Abstract
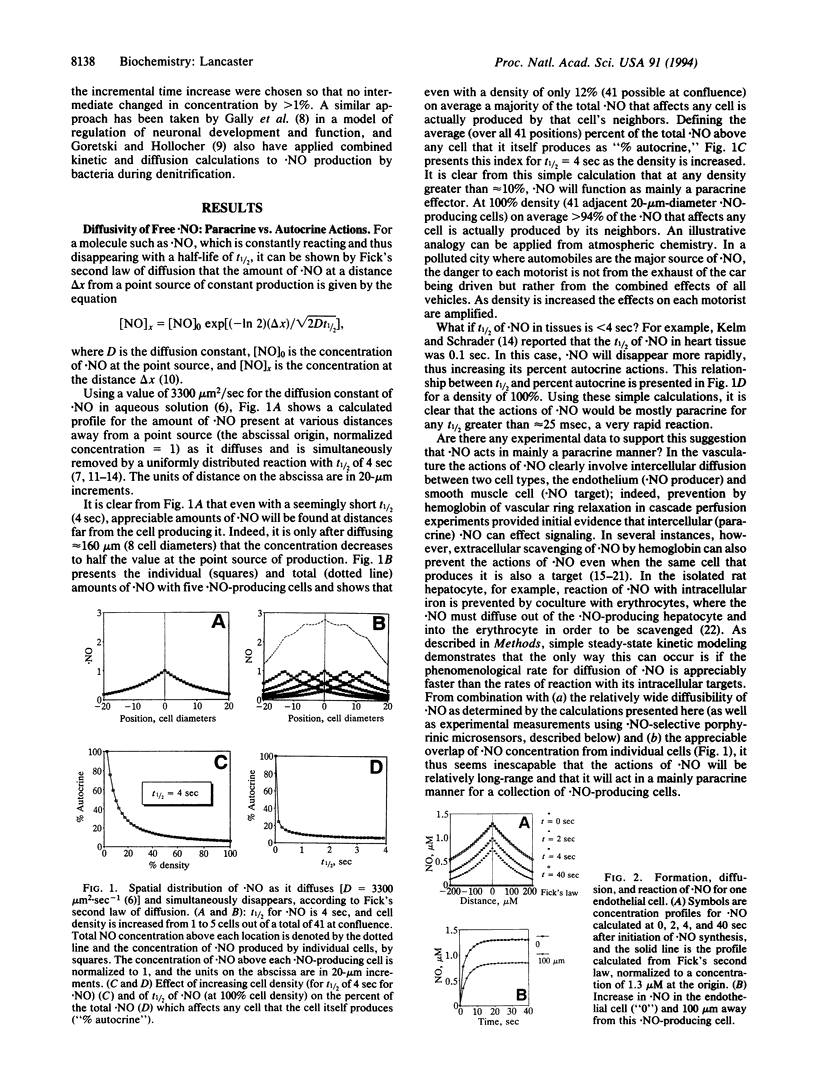
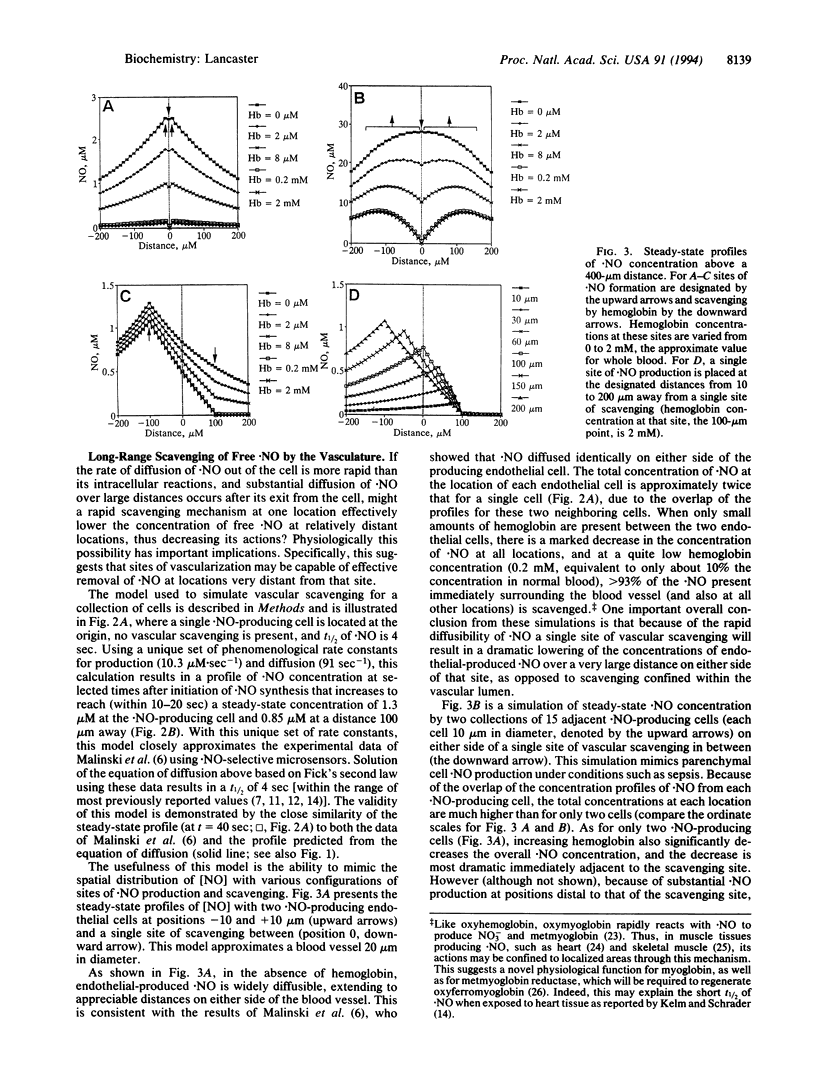
In spite of intense recent investigation of the physiological and pathophysiological roles of endogenously produced nitric oxide (.NO) in mammalian systems, little quantitative information exists concerning the diffusion of this small nonelectrolyte from its source (NO synthase) to its targets of action. I present here a conceptual framework for analyzing the intracellular and intercellular diffusion and reaction of free .NO, using kinetic modeling and calculations of the diffusibility of .NO and its reactions in aqueous solution based on published data. If the half-life of .NO is greater than approximately 25 msec and the rates of reaction of .NO with its targets are slower than its diffusion or reaction with O2 (for which there is experimental evidence in at least some systems), then (i) .NO acts in vivo in a mostly paracrine fashion for a collection of .NO-producing cells, (ii) .NO diffuses to significant concentrations at distances relatively far removed from a single .NO-producing cell, and (iii) localized sites of vascularization will scavenge .NO (and thus decrease its actions) at distances many cell diameters away from that site. These conclusions have important implications with regard to the mechanism of endothelium-dependent relaxation, the autocrine vs. paracrine actions of .NO, and the role of the spatial relationship between specific sites of .NO formation and neighboring blood vessels in .NO-effected and -affected neuronal signal transmission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. R., Driedzic W. R. Lack of correlation between cardiac myoglobin concentration and in vitro metmyoglobin reductase activity. J Exp Biol. 1992 Dec;173:301–306. doi: 10.1242/jeb.173.1.301. [DOI] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J. N., Harrison D. G., Myers P. R., Minor R. L. EDRF: nitrosylated compound or authentic nitric oxide. Basic Res Cardiol. 1991;86 (Suppl 2):17–26. doi: 10.1007/978-3-642-72461-9_3. [DOI] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Corasaniti M. T., Tartaglia R. L., Melino G., Nisticò G., Finazzi-Agrò A. Evidence that CHP100 neuroblastoma cell death induced by N-methyl-D-aspartate involves L-arginine-nitric oxide pathway activation. Neurosci Lett. 1992 Dec 7;147(2):221–223. doi: 10.1016/0304-3940(92)90600-c. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L., Snyder S. H. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992 Sep;32(3):297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerlé-Pallardy C., Lonchampt M. O., Chabrier P. E., Braquet P. Absence of implication of L-arginine/nitric oxide pathway on neuronal cell injury induced by L-glutamate or hypoxia. Biochem Biophys Res Commun. 1991 Nov 27;181(1):456–464. doi: 10.1016/s0006-291x(05)81441-x. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Hoekstra J. W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981 Jul;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gally J. A. Nitric oxide: linking space and time in the brain. