Abstract
An immunocytochemical comparison of vGluT1 and vGluT3 in the cochlear nucleus (CN) of deafened versus normal hearing rats showed the first example of vGluT3 immunostaining in the dorsal and ventral CN and revealed temporal and spatial changes in vGluT1 localization in the CN after cochlear injury. In normal hearing rats vGluT1 immunostaining was restricted to terminals on CN neurons while vGluT3 immunolabeled the somata of the neurons. This changed in the VCN three days following deafness, where vGluT1 immunostaining was no longer seen in large auditory nerve terminals but was instead found in somata of VCN neurons. In the DCN, while vGluT1 labeling of terminals decreased, there was no labeling of neuronal somata. Therefore, loss of peripheral excitatory input results in co-localization of vGluT1 and vGluT3 in VCN neuronal somata. Postsynaptic glutamatergic neurons can use retrograde signaling to control their presynaptic inputs and these results suggest vGluTs could play a role in regulating retrograde signaling in the CN under different conditions of excitatory input. Changes in vGluT gene expression in CN neurons were found three weeks following deafness using qRT-PCR with significant increases in vGluT1 gene expression in both ventral and dorsal CN while vGluT3 gene expression decreased in VCN but increased in DCN.
Keywords: cochlear nucleus, vGluT, calcium binding protein, real time PCR, cochlea, plasticity
Introduction
Maintaining the balance between excitation and inhibition is a key factor in the function of the central nervous system. Until the discovery of the vesicular glutamate transporters, detection and study of glutamatergic neurons was complex since glutamate is essential for protein synthesis in all cells and metabolic glutamate was not clearly separated from glutamate slated for synaptic release. The three different isoforms of vesicular glutamate transporters, encoded by three separate genes, transport glutamate into vesicles targeted for regulated release (Takamori et al., 2000; Fremeau et al., 2001; Takamori et al., 2001; Fremeau et al., 2002). The presence of any of the vGluTs indicates that a given neuron has glutamatergic properties (Takamori et al., 2000; Herzog et al., 2001; Takamori et al., 2001) and localization of vGluTs usually denotes sites of glutamate release (Takamori et al., 2000; Fremeau et al., 2001; Takamori et al., 2001; Fremeau et al., 2002). Also, increased levels of vGluTs results in more glutamate loaded into vesicles and ultimately more glutamate release (Wilson et al., 2005). Vesicular glutamate transporters 1 (vGluT1) and -2 (vGluT2) are primarily localized to axon terminals with a non-overlapping distribution in glutamatergic neurons. For example, in the cochlear nucleus (CN), vGluT1 and vGluT2 have been previously reported to be co-localized only sparsely. The vGluT1 axonal terminals appear to originate from somata within auditory pathways while vGluT2 terminals arise from somata in non-auditory regions (Zeng et al., 2009). Loss of glutamatergic axonal terminals containing vGluT1 mediated vesicle loading results in cross modal plasticity in which there is a compensatory increase in vGluT2 axon terminals usually associated with extra-auditory projections (Zeng et al., 2009).
In addition to being localized to axon terminals, the vGluT3 isoform has also been identified in cell bodies, principally in neurons that have been associated with other classical neurotransmitters such as GABA, serotonin, and dopamine (Fremeau et al., 2002; Gras et al., 2002; Schafer et al., 2002). Knockout of vGluT1 or vGluT2 results in animals with no major motor or sensory deficits at birth, but by three weeks animals showed increasing neurological pathologies including increased startle responses (Fremeau et al., 2004). However, vGluT3 knockout mice exhibit profound deafness, attributed to the inability of inner hair cells to load glutamate into vesicles (Seal et al., 2008). Recently, progressive nonsyndromic deafness, DFNA25, has been attributed to a mutation in the gene encoding for vGluT3 in humans (Ruel et al., 2008).
Although roles for vGluT1 and -2 in auditory brainstem plasticity have been suggested (Zhou et al., 2007; Altschuler et al., 2008; Zeng et al., 2009), vGluT3 function in this pathway has not been established. In addition to being profoundly deaf, mice with no vGluT3 expression exhibit decreased cochlear nucleus volumes and hyperexcitable neurons (Seal et al., 2008), similar to what is observed in the auditory pathways following hearing loss (Niparko and Finger, 1997; Kaltenbach and Afman, 2000; Kaltenbach et al., 2000; Mossop et al., 2000; Salvi et al., 2000; Brozoski et al., 2007; Bauer et al., 2008). Excitatory neurons often participate in retrograde signaling. Retrograde neurotransmission is a mechanism utilized throughout the central nervous system by which presynaptic neurotransmitter release is modulated (Zilberter et al., 1999; Pittman et al., 2000; Zilberter, 2000; Levenes et al., 2001; Yung et al., 2001). Specifically, postsynaptic glutamatergic neurons are known to provide feedback to presynaptic inputs via retrograde signaling (Llano et al., 1991; Pitler and Alger, 1992). Vesicular glutamate transporters may play a role in mediating this signaling mechanism as was suggested for the neocortex (Harkany et al., 2003; Harkany et al., 2004).
In the current study we demonstrate localization of vGluT1 in CN terminals and vGluT3 in CN somata under normal conditions. In addition we show deafness related co-localization of vGluT1 and -3 in CN somata and use calcium binding proteins to identify neuronal cell types that produce vGluT1. Finally, increased gene expression for vGluT1 following deafness supports our finding of increased vGluT1 immunloabeling in the ventral CN after hearing loss.
Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Wayne State University and conform to NIH guidelines. Adult, male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study.
Auditory Brainstem responses (ABRs)
Frequency specific hearing thresholds of ABRs were recorded at 4, 12, and 20 kHz using a Beyer sound source, Pyramid PA 600× stereo power amplifier hardware and the Data AcQusition and Real-Time Analysis (Daqarta 4.0) software package (Interstellar Research, Ann Arbor, MI). Briefly, rats were anesthetized with xylazine (8mg/kg) and ketamine (75 mg/kg), placed in a sound proof booth (Hamilton-Kinder, Poway, CA), and a transducer that delivered a tone at varying sound pressure levels (SPLs) was positioned directly into the ear canal. The reference needle electrode was inserted below the test ear, the ground electrode placed below the contralateral ear, and the active electrode was inserted at vertex (top of the head). Initially, the sound stimuli were presented at 80 dB SPL then, in hearing group, were decreased in 10 or 15 dB steps until near threshold, then 5 dB steps were used. In the deafened group, stimuli were elevated up to 100 dB. For each SPL, up to 1024 responses were averaged. The ABR hearing threshold was determined as the lowest level where the response could be detected at least twice (using ABR waveforms P1-P3).
Hearing was assessed by ABR measures in all animals at the beginning of the experiment and only those with hearing in the normal range (∼30 dB baseline ABR threshold at all three frequencies) were included in the study. A second ABR measure of hearing was done in the deafened group, just prior to perfusion or prior to dissection of tissue for PCR analyses. At least a 65 dB shift from the normal control baseline across the frequencies was necessary for inclusion of animals in the study.
Surgical procedures
The experimental group of rats was bilaterally deafened by ablation of cochlear hair cells. Each rat was anesthetized intramuscularly (i.m.) with a mixture of xylazine (8mg/kg) and ketamine (75mg/kg). Local injections of 1% lidocaine-HCl solution were made at the site of each surgical incision. Surgical procedures were performed under aseptic conditions. The skin incision was made through the post auricular region of the lateral neck. The bulla was opened without resection of any major muscles and mechanical ablation of cochlear hair cells was achieved using a dental pick, eye spud and a dissection microscope, then the bulla was sealed with dental cement (Durelon; 3M ESPE AG Dental Products, Seefeld, Germany) and the skin incision was closed with sutures. In all experiments, the ablation was performed bilaterally. After surgery, animals were injected (s.c.) with warm sterile 0.9% sodium chloride.
The extent of cochlear destruction was assessed by examining histological sections of the inner ear in ablated animals. In brief, after whole body perfusion and removal of brain, the temporal bones were removed and a local perfusion (from the base to the apex of cochlea) with 4% paraformaldehyde was performed. Following fixation the cochleae were decalcified in 0.12 M EDTA in PB pH 7.4 for 3 days at 4° C, cryoprotected in 30% sucrose in PB overnight, then embedded in M-1 Embedding Matrix (Anatomical Pathology, Pittsburgh, PA), and sectioned parallel to the modiolus (10-15 μm) using a cryostat. Sections were collected on Histo-Bond slides (Fisher Scientific, Pittsburgh, PA), stained with Toluidine Blue, dehydrated through graded alcohols, and mounted with Permount. Examination of cochleae with particular emphasis on the organ of Corti was performed under Leica DM 5000 light microscope (see Results).
Fixation and tissue preparation for immunocytochemistry
Following the ABR measures, rats were divided into five experimental groups: age matched normal hearing (n=12), 3 day deaf (n=4), 3 week deaf (n=3), 2 month deaf (n=3) and 1 year deaf (n=2) groups. Control and deafened rats were injected intra peritoneally (i.p.) with an overdose (0.22ml/kg) of Fatal-Plus (Vortech Pharmaceutical, Dearborn, MI) and perfused transcardially using the Perfusion Two automated perfusion system (myNeuroLab, St. Louis, MO). The perfusion consisted of a vascular rinse (9.25% sucrose in 0.1 M phosphate buffer pH 6.8) followed by fixation with 4.0% freshly depolymerized paraformaldehyde in phosphate buffer (PB). After perfusion brains were dissected and postfixed in the same fixative for one hour at room temperature and then cryoprotected in 30% sucrose in PB for 2 days at 4°C. Each brain, except the 3 day deafened group, was sectioned coronally at 40 μm using a freezing microtome. Sections were collected serially into 8 vials containing PB; pH 7.4, washed several times and processed for free floating fluorescence microscopy. In the case of 3 day deaf animals, in addition to the coronal sections, serial sagittal (n=1) 25 μm sections were also collected on Histo-Bond slides (Fisher Scientific).
Antibodies
All of the primary antibodies used in this study have been characterized by the manufacturers and in previous reports (Table 1). Details regarding sources of the antisera, the immunogens employed, and the dilutions used in the present series of experiments are provided in Table 1. The monoclonal antibody SV2 developed by Kathleen Buckley, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Dept. of Biological Sciences (Iowa City, IA 52242). The monoclonal antibody vGluT3 was obtained from the UC Davis/NIH NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California, Davis, CA 95616. We performed several controls for vGluT immunocytochemistry. 1. Each vGluT antibody was preadsorbed with a ten-fold excess (by weight) of the corresponding antigenic peptide and then applied into tissue. Specific immunoreactivity was abolished or greatly reduced. 2. Tests using two vGluT3 antibodies that were raised against different regions (Table 1) of rat vGluT3 (mouse anti-vGluT3: amino acids 546-588 of rat vGluT3 and guinea pig anti-VGluT3: amino acids 522-588 of rat vGluT3) produced identical labeling patterns. 3. None of the secondary antisera used alone produced labeling. 4. Western blot analyses were carried out to confirm vGluT antisera specificity in the rat cochlear nucleus (see Results).
