Abstract
Parkinson’s disease (PD) is a neurodegenerative movement disorder presenting with subcortical pathology and characterized by motor deficits. However, as is frequently reported in the literature, patients with PD can also exhibit cognitive and behavioral (i.e., nonmotor) impairments, cognitive executive deficits and depression being the most prominent. Considerable attention has addressed the role that disruption to frontostriatal circuitry can play in mediating nonmotor dysfunction in PD. The three nonmotor frontostriatal circuits, which connect frontal cortical regions to the basal ganglia, originate from the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC). The objective of the current study was to use our understanding of frontostriatal circuit function (via literature review) to categorize neuropsychological measures of cognitive and behavioral executive functions by circuit. To our knowledge, such an approach has not been previously attempted in the study of executive dysfunction in PD. Neuropsychological measures of executive functions and self-report behavioral inventories, categorized by circuit function, were administered to 32 nondemented patients with Parkinson’s disease (NDPD) and to 29 demographically matched, healthy normal control participants (NC). Our findings revealed significant group differences for each circuit, with the PD group performing worse than the NC group. Among the patients with PD, indices of impairment were greater for tasks associated with DLPFC function than with OFC function. Further, only an index of DLPFC test performance was demonstrated to significantly discriminate individuals with and without PD. In conclusion, our findings suggest that nondemented patients with PD exhibit greater impairment on neuropsychological measures associated with DLPFC than with ACC or OFC circuit function.
Introduction
Despite the early notion that sensory and intellectual abilities are unaffected in patients with PD (Parkinson, 1817), cognitive and behavioral sequelae in this patient population have been well documented in the literature (Barbosa, Limongi, & Cummings, 1997; Dubois & Pillon, 1997; Emre, 2003; Kremer & Starkstein, 2000; Morrison et al., 2000; Pahwa, Paolo, Tröster, & Koller, 1998; Raskin, Borod, & Tweedy, 1990; Zgaljardic, Borod, Foldi, & Mattis, 2003). The nonmotor impairments exhibited by these patients appear to represent a manifestation of PD separate from the disease pathology associated with the characteristic motor symptoms (i.e., tremor, bradykinesia, and rigidity).
Evidence concerning the etiology of the cognitive and behavioral sequelae associated with PD comes from different lines of research. First, as the neuropathological hallmark of PD is a reduction of dopaminergic neurons in the substantia nigra, it has been suggested that increasing available cortical dopamine would improve cognitive and behavioral sequelae in addition to the characteristic motor deficits. Nonetheless, as has been demonstrated in several reports, nonmotor deficits are not typically alleviated following dopamine therapy (e.g., Carbon et al., 2003; Cooper et al., 1992; Feigin et al., 2003; Gotham, Brown, & Marsden, 1988; Growdon et al., 1998). Moreover, despite the focus on the dopaminergic system in PD, other neurotransmitter systems (e.g., acetylcholine, norepinephrine, and serotonin), as well as Lewy Body inclusions, can play a contributory role in the etiology of nonmotor deficits exhibited by these patients (Agid, Javoy-Agid, & Ruberg, 1987; Emre, 2003; Przuntek, 2000; Zgaljardic, Foldi, & Borod, 2004). Second, similar cognitive and behavioral impairments are exhibited by patients with focal frontal system lesions and by those with PD (Starkstein & Robinson, 1993; Taylor, Saint-Cyr, & Lang, 1986). Thus, disruption to select prefrontal systems (including their connections to the basal ganglia [i.e., frontostriatal circuitry]), independent of those modulating motor programs, have been implicated in the cognitive and behavioral sequelae of PD (e.g., Carbon & Marie, 2003; Cummings, 1993; Fukuda, Edwards, & Eidelberg, 2001; Gotham et al., 1988; Lewis, Dove, Robbins, Barker, & Owen, 2003; Taylor et al., 1986; Tekin & Cummings, 2002; Zgaljardic et al., 2003).
The frontostriatal circuits are grouped into motor (i.e., the motor circuit & oculomotor circuit) and complex (i.e., nonmotor) circuits (Alexander, DeLong, & Strick, 1986; Middleton & Strick, 2000). The latter originate from (a) the dorsolateral prefrontal cortex (DLPFC), (b) the anterior cingulate cortex (ACC), and (c) the orbitofrontal cortex (OFC). Each circuit projects to specific striatal regions in a topographical fashion and remains segregated throughout the basal ganglia and thalamus, allowing other areas of the brain to communicate with each circuit along their respective pathways (Middleton & Strick, 2000; Zgaljardic & Eidelberg, 2003). The frontostriatal circuits also receive inputs from dopaminergic, noradrenergic, serotonergic, and cholinergic cell groups that modulate information processing (Tekin & Cummings, 2002).
Previous research in brain-damaged individuals, healthy adults, and non-human subjects (i.e., primates) has been able to specify associations between each of the three prefrontal cortical regions and particular cognitive and behavioral functions: (a) the ACC is believed to regulate attentional processes, such as response initiation, intention, inhibition, and conflict monitoring; (b) the DLPFC is involved in mediating cognitive executive functions, such as set-shifting, complex problem-solving, retrieval abilities, organizational strategies, concept-formation, and working memory; and (c) the OFC has been associated with aspects of decision-making based upon a reinforcement/reward schedule, impulse control, perseveration, and mood (for review, see Zgaljardic et al., 2003).
The current study is unique in its use of neural substrates to categorize neuropsychological measures in order to assess executive functioning in nondemented patients with Parkinson’s disease (NDPD). This aim was accomplished through the development of a test battery that incorporated findings pertaining to regional prefrontal function (i.e., DLPFC, ACC, and OFC) from PD, brain-damaged, healthy adult, animal (primate), and neuroimaging literatures. Performance on each test in our battery has been shown to be associated with functioning of one of the three aforementioned prefrontal regions (see review in Zgaljardic et al., 2003).
Methods
Participants
Participants were 61 right-handed adults, including 32 NDPD patients (59% men) and 29 NC individuals (48% men). Handedness was determined by self-report and confirmed by the Coren, Porac, and Duncan (1979) lateral preference inventory. All participants were native speakers of English; were between the ages of 50 and 79 (overall M = 66.8, SD = 7.0); and had an overall mean education level of 15.8 years (SD = 2.5) and an overall mean occupational level of 7.4 (SD = 1.5) on the Hollingshead Scale (Hollingshead, 1977), ranging from “1” (unskilled service worker) to “9” (major professional). The participant groups did not differ significantly across demographic variables (Table 1). In addition, no significant group differences were found on an estimate of premorbid level of intellectual functioning (Barona, Reyolds, & Chastain, 1984). Further, men and women were similarly distributed across the participant groups, X2 = .76, p = .385. See Table 1 for group means and standard deviations on these demographic variables.
Table 1.
