Abstract
Left atrial (LA) perfusion during disease states has been a topic of much interest, as the clinical implications and detrimental effects of lack of blood flow to the atria are numerous. In the chronic setting, perfusion changes may lead to LA ischemia and structural remodeling, a factor implicated in the self-perpetuation of chronic atrial fibrillation (AF). The association between AF and altered LA perfusion has been studied; however, a direct causal association between perfusion changes and AF has not been established. A comprehensive literature search of Medline, Embase and Google Scholar databases was conducted from 1960 to February 2014. We systematically analyzed reference lists of physiological articles and reviews for other possible relevant studies.
The aim of this review is to provide a comprehensive discussion of the AF-mediated changes in LA perfusion and the potential mechanisms underlying the alterations in coronary flow to the LA in this setting. In addition, we discuss the clinical contexts in which changes in LA perfusion may be relevant. Finally, this article highlights the need for longitudinal AF studies that would elucidate the changes in LA perfusion resulting from chronic AF and lead to advancements in effective treatments to prevent progression of this disease.
Keywords: myocardium, perfusion, sympathetic activity
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice and is growing as the population ages. AF is associated with increased mortality (Benjamin et al., 1994), however the etiology of AF is poorly understood. It is possible that LA ischemia and remodeling play important roles in the pathophysiology of AF. These factors may initiate and perpetuate AF and may also represent conditions resulting from this arrhythmia. This review paper will focus on the evidence for LA perfusion abnormalities during AF and examine possible mechanisms for altered perfusion in this setting. The clinical importance of the findings to date and future direction of research in this arena will also be discussed.
Atrial hemodynamics and perfusion during acute AF
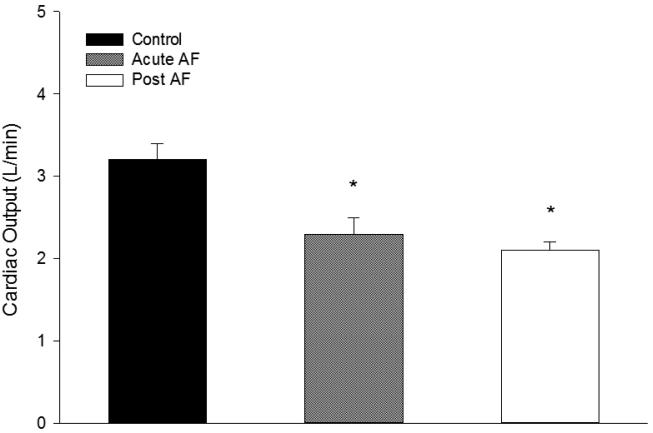
One of the hallmarks of atrial fibrillation, the irregularity of the ventricular response, has been implicated as an independent contributor to hemodynamic abnormalities observed during AF. Several investigators have shown that cardiac output is reduced during acute AF (McHale et al., 1983; Friedman et al., 1987) (Fig. 1). In addition, studies show that acute AF may cause increased atrial pressure, decreased compliance, and increased atrial metabolic demand. In the 1980s, several groups examined the effects of AF on atrial blood flow. In a dog model of acute AF, White et al. (White et al., 1986) used radioactive microspheres to measure atrial blood flow. At rest, pacing-induced AF increased flow to both atria 2.3-fold compared to sinus rhythm. Total flow to both atria increased from 6% of total coronary flow during sinus rhythm to 13% during acute AF. These changes were similar between electrically maintained and spontaneous AF but were not observed during rapid atrial pacing without AF (White et al., 1986). McHale and colleagues (McHale et al., 1983) used microspheres to study the effects of AF on atrial blood flow in conscious dogs with heart block. They reported a 180% increase in atrial blood flow during AF compared to sinus rhythm. The authors concluded acute AF induced by electrical stimulation significantly increases atrial blood flow and this increase may be due in part to the high energy demands of the fibrillating atria, however metabolic parameters were not examined by the investigators.
Fig 1.
Mean cardiac output during control, acute atrial fibrillation (AF) and after atrial fibrillation (post AF) in 18 dogs. *Significant difference compared to control (p<0.05). Adapted with permission from Friedman et al (1987).