Table 1. Antibodies.
| Antiserum/Immunogen | Species | Source/Cat. No. | Dilution | References |
|---|---|---|---|---|
| vGluT1 Amino acids 541-560 of rat vGluT1 | Guinea pig | Chemicon, Temecula, CA AB5905 | 1:1000 | (D'Sa et al., 2007; Brooke et al., 2010) |
| vGluT2 Synthetic peptide from rat vGluT2 | Guinea pig | Chemicon, Temecula, CA AB5907 | 1:1000 | (Altschuler et al., 2008; Billups et al., 2005; Brooke et al., 2010) |
| vGluT2 Recombinant protein from rat vGluT2 | Mouse | Chemicon, Temecula, CA MAB5504 | 1:500 | (Gomez-Nieto and Rubio, 2009) |
| vGluT3 Synthetic peptide from rat vGluT3 | Guinea pig | Chemicon, Temecula, CA AB55421 | 1:1000 | (Seal et al., 2008; Henny et al., 2010) |
| vGluT3 Amino acids 546-588 of rat vGluT3 | Mouse/Clone N34/34 | NeuroMab, Davis, CA 75-073 | 1:100 | Characterized by the manufacturer and present studies (see Methods and Results). |
| Glutamic acid decarboxylase (GAD65) Rat brain GAD65 | Mouse | Chemicon, Temecula, CA MAB351 | 1:1000 | (Caminos et al., 2007) |
| Glutamic acid decarboxylase (GAD67) Recombinant GAD67 protein | Mouse | Chemicon, Temecula, CA MAB5406 | 1:1000 | (Burianova et al., 2009; Brooke et al., 2010) |
| Glycine transporter 2 Synthetic peptide from rat C-terminus GlyT2 | Guinea pig | Chemicon, Temecula, CA AB1773 | 1:1000 | (Caminos et al., 2007; Brooke et al., 2010) |
| MAP2 Bovine brain microtubule protein | Mouse | Chemicon, Temacula, CA MAB3418 | 1:200 | (Altschuler et al., 2008) |
| SV2 Synaptic vesicles from Ommata electric organ | Mouse | Hybridoma Bank, Iowa City, IA | 1:100 | (D'Sa et al., 2007; Brooke et al., 2010) |
| Calretinin Recombinant rat calretinin | Rabbit | Chemicon, Temecula, CA AB149 (replaced by AB5054) | 1:2000 | (Bazwinsky et al., 2008) |
| Parvalbumin Carp muscle parvalbumin | Mouse/Clone PA-235 | Sigma, Saint Louis, MO P3171 | 1:500 | (Fredrich et al., 2009; Por et al., 2005) |
| Calbindin D-28K Chicken gut calbindin | Mouse/Clone CL-300 | Sigma, Saint Louis, MO C8666 | 1:1000 | (Fredrich et al., 2009) |
Fluorescence microscopy
Sections were washed several times in 0.1 M phosphate-buffered saline (PBS) with 0.3% Triton X-100 (PBST) and preincubated in blocking solution consisting of 3% normal serum from the species corresponding with the secondary antibodies in PBST for 3 hours at room temperature. Primary antibody or, in the double-labeling experiments, a mixture of two antibodies generated in different host species was diluted in PBST with 1% normal serum and applied for 3 days at 4°C. After several rinses, tissue was incubated in a species-specific secondary antibody or in a mixture of secondary antibodies (double-labeling experiments) conjugated to Alexa 488 or fluorescein isothiocyanate (FITC; green fluorescence) or Cy3 (red fluorescence) for 2 hours at room temperature. Alexa 488-(Molecular Probes, Eugene, OR) and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were both used at a 1:500 dilution but FITC-conjugated antibody (Jackson ImmunoResearch Laboratories) was diluted 1:200. After intensive washing, the sections were coverslipped using Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA). In some experiments, DAPI (4,6-diamino-2-phylindole) was added to counterstain nuclei. Fluorescent specimens were viewed on a Leica DM5000 microscope equipped with a fluorescence filter set. The brightness and contrast of the final images were adjusted in Adobe Photoshop.
Electron Microscopy
Animals were perfused transcardially (n = 2) first with a Ca2+ free Ringer's solution variant followed by a fixative comprising 1.25% glutaraldehyde and 2.0% freshly depolymerized paraformaldehyde. After perfusion, brains were postfixed in the same glutaraldehyde/paraformaldehyde solution for 90 minutes, and then tissue blocks containing the cochlear nucleus were then cut into 150 μm sections using a vibratome (Vibratome 1000, Ted Pella, Inc.) and placed serially into four vials containing 0.12M PB buffer.
Vibratome sections were incubated for one hour in 1.0% osmium tetroxide followed by 2.0% uranyl acetate. Tissues were then dehydrated in graded alcohols and propylene oxide and subsequently embedded in Epon812.
Ultrathin sections (70 – 80 nm) were cut on a Reichert ultramicrotome and collected on Formvar coated copper grids, contrasted with uranyl acetate and lead citrate then photographed on a JOEL 1010 TEM.
Protein isolation and Western blot
Freshly dissected tissue from cochlear nucleus subdivisions: ventral (VCN) and dorsal (DCN) cochlear nuclei were homogenized in ice-cold PBS buffer containing 0.1% Tween 20, 0.3% CHAPS and protease inhibitor cocktail (Sigma, St. Louis, MO). Protein concentrations were determined using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA) then samples were diluted 3:1 in 4× sample buffer. Proteins were run on a 10% acrylamide gel (12.5 μg protein/lane for vGluT1 as well as vGluT2 and 75 μg protein/lane for vGluT3). After electrophoretic separation, proteins were transferred onto Immobilon membrane (Bio-Rad Laboratories, Hercules, CA) for 90 minutes at a constant voltage of 150 V.
Membranes were blocked in 0.1 M Tris, pH 7.6, with 0.15 M NaCl and 0.05% Tween 20 (TBST) with 5% dry milk and 3 % normal donkey serum (NDS) for 1 hour at room temperature, then incubated in primary antiserum overnight at 4°C. The antisera were diluted (guinea pig vGluT1, 1:10,000; mouse vGluT2, 1:1000; guinea pig vGluT3, 1:500; mouse vGluT3, 1:100) in TBST containing 5% dry milk and 1% NDS (TBST/NDS). After several washes, membranes were incubated in a species-specific secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories; diluted 1:10,000 in TBST/NDS) for 2 hours at room temperature. Protein bands were visualized with Western Lightning Enhanced Chemiluminescence (ECL) detection reagents (Perkin Elmer, Boston, MA) and film autoradiography.
PCR for vGluT3
Using primers created against the coding region of rat vesicular glutamate transporter 3 (Forward 5′-GGCTGGAGGTTCGAGAGGATGAC-3′ Reverse 5′ GGGAAAAGCAATGGGTGTGGAGA-3′), PCR was performed on samples from DCN, VCN and auditory cortex (AC). For PCR the Advantage 2 Polymerase Mix (Clontech Mountainview, CA) was used with standard thermocycling conditions (95°C for 1 minute followed by 40 cycles of amplification at 95°C for 15 seconds, 65°C for 30 seconds and 72°C for 3 minutes). The PCR fragments were cloned and sequence verified.
Quantitative Real Time PCR (qRT-PCR)
The qRT-PCR methods have been described in detail previously (Holt et al., 2005; Holt et al., 2006; Cui et al., 2007). Following ABR recordings, rats were divided into a control group: age matched normal hearing (n=9), and two experimental groups: 3 day-deaf (n=3) and 3 week-deaf (n=3). The experimental groups were deafened by cochlear ablation, and prior to tissue dissection ABR measures were carried out to confirm deafness.
RNA extraction and assessment of quality
Rats were anesthetized with a lethal dose (0.22 ml/kg) of Fatal-Plus (Vortech Pharmaceuticals) and decapitated. Brains were immediately removed and placed in cold PBS. The auditory regions of interest: the dorsal (DCN) and ventral (VCN) cochlear nucleus were dissected and placed in RNAlater (Applied Biosystems, Foster City, CA). A pair of DCNs or VCNs from an individual animal was considered one pool and for each condition we collected three pools. Total RNA was isolated from tissues that were homogenized in TRI Reagent Solution (Applied Biosystems) using a modification of the standard TRIZOL protocol. In brief, chloroform was added to the homogenate and following centrifugation, the colorless aqueous phase was transferred to a Phase Lock Gel-Heavy tube (Eppendorf, Westbury, NY). RNA was extracted again with acid phenol and then precipitated with isopropanol. The RNA pellet was dissolved in DEPC water and stored at -80°C. RNA concentration was determined by UV spectrophotometry (A260) using a BioPhotometer (Eppendorf). The A260/A280 ratio was used to estimate the purity of RNA samples and for qRT-PCR only the samples with ratios of 1.8-2.0 were selected.
RNA quality was performed on an RNA 6000 Nano LabChip (Agilent Technologies, Palo Alto, CA) using an Agilent 2100 Bioanalyzer. The 28S:18S ribosomal RNA (rRNA) ratio of 1.4 or greater, as well as an RNA Integrity Number (RIN) of 8.4 or higher were both factors for sample selection (see Results).
qRT-PCR reverse transcription
In brief, total RNA (2 μg) extracted from normal and deafened animals was used to generate single-stranded cDNA using the high-Capacity cDNA Archive Kit (Applied Biosystems) that utilizes 250 units of MultiScribe reverse transcriptase and random primers in the presence of RNase inhibitor (20 units; Promega, Madison, WI) in a total reaction volume of 100 μl. The reactions were performed at 25°C for 10 minutes followed by 37°C for 2h using a thermal cycler (Eppendorf).
qRT-PCR denaturation and amplification
Briefly, for each gene of interest qRT-PCR was performed on three cDNA samples (in triplicate for each sample) using the Mastercycler ep realplex machine (Eppendorf), which allows the PCR product in each cycle to be measured in a quantifiable fashion. The sequence specific primers for individual vGluT genes (vGluT1, vGluT2, vGluT3), as well as the HPRT (hypoxanthine guanine phosphoribosyl transferase) were acquired through Assays-on-Demand (TaqMan Gene Expression Assays, Applied Biosystems). TaqMan gene expression assays employ 5′ nuclease chemistry with forward and reverse unlabeled PCR primers (900 μM each) and the TaqMan Minor Groove Binder (MGB) probe (6-FAM dye-labeled) (250 μM). Each PCR reaction mix contained 1 μl of cDNA sample (20 ng of RNA converted to cDNA) in TaqMan Universal PCR Master Mix (Applied Biosystems) with specific TaqMan gene expression assay in total reaction volume of 25 μl. 96-well PCR plates were read on a PCR machine using the following program: 50°C for 2min, 95°C for 10min (1 cycle) followed by 92°C for 15s, 60°C for 1min (40 cycles), and the data were analyzed using Realplex software (Eppendorf).
Analysis of qRT-PCR data. To analyze the relative changes in gene expression from qRT-PCR experiments, we used the 2-(Δ1Δ2Ct) method (Livak and Schmittgen, 2001). First, for each reaction we determined the cycle threshold (Ct) that indicates the cycle number at which the PCR product reaches a fixed threshold. Then to normalize the data, the average Ct value for the housekeeping gene (HPRT) was subtracted from the average Ct values for each gene of interest (Δ1Ct) in both control and experimental groups. Then the Δ1Ct value for each gene of interest in control group was subtracted from the corresponding Δ1Ct value in experimental group to derive Δ2Ct. These calculations were completed for each time point (3 day- and 3 week-deaf group). The final value was then expressed as logarithm to base 2. Using this formula (2-(Δ1Δ2Ct)), we calculated relative changes in gene expression between control and experimental groups. The significance of changes was evaluated by Student's T-test with a p value ≤ 0.05 considered statistically significant.
Cell and Terminal Analysis
To determine the number and range of sizes for vGluT1 and -2 labeled terminals in the VCN, each labeled terminal in a 160,000 μm2 area was counted (NIS Elements software, Nikon Instruments Inc., Melville, NY) from the right and left side of 2 – 3 sections from normal hearing and deaf animals. To determine whether the number of vGluT3 labeled neurons in the DCN were different in normal hearing and deafened animals, the number of labeled neurons in were counted in the DCN of from normal hearing and deafened animals. All of the neurons with visible nuclei in regions from the mid-rostrocaudal DCN were counted. The DCN was measured and the number of neurons was normalized based upon area. A Student's T-test was performed to determine differences, with a p value < 0.05 significant.