Between-group comparisons for demographic and screening variables
| PD (Mean/SD) N = 32 |
NC (Mean/SD) N = 29 |
t-value | p-value | |
|---|---|---|---|---|
| Age | 66.9/8.1 | 66.7/5.7 | −0.10 | .919 |
| Education | 15.4/2.7 | 16.2/2.2 | 1.24 | .220 |
| Hollingshead | 7.3/1.7 | 7.5/1.4 | 0.59 | .560 |
| Estimated premorbid IQ | 113.6/7.4 | 115.4/5.0 | 1.11 | .270 |
| Purdue Pegboard (dominant hand) | 9.3/3.0 | 12.8/1.9 | 5.39 | .001* |
| Purdue Pegboard (nondominant hand) | 9.1/2.2 | 12.2/1.5 | 6.46 | .001* |
| Purdue Pegboard (both hands) | 6.8/2.0 | 10.3/1.6 | 7.49 | .001* |
| Mattis Dementia Rating Scale (total) | 140.9/2.5 | 141.8/2.3 | 1.34 | .185 |
| Beck Depression Inventory | 7.7/6.0 | 5.5/4.5 | −1.62 | .112 |
| Brief Test of Attention | 17.1/1.9 | 17.8/1.3 | 1.87 | .066 |
| Form Discrimination Test | 30.6/1.6 | 30.5/1.4 | −0.29 | .774 |
p < .001.
All relevant medical history and demographic information was obtained via medical record review and a structured interview. A diagnosis of PD, as well as disease severity ratings (Modified Hoehn and Yahr Staging [0–5]; Hoehn & Yahr, 1997), were verified by a neurologist at the Movement Disorders Center of the North Shore/Long Island Jewish Health System in Long Island, New York, as part of the patient’s clinical visit. Clinical severity of PD was limited to mild-to-moderate levels (Modified Hoehn and Yahr stages ranging from 1.5 to 3) in order to minimize confounding factors (e.g., incoherent speech or immobility). The sample included five Stage 1.5 patients, fifteen Stage 2 patients, one Stage 2.5 patient, and eleven Stage 3 patients (M = 1.92, SD = .99).1 All patients continued their anti-Parkinsonian drug regimen on the day of testing.
Participants were excluded from the study if they (1) were severely depressed (Beck Depression Inventory score of ≥ 30 [Spreen & Strauss, 1991]), (2) were demented (Mattis Dementia Rating Scale total score ≤ 123 [Montgomery & Costa, 1983]), (3) had undergone a surgical procedure for the treatment of PD, (4) had a history of neurological disorder (other than PD) and/or traumatic brain injury, (5) presented with Parkinson-plus symptomatology (e.g., myoclonus, apraxia, oculomotor abnormalities, ataxia, or sensory loss), (6) were on anticholinergic therapy (e.g., trihexyphenidyl or benztropine), (7) were taking medications that can directly or indirectly impact cognitive functioning (e.g., sedatives, anti-convulsants, and/or neuroleptics), and/or (8) indicated a prior history of alcohol/drug dependence or psychiatric disorder.
Materials
The measures selected for the current test battery have each been shown to highlight impairment and/or function of one of the three previously described prefrontal cortical regions (see Table 2). In our development of this test battery (described in Zgaljardic et al., 2003), we performed a comprehensive literature review in order to select tasks that would be most relevant for the study of executive deficits in PD and associated frontostriatal circuitry. This test battery should not be viewed as an all-inclusive assessment of prefrontal cortical functioning, as we only utilized a select number of neuropsychological measures. In a similar vein, we are not suggesting that performance on individual tests from this battery is mediated solely by the three select prefrontal regions (i.e., DLPFC, ACC, and OFC).
Table 2.
Group means and standard deviations for experimental variables by circuit category
| PD (Mean/SD) N = 32 |
NC (Mean/SD) N = 29 |
|
|---|---|---|
| ACC | ||
| Apathy Scale | 29.5/9.5 | 23.4/5.2 |
| Initial Fluency | 34.9/8.0 | 41.9/8.4 |
| Stroop Color/Word (Incongruent) | 43.1/8.2 | 49.2/7.5 |
| Stroop Interference Index | 2.6/6.1 | 5.8/6.2 |
| DLPFC | ||
| Category Fluency | 33.8/10.2 | 41.8/12.6 |
| Digit Span | 5.7/1.8 | 7.0/2.4 |
| Executive Scale | 34.7/10.4 | 29.2/7.5 |
| Phonemic Fluency | 37.0/13.1 | 45.9/15.7 |
| Odd Man Out Test | 30.6/6.4 | 37.2/5.3 |
| Petrides Conditional Associate Learning – Criterion (no. trials) | 60.0/14.9 | 45.2/17.7 |
| Petrides Conditional Associate Learning – Errors (no.) | 43.4/24.9 | 27.2/19.1 |
| Spatial Span | 5.1/1.9 | 7.1/1.5 |
| Switching Fluency | 12.4/4.2 | 14.6/2.9 |
| Switching Fluency Accuracy | 11.1/4.2 | 14.0/3.3 |
| OFC | ||
| Beck Depression Inventory | 7.7/6.0 | 5.5/4.5 |
| Disinhibition Scale | 23.5/5.4 | 23.4/6.2 |
| Alternating Loops (no. errors) | .8/2.7 | .00/.00 |
| Twenty Questions Test – Abstraction Score | 23.2/11.7 | 33.5/13.9 |
| Twenty Questions Test – Total Questions | 33.8/11.6 | 26.2/5.9 |
| Twenty Questions Test – Weighted Score | 13.7/4.1 | 15.7/3.0 |
ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex.
A rationale and detailed explanation for the assignment of each task to its particular circuit category are provided in this section. The neuropsychological tasks utilized are categorized according to (1) screening measures, (2) DLPFC measures, (3) ACC measures, and (4) OFC measures. The test battery incorporated six screening measures, six DLPFC measures, three ACC measures, and four OFC measures. In order to compensate for PD motor symptomatology, specific considerations were made in selecting the standardized tasks to minimize motor involvement and fatigue. Each participant was administered one of three counter-balanced test batteries during a single testing session that lasted approximately two hours. Informed consent was obtained.
Screening Measures
Screening measures included gross intellectual functioning—Mattis Dementia Rating scale (Mattis, 1988); estimate of premorbid level of functioning—method of Barona et al. (1984); visual discrimination—Visual Form Discrimination Test (Benton, Hamsher, Varney, & Spreen, 1983); sustained attention—Brief Test of Attention (Schretlen, 1989); mood—Beck Depression Inventory (Beck, 1987)2, motor speed and dexterity—Purdue Peg Board (Tiffin, 1968).3
DLPFC Measures
The following measures were selected based on previous research demonstrating a relationship between test performance and abilities associated with set-shifting, working memory, intrinsic response generation, and conditional associate learning.