Atrial perfusion reserve during acute AF
Most LA perfusion studies measure the flow in the coronary vessels feeding the left ventricle; to our knowledge, there are very few studies that have examined perfusion reserve or reactive hyperemia in the LA during AF. White et al. found a 3.9-fold increase in LA blood flow during AF under vasodilation with chromonar (White et al., 1986). McHale et al. (McHale et al., 1983) measured left atrial perfusion reserve in 11 dogs with heart block using radiolabeled microspheres. The authors found atrial blood flow increased by 146% during AF with adenosine challenge compared to AF alone and concluded that the atrial blood flow during resting AF does not represent maximal flow; LA blood flow is regulated at a level consistent with its metabolic demand. These conclusions are in contrast to those made by van Braght et al. (van Bragt et al., 2013) who found an increase in lactate production during acute AF, which suggests blood flow to the LA is not sufficient to meet its metabolic demands. Future AF studies measuring atrial perfusion reserve in conjunction with atrial metabolites are warranted, since a reduction in blood flow to the atria does not necessarily mean there are ischemic conditions.
Mechanisms of perfusion regulation during AF
It is well established that the regulation of coronary flow to the metabolic need of the atrial myocardium is altered during AF (Neill et al., 1980; White et al., 1982). The mechanisms regulating coronary flow during AF are multifaceted and less understood. In contrast to the human studies, some investigators report a decrease in coronary vascular resistance (CVR) during AF and a concomitant increase or maintenance of local blood flow to the myocardium in the face of decreased arterial pressure. For example, Wichmann et al. (Wichmann et al., 1983b) measured the effects of acute AF on left circumflex (LCX) coronary flow using a flow probe in 21 anesthetized open-chest dogs. They reported preservation of coronary flow concomitant with a decrease in CVR and an increase in coronary oxygen extraction by the myocardial tissue during acute AF. However, when maximal dilation was induced during AF, CVR was significantly higher and LCX coronary flow was reduced (Fig. 2) suggesting the coronary regulation of blood flow is impaired. These results may be attributed to a reflexive neural constrictive component that occurs concomitantly with coronary vasodilation during acute AF, but may only be unmasked during maximal coronary dilation.
Fig 2.
A: Left circumflex coronary blood flow (CBF) and coronary vascular resistance (CVR) before (control) maximal coronary dilatation in 21 dogs; B: CBF and CVR after (carbochromen) maximal coronary dilatation. Adapted with permission from Wichmann et al (1983b).
Neural Mechanisms – sympathetic effects on coronary vasculature
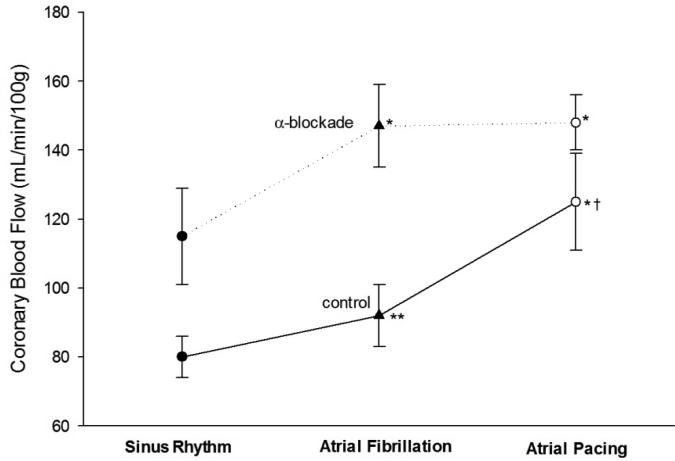
Similar to ischemic events associated with sympathetic activity during sinus rhythm (Heusch et al., 2000), the sympathetic nervous system has been implicated in the mechanisms for coronary vasoconstriction during acute AF (Wichmann et al., 1983a; Kochiadakis et al., 2001). The fall in blood pressure accompanying AF may be offset by a reflexive vasoconstriction to maintain pressure (Skinner et al., 1964; Wichmann et al., 1983a). For example, (Wichmann et al., 1983a) examined the effects of acute AF and atrial pacing on coronary flow before and after α-receptor blockade in 14 dogs with acute AF. They found an increase in plasma catecholamines and coronary blood flow and an increase in CVR during acute AF. Following α-receptor blockade, the differences in coronary blood flow and coronary vascular resistance between AF and atrial pacing were abolished, suggesting that a reflexive increase in sympathetic activity mediates coronary vasoconstriction during acute AF but not during atrial tachycardia alone (Fig. 3) (Wichmann et al., 1983a). In addition, previous work from investigators in our group has shown that sympathetic nerve activity is elevated in patients with chronic AF (Wasmund et al., 2003) and that this elevation is dependent on the irregularity of the RR intervals during AF (Segerson et al., 2007).
