170:987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 11.Peng X, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 12. Niessen F, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. This paper defined a novel mechanism for an S1P receptor on dendritic cells linking inflammation to coagulation during sepsis
- 13.Niessen F, et al. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009 Jan 13; doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Means CK, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 15.Awad AS, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am. J. Physiol. Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 16.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 17.Pan S, et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu H, et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111:222–229. doi: 10.1161/01.CIR.0000152101.41037.AB. [DOI] [PubMed] [Google Scholar]
- 19.Foster CA, et al. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2008 Jun 4; doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujino M, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J. Pharmacol. Exp. Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 21. Kappos L, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. A pivotal clinical trial that validated S1P receptors as targets for multiple sclerosis
- 22.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch. Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 23.Kihara A, Anada Y, Igarashi Y. Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J. Biol. Chem. 2006;281:4532–4539. doi: 10.1074/jbc.M510308200. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman K, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billich A, et al. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 27.Chun J, et al. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 28.Uhlenbrock K, Gassenhuber H, Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal. 2002;14:941–953. doi: 10.1016/s0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 29.Murata N, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 2000;352:809–815. [PMC free article] [PubMed] [Google Scholar]
- 30.Ito K, et al. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 31.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB. J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 32. Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. This study provided insights into the sources and regulation of S1P in plasma that are crucial for the maintenance of tonic receptor signalling
- 33.Lee MJ, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herr DR, et al. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J. Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kono M, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 38.Ishii I, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG-3. J. Biol. Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 39. Wei SH, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nature Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. The first direct two-photon visualization of an endothelial barrier that is regulated by the S1P1 receptor in living tissues
- 40. Sanna MG, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chem. Biol. 2006;2:434–441. doi: 10.1038/nchembio804. This paper provided formal biochemical and intravital two-photon imaging evidence for the S1P-S1P1 rheostat in the maintenance of endothelial integrity and tone, through the discovery and use of selective in vivo active antagonists of S1P1 to modulate receptor activation
- 41.Foss FW, Jr, et al. Synthesis and biological evaluation of γ-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg. Med. Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garcia JG, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 2001;108:689–701. doi: 10.1172/JCI12450. This was the seminal experimental and conceptual contribution to the field that first defined a role for S1P in barrier function
- 43.Schaphorst KL, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez T, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 45.Guo Y, et al. Quantitative proteomic analysis of human endothelial cell membrane rafts: evidence of MARCKS and MRP regulation in the sphingosine 1-phosphate-induced barrier enhancement. Mol. Cell Proteomics. 2007;6:689–696. doi: 10.1074/mcp.M600398-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dudek SM, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J. Biol. Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 47.Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J. Appl. Physiol. 2003;94:1193–1203. doi: 10.1152/japplphysiol.00690.2002. [DOI] [PubMed] [Google Scholar]
- 48.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell. Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez T, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 51.Singleton PA, et al. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am. J. Respir. Cell Mol. Biol. 2007;37:222–231. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 52.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J. Biol. Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 53.Van Brocklyn JR, Behbahani B, Lee NH. Homodimerization and heterodimerization of S1P/ EDG sphingosine-1-phosphate receptors. Biochim. Biophys. Acta. 2002;1582:89–93. doi: 10.1016/s1388-1981(02)00141-5. [DOI] [PubMed] [Google Scholar]
- 54.Zaslavsky A, et al. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim. Biophys. Acta. 2006;1761:1200–1212. doi: 10.1016/j.bbalip.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Rahaman M, Costello RW, Belmonte KE, Gendy SS, Walsh MT. Neutrophil sphingosine 1-phosphate and lysophosphatidic acid receptors in pneumonia. Am. J. Respir. Cell Mol. Biol. 2006;34:233–241. doi: 10.1165/rcmb.2005-0126OC. [DOI] [PubMed] [Google Scholar]
- 56.Skoura A, et al. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang AH, Ishii I, Chun J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta. 2002;1582:197–203. doi: 10.1016/s1388-1981(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 58.Okazaki M, et al. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am. J. Transplant. 2007;7:751–758. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 59.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell. Mol. Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ware LB, Matthay MA. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 61.Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 62.Vincent JL, et al. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit. Care Med. 2003;31:834–840. doi: 10.1097/01.CCM.0000051515.56179.E1. [DOI] [PubMed] [Google Scholar]
- 63.Liu KD, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am. J. Respir. Crit. Care Med. 2008;178:618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dudek SM, et al. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell. Signal. 2007;19:1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am. J. Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 66.Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1191–L1198. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- 67.Peiris M. Pathogenesis of avian flu H5N1 and SARS. Novartis Found. Symp. 2006;279:56–60. discussion 60-65, 216-219. [PubMed] [Google Scholar]
- 68.Beaty SR, Rose CE, Jr, Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 69.Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J. Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 70.Pober JS, Min W. Endothelial cell dysfunction, injury and death. Handb. Exp. Pharmacol. 2006;176:135–156. doi: 10.1007/3-540-36028-x_5. [DOI] [PubMed] [Google Scholar]