The Odd Man Out Test (OMOT; Richards, Cote, & Stern, 1993) assesses aspects of cognitive set-shifting. The clinical utility of the OMOT in detecting set-shifting impairments in PD has been previously demonstrated (e.g., Morrison et al., 2000; Raskin et al., 1990, 1992; Richards et al., 1993). Presumably, impaired set-shifting in patients with PD results from an inability to efficiently inhibit competing responses (Taylor & Saint-Cyr, 1995). With regard to underlying cortical function, set-shifting abilities were previously described to be associated with DLPFC function (e.g., Nagahama et al., 1998). The dependent variable was total raw score.
The backward trial of the Spatial Span Test (WMS; Wechsler, 1997) is a measure of spatial working memory, and the backward trial of the Digit Span Test (WMS; Wechsler, 1997) is a measure of verbal working memory. Previous imaging studies have demonstrated that spatial working memory task performance generally activates the right DLPFC (Jonides et al., 1993), whereas verbal/auditory working memory task performance activates the left DLPFC (Paulesu, Frith, & Frackowiak, 1993). The dependent variables were total raw score for the spatial and digit span tests, separately.
The Verbal Fluency Test (Delis, Kaplan, & Kramer, 2001) assesses intrinsic word-list generation abilities with phonemic and semantic cueing. In addition, the test incorporates a set-shifting component (i.e., alternating fluency) for the semantic fluency trial. Set-shifting performance and maintaining word-list generation over time (i.e., holding task information online) have been associated with DLPFC function (Dias, Robbins, & Roberts, 1996; MacDonald, Cohen, Stenger, & Carter, 2000; Nagahama et al., 1998; Stuss et al., 1998). Successful retrieval in a verbal fluency paradigm is dependent upon an individual’s ability to monitor previous responses and to update retrieved items after each response. Thus, disruption to this system (i.e., DLPFC) would lead to a failure in updating cognitive set (i.e., phonemic or semantic cue) resulting in impaired set-maintenance (e.g., rule violations and repetition errors) and/or paucity in responding. The dependent variables included: (a) letter fluency (total correct), (b) category fluency (total correct), (c) category switching (total correct), and (d) switching accuracy (number of correct consecutive shifts between categories).
The modified version of the Petrides Conditional Associate Learning Test (Petrides, 1985) requires the learning of arbitrary associations between pairs of spatial locations using a trial-and-error strategy. Conditional associate learning (CAL) tests were originally designed for animal studies, where poor performance was associated with damage to the prefrontal cortex (e.g., Petrides, 1982). The test was modified for humans, and its sensitivity to DLPFC damage has been established. For example, Levine et al. (1997) assessed patients with focal frontal and posterior lesions and healthy young and old adults using a CAL task. The authors were able to demonstrate that aging and focal frontal damage produced similar deficits on their version of a CAL task. Here, impaired performance was attributed to strategic rather than basic associative processes due to its trial-and-error format. Performance errors were described in terms of the difficulties the participants experienced in using past information to guide on-line performance. Poor CAL performance in Levine et al.’s elderly and brain-damaged groups was described as being related to DLPFC dysfunction.
For modified CAL administration in the current study, four identical blocks were placed in specified locations in front of the participant. In addition, four identical blank white index cards were positioned next to each other in a row in front of the blocks. The blocks are labeled with numbers 1 through 4 and can only be viewed by the examiner for scoring purposes. The participants were provided with minimal instruction and were told that each index card is associated with one of the four blocks. When the examiner points to a block, the participant is instructed to point to the card they believe is associated with that particular block. The only feedback the examiner provides is to indicate whether the participant’s response is “correct” or “incorrect”. Participants must utilize a trial-and-error approach in order to learn the associations. The dependent variables include the (a) total number of trials to criterion (i.e., 12 consecutive correct responses), and (b) total number of errors.
The Frontal Systems Behavior Scale (FrSBe; Grace & Malloy, 2001) is a self-report rating scale consisting of 46 items designed to measure behaviors associated with damage to specified regions of the prefrontal cortex. The FrSBe incorporates three subscales: the (A) Apathy Scale; (D) Disinhibition Scale; and (E) Executive Dysfunction Scale. Each item is rated on a scale ranging from 1–5 (1 = almost never, 2 = seldom, 3 = sometimes, 4 = frequently, and 5 = almost always). The scale was standardized so that individual scores can be obtained for each of the three aforementioned scales. Elevated scores on the Executive Dysfunction (E) scale, in particular, have been demonstrated to represent personality traits indicative of DLPFC disruption such as poor sustained attention, working memory, organization, planning, sequencing, and problem-solving abilities. An example of a statement for this scale is as follows “Cannot do two things at once”. There are 17 items on this scale, and the dependent variable was the total Executive Dysfunction score.
ACC Measures
The following measures were selected based on previous research demonstrating a relationship between test performance and abilities associated with cognitive and behavioral functions such as response-monitoring, inhibition, initiation, and apathy.
The Stroop Color-Word Interference Test (SCWT; Golden, 1978) measures one’s cognitive flexibility and the ability to conform to changing demands and to suppress a habitual response in favor of a novel one. On the incongruent color/word trials, the participant sees color names (blue, green, and red) printed in colored ink (blue, green, and red), but must state the ink color and disregard the verbal content. Increased reaction time due to heightened conflict monitoring on incongruent color/word trials on a modified version of the Stroop task was shown to correlate with increased anterior cingulate activation in healthy adults (MacDonald et al., 2000). The dependent variables used for the current study were total score for the incongruent color/word trial and interference index.
The Verbal Fluency Test (Delis et al., 2001) was used to assess response initiation in the current study. Using a task of phonemic and semantic word-list generation, Stuss et al. (1998) assessed verbal fluency in 74 brain-damaged patients, as well as a comparison group of age-matched healthy adults. Patients with superior medial frontal lesions (including the ACC), both alone or in conjunction with other cortical lesions, were moderately impaired on a task of word-list generation and produced significantly fewer words during the initial 15 seconds than did the control group. Stuss et al.’s findings complement other work that associates ACC function with response initiation on tasks involving spontaneous verb generation, design fluency, and object construction in healthy controls (Cohen, Kaplan, Moser, Jenjins, & Wilkinson, 1999; Frith, Friston, Liddle, & Frackowiak, 1991; Petersen, Fox, Posner, Mintum, & Raichle, 1988). The dependent variable was total raw score, which was derived from the total number of words generated within the first 15 seconds of the task across phonemic, category, and category/switching conditions.
The Apathy (A) scale of the Frontal Systems Behavioral Scale (Grace & Malloy, 2001) was used as a measure of ACC function in the current study. Here, apathy is described as having diminished impulse for speech, action, and psychic initiative (Lichter & Cummings, 2000). Elevated scores on the Apathy scale have been reported to indicate behavioral and/or personality characteristics involving decreases in motivation, spontaneity, and task persistence with blunted affective expression and a lack of concern for self-care. Previous work has established a relationship between apathy and ACC dysfunction (e.g., Cohen et al., 1999). An example of an item for this scale is as follows: “I sit around doing nothing”. This scale has 14 items, and the dependent variable was total Apathy score.