Results: Vesicular Glutamate Transporter Expression and Production in the Cochlear Nucleus
Cochlear ablation results in profound hearing loss
To determine the hearing status of each of the animals, auditory brainstem responses were analyzed prior to and after cochlear hair cell ablation. Normal hearing animals showed average thresholds of 30 dB at 4 kHz, 28 dB at 12 kHz and 30 dB at 20 kHz. In no case did any animal have detectable thresholds (up to 100 dB) at 3 days, 3 weeks, or 2 months following cochlear hair cell ablation (Figure 1A) at any frequency tested. The absence of ABR thresholds following cochlear hair cell ablation suggests that the surgery to mechanically destroy hair cells was successful. We also prepared mid-modiolar sections through the cochlea of normal hearing and profoundly deaf animals to histologically examine turns of the cochlea from apex to base. In deaf animals we observed the presence of spiral ganglion cells as well as the absence of more than 95% of hair cells with only 1- 2% of hair cells missing in normal hearing animals (data not shown).
Figure 1.
Confirmation of hearing status, the presence of vGluT1-3 in the CN and differential gene expression in DCN and VCN following deafeness. Auditory brainstem responses were recorded from normal hearing and deafened animals across three different frequencies, 4, 12, and 20 kHz (A). Analysis of RNA quality Agilent bioanalyzer scans of the total RNA from the rat DCN and VCN (B). Ratios of 28S/18S ribosomal RNA are calculated from the area under each curve. Virtual gels to the right of each sample show bands representing 18S and 28S ribosomal RNAs. The DCN-hearing group sample has 28S/18S ribosomal RNA (rRNA) ratio of 1.6 and RNA Integrity Number (RIN) 8.9 and the ratio of the DCN-bilaterally deafened sample is 1.4 with a RIN of 9.2. The VCN normal and deafened samples have 28S/18S ratio of 1.4 and RINs of 8.4 and 8.8 respectively. All profiles have small spikes (arrows) in fluorescence between 23 and 28 seconds, corresponding to the 5S rRNA and other small RNAs. The fact that small RNAs are not buried by breakdown products, suggests that the RNAs are largely intact. Following deafness hearing thresholds were more than 100 decibels (arrows). Deafness-related changes in vGluT1-3 gene expression within the rat VCN (C) and DCN (D), 3 days and 3 weeks after bilateral cochlear hair cell ablation. Asterisks indicate significant changes (p ≤ 0.05). Error bars indicate standard deviation. Western immunoblots of rat ventral (VCN) and dorsal (DCN) cochlear nucleus were processed for vGluT1, vGluT2, and vGluT3 isoforms (E). Robust levels of the vGluT1 and -2 proteins are present in both VCN and DCN, with vGluT3 detected at a lower level, particularly in the DCN samples. Each antibody recognizes only one band: gp vGluT1 ∼60 kDa, m vGluT2 ∼62 kDa, and both m vGluT3 and gp vGluT3 ∼60 kDa (arrowheads). This labeling is consistent with reported molecular weights for each vGluT isoform. PCR showing vGluT3 gene expression in AC, DCN and VCN samples. Arrowhead indicates a single band of approximately 1.9 kb seen in all three auditory regions (F). AC - auditory cortex, gp vGluT1 - guinea pig anti-vGluT1, m vGluT2 - mouse anti-vGluT2, m vGluT3 - mouse anti-vGluT3 and gp vGluT3 - guinea pig anti-vGluT3
Hearing loss results in differential vGluT expression both spatially and temporally
Controls
We used Agilent analysis (Agilent Bioanalyzer, Applied Biosystems) to determine the integrity of our RNA. Agilent analysis, based upon electrophoretic RNA separation, allows for determination of RNA concentration, ribosomal RNA (rRNA) ratios, and RNA integrity number (RIN) calculation. Both rRNA ratios and RIN were used in our assessment. The 28S:18S ratio is one widely accepted measure used to assess RNA integrity (Maniatis et al., 1982). However, rRNA comparison can often yield results that are not reproducible. The recent development of an algorithm that compares characteristics of several regions from the recorded electropherogram resulting from electrophoretic RNA separation and generates a number from 1 - 10 with 10 being the most intact RNA (the RIN) allows for the assessment of RNA samples that is not dependent on the instrument or concentration of the sample (down to 25 ng/μl). While the 28S:18S rRNA ratio from the hearing and deaf group from DCN samples ranged from 1.4 - 1.6 (Figure 1B) the RIN on average was 8.9 for normal hearing samples and 9.2 for samples from the deafened group (Figure 1B) indicating that dissection and RNA isolation resulted in RNA with little degradation. Samples from the VCN, regardless of whether they were obtained from normal or deafened subjects had 28S : 18S ratios of 1.4 and RINs ranging from 8.4 - 8.8 (Figure 1B) indicating good RNA integrity.
Changes in gene expression level
The cochlear nucleus can be dissected into two primary functional subdivisions, the ventral cochlear nucleus (VCN) that receives the majority of the excitatory input from the periphery and the dorsal cochlear nucleus (DCN) that receives some excitatory projections from the periphery as well as some excitatory projections from the VCN. In the VCN significant differential expression was observed for each of the vGluTs, but only at the three week time point (Figure 1C). For vGluT1, gene expression was significantly increased 3.2 fold (220% p ≤ 0.05) 3 weeks after hearing loss while vGluT2 and vGluT3 were significantly decreased 0.70 and 0.68 fold (30% and 32% respectively, p ≤ 0.05) compared to normal hearing controls. To determine whether these temporal changes in vGluT expression were spatially specific to the VCN, the primary target of the cochlear nerve, we also examined the DCN for changes in vGluT expression (Figure 1D). In the DCN, once again hearing loss resulted in a significant, 3.7 fold, increase in the expression of vGluT1 (270% p = 0.016) only at the three week time point. While there was no statistically significant change in the expression of vGluT2 1.32 fold (32% p = 0.096) in the DCN there was, however, a significant increase in vGluT3 expression, 1.81 fold (81% p ≤ 0.05) suggesting that the most robust and consistent changes in regulation of vGluT expression occur after three weeks of hearing loss regardless of the source of primary synaptic input.
Both spatial and temporal localization of vGluTs change following hearing loss
Controls
Antibodies for calcium binding proteins (CaBP) in the rat CN have previously been verified (Fredrich et al., 2009). To verify the specificity of vGluT antibodies in the rat CN several methods were employed. First, BLAST analysis of the sequence against which the antibodies were generated returned no other genes with sequence similarity. Second, Western blotting was performed on samples from the VCN and DCN using antibodies against vGluT1, vGluT2, and vGluT3 (Figure 1E). In each lane a single band of the expected size (∼62 kD) was observed for vGluT1 in both the VCN and DCN with the VCN showing more intense labeling. For vGluT2 labeling of bands at ∼56 kD was equally intense in the VCN and DCN. Two different antibodies were used to verify the presence of vGluT3 protein in the VCN and DCN. Both antibodies resulted in the same pattern of labeling with a labeled band (60 kD for mouse and 65 kD for guinea pig) in both the VCN and DCN with the labeling in the VCN being much more robust. In addition, using the specific antigen against which the antibodies were targeted to for preadsorption of each of the primary antibodies resulted in complete loss (vGluT1 and -2) or great diminution of labeling (vGluT3). In each case the exclusion of the primary antibody resulted in no labeling. Taken together, these results suggest that each antibody is specific for the particular vGluT labeling pattern.
Finally, since vGluT3 had not been previously reported in the cochlear nucleus, we also designed specific primers for PCR verification of vGluT3. We found a band of predicted size (∼1.9 kb) spanning the entire coding region (Figure 1F) in the VCN, DCN, and auditory cortex (AC). The PCR products were cloned and sequence verified. No splice variants were identified. This suggests that a single vGluT3 isoform is present in cochlear nucleus and the auditory cortex. Taken together our gene expression and Western blot data provide evidence that neurons of the CN express and produce vGluT1, -2, and -3. Since proteins for all three of the vGluTs were identified in both regions of the CN and loss of vGluT3, but not vGluT1 and-2 leads to deafness, we wanted to compare the spatial and temporal relationship between the vGluTs in normal hearing and deafened rats.
Axon terminal labeling of vGluT1 and -2, has previously been identified in the CN and their association with the granule vs core regions of the VCN and DCN has been reported for normal and deaf animals (Zhou et al., 2007; Zeng et al., 2009). However, the relationship of vGluT1 and -2 labeled axon terminals with known cell types within the VCN and DCN and their changes following hearing loss have not been reported. In addition, there are no previous reports of the distribution and localization of vGluT3 in the CN. We have used immunocytochemistry for vGluTs to determine the distribution of the transporters in the CN as well as the targeting of the transporters to axon terminals and/or somata and proximal dendrites. As recent studies have reported, specific calcium binding proteins can be used to identify different cell types in the CN (Por et al., 2005; Fredrich et al., 2009). We have therefore used double labeling for vGluTs and CaBP to learn more about the relationship of vGluTs with specific cells in the CN with primary emphasis being placed on vGluT1 in the VCN before and after hearing loss.
vGluT1 is associated with specific cell types in the VCN under basal conditions
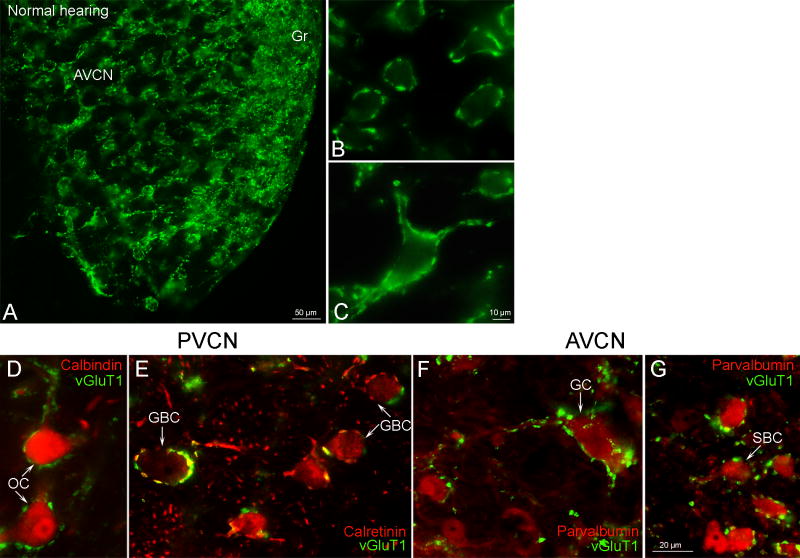
Robust immunolabeling for vGluT1 was found in both the core and the granule cell domain of the VCN (Figures 2A & 3A). We found vGluT1 labeling located in large terminals (1.66 μm – 2.51 μm) many of which were axosomatic. Smaller terminals were found to be both axosomatic and axodendritic (0.54 μm – 1.44 μm) with small axodendritic terminals more prevalent than small axosomatic (Figure 2B-C & 3A′). This localization to axon terminals fits well with that previously described in the central nervous system at large and specifically in the cochlear nucleus (Zhou et al., 2007; Gomez-Nieto and Rubio, 2009). Since CaBP have been determined to be specific for certain cells within the CN (Por et al., 2005; Fredrich et al., 2009) and many of the vGluT1 immunolabeled terminals outlined somatic profiles we combined immunocytochemistry for CaBP and vGluT1 to determine the cells with which vGluT1 labeled terminals form synapses. We summarize results from the current and other studies that utilize CaBP to identify specific cell types in the rat CN (Table 2). In the octopus cell region of the posterior ventral cochlear nucleus (PVCN) the CaBP, calbindin labels octopus cells and vGluT1 labeled terminals are in very close association with octopus cells, outlining somata and dendrites (Figure 2D). In the core of PVCN immunolabeling for the CaBP, calretinin is in somata of globular bushy cells. The somata of globular bushy cells are surrounded by calretinin and vGluT1 labeled axon terminals some of which are co-localized (Figure 2E). In the anterior ventral cochlear nucleus (AVCN) the CaBP parvalbumin labels both giant cells (large multipolar cells) and spherical bushy cells. Immunolabeling for vGluT1 is found in axon terminals around both giant and spherical bushy cells (Figure 2F-G) with larger vGluT1 immunolabeled terminals on giant cell somata and dendrites with smaller terminals around spherical bushy cells. Using three different CaBP we were able to identify four cell types that closely appose and may form synapses with vGluT1 immunolabeled terminals. This led us to ask whether hearing loss resulted in a loss of the relationship between vGluT1 labeled terminals and specific neurons in the CN.
