Abstract
Background
Photodynamic therapy with aminolevulinic acid (ALA PDT) for oral leukoplakia has shown promising effects in regression of oral leukoplakia. Although ALA has been extensively studied and is an ideal photosensitizer, the optimal light dose for treatment of oral leukoplakia has not been determined. We conducted a phase I study to determine MTD and DLT of PDT in patients treated with ALA for leukoplakia.
Methods
Patients with histologically confirmed oral leukoplakia received a single treatment of ALA PDT in cohorts with escalating doses of light (585 nm). Clinical, histologic, and biologic markers were assessed.
Results
Analysis of eleven participants is reported. No significant toxicity from ALA PDT was observed in patients who received ALA with a light dose of up to 4 J/cm2. One participant experienced transient grade 3 transaminase elevation due to ALA. One participant had a partial clinical response 3 months after treatment. Biologic mucosal risk markers showed no significant associations. Determination of MTD could not be accomplished within a feasible timeframe for completion of the study.
Conclusions
ALA PDT could be safely administered with a light dose up to 4 J/cm2 and demonstrated activity. Larger studies are needed to fully elucidate the MTD and efficacy of ALA-PDT.
Keywords: leukoplakia, aminolevulinic acid, photodynamic therapy, phase I, laser
Introduction
Each year in the United States over 40,000 new cases of head and neck squamous cell cancer (HNSCC) are diagnosed, with approximately 27,000 of these cases occurring in the oral cavity or pharynx. 1 Oral cavity cancer is associated with a poor prognosis with 5 year survival rates of less than 50%. 1 While incremental advances have been achieved in the management of SCCHN, our failure to achieve substantial improvements in prognosis for the majority of SCCHN patients underscores the need to investigate effective strategies for cancer prevention.
The presentation of potentially malignant disorders of the oral cavity is highly variable. Cytologic dysplasia, which occurs when architectural disturbance is accompanied by cytologic atypia, correlates well with subsequent development of invasive squamous cell carcinoma.2 Leukoplakia is a condition characterized by a white patch or plaque involving the oral mucosa. Leukoplakia may coexist with varying grades of dysplasia and is generally also considered to be a potentially malignant disorder. Oral leukoplakia is therefore an excellent clinical model for examining the cancer prevention strategies.
Photodynamic therapy (PDT) involves the topical or systemic administration of a photosensitizing agent that, in the presence of light corresponding to an optimal wavelength, creates reactive oxygen species capable of inducing cytotoxic damage. Aminolevulinic acid (ALA) is a photosensitizing agent that is an endogenous metabolite present in virtually all mammalian cells. It is the product of the first committed step in heme biosynthesis, and is a metabolic precursor of the endogenously formed photosensitizer, protoporphyrin IX (PpIX). Upon exposure to light within one of the absorption peaks of PpIX (410-635 nm), cytotoxic free radical species are generated. Based upon its biochemical properties, ALA has a number of potential advantages compared to other photosensitizing agents. ALA is excreted rapidly, which limits its potential phototoxic side effects. Administration of ALA bypasses the negative feedback control of its metabolic pathway, and leads to selective accumulation of PpIX in mucosal and epithelial tissues. 3 By preferentially accumulating in the mucosa, rather than the underlying stroma, PpIX thereby reduces damage to deeper layers. In addition, several reports suggest that PpIX may accumulate preferentially in dysplasia and tumor rather than normal tissue. 4, 5
Use of PDT for Barrett's esophagus is well established. A considerable number of published reports have examined the use of ALA as a photosensitizer for PDT in Barrett's mucosa. 6-10 Significantly less information exists regarding ALA PDT for oral leukoplakia. However, available data on ALA PDT for oral leukoplakia is promising. 11-13 Small single institution studies have examined various doses of both topical and orally administered ALA, followed by light therapy of varying light intensities from 100 to 200 J/cm2. These studies have had in common observations of high response rates, defined by complete and partial resolution of macroscopic disease, but also frequent observations of acute pain of sufficient degree to require analgesia. Unfortunately, these trials did not rigorously examine toxicity in relation to the optimal dose or schedule of light intensity. In the present trial we examined ALA PDT in oral leukoplakia to determine the optimal light intensity when given with a fixed dose of orally administered ALA, as well as toxicity and tolerability and association with mucosal risk markers.