OFC Measures
The following measures were selected based on previous research demonstrating a relationship between test performance and abilities associated with functions such as disinhibition, decision-making, impulsivity, and perseveration, as well as depressive symptomatology.
The Beck Depression Inventory (BDI; Beck, 1987) was used as an investigational measure addressing OFC function. Depression in PD has been associated with decreased OFC activation (for reviews, see Cummings, 1993; Masterman & Cummings, 1997; Mayberg et al., 1990). For instance, fluorodeoxyglucose (FDG) PET scanning studies have reported significant hypometabolism in the ventrodorsal caudate and orbitofrontal region of the frontal lobe in depressed patients with PD relative to nondepressed patients with PD and to NCs (for review, see Mayberg, 2000).
Each item on the BDI has four choices indicating the level of severity in which one agrees with the presenting statement. For example, the range of statements under self-hate is “3 = I hate myself,” “2 = I am disgusted in myself,” “1 = I am disappointed in myself,” “0 = I don’t feel disappointed in myself.” There are 21 items on the BDI, and the highest score obtainable for each item is 3 points. Classification of depression severity by BDI scores has been defined as 10–15 = mild; 16–19 = mild/moderate; 20–29 = moderate/severe; 30–63 = severe (Spreen & Strauss, 1991). The dependent variable was total raw score.
Elevated scores on the Disinhibition Scale (D) of the Frontal Systems Behavioral Scale (Grace & Malloy, 2001) are indicative of behavioral problems associated with disruption to the OFC. In humans, OFC disruption can lead to emotional instability (e.g., disinhibition and obsessive-compulsive behaviors) due to a dissociation of frontal monitoring systems from limbic input (for reviews, see Eslinger & Damasio, 1985; Lichter, 2000; Masterman & Cummings, 1997). Patients may exhibit social and behavioral deficits such as euphoria, diminished affect, impulsivity, social irresponsibility, and poor reasoning and decision-making abilities with relatively preserved intellect (Damasio, 1994; Rahman, Sahakian, Cardinal, Rogers, & Robbins, 2001; Rolls, 2000; Stuss et al., 1982; Stuss et al., 1983; Upton & Thompson, 1999). An example of an item for this scale is as follows: “I do things impulsively”. This scale has 15 items, and the dependent variable was total Disinhibition score.
The Alternating Loops and Letters Test (Luria, 1966) was used as a measure of motor perseveration. Participants were asked to make 10 copies of alternating letters and patterns. The dependent variable was total number of errors.
The Twenty Questions Test (Delis et al., 2001) requires participants to guess a previously designated object from a display of 30 living and non-living objects. The OFC is known to mediate reward-punishment contingencies in regards to decision-making and hypothesis-testing (Upton & Thompson, 1999). Previous reports have demonstrated that patients with OFC damage tend to exhibit poor decision-making and reasoning abilities, indicating impulsive and/or careless response generation (Beehara, Damasio, Damasio, & Anderson, 1994; Damasio, 1994; Upton & Thompson, 1996, 1999). Dependent measures of the Twenty Questions Test include (1) an initial abstraction score, (2) the number of total questions asked, and (3) the total weighted achievement score. The initial abstraction score quantifies the level of abstract thinking represented in the first question asked by the participant for each item. The most resourceful first question will eliminate the greatest number of objects from the display. A low initial abstraction score would reflect impaired categorical processing and impulsive responding behavior. The total number of questions asked serves as a global achievement measure. The fewer questions the participant asks before correctly guessing the object, the more efficient he/she is in their line of questioning, suggestive of preserved abstract thinking with a lack of impulsivity. During this test, participants are awarded bonus points for questions that are not concrete and that eliminate the most number of items. Thus, the more concrete a participant is in their responding, the fewer bonus points they will receive, hence a lower total weighted achievement score.
Results
Group Comparisons by Circuit Test Category
In order to assess the extent of group performance differences across the three neuropsychological test categories, multivariate Hotelling’s T2 test comparisons were performed. Each comparison was found to be statistically significant—DLPFC, F (10, 50) = 3.61, p = .001; ACC, F (4, 56) = 4.53, p = .003; OFC, F (6, 54) = 3.09, p = .011, indicating that NC participants performed significantly better than PD participants for each test category. Subsequent univariate between-group comparisons for each task revealed the following: for DLPFC, all 10 variables revealed significant differences (p < .05); for ACC, all 4 variables revealed significant differences (p < .05); and for OFC, only the 3 variables from the Twenty Questions Test revealed significant differences (p < .05). Indices of depression (p = .112), disinhibition (p = .954), and motor perseveration (p = .119) did not achieve statistical significance. Due to the large majority of significant findings for each circuit, effect sizes were computed to establish the magnitude of these differences. The following values were obtained: DLPFC = .419, ACC = .244, and OFC = .256. According to pre-established criteria (Cohen, 1988), these effect sizes can be defined as small. Further, effect size differences among test categories were not significant for a two-tailed test.
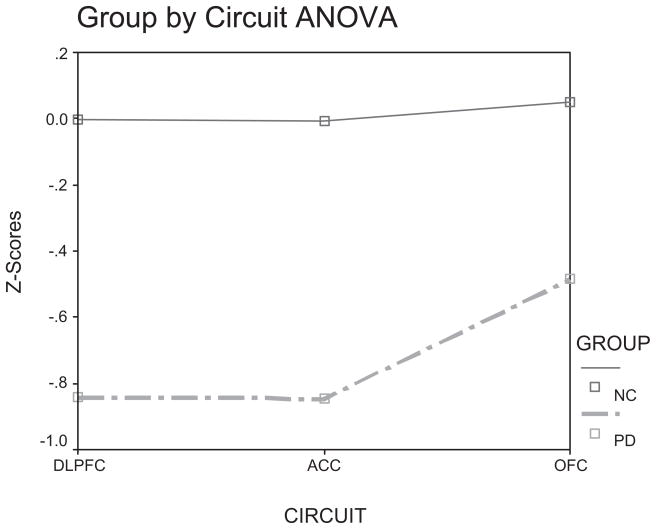
Next, in order to assess whether an interaction existed between group and circuit, a 2 (Group [PD, NC]) X 3 (Circuit [DLPFC, ACC, & OFC]) factorial Analysis of Variance (ANOVA) was performed. In order to enter all 20 experimental variables into the same analysis, all raw score data were normalized and entered as composite scores. The procedure was as follows: (a) z-scores were first generated for all NC participants, (b) z-scores for the PD participants were then obtained by utilizing the NC group as a normative sample; and (c) for each participant, z-scores were summed across each variable and then divided by the number of variables within each test category (i.e., 10 for the DLPFC, 4 for the ACC, and 6 for the OFC). The Kolmogorov-Smirnov test revealed that the z-score distributions for each measure were normal. Using this z-score transformation procedure, each participant had three composite scores, one for each circuit. The ANOVA revealed a significant Main effect of circuit, F (2, 118) = 4.63, p = .012. Post-hoc tests revealed that the DLPFC composite scores (M = −.443, SD = .78) were significantly lower (Least Significant Difference [LSD]; p = .012) than the OFC composite scores (M = −.232, SD = .61). Further, the ACC composite scores (M = −.448, SD = .86) were significantly lower than the OFC composite scores (LSD; p = .012). There was no significant difference between DLPFC and ACC composite scores (LSD; p = .949). The Main effect of Group was significant, F (1, 59) = 26.4, p < .001, with the PD group circuit composite scores (M = −.725, SD = .10) lower than those for the NC group (M = .012, SD = .104). The Group X Circuit interaction was not significant, F (1, 118) = 2.50, p = .086, but it did reveal a trend. As can be seen in Figure 1, the extent of the differences between the PD and NC groups appeared to be reduced only for the OFC composite scores.