Figure 2.
Immunoreactivity for vGluT1 in the AVCN (A-C) and the association of vGluT1-immunoreactive terminals with the specific cell types immunolabeled for calcium binding proteins (CaBP) in the PVCN (D, E) and the AVCN (F, G) from normal hearing rats. vGluT1 is seen in large synaptic terminals surrounding somata (A-C: axo-somatic endings), and proximal dendrites (A and C: axo-dendritic endings). The somata and proximal dendrites of CB-immunoreactive OCs are covered by many vGluT1-positive terminals (D). CR-positive GBC somata are surrounded by glutamatergic vGluT1 (green) and CR-positive (red) terminals (E). GC and SBCs in the AVCN show immunoreactivity for PV. VGluT1 terminals (green) are apposed to somata and proximal dendrites of these cells (F-G). Arrows in D-G point to specific cell types. PVCN - posterior ventral cochlear nucleus, AVCN - anterior ventral cochlear nucleus, Gr - AVCN granule cell domain, CB - calbindin, CR - calretinin, PV - parvalbumin, OC - octopus cell, GBC - globular bushy cell, GC - giant cell, SBC - spherical bushy cell. Scale bars shown in C is valid for B while scale bar in G applies to D-F as well.
Figure 3.
Deafness-related changes in vGluT1 expression within the AVCN. Immunoreactivity for vGluT1 in normal hearing animals is seen in large synaptic terminals surrounding somatic profiles (asterisks in A′) defined as axo-somatic endings (A, A′). Bilateral deafening by cochlear ablation produced changes in the localization of vGluT1. In 3 day-deafened animals glutamatergic terminals are labeled in the ventral AVCN (B, B″), but in the dorsal AVCN vGluT1 is almost exclusively localized to cell bodies (B, arrows in B′). Arrowheads (B) designate the border between the dorsal and ventral portion of AVCN and asterisks (B″) the somata surrounded by vGluT1 positive terminals. In 2 month-deafened animals, vGluT1 labeling is localized to somata and only a few small terminals (C, C′). AVCN-granule cell domain (Gr) shows heavier labeling when compared to the hearing group (compare Fig. 3C and Fig. 3A). In 1 year-deafened animals (D- E′), vGluT1 immunolabeling is still observed in the cell bodies (arrows in D′), however new vGluT1-positive synaptic endings were found, often around somata (asterisks in D′ and E′). Those terminals are smaller and fewer in comparison with the normal hearing group. In the DCN vGluT1 immunolabeling is observed in axon terminals in each layer (F). After 2 months of deafness vGluT1 immunolabeling appears diminished in DCN-F, but is still restricted to terminals (G). DCN-M; molecular layer of DCN, DCN-F; fusiform cell layer of DCN, DCN-D; deep core layer of DCN. Scale bars shown in E and E′ valid for A-E and A′-E′ respectively. Scale bar in G is valid for F. Auditory brainstem responses (ABRs) evoked with 20 kHz stimuli at different intensities (H). ABRs recorded in normal hearing animals show the typical five ABR peaks (1-5) at the intensity of 80 dB. Each peak is generated by a defined brainstem region. Following bilateral cochlear ablation (2 months and 1 year) ABR peaks are not generated at intensities of 80 dB and higher (H). The association of vGluT1-positive somata with the distinct cell types immunolabeled for CaBP in the PVCN (I-K) and the AVCN (L and M) in bilaterally deafened rats. The somata of OCs immunolabeled for CR are co-localized with vGluT1 as indicated in yellow (I). A morphologically distinctive GC with a large (∼30 μm), angular soma and relatively small nucleus is immunolabeled for CR and vGluT1 (J). Globular bushy cells (GBC) distinguished by an ovoid cell body with eccentrically placed nucleus and a single primary dendrite also show immunoreactivity for vGluT1 (J, K). Both vGluT1 and PV are present in SBCs within the AVCN (L, M). PV immunoreactive SBCs with elongated somas and one or two primary dendrites branching into a dense “bush” (see arrowhead in L) are seen in the insets. Arrows in I-M point to specific cell types. OC - Octopus Cell, GC – Giant Cell, SBC - Spherical Bushy Cell, CR – Calretinin, PV – Parvalbumin. Scale bar shown in M is also valid for I-L.
Table 2. Comparison of calcium binding proteins and associated cell types within the rat CN.
GBC; globular bushy cell, SBC; spherical bushy cell, OC; octopus cell, FC; fusiform cell, PC; pyramidal cell, CWC; cartwheel cell, mGC; multipolar giant cell, MC; multipolar cell, PV; parvalbumin, CB; calbindin, CR; calretinin.
| Brain region | PVCN | AVCN | DCN |
|---|---|---|---|
| Cell type | |||
| GBC | PV+, CR+ (Por et al., 2005; present study) | - | - |
| SBC | - | PV+ (Por et al., 2005, present study) | - |
| OC | CB+, CR+ (Por et al., 2005; Fredrich et al., 2009; present study) | - | - |
| FC (= PC) | - | - | PV+, CB+ (Por et al., 2005, present study) |
| CWC | - | - | PV+ (present study) |
| mGC (Smith and Rhode, 1989; Oertel et al., 1990)) | CR+ (present study, deaf) | PV+ (present study) | CB+ (Por et al., 2005; Fredrich et al., 2009) |
| T stellate (=MC type I; Doucet and Ryugo, 2006) | PV+ (Fredrich et al., 2009) | - | - |
| D stellate (=MC type II; Doucet and Ryugo, 2006) | CR+ (Fredrich et al., 2009; present study, deaf) | - | - |
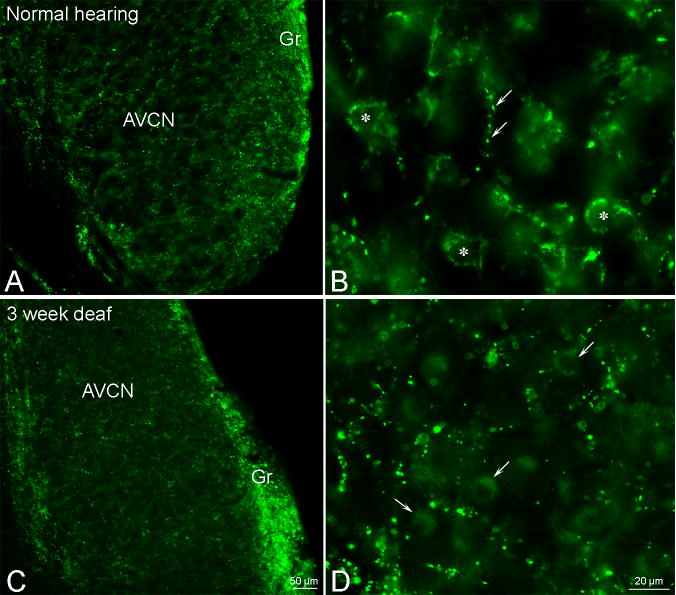
Hearing loss results in a progressive spatio-temporal change in vGluT1 localization
To determine the effect of hearing loss on the association of vGluT1 positive terminals with specific cells in the VCN, we examined sections from three day, two month and one year deafened animals. The size of small vGluT1 labeled terminals was decreased in deafened animals with small terminals ranging in size from 0.32 μm – 0.72 μm (41% - 50% decrease from normal hearing) and large terminals ranging from 0.82 μm – 2.11 μm with the smallest of the large terminals showing a 51% reduction in size when compared to large vGluT1 terminals in normal hearing animals. When compared to vGluT1 labeled terminals from normal hearing rats (Figure 3A - A′) the AVCN from three day deafened rats (Figure 3B - B′) contained a dorsal region in which there were small labeled terminals, but the majority of large vGluT1 labeled terminals observed in hearing animals were absent. Surprisingly, deafness resulted in vGluT1 labeled somata in the dorsal AVCN. However, ventral to this zone of labeled somata in the AVCN robust vGluT1 labeled terminals were still present (Figure 3B - B″).
Two months after hearing loss only a few small vGluT1 labeled terminals remained in the core of the AVCN with the majority of immunolabeling reflecting a shift in localization from terminals to somata throughout the core region (Figure 3C - C′). At the same time point there appears to be more vGluT1 labeled elements in the granule cell region of the AVCN (an observation that needs additional confirmation) with a further shift in vGluT1 labeling to the somata.
One year of hearing loss resulted in a predominance of vGluT1 labeled somata with labeled small terminals in the rostral AVCN (Figure 3D - E′). However, more caudal sections contained a re-emergence of axosomatic vGluT1 labeled terminals (Figure 3E-E′) though not as large as axosomatic vGluT1 labeled terminals in the AVCN from normal hearing animals. While vGluT1 labeling remained localized to the terminals in the granule cell region of the AVCN the labeling returned to near normal levels one year after hearing loss. In all three layer of the DCN vGluT1 was also localized to terminals in normal hearing animals (Figure 3F). While there was diminished labeling of vGluT1 labeled terminals following two months of deafness, primarily in the fusiform layer, no deafness associated increases in vGluT1 were observed in somata (Figure 3G).
To determine whether the progressive loss of and ultimate return of axosomatic vGluT1 labeled terminals coincided with a sequential loss and perhaps return (even with virtually no hair cells present) of hearing, auditory brainstem responses (ABRs) were tested. Even at 100 dB, no ABR responses were generated from rats that were deafened for two months or one year (Figure 3H) suggesting that the new vGluT1 axosomatic terminals were not functional in the detection of sound. Since in conjunction with loss of terminals, labeling of vGluT1 was observed in somata after hearing loss we wanted to determine which cell types were associated with the deafness induced vGluT1 labeled somata. We used CaBP (Figure 3I-M) as well as the morphology and location of the cell within the CN to identify the cell types. In the somata of octopus cells, giant cells, and globular bushy cells in the PVCN calretinin and vGluT1 were co-localized (Figure 3I-J). Parvalbumin was used to identify spherical bushy cells in the AVCN. Following deafness parvalbumin and vGluT1 were co-localized in the somata of spherical bushy cells (Figure 3L and M). Since the loss, but not the re-emergence, of vGluT1 terminals seemed to progress from dorsal to ventral during the initial phase of deafness, we examined the rostro-caudal extent of the CN for vGluT1 labeling after 3 days of deafness to better assess the topography of the loss.
To localize the progression of loss of vGluT1 axosomatic terminals from the CN we examined sagittal sections throughout the rostrocaudal extent of the nucleus from three day deafened animals (Figure 4), a time at which we find vGluT1 labeling in both axosomatic terminals and within somata. The most anterior lateral portions of the nucleus consist of AVCN (AVCN-A) and the granule cell region (Figure 4A). At this early time point the AVCN-A contains a few small labeled terminals in the core, but primarily vGluT1 localized to cell bodies with very robust terminal labeling in the granule cell region (Figure 4B-B′). Unlike AVCN-A, three days following hearing loss the posterior AVCN (AVCN-P; Figure 4C) contains vGluT1 labeled axosomatic terminals (Figure 4D-D′) that are morphologically indistinguishable from those found in normal hearing animals (compare Figures 2A and 3A with Figure 4D-D′). In the anterior PVCN (PVCN-A), the octopus cell region still contains strong vGluT1 terminal labeling (Figure 4E-F′), but sections through the posterior PVCN (PVCN-P; Figure 4G) contain vGluT1 labeled somata (Figure 4H-H′). These results suggest that hearing loss produces no loss in vGluT1 labeled terminals in the granule cell regions with a gradual, but dramatic loss of large axosomatic terminals beginning in the most anterior lateral AVCN and posterior PVCN with a spread to the other regions of the CN within two months.