Fig 3.
Coronary blood flow before (control) and after alpha-receptor blockade with phenoxybenzamine (10 mg/kg, intravenously) in 14 dogs with acute AF. Adapted with permission from Wichmann et al (1983a).
Humoral Mechanisms
Both Angiotensin-II (Ang-II) and Endothelin-1 (ET-1) play important roles in the regulation of coronary blood flow in normal and cardiovascular disease states and may be a potential link between AF and abnormal LA perfusion. Ang-II activates a cascade of events, including oxidative stress (Doughan et al., 2008), that may impair LA blood flow and induce ischemia (Goette et al., 2009). Goette et al. (Goette et al., 2008) found that plasma concentrations of Ang-II were significantly elevated in patients with permanent and paroxysmal AF compared to controls. These data suggest Ang-II may play a role in the coronary vasoconstriction observed during acute AF. Cardin et al. (Cardin et al., 2003) measured tissue concentrations of Ang-II in a canine model of congestive heart failure that produced AF and atrial fibrosis similar to that observed in clinical AF. They found elevated tissue concentrations of Ang-II during AF. However, after inhibition of Ang-II, structural remodeling was only partially prevented. These results suggest AF-mediated changes in LA structure may involve more than one humoral pathway.
ET-1 is a strong vasoconstrictor which may cause a decrease in LA perfusion during maximal dilation and induce ischemia (Goette et al., 2012). Mayyas et al. (Mayyas et al., 2010) examined left atrial appendage tissue in patients undergoing cardiac surgery. They found an association between ET-1 and fibrosis and a positive correlation between ET-1 content and LA size and AF persistence. Literature also suggests a vicious cycle between perfusion abnormalities and ET-1 release during AF such that AF-mediated changes in perfusion enhance release of ET-1 which in turn aggravates coronary vasoconstriction and flow abnormalities (Neubauer et al., 1991; Hiller et al., 1997; Goette et al., 2012).
The relative contributions of altered hemodynamics, neural and humoral factors affecting coronary flow during AF remain unclear. These data highlight the complexity of the regulation of coronary blood flow during AF; there is most likely an interaction between humoral and neural factors mediating coronary flow, vascular resistance and LA perfusion during AF. It is possible that during acute AF, vasodilatory factors mask vasoconstrictive mechanisms leading to a net increase in perfusion. It is also possible that during chronic AF, control of coronary flow is altered, creating a net vasoconstrictive effect and thus decreasing perfusion.
Pathophysiologic implications of altered perfusion during AF
Many researchers are working to increase our understanding of the consequences of AF. AF is thought to be a self-perpetuating disease (Kopecky et al., 1987; Wijffels et al., 1995), with ischemia and fibrosis as the major culprits (Thijssen et al., 2000; Thijssen et al., 2001; Allessie et al., 2002; Kostin et al., 2002). It is plausible that AF creates a milieu favoring ischemia and structural changes such as fibrosis (Ausma et al., 1997). It has been postulated that AF causes an increase in coronary blood flow demand exceeding the perfusion reserve such that there is a supply/demand mismatch of the atrial myocardium (van Bragt et al., 2013). In the chronic state, this mismatch might result in recurrent bouts of atrial ischemia. A deficient oxygen supply to the LA can result in electrical and structural remodeling such as fibrosis (De Boer et al., 2003), an irreversible structural change that causes an exacerbation of ischemia due to longer oxygen diffusion pathways through the tissue. Fibrosis also leads to electrophysiologic changes favoring re-entry pathways (De Boer et al., 2003).