Figure 1.
Group (PD, NC) by Circuit (DLPFC, ACC, & OFC) Analysis of Variance.
PD Performance Differences by Circuit Test Category
The binomial sign test, based on a nominal scale of measurement, was performed to assess task performance (categorized by circuit) for the PD group only using a procedure adapted from Raskin, Borod, and Tweedy (1992). This set of statistical procedures was conducted in order to control for any unusual confounding factors attributed to our sample of NC participants (e.g., endorsement of depression). First, cut-off scores (5th percentile [i.e., two standard deviations below the mean]) for each measure were obtained using standardized normative data that were subsequently dichotomized to an index score of “1” or “0”. A score of “1” was assigned to indicate performance below or equal to the cut-off (impaired performance) for a particular task, whereas a score of “0” was assigned to indicate performance above the cut-off (unimpaired performance). For this analysis, participants’ index scores (i.e., 0 or 1) were summed and averaged separately for each of the three circuits (i.e., 10 for DLPFC, 4 for ACC, and 6 for OFC). The three binomial sign test comparisons were as follows: DLPFC vs. ACC, DLPFC vs. OFC, and ACC vs. OFC. No significant index score differences were revealed when comparing the DLPFC versus the ACC, observed probability = .54, p = .845, or the ACC versus the OFC, observed probability = .68, p = .134. For the DLPFC versus the OFC comparison, a significant difference was found, observed probability = .74, p = .021, indicating that patients with PD demonstrated a greater incidence of impairment on tasks categorized as DLPFC than OFC.
Group Classification by Circuit Test Category
As our test battery was developed with three distinct subsets of measures, we felt it would be meaningful to investigate whether or not individual participant performance could predict group membership. This would allow us to gain better insight regarding the specific type of neuropsychological executive impairments in our sample of patients with PD. A Logistic Regression analysis was performed using our circuit composite scores. An overall test of the model indicated that the three circuit composite scores that were entered into the equation significantly impacted the dependent variable (X2 = 26.02; p < .001) and that 46.3% of the total variance was accounted for by the three variables entered. The Hosmer and Lemeshow Test was not significant (X2 = 5.27, p = .728), suggesting a goodness-of-fit for the model. The analysis specified that the DLPFC composite scores significantly contributed to the prediction of group membership (WALD = 3.74 [1], p = .05). The expected probabilities generated by the analysis indicated that participants who obtained a high DLPFC composite score were four times more likely to be in the NC group than in the PD group. The ACC (WALD = 1.00 [1], p = .318) and OFC (WALD = 2.80 [1], p = .094) composite score probabilities did not reach statistical significance. Classification results from this analysis revealed that of the 32 PD participants in the study, 8 had performance profiles more similar to NC participants, whereas the remaining 24 participants were correctly identified as having a diagnosis of PD.
Discussion
In the current study, we examined neuropsychological performance using standardized measures of cognitive executive functions and behavioral inventories, categorized by nonmotor frontostriatal circuits, in NDPD patients and demographically matched NC participants. First, as expected, group comparisons across neuropsychological tests revealed that our PD group performed worse than the NC group. Second, although a Group X Circuit interaction was not statistically significant, a trend suggested that the PD group’s performance on measures categorized by DLPFC and ACC functions appeared to be more severely compromised than performance on measures categorized by OFC function. Third, for the PD group alone, our findings revealed that indices of impairment appeared to be greatest for tasks categorized by DLPFC function. Fourth, an index of DLPFC circuit performance was found to be the only significant factor that discriminated individuals with PD from those without PD.
In retrospect, the lack of a statistically significant Group X Circuit interaction appears to mirror the findings from the univariate group comparisons, as the majority of group differences across the experimental tasks were significant. The statistical trend may have been influenced by a decrease in the superiority of the NC group, relative to the PD group, on OFC measures. Moreover, when assessing PD patients alone, we discovered that performance on DLPFC measures was more frequently associated with impaired performance relative to OFC measures. Task performance differences between OFC and ACC indices of impairment, as well as between ACC and DLPFC, did not achieve statistical significance. From a neuropsychological perspective, it can be speculated that the lack of a significant difference among indices of impairment for the ACC test category relative to the DLPFC or OFC test category may be attributed to the reported “dual” role of this region in mediating both cognitive and behavioral functions in humans (Cummings, 1993; Masterman & Cummings, 1997; Zgaljardic et al., 2003), whereas DLPFC and OFC functioning each appear to be more specific to cognition or behavior, respectively. Thus, it can be speculated that our findings may reflect a neuroanatomical dichotomy between cognitive and behavioral dysfunction in NDPD patients (e.g., Sarazin et al., 1998).
Aside from motor deficits, performance on measures categorized by DLPFC function may serve as a useful diagnostic indicator for PD as suggested by the findings from the current study. The logistic regression analysis revealed that an index of DLPFC category test performance was the only significant factor that optimally differentiated PDs from NCs. Within the PD group, we wanted to identify any differences between the 8 NC-like PD patients and the 24 correctly identified PD patients classified by our analysis. We performed multiple independent sample t-tests (using the Bonferroni correction), on an exploratory basis, in order to compare these two subgroups across demographic, screening, and experimental variables. Our findings revealed that the 8 NC-like participants were younger at the time of the current study and at initial PD diagnosis had higher levels of education than the correctly identified PD subgroup. For screening measures, the correctly identified PD subgroup endorsed significantly greater symptoms related to depression4, performed worse on a measure of sustained attention, and had slower reaction times on a measure of manual dexterity and speed. Of note, modified Hoehn and Yahr scores did not differ significantly between the two PD subgroups. For experimental variables, the correctly identified PD subgroup performed worse on the Spatial Span test, the Digit Span test, the modified version of the CAL test (number of trials and errors), the Odd Man Out Test, and the Executive Dysfunction scale of the FrSBe than did the NC-like PD subgroup (see Table 3). Thus, exploratory findings appear to suggest that our patient sample may have been varied with regards to disease progression, as the correctly identified PD subgroup, in general, performed worse on measures categorized by DLPFC function. Subgroup differences were not found for ACC and OFC measures.
Table 3.