Figure 4.
Serial sagittal sections through the rat CN stained for cresyl violet sections (A-G) and VGluT1 three days following bilateral cochlear ablation (B-H and B′-H′). In the anterior part of AVCN (B and B′) and the posterior part of PVCN (H and H′), vGluT1 immunoreactivity is almost exclusively localized to cell bodies. The heavy vGluT1 terminal labeling is still seen in the octopus cell region (F and F′) and less prominent in the posterior part of AVCN (D and D′). DCN-M; molecular layer of DCN, DCN-F; fusiform cell layer of DCN, DCN-D; deep core layer of DCN, OCR; octopus cell region. Scale bars shown in B and B′ valid for A-H and B′, D′, F′, H′ respectively.
To determine whether this “slow” spread of terminal loss was due to incomplete ablation of the cochlea, mid-modiolar sections through the cochlea were examined for the presence of hair cells in three day deafened animals. In each case, the overwhelming majority of the hair cells were absent with an occasional inner or outer hair cell present in a basal turn of the cochlea.
Consistent with our RT-PCR data these results suggest that following loss of vGluT1 excitatory input, the targets of those terminals up-regulate vGluT1. Since vGluT2 has been reported to have a complementary distribution when compared to vGluT1, we first wanted to determine whether the localization of vGluT2 was in terminals or somata and second whether there were changes with deafness.
Deafness related spatial changes in vGluT2
Label for vGluT2 in the CN of normal hearing rats was localized to both axosomatic and axodendritic terminals. Axosomatic terminals in the AVCN appear to make contact with somata that are relatively small in diameter (Figure 5A-B). Following deafness there is an almost complete loss of vGluT2 in larger terminals (0.8 – 1.2 μm) with an increase in the number of smaller terminals (∼0.4 μm; 20%; Figure 5C-D). Although the increase in vGluT2 labeled small terminals is found in both the core and the granule cell region, increased labeling is especially evident in the granule cell domain (Figure 5C). Similar to vGluT1, albeit less pronounced, labeling for vGluT2 found in the core of the CN is observed in somata following hearing loss (Figure 5D).
Figure 5.
Immunoreactivity for vGluT2 in normal hearing (A, B) and three weeks following bilateral deafness (C, D) in the rat AVCN. In control group, vGluT2-IR is particularly intense in the granule cell region (Gr) (A) and is less impressive in the core of AVCN, with labeling seen in terminals contacting somata (asterisks in B) and small terminals with diffuse distribution (arrows in B). In the deafened group, the intensity of vGluT2 labeling is greatly diminished (C, D) compare with hearing group, except the Gr region that shows very strong vGluT2 labeling (C). Within the AVCN immunolabeling is seen in small terminals and is also evident in cell bodies (arrows in D). Scale bar shown in C is valid for A and scale bar shown in D is valid for B.
Since vGluT1 and −2 are both localized to terminals in the CN of normal hearing rats and since hearing loss gives rise to decreased labeling of terminals in the core, increased labeling in the granule cell region and the ability to discern labeling in somata, we asked whether vGluT1 and vGluT2 are localized to the same terminals in the CN of normal hearing rats. While some co-labeling of vGluT1 and -2 was detected throughout the CN, the majority of co-localization was found in the granule cell domain of the VCN (Figure 6A-A‴) and the fusiform and deep layers of the DCN (Figure 6B-B‴). These results suggest that some terminals may have access to both vGluT1 and -2 for the loading of glutamatergic vesicles. The vGluT1 and -2 proteins are localized to terminals under normal hearing conditions and are found in somata following deafness. However, there are currently no reports of vGluT3 localization in the CN. Therefore we have used immunocytochemistry to localize vGluT3 in the CN in normal hearing and deafened rats.
Figure 6.

Double labeling immunofluorescence showing vGluT1 (green) and vGluT2 (red) in the hearing rat PVCN granule cell domain (A-A‴) and in the DCN (B-B‴). In the PVCN-Gr, many terminals are labeled for both vGluTs as indicated in yellow (arrows in A′-A‴). Within the DCN, the majority of vGluT1 and vGluT2 terminals show complementary distribution, however glutamatergic axon terminals coexpressing both isoforms are frequently seen in the DCN-F and the DCN-D layers (arrows in B′-B‴). PVCN-Gr; PVCN granule cell domain, DCN-M; molecular layer of DCN, DCN-F; fusiform cell layer of DCN, DCN-D; deep core layer of DCN. Scale bars shown in A‴ and B‴ valid for A′-A‴ and B′-B‴ respectively.
Distinctive localization of vGluT3 and close association with inhibitory terminals
Labeling for vGluT3 was found throughout the VCN and DCN in normal hearing animals. Unlike vGluT1 and −2, vGluT3 in normal hearing rats was localized largely to somata and dendrites (Figure 7A-B). Although some vGluT3 labeling was identified in the small neurons of the granule cell domain (terminals and somata), compared to vGluT1 and −2, labeling was sparse. The molecular layer of the DCN contained some labeled axon terminals with the most dorsal portion showing the heaviest labeling (Figure 7B). While labeled vGluT3 axon terminals could be identified in the fusiform and deep core layers of the DCN, the labeled somata were predominant. Following hearing loss vGluT3 labeling remained localized to somata in both the VCN and DCN (Figure 7C-D) with vGluT3 labeling in the granule region of the VCN appearing more sparse when compared to normal hearing controls. In the DCN by the third week of hearing loss, vGluT3 labeling remained very robust, especially in DCN-F, the fusiform layer (Figure 7D). When we counted the number of neurons labeled for vGluT3 in the DCN there was no significant difference across groups (4.03 neurons/40,000 μm2 ± 0.81 SEM for normal hearing animals and 6.84 neurons/40,000 μm2 ± 1.15 SEM for animals with hearing loss; p = 0.117)
Figure 7.
vGluT3 immunolabeling in normal hearing (A-B) and bilateral deafened (C-D) rats. In control group, vGluT3 is particularly localized to cell bodies in both the AVCN (A, A′) and the DCN layers (B). Three weeks following deafness, there is no change in the localization of vGluT3 and vGluT3 immunoreactivity is still seen in somata. Arrows indicate vGluT3 positive cells with labeling in soma as well as in proximal dendrite. In both hearing and deafened animals, within the AVCN region, vGluT3 is less prominent in the Gr (A, C) but in the DCN, the molecular layer (DCN - M) shows modest vGluT3 labeling (B, D). Gr; AVCN granule cell domain, DCN – F; fusiform cell layer of DCN, DCN – D; deep core layer of DCN. Scale bars shown in C, C′, D valid for A, A′, B respectively.
We also examined the relationship between vGluT3 labeled somata in the VCN and DCN and excitatory versus inhibitory axosomatic terminals (Figure 8). Robust labeling of synaptic terminals in close apposition to vGluT3 labeled somata was observed in both the VCN and DCN when using the synaptic vesicle marker SV2 (Figure 8A, D – D″). Many of the vGluT3 labeled somata in the VCN appeared to make contact with both vGluT1 (Figure 8B - B″) and glycinergic (Figure 8C) terminals. However, in the DCN vGluT3 labeled somata in the fusiform layer (DCN-F) appeared to receive very few vGluT1 axosomatic contacts (Figure 8E - E″). While inhibitory terminals (GAD67) surrounding vGluT3 positive somata was observed in the DCN-F layer (Figure 8F).
Figure 8.
The association of vGluT3-positive neurons with inhibitory and excitatory terminals in normal hearing rats. Triple immunolabeling for vGluT3 (red A - D; green F), (blue) DAPI, and (green) either SV2 (A, D) vGluT1 (B, E), and GlyT2 (C) is found within the PVCN (A, B, C) and DCN (D, E). Double labeling for (green) vGluT3 and (red) GAD67 in the DCN (F). Many vGluT3 positive cell bodies are decorated by glycinergic terminals in the PVCN (C) and GABAergic terminals (arrowheads) in the DCN (F). Asterisks indicate cells covered by GAD67 positive terminals but not labeled for vGluT3. DCN-M: molecular layer of DCN, DCN-F: fusiform cell layer of DCN.
Since vGluT1 and -2 distribution appeared to be complementary in the CN core regions, but co-localized in the granule cell domain, we asked whether vGluT3 labeling would show co-localization with vGluT1 or be complementary.
Cochlear distribution of vGluT1 and -3 is both co-localized and complementary
Transcripts for vGluT1, -2, and -3 have been reported in the cochlea with only vGluT3 localized to inner hair cells (Seal et al., 2008). To more precisely determine the distribution of the vGluTs in the organ of corti we examined mid-modiolar sections from adult normal hearing animals labeled with either vGluT1 or -3 (Figure 9A – C′). Labeling for vGluT1 was present in all turns of cochlea both within the organ of corti (Figure 9A′-1) and the spiral ganglion (Figure 9A′-2). Within the organ of corti punctate labeling was found in the region of the subcuticular plate of outer hair cells and around the inner hair cells (Figure 9A′-1a). Labeling for vGluT1 was also found in the inner pillar cells as well as phalangeal cells. Within the spiral ganglion, robust labeling for vGluT1 was found within spiral ganglion cells (SGCs) with punctate labeled profiles on and between SGCs (Figure 9A′-2a).
Figure 9.
The Organ of Corti from normal hearing rats immunolabels for vGluT1 (A-A′-2) and -3 (B-B′-2). Organ of Corti from the middle turn (A-1 and A-1a) with vGluT1 labeling present in pillar cells, inner phalangeal cells and punctate terminal like labeling around inner (filled asterisk) and outer hair cells (open asterisks). Arrowheads indicate punctate labeling in the region of subcuticular plate of OHCs (A′-1a). vGluT1 labeling is also in SGCs as well as in terminals in and around SGCs (A′-2, A′-2a). Basal turn from rat Organ of Corti with vGluT3 immuno-labeling in inner hair cells (B′-1, filled asterisk in B′-1a), outer hair cells (open asterisks in B′ -1a). vGluT3 labeling is also in SGCs as well as in terminals in and around SGCs (B′-2, arrowheads in B′-2a). A mid modialar section from a normal hearing rat immunolabeled for vGluT3. Each turn of the cochlea shows labeling for vGluT3 in the Organ of Corti, specifically inner hair cells, (C-C′). The rectangle containing the Organ of Corti in the basal turn is further illustrated in B′. Circles surround Organ of Corti from apical and basal turns. SGCs; spiral ganglion cells, IHC; inner hair cell, OHCs; outer hair cells. Scale bar shown in B′ is valid for A, A′, B. Scale bar shown in B′-1a is valid for all images containing a lower case “a” label and scale bar shown in in C′ is valid for C.
As with vGluT1, vGluT3 labeling was found within all cochlear turns (Figure 9C-C′) with labeling observed in both the organ of corti and the spiral ganglion (Figure 9B′). Within the organ of Corti only the inner hair cells (Figure 9B′-1a), stria vascularis and spiral ligament appeared to be labeled (Figure 9C-C′). Within the spiral ganglion, SGCs were moderately labeled for vGluT3 with prominent punctate labeling on SGC somata (Figure 9B′-2a). Taken together these results suggest both vGluT1 and vGluT3 are both found in SGCs, neurons that project to the CN. Since vGluT1 and -3 could be localized to the same cells and the localization of both vGluT1 and -3 is in CN somata following deafness we asked whether vGluT1 and -3 were co-localized in the CN following cochlear hair cell ablation.