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11651–11652. doi: 10.1073/pnas.89.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P. J., Kaube H., Hoskin K. L. Nitric oxide synthesis couples cerebral blood flow and metabolism. Brain Res. 1992 Nov 6;595(1):167–170. doi: 10.1016/0006-8993(92)91470-y. [DOI] [PubMed] [Google Scholar]
- Goretski J., Hollocher T. C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988 Feb 15;263(5):2316–2323. [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Chaudhuri G. EDRF generation and release from perfused bovine pulmonary artery and vein. Eur J Pharmacol. 1988 Apr 27;149(1-2):79–88. doi: 10.1016/0014-2999(88)90045-3. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Benz A. M., Clifford D. B., Zorumski C. F. Nitric oxide inhibitors attenuate N-methyl-D-aspartate excitotoxicity in rat hippocampal slices. Neurosci Lett. 1992 Feb 3;135(2):227–230. doi: 10.1016/0304-3940(92)90442-a. [DOI] [PubMed] [Google Scholar]
- Jensen A. M., Chiu S. Y. Fluorescence measurement of changes in intracellular calcium induced by excitatory amino acids in cultured cortical astrocytes. J Neurosci. 1990 Apr;10(4):1165–1175. doi: 10.1523/JNEUROSCI.10-04-01165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L., Manev H., Costa E., Wroblewski J. T. Inhibition of glutamate-induced cell death by sodium nitroprusside is not mediated by nitric oxide. Neuropharmacology. 1991 Nov;30(11):1241–1243. doi: 10.1016/0028-3908(91)90171-7. [DOI] [PubMed] [Google Scholar]
- Kollegger H., McBean G. J., Tipton K. F. Reduction of striatal N-methyl-D-aspartate toxicity by inhibition of nitric oxide synthase. Biochem Pharmacol. 1993 Jan 7;45(1):260–264. doi: 10.1016/0006-2952(93)90401-h. [DOI] [PubMed] [Google Scholar]
- Lerner-Natoli M., Rondouin G., de Bock F., Bockaert J. Chronic NO synthase inhibition fails to protect hippocampal neurones against NMDA toxicity. Neuroreport. 1992 Dec;3(12):1109–1112. doi: 10.1097/00001756-199212000-00019. [DOI] [PubMed] [Google Scholar]
- Lo T. M., Fallert C. J., Piser T. M., Thayer S. A. HIV-1 envelope protein evokes intracellular calcium oscillations in rat hippocampal neurons. Brain Res. 1992 Oct 30;594(2):189–196. doi: 10.1016/0006-8993(92)91125-x. [DOI] [PubMed] [Google Scholar]
- Malinski T., Taha Z., Grunfeld S., Patton S., Kapturczak M., Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–12234. [PubMed] [Google Scholar]
- Marsden P. A., Ballermann B. J. Tumor necrosis factor alpha activates soluble guanylate cyclase in bovine glomerular mesangial cells via an L-arginine-dependent mechanism. J Exp Med. 1990 Dec 1;172(6):1843–1852. doi: 10.1084/jem.172.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Mordvintcev P., Vanin A. F., Busse R. The potent vasodilating and guanylyl cyclase activating dinitrosyl-iron(II) complex is stored in a protein-bound form in vascular tissue and is released by thiols. FEBS Lett. 1991 Dec 9;294(3):252–256. doi: 10.1016/0014-5793(91)81441-a. [DOI] [PubMed] [Google Scholar]