Univariate comparisons for PD subgroup performance based on logistic regression analysis
| PD (N = 24) (Mean/SD) | PD/NC (N = 8) (Mean/SD) | t-value | |
|---|---|---|---|
| Age | 68.7/6.7 | 61.5/10.1 | −2.30* |
| Age at PD Dx | 63.8/8.1 | 55.8/9.3 | −2.37* |
| Education | 14.8/2.7 | 17.0/2.4 | 2.04* |
| Beck Depression Inventory | 9.0/6.0 | 3.8/4.2 | −2.32* |
| Brief Test of Attention | 16.6/1.7 | 18.4/1.8 | 2.51* |
| Purdue Pegboard (right) | 8.7/2.5 | 11.1/3.7 | 2.13* |
| Purdue Pegboard (bilateral) | 6.4/1.7 | 8.1/2.4 | 2.28* |
| Spatial Span Backwards | 4.6/1.6 | 6.8/1.7 | 3.22** |
| Petrides Conditional Associate Learning (no. trials to criterion) | 64.7/8.0 | 45.9/21.5 | −3.68** |
| Petrides Conditional Associate Learning (no. errors) | 52.2/22.2 | 17.3/9.1 | −4.28** |
| Digit Span Backwards | 5.2/1.5 | 7.3/1.8 | 3.15** |
| Executive Dysfunction. Scale | 38.2/9.7 | 24.4/2.8 | −3.91** |
| Odd Man Out Test | 28.4/5.9 | 37.1/1.6 | 4.11** |
p < .05;
p < .001.
PD/NC = PD participants classified as NC by logistic regression analysis.
Our subgroup findings are relatively consistent with prior work. For instance, Lewis, Cools, et al. (2003) reported that NDPD patients who performed worse on the Tower of London Task (designated as an indicator of impaired executive abilities) than a subgroup of NDPD patients with “preserved” executive abilities also performed significantly worse in manipulating information on a task of verbal working memory. Their two subgroups were comparable for clinical severity and on demographic and screening variables. In a smaller sample of this cohort, neuropsychological performances were associated with subgroup differences in striatal and frontal activation as assessed with functional magnetic resonance imaging (Lewis, Dove, et al., 2003).
The association between clinical disease severity and neurodegeneration in PD has been extensively monitored (for reviews, see Trost, Dhawan, Feigin, & Eidelberg, 2005; Zgaljardic & Feigin, 2004) and appears to demonstrate significantly greater DLPFC circuit disruption relative to that of the ACC and OFC circuitry. Rinne, Rummukainen, Paljarui, and Rinne (1989) reported a significant correlation between dementia severity in PD and neuronal loss in select regions of the substantia nigra that have specific projections to the caudate nucleus, suggesting that this particular subcortical region may be critical in the cognitive profile exhibited by PD patients. Dopamine depletion in the caudate nucleus (as measured by positron emission tomography [PET]) has been associated with cognitive executive declines in early-stage patients with PD (Carbon et al., 2004). This depletion appears to be greatest in the anterodorsal head of the caudate which has substantial connections with the DLPFC (Alexander, Crutcher, & DeLong, 1990; Kish et al., 1986; Rosvold, 1972; Yeterian & Pandya, 1991). Moreover, Kaasinen et al. (2003) discovered significant extrastriatal declines in D2/D3 receptor availability in early stage PD patients using PET over a 3-year period. Significant declines were noted in the left dorsolateral prefrontal cortex, left lateral temporal cortex, and the left and right thalamus. The DLPFC region exhibited the greatest decline in receptor affinity.
Alternatively, ventral regions of the caudate, which have more extensive connections with ventral regions of the frontal lobe (i.e., OFC), are relatively preserved in the early stages of PD, sparing behavioral (i.e., non-cognitive) functions maximally dependent on this neural circuitry (Cools, Barker, Sahakian & Robbins, 2001; Lewis, Cools, et al., 2003; Swainson et al., 2000). Thus, it can be inferred from previous research, especially from neuroimaging findings, that the ACC and OFC circuits would appear to be less compromised relative to the DLPFC circuit, especially in the earlier stages of the disease process. Lending further support to the notion of greater DLPFC than OFC dysfunction in NDPD patients is the fact that cognitive deficits are typically more prevalent than behavioral deficits (e.g., Green et al., 2002; Pahwa et al., 1998; Zgaljardic et al., 2003).
Methodological and statistical limitations were evident in the current study and would need to be addressed before further work in assessing the relationship between neuropsychological performance and frontostriatal circuitry can be conducted. First, one concern is content validity: essentially, do our circuit composite scores actually reflect what they were purported to measure? Here, the internal consistency among the measures within each circuit test category is a concern. Due to the preliminary nature of the current study, as well as our small sample size, factor analytic procedures were not performed. Although internal consistency would be important to consider in future work, it may be difficult to achieve as some neuropsychological executive measures are multi-faceted and may lack a defined unitary executive function (Stuss, 1993; Stuss & Alexander, 2000). In fact, it has been suggested that the various neuropsychological executive measures used in the clinical setting may not be consistent in their ability to assess cognition (e.g., Derouesne, 2003; Miyake, Friedman, Emerson, Witzki, & Howerter, 2000; Stuss, 1993). This may help explain why low correlations have been reported to exist among frontal lobe assessment measures (Stuss & Alexander, 2000). Second, the current findings are limited to individuals with PD when, in fact, patients with other neurodegenerative disorders (e.g., Alzheimer’s disease and/or Huntington’s disease) may demonstrate a similar cognitive and behavioral profile to the one produced by our patient sample. In other words, while frontostriatal circuit disruption, per se, may not be specific to PD, the relative pattern of disruption among the three circuits may be; hence, future studies in this area should include patients from other neurological populations in addition to healthy adults. Lastly, our findings appear to reflect one particular point on the disease timeline in our sample, as NDPD patients were only included if they demonstrated mild-to-moderate clinical severity. Thus, we did not assess patients at the more impaired end of the clinical spectrum. We can only speculate that patients in the later stages of PD might have exhibited greater levels of executive impairments (indicative of further disease progression) than demonstrated by our sample. However, the rate and severity of neurodegeneration in patients with PD would need to be supported by neuroimaging data (e.g., position emission tomography).
Conclusions
The current preliminary study was performed as an initial step in assessing the association between clinical neuropsychological measures and functional neuroanatomy in individuals with NDPD. Our findings, which appear to be consistent to some extent with neuropathological evidence, suggest that the DLPFC lends greater influence to the overall executive impairment found in NDPD patients, whereas cognitive and behavioral sequelae associated with ACC and OFC functions appear to be less prevalent. The relationship between prefrontal neurodegeneration in patients with PD and their neuropsychological task performance needs further delineation. Future work should attempt to validate neuropsychological measures of executive functioning by correlating indices of circuit performance with functional imaging data that have established neuroanatomically related patterns in NDPD patients. The battery of tests proposed in the current study may ultimately be used in comprehensive neuropsychological assessment of executive functions, as categorized by circuit, in PD patients within the clinical setting.