In the CN vGluT1 and -3 co-localize following deafness
To better determine the localization of vGluT1 and -3 within the CN, sections triple labeled for MAP2 (somata and dendrites), DAPI (nuclei) and either vGluT1 or -3 were examined (Figure 10). Labeling for vGluT1 was clearly observed primarily in axosomatic terminals with some axodendritic terminal labeling (Figure 10A). While vGluT3 labeling was primarily observed within somata with some labeling of terminals distributed throughout the neuropil (Figure 10D - E). Unlike hearing animals, following deafness vGluT1 labeling is localized to somata and is co-localized with vGluT3 (Figure 10I - I″) suggesting plasticity in the form of deafness induced convergence of two available pools of synaptic vesicles into one neuronal compartment, ultimately impacting neuronal excitability.
Figure 10.
Mechanisms of glutamate signaling within the CN of normal hearing (A-H″) and deafened (I-L″) rats. Co-localization of vGluT1 with a neuronal marker (MAP2) and DAPI, a nuclear marker. Large vGluT1-positive terminals (green) are seen as axo-somatic (asterisks) as well as axo-dendritic (arrowheads) endings (A). Electron micrograph of an axo-somatic synapse. Excitatory terminal (ET) is packed with small round synaptic vesicles (B) synapses with the soma of an excitatory neuron (CB). Arrowheads designate the asymmetric postsynaptic density. Schematic of conventional neuronal signaling of an excitatory CN neuron under normal hearing conditions with synaptic endings containing either vGluT1 loaded vesicles (C′), vGluT2 loaded vesicles (C″) or vesicles loaded by both vGluT1 and -2 (C‴). Increased calcium pre-synaptically results in the release of excitatory neurotransmitter from terminals with glutamate binding to a variety of glutamate receptors post-synaptically. VGluT3 labeling (green) is frequently present in the somata of CN neurons as indicated by co-labeling with MAP2 (red; D, E). Asterisk indicates a cell expressing vGluT3 in the soma as well as in proximal dendrites. VGluT3 immunolabeling is also occasionally seen in synaptic terminals (arrowheads) in the neuropil. DAPI (blue) is used as a nuclear marker. SBC - spherical bushy cell. Scale bar shown in E is valid for D. Electron micrograph of a soma from an excitatory neuron (F) with arrowheads indicating coated vesicles near the golgi apparatus (GA) as well as a multivesicular body (VB). An axo-somatic synapse with pleomorphic vesicles contained within the inhibitory terminal (IT) at symmetric synapses (G). Arrowheads indicate vesicles near the postsynaptic membrane. CB - soma of an excitatory neuron. Using the synaptic vesicle marker SV2 both inhibitory and excitatory terminals apposed to vGluT3 labeled somata are observed (H′, H′) in the VCN. Specifically, inhibitory glycinergic terminals (H′) and vGluT1 labeled terminals (H′) appear to contact vGluT3 labeled somata in the VCN. Schematic of non-conventional, retrograde synaptic signaling in an excitatory CN neuron under conditions of high activity with vGluT3 loaded vesicles in the somata apposed either by inhibitory terminals containing vesicles with GABA or glycine (H-H′) or excitatory vesicles with glutamate (H and H″). When calcium levels are inceased postsynaptically vGluT3 loaded vesicles release glutamate from somata/dendrites which binds to metabotropic glutamate receptors either on the inhibitory terminal (H′) causing a local increase in calcium thereby releasing inhibitory transmitter which binds to inhibitory receptors on the soma decreasing neuronal activity of the excitatory neuron, or on the excitatory terminal (H″) causing inactivation of voltage gated calcium channels resulting in a decrease in calcium and blockage of release of excitatory transmitter ultimately decreasing neuronal activity of the excitatory neuron. Immunofluorescence for vGluT1 (green) and vGluT3 (red) in the CN of the deafened rat shows labeling within somata (I-I″) with the majority of somata showing co-localization of vGluT1 and vGluT3 as indicated in yellow (I″). Co-immunolabeling for vGluT1 (green) and GAD65 (red; J-J″) or SV2 (red; K-K′) in the CN. Somata are positive for vGluT1 (J and K) while terminals label for GAD65 (J′) or SV2 (K). Double labeling demonstrates that while there are few labeled vGluT1 terminals, labeled somata are still apposed by some GABAergic (J″) and some other non - vGluT1 positive terminals following deafness. Schematic of neuronal signaling in an excitatory CN neuron under deafened conditions with portions of the postsynaptic membrane apposed by inhibitory terminals (L-L′) or no terminal (L and L″). Following deafness the three proteins for loading glutamate into vesicles (vGluT1-3) are now localized to somata/dendrites. Increased calcium can result in retrograde release of glutamate from somata/dendrites with either weak inhibition from activation of inhibitory terminals (L′) or an upregulation of excitatory receptors combined with spillover of glutamate to sites enriched in glutamate receptors increasing sensitivity to glutamate and providing self-stimulation (L″).
Previous studies have postulated a unique role in synaptic signaling for vGluT3 (Harkany et al., 2003; Harkany et al., 2004). Our results suggest that the unique localization of vGluT3 within CN somata combined with the juxtaposition of vGluT3 somata and inhibitory terminals (Figure 10H −H‴ and J - K‴) provide a basis for considering retrograde signaling in CN neurons. To begin to explore this possibility we used electron microscopy to determine whether excitatory somata/dendrites synapsing with inhibitory terminals in the CN contain vesicle like structures.
Ultrastructure of CN neurons suggest retrograde neurotransmission
Like most highly active neurons in the central nervous system, typical CN neurons have many mitochondria, a prominent Golgi apparatus, moderate to high levels of rough endoplasmic reticulum and polysomes (Figures 10B & F-G). Unlike the majority of neurons in the central auditory system, normal VCN neurons receive the majority of excitatory inputs onto somata instead of dendrites (Figures 10A - B). These excitatory terminals can be visualized using labeling for vGluTs with vGluT1 localized to large synaptic endings with round vesicles, indicative of the endbulbs of Held originating in the Spiral Ganglion (SG). In addition to vGluT1, we found vGluT3 within CN somata and dendrites (Figures 10D-E). Ultrastructure of CN neurons reveal clathrin coated and non-coated vesicles as well as multivesicular bodies within somata (Figure 10F-G). Vesicles were observed both near the golgi apparatus (GA) and near the cytoplasmic membrane within the somata. Multivesicular bodies (VBs) are organelles that have a thick single membrane and contain coated and non-coated vesicles. In other systems these vesicles in neurons do not have an endocytic or lysosomal origin, but appear to be similar to vesicles found near the outer limits of the Golgi apparatus (Friend, 1969). More recently, VBs in the striatum have been shown to be positive for vGluT3, (Fremeau et al., 2002) suggesting a role for vesicles within VBs in transport and release of glutamate.
Discussion
In the current study we report the distribution of vGluT3 in the CN. We also demonstrate spatial and temporal changes in gene expression for all three vGluTs as a consequence of deafness. Previous studies of other systems support the idea of a complementary distribution of vGluTs (one neuron produces one vGluT) with vGluT1 reported primarily in cortex, hippocampus, and cerebellar cortex, vGluT2 reported in neurons of the thalamus and brainstem and vGluT3 reported in the caudate-putamen, the olfactory tubercle, the nucleus accumbens, the hippocampus, the interpeduncular nucleus and raphe nuclei (Fremeau et al., 2001; Herzog et al., 2001; Fremeau et al., 2002; Gras et al., 2002; Schafer et al., 2002; Varoqui et al., 2002; Herzog et al., 2004). In the auditory system, the expression of vGluT1 and -2 seemed to be largely complementary within the CN of normal hearing animals (Zhou et al., 2007; Zeng et al., 2009), suggesting that excitatory synapses containing vGluT1 and those with vGluT2 may have differential synaptic properties. However, in the present study glutamatergic axon terminals co-expressing both isoforms were frequently seen in the granule cell domain of VCN as well as in the DCN (fusiform layer and deep layer) and occasionally in the core of VCN. These results fit well with results from another study reporting more than 80% co-localization of vGluT1 and -2 in the rat trigeminal ganglion, a region which projects heavily to the rat CN granule cell domain (Li et al., 2003). The discrepancy of vGluT1 and -2 co-localization in our study and the complementary distribution of vGluT1 and -2 in the CN granule cell region observed by others may be due to species differences (guinea pig in previous studies and rat in the current study). Recently, several studies of the auditory system suggest co-production of vGluT1 and -2 in the SOC (Billups, 2005; Blaesse et al., 2005) and the IC (Altschuler et al., 2008) under normal hearing conditions.
The functional significance of such co-localization is unknown. However, we do know that both vGluT1 and vGluT2 are ATP dependent, require chloride stimulation and are specific for glutamate in terms of vesicle loading. While vGluT1 function is dependent upon membrane potential vGluT2 function is dependent upon both membrane potential and pH gradient (Bai et al., 2001). In the neocortex recent studies suggest that axon terminals showing co-localization of vGluT1 and -2 might change their synaptic properties, including the speed of vesicle recycling, in an activity-dependent manner depending upon the vGluT used to load synaptic vesicles (Nakamura et al., 2007). This suggests that some neurons may have the ability to adapt to changes in external stimuli e.g. hearing loss, via changes in the vGluT selected for loading of vesicles.
In the current study, the loss of hearing results in significant changes in gene expression levels for all three isoforms in the VCN while only vGluT1 and −3 show significant changes in the DCN. In both regions gene expression is affected for each of the vGluTs at the 3 week deaf time point and may reflect the strength of the loss of synaptic input following cochlear ablation. In the VCN, we demonstrate decreases in both vGluT2 and -3 with perhaps a compensatory increase in vGluT1 expression. In the DCN, while there is an increase in vGluT1, there is also an increase in the expression level of vGluT3. These differential changes in gene expression suggest multiple modes of excitatory signaling in the VCN and DCN following deafness.
These plastic changes in gene expression are accompanied by a robust loss of large vGluT1 labeled axosomatic terminals in the CN, presumably from the spiral ganglion. Another dramatic change was the appearance of vGluT1 labeling in somata as early as three days after hearing loss with a progressive switch from axonal labeling to somatic labeling in the VCN over time. The appearance of vGluT1 labeling in somata following hearing loss could represent a loss of the ability of the transporter to be targeted to axon terminals. However, globular and spherical bushy cells as well as octopus cells, each of which has somatic vGluT1 labeling following hearing loss, send projections from the CN to the superior olivary complex (SOC) and vGluT1 labeling in the SOC was still observed in terminals following deafness (data not shown). Another possibility for deafness related somatic labeling for vGluT1 in the VCN could have to do with the uptake and repackaging of glutamate by vGluT1 into cells (neurons and/or other cells) following loss or leakage from damaged cells. While this might be possible, given that re-uptake mechanisms often include glia and can involve endocytosis and vesicle recycling to stop transmitter action, our gene expression and immunocytochemistry studies argue against this case. First, our gene expression results show an increase in vGluT1 gene expression in the CN with hearing loss suggesting the somatic labeling observed is due to increased protein production. Second, we have used calcium binding proteins, as well as a neuronal marker (MAP2), and morphological characteristics to demonstrate that the somatic labeling observed following deafness occurs in specific neurons and not in glia. Still, somatic labeling could be due to autofluorescence. If this were the case we would expect that the fluorescence would not be wavelength specific and would be observed even without the presence of primary and/or secondary antibodies. We have been able to demonstrate that immunofluorescence for vGluT1 positive somata is specific for the wavelength of the secondary antibody used, is not present in the absence of the primary or secondary antibody, and is still observed when immunolabeling is performed using the non-fluorescent chromagen, diaminobenzidine.