Participants and Methods
Participants
Subjects eligible for the study were required to have histologically confirmed oral leukoplakia with dysplasia OR with hyperplasia in a high-risk area (floor of mouth, tongue or oropharynx). Subjects with oral leukoplakia with hyperplasia in a NON-high-risk location (such as frictional keratoses in the buccal mucosa from ill-fitting dentures) were not eligible. Patients with previous early stage (I and II) head and neck cancer were eligible if disease free for 2 years following definitive treatment. Prior treatment, including experimental therapy was permissible if experimental treatment was completed ≥ 3 months prior to study entry. Other eligibility criteria were as follows: age ≥ 18 years; Zubrod ≤ 1; adequate organ function defined by: Hgb > 12 gm/dl, platelets > 100,000/μL, ANC ≥ 1500/μL, creatinine ≤ 1.5 mg/dl, SGPT and SGOT≤ 1.5× the institutional upper limit of normal (ULN), total bilirubin ≤1.5× the institutional ULN; life expectancy > 2years; signed written informed consent.
Treatment and Dose Escalation Schedule
ALA was provided by DUSA Pharmaceuticals Inc. (Wilmington, MA) with long pulse dye laser 585 nanometer (nm) using Cynosure PhotoGenica SV pulsed dye laser system. ALA powder was administered by mouth as a single dose dissolved in 50 ml of water 3-4 hours before light treatment. The initial dose of ALA was 60 mg, but this was amended to 30 mg after a participant experienced grade 3 transaminase elevation ascribed to ALA. Participants were given strict instructions for light protection for 24 hours following ALA administration. A laser spot size of 10 mm was used with a specific laser setting (Joules) to achieve the desired fluence (Joules/cm2).
Dose escalation cohorts were planned with 4 participants per cohort--each corresponding to a light dose from 2 to 8 J/cm2 by increments of 2 J/cm2. The first cohort received no ALA. Subsequent cohorts received a fixed dose of ALA without escalation. Light dose escalation was performed according to the following plan: if dose limiting toxicity (DLT), defined as any grade 3 toxicity, was experienced in at least 1 of 4 participant in a cohort, 2 more participants were to be treated on the next lower dose cohort (N = 6). If an additional participant on the lower dose cohort experienced one or more DLTs, additional participants were to be enrolled on the next lower cohort until either a dose cohort had 0/6 participants with DLTs or the lowest dose cohort had treated 6 participants. If 0/4 participants experienced DLT(s) at the highest dose cohort, 2 additional participants were to be enrolled at that cohort. The maximimum tolerated dose, MTD, was defined as the highest dose at which 0 of 6 experienced grade 3 or greater toxicity.
All four subjects in a dose level had to complete one month of follow-up without dose limiting toxicity (DLT) prior to enrollment of subjects in the next dose level. Only one subject was to be treated at a time; the next subject on the same dose level was not to be treated until the prior subject had completed safety assessment at 24-48 hours following treatment. Safety assessment was performed at 24-48 hrs, 14 days, and 1 and 3 months post-PDT. Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3. As participants only received ALA and PDT one time, no dose modification was planned for any given participant. DLT was defined as being any grade 3 toxicity, with the exception of asymptomatic LFTs ≤15× the upper limit of normal (ULN) that resolved to grade 2 or lower by day 14. Dose escalation was planned to continue in the presence of grade 2 toxicity. If a DLT was experienced in at least 1 of 4 participants in a given cohort, 2 more individuals were to be treated on the next lower dose cohort (N = 6). If an additional participant on the lower dose cohort experienced one or more DLTs, additional subjects were to be enrolled on the next lower cohort until either a dose cohort had 0/6 subjects with DLTs or the lowest dose cohort had treated 6 patients. If 0/4 subjects were to experience DLT(s) at the highest dose cohort, 2 additional patients were to be enrolled in that cohort. The maximum tolerated dose (MTD) was defined as the highest dose at which 0 of 6 participants experienced grade 3 or greater toxicity.
Definitions of Response
Clinical response was defined as follows: Complete response—disappearance of all measurable disease; Partial response—decrease in the cross-sectional areas of a measurable leukoplakia lesion by at least 50% in the product of the two longest diameters of a single lesion in the absence of new “in field” lesions.; Stable disease—decrease in the cross-sectional area of a measurable leukoplakia lesion by <50% of the product (or sum of products) of measured lesion(s) diameters in the absence of new “in field” lesions; Progressive disease—increase in the product (or sum of products) of the measured lesion diameters by ≥25% or development of a new “in field” leukoplakia lesion. If multiple lesions were treated, then the sum of all lesions was used. “Out of field” progression was documented but not used in response analysis. Response was defined based upon the status of disease at the 3 month time point.
Histologic evaluation of all biopsies was performed independently by two pathologists who were blinded to clinical data. Biopsy samples were scored according to the extent of dysplasia on a 5-point ordinal scale (no dysplasia, mild, moderate, or severe dysplasia, and carcinoma in situ) as previously described. 14 Graders used a scoring system for hyperplasia based upon established ranges of normal mucosal thickness from the literature at specific anatomic sites. Hyperplasia was graded as mild, moderate, or extensive. Histologic response was determined by comparison of pre- and post-treatment biopsies for both severity of dysplasia and extent of hyperplasia.