Acknowledgments
This study was based, in part, on a doctoral thesis conducted by Dennis J. Zgaljardic at Queens College and The Graduate Center of CUNY. Dennis J. Zgaljardic is now at the Department of Neuropsychology, Transitional Learning Center, Galveston, TX. This research was funded by NIH R01 NS35069 and the Susan and Leonard Feinstein Endowment for the Neuroscience at the Institute for Medical Research, North Shore-Long Island Jewish Health System. This work was supported, in part, by K24 NS02101 to Dr. Eidelberg; by K08 NS02011 to Dr. Feigin; by Professional Staff Congress (PSC)-CUNY Award 64299-0033, R01 DC01150 subcontract, and R01 MH42172 to Dr. Borod; and by PSC-CUNY Award 65288-0034 to Dr. Foldi.
Footnotes
Laterality of motor symptoms was not considered in light of previous work (St. Clair, Borod, Sliwinski, Cote, & Stern, 1998) that revealed nonsignificant differences between PD patients with either left- or right-sided motor symptoms on numerous neuropsychological measures.
The Beck Depression Inventory was used as a screening measure to exclude severely depressed participants as severe mood symptoms can compromise cognitive functioning. However, as research has demonstrated that depressive symptomatology is likely related to OFC dysfunction, the BDI was utilized as an experimental variable, as well. None of the participants in the current study met the criterion for severe depression.
Performance on the Purdue Pegboard was not used for inclusion/exclusion purposes in the current study; instead, it was utilized to gauge motor performance in our PD sample. Whereas motor severity in patients with PD was assessed using the Hoehn and Yahr scale (1997) in the current study, we wanted to incorporate an additional measure of motor dexterity and speed for both participant groups for the purpose of conducting exploratory comparisons among motor, cognitive, and behavioral task performances.
In spite of significant subgroup differences on the BDI, mean scores for the NC-like (M = 3.8) and the correctly identified (M = 9.0) PD subgroups were below the cutoff for mild depression.
Initial data from this study were presented at the annual meeting of the International Neuropsychological Society, Baltimore, MD, February 2004.
References
- Agid Y, Javoy-Agid F, Ruberg M. Biochemistry of neurotransmitters in Parkinson’s disease. In: Marsden CD, Fahn S, editors. Movement disorders. Vol. 2. London: Butterworth; 1987. pp. 166–230. [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Barbosa ER, Limongi JCP, Cummings JL. Parkinson’s disease. The Psychiatric Clinics of North America. 1997;20:769–790. doi: 10.1016/s0193-953x(05)70344-0. [DOI] [PubMed] [Google Scholar]
- Barona A, Reynolds CR, Chastain R. A demographically based index of premorbid intelligence for the WAIS-R. Journal of Consulting and Clinical Psychology. 1984;52:885–887. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to the human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory: Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Benton A, Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment. New York: Oxford University Press; 1983. [Google Scholar]
- Carbon M, Ghilardi MF, Feigin A, Fukuda M, Silvestri G, Mentis MJ, et al. Learning networks in healthy and Parkinson’s disease: Reproducibility and treatment effects. Human Brain Mapping. 2003;11:197–211. doi: 10.1002/hbm.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, et al. Caudate nucleus: Influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neurolmage. 2004;21:1384–1390. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Carbon M, Marie RM. Functional imaging of cognition in Parkinson’s disease. Current Opinion in Neurology. 2003;16:475–480. doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenjins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53:819–824. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task commands. Cerebral Cortex. 2001;12:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. Brain. 1992;115:1701–1725. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C, Duncan P. A behaviorally validated self-report inventory to assess 4 types of lateral preferences. Journal of Clinical Neuropsychology. 1979;1:55–64. [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error. New York: Putnam; 1994. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) USA: Psychological Corporation; 2001. [Google Scholar]
- Derouesne C. Semiology of executive functions. La Revue du Praticien. 2003;15:388–393. [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. Journal of Neurology. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Emre M. What causes mental dysfunction in Parkinson’s disease? Movement Disorders. 2003;18:S63–S71. doi: 10.1002/mds.10565. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Feigin A, Ghilardi MF, Carbon M, Edwards C, Fukuda M, Dhawan V, et al. Effects of levodopa on motor sequence learning in Parkinson’s disease. Neurology. 2003;60:1744–1749. doi: 10.1212/01.wnl.0000072263.03608.42. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RSJ. Willed action and the prefrontal cortex in man: A study with PET. Proceedings Biological Sciences/The Royal Society. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Edwards C, Eidelberg D. Functional brain networks in Parkinson’s disease. Parkinsonism and Related Disorders. 2001;8:91–94. doi: 10.1016/s1353-8020(01)00022-0. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Diagnosis and rehabilitation in clinical neuropsychology. Springfield, IL: Charles C. Thomas; 1978. [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy PF. Frontal Systems Behavioral Scale. Professional Manual. USA: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Green J, McDonald WM, Vitek MD, Evatt M, Freeman A, Haber M, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- Growdon JH, Kieburtz K, McDermott MP, Panisset M, Friedman JH the Parkinson Study Group. Levodopa improves motor function without impairing cognition in mild non-demented Parkinson’s disease patients. Neurology. 1998;50:1327–1331. doi: 10.1212/wnl.50.5.1327. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Unified Parkinson’s disease rating scale [UPDRS]: Modified Hoehn and Yahr staging. In: Herndon RM, editor. Handbook of clinical neurologic scales. New York: Dernos Vermande; 1997. pp. 81–91. [Google Scholar]
- Hollingshead A. Four-factor index of social status. Yale University; New Haven, CT: 1977. Unpublished manuscript. [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintum MA. Spatial working memory in humans revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Aalto S, Nagren K, Hietala J, Sonninen P, Rinne JO. Extrastriatal dopamine D(2) receptors in Parkinson’s disease: A longitudinal study. Journal of Neural Transmission. 2003;110:591–601. doi: 10.1007/s00702-003-0816-x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Rajput A, Gilbert J, Rozdilsky B, Chang LJ, Shannak K, et al. Elevated aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson’s disease: Correlation with striatal dopamine loss. Annals of Neurology. 1986;20:26–31. doi: 10.1002/ana.410200106. [DOI] [PubMed] [Google Scholar]
- Kremer J, Starkstein SE. Affective disorders in Parkinson’s disease. International Review of Psychiatry. 2000;12:290–297. [Google Scholar]
- Levine B, Stuss DT, Milberg WP. Effects of aging on conditional associate learning process analyses and comparison with focal frontal lesions. Neuropsychology. 1997;11:367–381. doi: 10.1037//0894-4105.11.3.367. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. The Journal of Neuroscience. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter DG. Movement disorders and frontal-subcortical circuits. In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: The Guildford Press; 2000. pp. 260–313. [Google Scholar]