- 71.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch. Pharmacol. 2007;376:1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 72.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 74.Scalia R. Statins and the response to myocardial injury. Am. J. Cardiovasc. Drugs. 2005;5:163–170. doi: 10.2165/00129784-200505030-00003. [DOI] [PubMed] [Google Scholar]
- 75.Martino A, et al. Sphingosine 1-phosphate interferes on the differentiation of human monocytes into competent dendritic cells. Scand. J. Immunol. 2007;65:84–91. doi: 10.1111/j.1365-3083.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 76.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J. Biol. Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panetti TS, Nowlen J, Mosher DF. Sphingosine-1-phosphate and lysophosphatidic acid stimulate endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 2000;20:1013–1019. doi: 10.1161/01.atv.20.4.1013. [DOI] [PubMed] [Google Scholar]
- 78.Lan YY, et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am. J. Transplant. 2005;5:2649–2659. doi: 10.1111/j.1600-6143.2005.01085.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFα in primary human monocytes. J. Cell. Physiol. 2006;208:109–115. doi: 10.1002/jcp.20646. [DOI] [PubMed] [Google Scholar]
- 80.Lee C, et al. Attenuation of shock-induced acute lung injury by sphingosine kinase inhibition. J. Trauma. 2004;57:955–960. doi: 10.1097/01.ta.0000149495.44582.76. [DOI] [PubMed] [Google Scholar]
- 81.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 82.Bolick DT, et al. Sphingosine-1-phosphate prevents tumor necrosis factor-α-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- 83.Whetzel AM, et al. Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ. Res. 2006;99:731–739. doi: 10.1161/01.RES.0000244088.33375.52. [DOI] [PubMed] [Google Scholar]
- 84.Aoki S, et al. The suppressive effect of sphingosine 1-phosphate on monocyte-endothelium adhesion may be mediated by the rearrangement of the endothelial integrins α5β1 and αvβ3. J. Thromb. Haemost. 2007;5:1292–1301. doi: 10.1111/j.1538-7836.2007.02559.x. [DOI] [PubMed] [Google Scholar]
- 85.Lin CI, Chen CN, Chen JH, Lee H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J. Cell. Biochem. 2006;99:1216–1232. doi: 10.1002/jcb.20963. [DOI] [PubMed] [Google Scholar]
- 86.Dev KK, et al. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol. Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N. Engl. J. Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 88.Rausch M, et al. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: clinical implications for ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. J. Magn. Reson. Imaging. 2004;20:16–24. doi: 10.1002/jmri.20057. [DOI] [PubMed] [Google Scholar]
- 89.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 90. Foster CA, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J. Pharmacol. Exp. Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. This paper described mechanisms for S1P receptor modulation of BBB function, neuronal function and lymphocytes in models of EAE
- 91.Pilorget A, et al. Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J. Neurochem. 2007;100:1203–1210. doi: 10.1111/j.1471-4159.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- 92.Yopp AC, et al. Sphingosine 1-phosphate receptors regulate chemokine-driven transendothelial migration of lymph node but not splenic T cells. J. Immunol. 2005;175:2913–2924. doi: 10.4049/jimmunol.175.5.2913. [DOI] [PubMed] [Google Scholar]
- 93.Singer I, et al. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J. Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- 94.Webb M, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J. Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 95.Kataoka H, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental auto immune encephalomyelitis by inhibition of T cell infiltration. Cell. Mol. Immunol. 2005;2:439–448. [PubMed] [Google Scholar]
- 96.Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain Res. Rev. 2001;38:140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 97.Prados MD, et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro.Oncol. 2003;5:96–103. doi: 10.1093/neuonc/5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Warren K, et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children’s Oncology Group. Cancer Chemother. Pharmacol. 2006;58:343–347. doi: 10.1007/s00280-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 99.Sanna MG, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 100.Tsukada YT, Sanna MG, Rosen H, Gottlieb RA. S1P1-selective agonist SEW2871 exacerbates reperfusion arrhythmias. J. Cardiovasc. Pharmacol. 2007;50:660–669. doi: 10.1097/FJC.0b013e318157a5fe. [DOI] [PubMed] [Google Scholar]
- 101.Gonzalez-Cabrera PJ, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like head-group interactions. Mol. Pharmacol. 2008 Aug 15; doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marsolais D, et al. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol. Pharmacol. 2008;74:896–903. doi: 10.1124/mol.108.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jo E, et al. S1P1-selective in vivo -active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem. Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 104.Sensken SC, et al. Selective activation of G alpha i mediated signalling of S1P3 by FTY720-phosphate. Cell. Signal. 2008;20:1125–1133. doi: 10.1016/j.cellsig.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 105.Mechtcheriakova D, et al. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 2007;581:3063–3068. doi: 10.1016/j.febslet.2007.05.069. [DOI] [PubMed] [Google Scholar]
- 106.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 107.Mitra P, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl Acad. Sci. USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawahara A, et al. The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science. 2008;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 109.Le Stunff H, Peterson C, Liu H, Milstien S, Spiegel S. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta. 2002;1582:8–17. doi: 10.1016/s1388-1981(02)00132-4. [DOI] [PubMed] [Google Scholar]
- 110.Levkau B. Sphingosine-1-phosphate in the regulation of vascular tone: a finely tuned integration system of S1P sources, receptors, and vascular responsiveness. Circ. Res. 2008;103:231–233. doi: 10.1161/CIRCRESAHA.108.181610. [DOI] [PubMed] [Google Scholar]
- 111.Roviezzo F, et al. Sphingosine-1-phosphate/ sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am. J. Respir. Cell. Mol. Biol. 2007;36:757–762. doi: 10.1165/rcmb.2006-0383OC. [DOI] [PubMed] [Google Scholar]
- 112.Theilmeier G, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 113.Tani M, et al. Sphingosine 1-phosphate (S1P) inhibits monocyte-endothelial cell interaction by regulating of RhoA activity. FEBS Lett. 2007;581:4621–4626. doi: 10.1016/j.febslet.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 114.Song J, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J. Pharmacol. Exp. Ther. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 115.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 116.Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem. Biophys. Res. Commun. 2002;299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]