Autopsy studies report a correlation between the degree of structural damage of the atria and the duration of AF (Davies & Pomerance, 1972). In addition, new research from our group has shown that LA fibrosis assessment using magnetic resonance imaging is predictive of successful ablation such that AF patients with minimal fibrosis have a better prognosis of lack of recurrence following ablation whereas those with extensive fibrosis have a greater likelihood of recurrence (McGann et al., 2014). Thus, it is clinically important to study the effects of AF on LA perfusion, as this disease creates a vicious cycle of detrimental effects to the myocardium.
Clinical Implications
The alterations in LA perfusion have been implicated as one of the consequences of acute AF. However, short-term experiments do not elucidate whether chronic AF is associated with similar changes. Longitudinal AF studies are necessary to follow the development of perfusion and structural changes and produce clinically relevant data. Importantly, understanding the changes in LA perfusion longitudinally may lead to a better understanding of the cascade of events leading to ischemia and structural changes observed in patients with chronic AF. It may also lead to better coronary disease risk assessment and AF prognosis. Finally, the degree to which perfusion abnormalities due to AF are reversible is an important question in understanding the perpetuation of AF and may lead to therapies that prevent progression of AF.
Conclusions
Many facets of AF remain unknown, including the consequences of chronic AF on LA perfusion. Whether AF causes the LA myocardium to become ischemic over time is unknown. While perfusion abnormalities and atrial ischemia have been implicated in the detrimental effects of chronic AF on the myocardium, there is a paucity of data supporting this notion. The etiology of AF is complex and research requires longitudinal studies to follow the evolution of perfusion abnormalities and their consequences and produce clinically relevant data. Experimental models make it possible to study changes in LA perfusion over time from a multidisciplinary perspective. Using this approach will prove essential to uncover the mechanisms of the perpetuation of AF and aid in the development of appropriate therapies for its management.
NEW FINDINGS.
The topic of this review is the effects of atrial fibrillation (AF) on left atrial perfusion.
This review highlights the interplay between AF, ischemia and fibrosis. Advances in fibrosis detection can better predict AF recurrence and effectiveness of ablative techniques; perfusion abnormalities may hold more clinical relevance than once thought.
REFERENCES
- Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J. 1972;34:520–525. doi: 10.1136/hrt.34.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer RA, Pinto YM, Van Veldhuisen DJ. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation. 2003;10:113–126. doi: 10.1038/sj.mn.7800188. [DOI] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Friedman HS, O'Connor J, Kottmeier S, Shaughnessy E, McGuinn R. The effects of atrial fibrillation on regional blood flow in the awake dog. Can J Cardiol. 1987;3:240–245. [PubMed] [Google Scholar]
- Goette A, Bukowska A, Dobrev D, Pfeiffenberger J, Morawietz H, Strugala D, Wiswedel I, Rohl FW, Wolke C, Bergmann S, Bramlage P, Ravens U, Lendeckel U. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30:1411–1420. doi: 10.1093/eurheartj/ehp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goette A, Bukowska A, Lendeckel U, Erxleben M, Hammwohner M, Strugala D, Pfeiffenberger J, Rohl FW, Huth C, Ebert MP, Klein HU, Rocken C. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation. 2008;117:732–742. doi: 10.1161/CIRCULATIONAHA.107.730101. [DOI] [PubMed] [Google Scholar]
- Goette A, Bukowska A, Lillig CH, Lendeckel U. Oxidative Stress and Microcirculatory Flow Abnormalities in the Ventricles during Atrial Fibrillation. Front Physiol. 2012;3:236. doi: 10.3389/fphys.2012.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101:689–694. doi: 10.1161/01.cir.101.6.689. [DOI] [PubMed] [Google Scholar]