- Lichter DG, Cummings JL. Introduction and Overview. In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: The Guildford Press; 2000. pp. 1–43. [Google Scholar]
- Luria AR. Higher cortical functions in man. New York: Basic Books; 1966. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Masterman DL, Cummings JL. Frontal-subcortical circuits: The anatomic basis of executive, social, and motivated behaviors. Journal of Psychopharmacology. 1997;11:107–114. doi: 10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Mayberg HS. Depression and frontal-subcortical circuits: Focus on prefrontal limbic interactions. In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: The Guildford Press; 2000. pp. 177–206. [Google Scholar]
- Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, et al. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Annals of Neurology. 1990;28:57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. A revised neuroanatomy of the fronto-subcortical circuits. In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: The Guildford Press; 2000. pp. 44–58. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Montgomery KM, Costa L. Neuropsychological test performance of a normal elderly sample. Paper presented at the annual meeting of the International Neuropsychological Society; Mexico City, Mexico. 1983. Feb, [Google Scholar]
- Morrison CE, Borod JC, Brin MF, Raskin SA, Germano IM, Weisz DJ, et al. A program for neuropsychological investigation of deep brain stimulation (PNIDBS) in movement disorder patients: Development, feasibility, and preliminary data. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2000;13:204–219. [PubMed] [Google Scholar]
- Nagahama Y, Sadato N, Yamauchi H, Katsumi Y, Hayashi T, Fukuyama H, et al. Neural activity during attention shifts between object features. NeuroReport. 1998;9:2633–2638. doi: 10.1097/00001756-199808030-00038. [DOI] [PubMed] [Google Scholar]
- Pahwa R, Paolo A, Tröster A, Koller W. Cognitive impairment in Parkinson’s disease. European Journal of Neurology. 1998;5:431–441. doi: 10.1046/j.1468-1331.1998.550431.x. [DOI] [PubMed] [Google Scholar]
- Parkinson J. Essay of the shaking palsy. London: Whittingham & Rowland for Sherwood, Neeley and Jones; 1817. [Google Scholar]
- Paulesu E, Frith CD, Frackowiack RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintum M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petrides M. Motor conditional associative-learning after selective prefrontal lesions in the monkey. Behavioral Brain Research. 1982;5:407–413. doi: 10.1016/0166-4328(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. Deficits on conditional associative-learning tasks after frontal and temporal lobe lesions in man. Neuropsychologia. 1985;23:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- Przuntek H. Non-dopaminergic therapy in Parkinson’s disease. Journal of Neurology. 2000;247(Suppl 2):II/19–II/24. doi: 10.1007/pl00007756. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Cardinal RN, Rogers RD, Robbins TW. Decision making and neuropsychiatry. Trends in Cognitive Sciences. 2001;5:271–277. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- Raskin SA, Borod JC, Tweedy J. Neuropsychological aspects of Parkinson’s disease. Neuropsychology Review. 1990;1:185–221. doi: 10.1007/BF01112571. [DOI] [PubMed] [Google Scholar]
- Raskin SA, Borod JC, Tweedy JR. Set-shifting and spatial orientation in patients with Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology. 1992;14:801–821. doi: 10.1080/01688639208402864. [DOI] [PubMed] [Google Scholar]
- Richards M, Cote LJ, Stern Y. Executive function in Parkinson’s disease: Set-shifting or set-maintenance? Journal of Clinical and Experimental Neuropsychology. 1993;15:266–279. doi: 10.1080/01688639308402562. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Rummukainen J, Paljarui L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Annals of Neurology. 1989;26:47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rosvold HE. The frontal lobe system: Cortical-subcortical interrelationships. Acta Neurobiologiae Experimentalis. 1972;32:439–460. [PubMed] [Google Scholar]
- Sarazin M, Pillon B, Giannakopoulos P, Rancurel G, Samson Y, Dubois B. Clinicometabolic dissociation of cognitive functions and social behavior in frontal lobe lesions. Neurology. 1998;51:142–148. doi: 10.1212/wnl.51.1.142. [DOI] [PubMed] [Google Scholar]
- Schretlen D. Brief test of attention. Odessa, FI: Psychological Assessment Resources; 1989. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1991. [Google Scholar]
- Starkstein SE, Robinson RG. Depression in neurologic disease. Baltimore: John Hopkins Univesity Press; 1993. [Google Scholar]
- St Clair J, Borod JC, Sliwinski M, Cote LJ, Stern Y. Cognitive and affective functioning in Parkinson’s disease patients with lateralized motor signs. Journal of Clinical and Experimental Neuropsychology. 1998;20:320–327. doi: 10.1076/jcen.20.3.320.820. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Assessment of neuropsychological dysfunction in frontal lobe degeneration. Dementia. 1993;4:220–225. doi: 10.1159/000107326. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:265–278. [PubMed] [Google Scholar]
- Stuss DT, Benson DF, Kaplan EF, Weir WS, Lieberman MA, Ferrill D. The involvement of the orbitofrontal cerebrum in cognitive tasks. Neuropsychologia. 1983;21:235–248. doi: 10.1016/0028-3932(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Kaplan EF, Benson DF, Weir WS, Chiulli S, Sarazin FF. Evidence for the involvement of orbitofrontal cortex in memory functions: An interference effect. Journal of Comparative and Physiological Psychology. 1982;96:913–925. doi: 10.1037/0735-7036.96.6.913. [DOI] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: Possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA. The neuropsychology of Parkinson’s disease. Brain and Cognition. 1995;28:281–296. doi: 10.1006/brcg.1995.1258. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. Brain. 1986;109:845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue Pegboard: Examiner Manual. Chicago: Science Research Associates; 1968. [Google Scholar]
- Trost M, Dhawan V, Feigin A, Eidelberg D. PET/SPECT. In: Beal MF, Lang A, Ludolph A, editors. Neurodegenerative diseases: Neurobiology, pathogenesis, and therapeutics. Cambridge, UK: Cambridge University Press; 2005. pp. 290–300. [Google Scholar]
- Upton D, Thompson PJ. Epilepsy in the frontal lobes: Neuropsychological characteristics. Journal of Epilepsy. 1996;9:215–222. doi: 10.1016/0920-1211(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Upton D, Thompson PJ. Twenty questions task and frontal lobe dysfunction. Archives of Clinical Neuropsychology. 1999;14:203–216. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale – III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. The Journal of Comparative Neurology. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Foldi NS, Borod JC. Cognitive and behavioral dysfunction in Parkinson’s disease: Neurochemical and clinicopathological contributions. Journal of Neural Transmission. 2004;111:1287–1301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Eidelberg D. Corpus striatum. In: Aminoff M, Daroff R, editors. Encyclopedia of the neurological sciences. San Diego: Academic Press; 2003. pp. 774–777. [Google Scholar]
- Zgaljardic DJ, Feigin A. Neuroimaging of PD and atypical parkinsonism. Current Neurological Neuroscience Reports. 2004;4:284–289. doi: 10.1007/s11910-004-0053-1. [DOI] [PubMed] [Google Scholar]