- Nakane M., Schmidt H. H., Pollock J. S., Förstermann U., Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993 Jan 25;316(2):175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Neylon C. B., Irvine R. F. Synchronized repetitive spikes in cytoplasmic calcium in confluent monolayers of human umbilical vein endothelial cells. FEBS Lett. 1990 Nov 26;275(1-2):173–176. doi: 10.1016/0014-5793(90)81465-z. [DOI] [PubMed] [Google Scholar]
- Pauwels P. J., Leysen J. E. Blockade of nitric oxide formation does not prevent glutamate-induced neurotoxicity in neuronal cultures from rat hippocampus. Neurosci Lett. 1992 Aug 31;143(1-2):27–30. doi: 10.1016/0304-3940(92)90225-v. [DOI] [PubMed] [Google Scholar]
- Reiser G. Mechanism of stimulation of cyclic-GMP level in a neuronal cell line mediated by serotonin (5-HT3) receptors. Involvement of nitric oxide, arachidonic-acid metabolism and cytosolic Ca2+. Eur J Biochem. 1990 May 20;189(3):547–552. doi: 10.1111/j.1432-1033.1990.tb15521.x. [DOI] [PubMed] [Google Scholar]
- Sandman C. A., O'Halloran J. P., Isenhart R. Is there an evoked vascular response? Science. 1984 Jun 22;224(4655):1355–1357. doi: 10.1126/science.6729458. [DOI] [PubMed] [Google Scholar]
- Scher W., Scher B. M. A possible role for nitric oxide in glutamate (MSG)-induced Chinese restaurant syndrome, glutamate-induced asthma, 'hot-dog headache', pugilistic Alzheimer's disease, and other disorders. Med Hypotheses. 1992 Jul;38(3):185–188. doi: 10.1016/0306-9877(92)90091-p. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Katusic Z. S., Vanhoutte P. M. Neurohypophyseal peptides and tachykinins stimulate the production of cyclic GMP in cultured porcine aortic endothelial cells. J Pharmacol Exp Ther. 1990 Dec;255(3):994–1000. [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. Locally distributed synaptic potentiation in the hippocampus. Science. 1994 Jan 28;263(5146):532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- Stadler J., Bergonia H. A., Di Silvio M., Sweetland M. A., Billiar T. R., Simmons R. L., Lancaster J. R., Jr Nonheme iron-nitrosyl complex formation in rat hepatocytes: detection by electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 1993 Apr;302(1):4–11. doi: 10.1006/abbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Tsukahara H., Gordienko D. V., Goligorsky M. S. Continuous monitoring of nitric oxide release from human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):722–729. doi: 10.1006/bbrc.1993.1685. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Mordvintcev P. I., Malenkova I. V., Vanin A. F. Similarity between the vasorelaxing activity of dinitrosyl iron cysteine complexes and endothelium-derived relaxing factor. Eur J Pharmacol. 1992 Feb 18;211(3):313–317. doi: 10.1016/0014-2999(92)90386-i. [DOI] [PubMed] [Google Scholar]