- Hiller KH, Roder F, Adami P, Voll S, Kowallik P, Haase A, Ertl G, Bauer WR. Study of microcirculation by coloured microspheres and NMR-microscopy in isolated rat heart: effect of ischaemia, endothelin-1 and endothelin-1 antagonist BQ 610. J Mol Cell Cardiol. 1997;29:3115–3122. doi: 10.1006/jmcc.1997.0538. [DOI] [PubMed] [Google Scholar]
- Kochiadakis GE, Skalidis EI, Kaleboubas MD, Igoumenidis NE, Hamilos MI, Parthenakis FI, Vardas PE. Effect of acute atrial fibrillation on coronary circulation. Am J Cardiol. 2001;88:1431–1433. A1438. doi: 10.1016/s0002-9149(01)02129-4. [DOI] [PubMed] [Google Scholar]
- Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr., Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, Van Wagoner DR. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–379. doi: 10.1161/CIRCEP.109.924985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, Burgon NS, Greene T, Kim D, Dibella EV, Parker D, Macleod RS, Marrouche NF. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale PA, Rembert JC, Greenfield JC., Jr. Effect of atrial fibrillation on atrial blood flow in conscious dogs. Am J Cardiol. 1983;51:1722–1727. doi: 10.1016/0002-9149(83)90218-7. [DOI] [PubMed] [Google Scholar]
- Neill WA, Sewell D, Gopal M, Oxendine J, Painter L. Independent regulation of atrial coronary blood flow by atrial contraction rate in conscious dogs. Pflugers Arch. 1980;388:193–195. doi: 10.1007/BF00584128. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Zimmermann S, Hirsch A, Pulzer F, Tian R, Bauer W, Bauer B, Ertl G. Effects of endothelin-1 in the isolated heart in ischemia/reperfusion and hypoxia/reoxygenation injury. J Mol Cell Cardiol. 1991;23:1397–1409. doi: 10.1016/0022-2828(91)90186-p. [DOI] [PubMed] [Google Scholar]
- Segerson NM, Sharma N, Smith ML, Wasmund SL, Kowal RC, Abedin M, Macgregor JF, Pai RK, Freedman RA, Klein RC, Wall TS, Stoddard G, Hamdan MH. The effects of rate and irregularity on sympathetic nerve activity in human subjects. Heart Rhythm. 2007;4:20–26. doi: 10.1016/j.hrthm.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Skinner NS, Jr., Mitchell JH, Wallace AG, Sarnoff SJ. Hemodynamic Consequences of Atrial Fibrillation at Constant Ventricular Rates. Am J Med. 1964;36:342–350. doi: 10.1016/0002-9343(64)90160-3. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Ausma J, Borgers M. Structural remodelling during chronic atrial fibrillation: act of programmed cell survival. Cardiovasc Res. 2001;52:14–24. doi: 10.1016/s0008-6363(01)00367-4. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M. Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol. 2000;9:17–28. doi: 10.1016/s1054-8807(99)00038-1. [DOI] [PubMed] [Google Scholar]
- van Bragt KA, Nasrallah HM, Kuiper M, Luiken JJ, Schotten U, Verheule S. Atrial supply-demand balance in healthy adult pigs: coronary blood flow, oxygen extraction, and lactate production during acute atrial fibrillation. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt239. [DOI] [PubMed] [Google Scholar]
- Wasmund SL, Li JM, Page RL, Joglar JA, Kowal RC, Smith ML, Hamdan MH. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation. 2003;107:2011–2015. doi: 10.1161/01.CIR.0000064900.76674.CC. [DOI] [PubMed] [Google Scholar]
- White CW, Holida MD, Marcus ML. Effects of acute atrial fibrillation on the vasodilator reserve of the canine atrium. Cardiovasc Res. 1986;20:683–689. doi: 10.1093/cvr/20.9.683. [DOI] [PubMed] [Google Scholar]
- White CW, Kerber RE, Weiss HR, Marcus ML. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res. 1982;51:205–215. doi: 10.1161/01.res.51.2.205. [DOI] [PubMed] [Google Scholar]
- Wichmann J, Ertl G, Hohne W, Schweisfurth H, Wernze H, Kochsiek K. Alpha-receptor restriction of coronary blood flow during atrial fibrillation. Am J Cardiol. 1983a;52:887–892. doi: 10.1016/0002-9149(83)90435-6. [DOI] [PubMed] [Google Scholar]
- Wichmann J, Ertl G, Rudolph G, Kochsiek K. Effect of experimentally induced atrial fibrillation on coronary circulation in dogs. Basic Res Cardiol. 1983b;78:473–491. doi: 10.1007/BF01906459. [DOI] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]