This still leaves the question of why trauma induced somatic labeling of vGluT1 does not occur all at once throughout the CN, but rather progresses in a path specific gradient. After deafness, in the AVCN, vGluT1 labeled terminals decrease and increased somatic vGluT1 labeling initially occurs rostrally while in the PVCN labeled somata first appear caudally. Since our method of cochlear ablation primarily destroys the organ of Corti, but not the spiral ganglion cells, perhaps the patterned loss of terminal labeling and increase in somatic labeling can be explained based on the projection pattern of spiral ganglion cells with changes occurring first in those regions furthest from the spiral ganglion. Previous reports have demonstrated that the rostral AVCN is further from the spiral ganglion than the caudal AVCN and that the caudal PVCN is further from the spiral ganglion than the rostral PVCN (Arnesen and Osen, 1978; Nó et al., 1981). In each region the larger terminals from the spiral ganglion are in the region furthest from the spiral ganglion -- rostrally for AVCN and caudally for PVCN (Sento and Ryugo, 1989). Based on our calcium binding studies before and after hearing loss we show that vGluT1 labeled terminals from the spiral ganglion impinge upon somata that label for vGluT1 following deafness. These results imply that as vGluT1 input from the spiral ganglion is lost, target cells up-regulate vGluT1 production, with loss of input and concurrent up regulation of vGluT1 occurring first in target regions furthest from the spiral ganglion. By two months of deafness very few terminals were present throughout the dorsoventral extent of the AVCN. In 1-year deafened animals, vGluT1 immunolabeling was often seen in perisomatic locations representing axo-dendritic terminals. Those synaptic endings were smaller and fewer in comparison with the hearing group, suggesting a deafness-related reorganization of synaptic connections within the CN and/or new axonal growth over time to establish a new homeostatic balance. The neuronal source of those new synaptic endings will require future study.
Labeling for vGluT3 has been reported in neurons that are not normally associated with glutamatergic signaling and has been associated with non-classical excitatory neurons eg., dopaminergic, serotonergic, glycinergic, GABAergic, (Fremeau et al., 2002; Gras et al., 2002; Schafer et al., 2002). In vGluT knock-out models only the knock-out of vGluT3 results in hearing loss with a decrease in the volume of the VCN (Seal et al., 2008). Recent reports also demonstrate that humans with profound hearing loss have a mutation in the vGluT3 gene (Ruel et al., 2008). Unlike vGluT1 and -2, vGluT3 has been reported to label somata under normal conditions (Fremeau et al., 2002; Gras et al., 2002). Indeed, in the present study, vGluT3 labeling was localized to somata, dendrites and a small number of terminals with little to no change in this localization after hearing loss. Since vGluT3 labeling has not been previously demonstrated in the spiral ganglion or the CN we asked whether vGluT1 and -3 labeling were complementary or co-localized in the cochlea and CN. We found vGluT3 in the organ of Corti localized to inner hair cells while vGluT1 appeared to label supporting cells as well as fibers and terminals around the hair cells, which is consistent with recent studies (Seal et al., 2008). Spiral ganglion cells were labeled for both vGluT1 and -3 (albeit more robustly for vGluT1). Therefore, while vGluT1 and -3 labeling in the organ of Corti appeared to be complementary, labeling for both transporters was present in spiral ganglion cells. Additional studies will be necessary to determine whether both transporters are present in the same SGCs.
Our results suggest that normally excitatory CN neurons participate in both conventional neuronal signaling (transmitter release from terminals) and un-conventional retrograde signaling (transmitter release from dendrites/somata) with perhaps, a dramatic shift towards a more atypical, retrograde signaling mechanism following trauma (Figure 10). Specifically, in the CN vGluT1 and -2 loaded vesicles appear to play a role in conventional anterograde signaling (Figure 10A-C‴). However, vGluT3 may indicate retrograde signaling (Figure 10D-H), based upon somatic localization. In the auditory system conventional signaling begins in the cochlea when sound triggers an increase in local calcium levels followed by release of glutamate from vGluT3 loaded vesicles within inner hair cells. The released transmitter excites SGCs resulting in an increase in calcium levels within terminals located in the cochlear nucleus. The increased Ca2+ can cause release of contents from either vGluT1 loaded vesicles, found closely apposed to somata and dendrites (Figures 10A C, & C′), vGluT2 loaded vesicles (Figures 10C, & C″) closely apposed to somata, or vGluT1/2 (Figure 10C-C‴) loaded vesicles. The type of vGluT loaded vesicle recruited for glutamate release is dependent upon the electrical gradient (vGluT1) or both the electrical and chemical gradient (vGluT2) as well as the activity level of the neuron. Excitatory synapses in the CN contain ionotropic glutamate receptors (AMPA, NMDA and Kainate) with subunit configurations optimized for rapid neuronal activation and deactivation (Rubio and Wenthold, 1997, 1999; Petralia et al., 2000; Rubio, 2000; Sato et al., 2000). The balance between the available pool of vGluT loaded vesicles and glutamate receptors is critical for appropriate neuronal signaling in the CN.
While other studies did not report vGluT3 expression in the CN with in situ (Herzog et al., 2004) more recent studies indicate that vGluT3 is much less abundant in comparison to vGluT1 and -2 and can be easily overlooked (reviewed in Seal and Edwards 2006). Our Western blotting data confirm these findings. In addition, we corroborate previous findings of vGluT3 in hair cells (Seal et al., 2008) and extend those studies to show vGluT3 expression and production in the CN using targeted gene expression, combined with Western blotting and immunocytochemistry. The localization of vGluT3 to somata/dendrites (Fremeau et al., 2002; Gras et al., 2002; Herzog et al., 2004) as well as functional studies (Harkany et al., 2004) implicate vGluT3 loaded vesicles in retrograde signaling. In the current study vGluT3 was found in the somata/dendrites within the CN and our electron microscopy study revealed vesicle like structures in the somata/dendrites of excitatory neurons (Figure 10F-G). The presence of vGluT3 in vesicle like structures has been reported in dendrites of pyramidal neurons of the rat neocortex with vGluT3 participating in retrograde signaling via glutamate release from dendrites (Harkany et al., 2004). Possible roles for retrograde signaling in the CN abound, including as a mechanism for dampening the effects of overstimulation. We have demonstrated that in addition to vGluT1 labeled terminals, glycinergic and GABAergic immunoreactive terminals appose vGluT3 neurons (Figure 8F). These axo-somatic synapses can comprise both ionotropic and metabotropic glutamate receptors (mGluRs). Previously, mGluRs have been localized both pre- and post-synaptically at CN synapses and can have long lasting (minutes) effects on activity (Bilak and Morest, 1998; Oertel and Young, 2004). In both the DCN (Molitor and Manis, 1997) and VCN (Sanes et al., 1998) activation of mGluRs results in depression of neuronal activity. Based on our findings and other reports, we reasoned that during times of increased neuronal excitation vGluT3 loaded vesicles in dendrites and somata may retrogradely release glutamate via local increases in calcium. The glutamate could then bind to mGluRs on inhibitory terminals triggering an increase in calcium and release of inhibitory transmitter into the synaptic cleft allowing for activation of inhibitory terminals and depression of neuronal activity (Figure 10H-H′). While we cannot definitively state that vGluT3 labeled somata in the CN have more inhibitory inputs when compared to excitatory inputs (Figure 8) our data are highly suggestive (compare Figure 8B – C and E –F) and support previous work in the DCN that demonstrate fewer excitatory axosomatic contacts onto fusiform cells (Rubio and Juiz, 2004) when compared to inhibitory axosomatic contacts. A calcium dependent mechanism for the enhancement of inhibition during prolonged spiking activity or briefly following a period of increased activity have recently been suggested in the CN (Kim and Trussell, 2009). Alternatively, retrograde release of glutamate could result in bound mGluRs on excitatory terminals reducing influx of Ca2+ through voltage gated calcium channels (CaVs; Figure 10H and 10H″). Voltage gated calcium channels have been identified in axons of CN neurons (Bender and Trussell, 2009) and activation of mGluR receptors in the CN decreases Ca2+ entry via CaVs (Lu and Rubel, 2005). In addition to retrograde signaling, increased neuronal activity can result in decreased surface expression of AMPA receptors (Chen et al., 2009) supporting the idea of a plurality of mechanisms in the CN for protection against excitotoxicity.
Although vGluT1 and -3 appear to be contained within the same neuron, under normal hearing conditions, each transporter is compartmentalized within different regions of the neuron with vGluT3 in somata and vGluT1 in terminals (Figure 10A & D). Following hearing loss labeling for both vGluT1 and -3 was somatic and co-localized in CN neurons (Figure 10I - I″). Other consequences of hearing loss include, a significant loss of labeled glycinergic terminals (Kim et al., 2004; Asako et al., 2005; Buras et al., 2006) with many GABAergic terminals remaining (Figure 10 J - K″), increased AMPA receptors placed in the cell membrane (Suneja et al., 2000; Rubio, 2006; Whiting et al., 2009) and increases in calretinin within the somata of CN neurons (Idrizbegovic et al., 2006). Calretinin levels have been shown to increase in the cochlear nucleus following hearing loss (see Idrizbegovic et al., 2006 for review). Since calretinin is a calcium binding protein, increased levels can indicate increased calcium (Lukas and Jones 1994; Chard et al., 1995; Heizmann and Braun 1995; Miller 1995; Verkhratsky and Toescu 1998) Idrizbegovic et al., 2006). The presence of increased calcium in CN neurons after hearing loss could result in increased spontaneous release of glutamate from vGluT1 or -3 loaded vesicles in somata and dendrites. Therefore, glutamate released from somata and/or dendrites following hearing loss could bind to mGluRs resulting in inhibition of neuronal firing (Figure 10L – L′). However, since fewer inhibitory terminals and more AMPA receptors are present after hearing loss, glutamate would be more likely to bind to AMPA receptors on somata than metabotropic glutamate receptors on inhibitory terminals (Figure 10 L and L″). The result then could be an increase in spontaneous neuronal activity, perhaps as is seen in fusiform cells of the DCN following hearing loss (Waller and Godfrey, 1994; Zhang and Kaltenbach, 1998).
Our results underscore the importance of vGluTs as critical players in neuronal signaling in the CN. Under conditions of normal hearing conditions vGluT1 and -2 appear to be primarily axosomatic in the CN with vGluT3 localized to somata. Following hearing loss, when all vGluTs are found in somata and inhibitory input is decreased, spontaneous release of glutamate from somata/dendrites may be a mechanism for maintenance of the neuron and downstream synaptic connections.
Acknowledgments
These studies were supported by R03 NIDCD Grant DC007733 to AGH, and P30 Grants (Anatomy and Cell Biology, Wayne State Univ; Kresge Hearing Research Institute, Univ. of MI). We would like to acknowledge the technical assistance of K. Vistisen with Western blot and Dr. R. Armant for assistance with Agilent analysis. We thank Dr. J. Erickson for providing control peptides for the mfusion-vGluT antibodies and Dr. M. Drescher for critical comments about the Organ of Corti analysis. We also thank Drs. W. Crossland, D. Godfrey and P. Walker for reading and providing critical feedback of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Altschuler RA, Tong L, Holt AG, Oliver DL. Immunolocalization of vesicular glutamate transporters 1 and 2 in the rat inferior colliculus. Neuroscience. 2008;154:226–232. doi: 10.1016/j.neuroscience.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnesen AR, Osen KK. The cochlear nerve in the cat: topography, cochleotopy, and fiber spectrum. J Comp Neurol. 1978;178:661–678. doi: 10.1002/cne.901780405. [DOI] [PubMed] [Google Scholar]
- Asako M, Holt AG, Griffith RD, Buras ED, Altschuler RA. Deafness-related decreases in glycine-immunoreactive labeling in the rat cochlear nucleus. J Neurosci Res. 2005;81:102–109. doi: 10.1002/jnr.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem. 2001;276:36764–36769. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazwinsky I, Hartig W, Rubsamen R. Characterization of cochlear nucleus principal cells of Meriones unguiculatus and Monodelphis domestica by use of calcium-binding protein immunolabeling. J Chem Neuroanat. 2008;35:158–174. doi: 10.1016/j.jchemneu.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–271. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak SR, Morest DK. Differential expression of the metabotropic glutamate receptor mGluR1alpha by neurons and axons in the cochlear nucleus: in situ hybridization and immunohistochemistry. Synapse. 1998;28:251–270. doi: 10.1002/(SICI)1098-2396(199804)28:4<251::AID-SYN1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Billups B. Colocalization of vesicular glutamate transporters in the rat superior olivary complex. Neurosci Lett. 2005;382:66–70. doi: 10.1016/j.neulet.2005.02.071. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Ehrhardt S, Friauf E, Nothwang HG. Developmental pattern of three vesicular glutamate transporters in the rat superior olivary complex. Cell Tissue Res. 2005;320:33–50. doi: 10.1007/s00441-004-1054-8. [DOI] [PubMed] [Google Scholar]
- Brooke RE, Corns L, Edwards IJ, Deuchars J. Kv3.3 immunoreactivity in the vestibular nuclear complex of the rat with focus on the medial vestibular nucleus: targeting of Kv3.3 neurones by terminals positive for vesicular glutamate transporter 1. Brain Res. 2010;1345:45–58. doi: 10.1016/j.brainres.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Buras ED, Holt AG, Griffith RD, Asako M, Altschuler RA. Changes in glycine immunoreactivity in the rat superior olivary complex following deafness. J Comp Neurol. 2006;494:179–189. doi: 10.1002/cne.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol. 2009;44:161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. J Comp Neurol. 2007;505:363–378. doi: 10.1002/cne.21497. [DOI] [PubMed] [Google Scholar]
- Chard PS, Jordan J, Marcuccilli CJ, Miller RJ, Leiden JM, Roos RP, Ghadge GD. Regulation of excitatory transmission at hippocampal synapses by calbindin D28k. Proc Natl Acad Sci U S A. 1995;92:5144–5148. doi: 10.1073/pnas.92.11.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Peppi M, Kujawa SG, Sewell WF. Regulated expression of surface AMPA receptors reduces excitotoxicity in auditory neurons. J Neurophysiol. 2009;102:1152–1159. doi: 10.1152/jn.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YL, Holt AG, Lomax CA, Altschuler RA. Deafness associated changes in two-pore domain potassium channels in the rat inferior colliculus. Neuroscience. 2007;149:421–433. doi: 10.1016/j.neuroscience.2007.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Structural and functional classes of multipolar cells in the ventral cochlear nucleus. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:331–344. doi: 10.1002/ar.a.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Sa C, Gross J, Francone VP, Morest DK. Plasticity of synaptic endings in the cochlear nucleus following noise-induced hearing loss is facilitated in the adult FGF2 overexpressor mouse. Eur J Neurosci. 2007;26:666–680. doi: 10.1111/j.1460-9568.2007.05695.x. [DOI] [PubMed] [Google Scholar]
- Fredrich M, Reisch A, Illing RB. Neuronal subtype identity in the rat auditory brainstem as defined by molecular profile and axonal projection. Exp Brain Res. 2009;195:241–260. doi: 10.1007/s00221-009-1776-7. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular Glutamate Transporters 1 and 2 Target to Functionally Distinct Synaptic Release Sites. Science. 2004;18:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Friend DS. Cytochemical staining of multivesicular body and golgi vesicles. J Cell Biol. 1969;41:269–279. doi: 10.1083/jcb.41.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nieto R, Rubio ME. A bushy cell network in the rat ventral cochlear nucleus. J Comp Neurol. 2009;516:241–263. doi: 10.1002/cne.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Hartig W, Berghuis P, Dobszay MB, Zilberter Y, Edwards RH, Mackie K, Ernfors P. Complementary distribution of type 1 cannabinoid receptors and vesicular glutamate transporter 3 in basal forebrain suggests input-specific retrograde signalling by cholinergic neurons. Eur J Neurosci. 2003;18:1979–1992. doi: 10.1046/j.1460-9568.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- Harkany T, Holmgren C, Hartig W, Qureshi T, Chaudhry FA, Storm-Mathisen J, Dobszay MB, Berghuis P, Schulte G, Sousa KM, Fremeau RT, Jr, Edwards RH, Mackie K, Ernfors P, Zilberter Y. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW, Braun K. Calcium regulation by calcium-binding proteins in neurodegenerative disorders. New York Austin: Springer-Verlag; R.G. Landes; 1995. [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–1157. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AG, Asako M, Duncan RK, Lomax CA, Juiz JM, Altschuler RA. Deafness associated changes in expression of two-pore domain potassium channels in the rat cochlear nucleus. Hear Res. 2006;216-217:146–153. doi: 10.1016/j.heares.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen S, Tietz EI. Immunocytochemical detection of regional protein changes in rat brain sections using computer-assisted image analysis. J Histochem Cytochem. 1996;44:981–987. doi: 10.1177/44.9.8773563. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Salman H, Niu X, Canlon B. Presbyacusis and calcium-binding protein immunoreactivity in the cochlear nucleus of BALB/c mice. Hear Res. 2006;216-217:198–206. doi: 10.1016/j.heares.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Morest DK, Potashner SJ. Quantitative study of degeneration and new growth of axons and synaptic endings in the chinchilla cochlear nucleus after acoustic overstimulation. J Neurosci Res. 2004;77:829–842. doi: 10.1002/jnr.20211. [DOI] [PubMed] [Google Scholar]
- Kim Y, Trussell LO. Negative shift in the glycine reversal potential mediated by a Ca2+- and pH-dependent mechanism in interneurons. J Neurosci. 2009;29:11495–11510. doi: 10.1523/JNEUROSCI.1086-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesperance MM, Helfert RH, Altschuler RA. Deafness induced cell size changes in rostral AVCN of the guinea pig. Hear Res. 1995;86:77–81. doi: 10.1016/0378-5955(95)00056-a. [DOI] [PubMed] [Google Scholar]
- Levenes C, Daniel H, Crepel F. Retrograde modulation of transmitter release by postsynaptic subtype 1 metabotropic glutamate receptors in the rat cerebellum. J Physiol. 2001;537:125–140. doi: 10.1111/j.1469-7793.2001.0125k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Xiong KH, Dong YL, Fujiyama F, Kaneko T, Mizuno N. Vesicular glutamate transporters, VGluT1 and VGluT2, in the trigeminal ganglion neurons of the rat, with special reference to coexpression. J Comp Neurol. 2003;463:212–220. doi: 10.1002/cne.10755. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Lu Y, Rubel EW. Activation of metabotropic glutamate receptors inhibits high-voltage-gated calcium channel currents of chicken nucleus magnocellularis neurons. J Neurophysiol. 2005;93:1418–1428. doi: 10.1152/jn.00659.2004. [DOI] [PubMed] [Google Scholar]
- Lukas W, Jones KA. Cortical neurons containing calretinin are selectively resistant to calcium overload and excitotoxicity in vitro. Neuroscience. 1994;61:307–316. doi: 10.1016/0306-4522(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning : a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- Miller RJ. Regulation of calcium homoeostasis in neurons: the role of calcium-binding proteins. Biochem Soc Trans. 1995;23:629–632. doi: 10.1042/bst0230629. [DOI] [PubMed] [Google Scholar]
- Molitor SC, Manis PB. Evidence for functional metabotropic glutamate receptors in the dorsal cochlear nucleus. J Neurophysiol. 1997;77:1889–1905. doi: 10.1152/jn.1997.77.4.1889. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Watakabe A, Hioki H, Fujiyama F, Tanaka Y, Yamamori T, Kaneko T. Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: a quantitative analysis with correlation coefficient. Eur J Neurosci. 2007;26:3054–3067. doi: 10.1111/j.1460-9568.2007.05868.x. [DOI] [PubMed] [Google Scholar]
- Niparko JK, Finger PA. Cochlear nucleus cell size changes in the dalmatian: model of congenital deafness. Otolaryngol Head Neck Surg. 1997;117:229–235. doi: 10.1016/s0194-5998(97)70179-7. [DOI] [PubMed] [Google Scholar]
- Nó Ld R. The primary acoustic nuclei. New York: Raven Press; 1981. [Google Scholar]
- Oertel D, Wu SH, Garb MW, Dizack C. Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J Comp Neurol. 1990;295:136–154. doi: 10.1002/cne.902950112. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Rubio ME, Wang YX, Wenthold RJ. Differential distribution of glutamate receptors in the cochlear nuclei. Hear Res. 2000;147:59–69. doi: 10.1016/s0378-5955(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol. 1992;450:127–142. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman QJ, Hirasawa M, Mouginot D, Kombian SB. Neurohypophysial peptides as retrograde transmitters in the supraoptic nucleus of the rat. Exp Physiol. 2000;85(Spec No: 13):9S–143S. doi: 10.1111/j.1469-445x.2000.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Por A, Pocsai K, Rusznak Z, Szucs G. Presence and distribution of three calcium binding proteins in projection neurons of the adult rat cochlear nucleus. Brain Res. 2005;1039:63–74. doi: 10.1016/j.brainres.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Rubio ME. Selective targeting of glutamate receptors in neurons. Mol Neurobiol. 2000;21:1–19. doi: 10.1385/MN:21:1-2:001. [DOI] [PubMed] [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in rats. Hear Res. 2006;216-217:154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Differential distribution of synaptic endings containing glutamate, glycine, and GABA in the rat dorsal cochlear nucleus. J Comp Neurol. 2004;477:253–272. doi: 10.1002/cne.20248. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Differential distribution of intracellular glutamate receptors in dendrites. J Neurosci. 1999;19:5549–5562. doi: 10.1523/JNEUROSCI.19-13-05549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJ, Lesperance MM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Sanes DH, McGee J, Walsh EJ. Metabotropic glutamate receptor activation modulates sound level processing in the cochlear nucleus. J Neurophysiol. 1998;80:209–217. doi: 10.1152/jn.1998.80.1.209. [DOI] [PubMed] [Google Scholar]
- Sato K, Shiraishi S, Nakagawa H, Kuriyama H, Altschuler RA. Diversity and plasticity in amino acid receptor subunits in the rat auditory brain stem. Hear Res. 2000;147:137–144. doi: 10.1016/s0378-5955(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sento S, Ryugo DK. Endbulbs of held and spherical bushy cells in cats: morphological correlates with physiological properties. J Comp Neurol. 1989;280:553–562. doi: 10.1002/cne.902800406. [DOI] [PubMed] [Google Scholar]
- Smith PH, Rhode WS. Structural and functional properties distinguish two types of multipolar cells in the ventral cochlear nucleus. J Comp Neurol. 1989;282:595–616. doi: 10.1002/cne.902820410. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. AMPA receptor binding in adult guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Exp Neurol. 2000;165:355–369. doi: 10.1006/exnr.2000.7471. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- Waller HJ, Godfrey DA. Functional characteristics of spontaneously active neurons in rat dorsal cochlear nucleus in vitro. J Neurophysiol. 1994;71:467–478. doi: 10.1152/jn.1994.71.2.467. [DOI] [PubMed] [Google Scholar]
- Whiting B, Moiseff A, Rubio ME. Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss. Neuroscience. 2009;163:1264–1276. doi: 10.1016/j.neuroscience.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WH, Leung PS, Ng SS, Zhang J, Chan SC, Chow BK. Secretin facilitates GABA transmission in the cerebellum. J Neurosci. 2001;21:7063–7068. doi: 10.1523/JNEUROSCI.21-18-07063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009;29:4210–4217. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250:197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Zilberter Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J Physiol. 2000;528:489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]