Mucosal Risk Markers
Punch biopsies of target lesions were performed at baseline (between 8 to 4 weeks before start of therapy) and at completion 3 months following therapy. Markers of proliferation (Ki-67), apoptosis (TUNEL, p53), and signal transduction modulation (cyclin D1) were performed by immunohistochemistry (IHC) on paraffin-embedded tissue. Mucosal risk was also assessed by DNA ploidy analysis.
Immunohistochemistry
The expression of p53, cyclin D1, Ki-67 and TUNEL were assessed in oral biopsy samples. Immunohistochemistry was performed using the Leica Bond Max and Polymer automated detection systems (Leica Microsystems, Wetzlar, Germany). The following antibodies were used for marker detection: For p53, mouse antihuman monoclonal Ab (DO-7), (PA0057; Leica Biosystems, United Kingdom), ready to use (RTU) antibody optimized for Bond Leica detection system; For cyclin D1, rabbit monoclonal Ab (EP 12)(AP-0017; Epitomics, Burlingame, CA), RTU antibody optimized for Bond Leica detection system; For Ki67 (MM1), RTU Bond antibody (PA0057; Leica Biosystems, United Kingdom) optimized for Bond Leica detection system; For TUNEL, Apoptosis Detection Kit (S7101; Millipore, Billerica, MA) was used. Finally, sections were slightly counterstained with hematoxylin for 7 min and blued in 0.4% ammonia water followed by rehydration and cover slip mounting.
All of the above markers were validated according to protocol standardization at Pathology Core Facility, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL. Appropriate known control tissue was used for positive control and primary Abs were omitted in negative controls.
Immunohistochemistry Evaluation
TUNEL staining was evaluated by counting positively-stained pyknotic bodies. All the cells of the squamous epithelium in each biopsy excluding cells of the Stratum corneum (cornified layer with flattened, fused cell remnants) were taken in to evaluation.
The staining score was performed based on the following classification:
0, No staining;
1, Weak staining;
2, Moderate staining; and
3, Strong stains.
The IHC biomarker score was assessed by counting positively stained nuclei with respect to all the squamous epithelial nuclei which include Stratum basale, S.spinosum and S.granulosal layers. Staining with a score of 2 and 3 are considered as positive. IHC score index is then converted to a percentage figure.
For the automated determination of Ki67, cyclin D1 and p53, stained slides were scanned using a TissueFAXS® (TissueGnostics®, Vienna, Austria) Image Acquisition and Management Software. Visualization and automated cell counts were carried out using the HistoQuest® system version 3.0.3 (TissueGnostics®, Vienna, Austria). Digitized slides were evaluated using the program's nuclear scoring algorithm, which quantifies nuclear staining within biopsy specimens and derives a counting score for each target area. 15 Nuclei stained with polymerized di-aminobenzidine and/or hematoxylin were identified and separated by a thresholding and segmentation algorithm. Using the HistoQuest the investigator was able to adjust the upper and lower limits of a range of acceptable nuclear areas, such that cells within the specified range were accepted for analysis, whereas those out of the range were rejected. Thus sub-epithelial stromal elements and inflammatory cells were excluded from the analysis through sampling and automated gating restrictions respectively. Only the lower 5 cell layers from basement membrane were considered for the automated evaluation. The final counts were represented as percentage of nuclei stained positive to total nuclei in the selected region. Several regions from each biopsy were averaged to get the final result. Cutoff parameters for nuclear staining were defined based on the negative control and background immunohistochemical staining. Nuclear staining was classified as positive or negative based on observer-specified intensity thresholds
DNA ploidy was performed as previously described. 14, 16, 17 Cytophotometric parameters were derived from formalin-fixed paraffin-embedded tissue, sectioned at 8μm thick and stained with blue Feulgen DNA ploidy analysis staining kit (DPK500-IFU, ScyTek Laboratories, UT). Internal non lesional control cells were selected manually as ACIS control reference to compare the sample cells of interest. The ACIS generated a histogram from the collected cells based on the raw integrated optical density (IOD) values of digital nuclear images. An internal control peak was assigned to internal non-lesional cells (>30 per biopsy) containing normal amount of DNA with which all biopsy cells (not lesion specific) were compared. Control nuclei were used to set the DNA Index (DI) to 1.0. (D.I = DNA unknown/DNA diploid control). This peak marked the position of diploid population. Tonsil tissue was used as an external run control for staining and IOD determination. The mean integrated optical density (IOD) of control cells was assigned a DNA Index (DI) of 1, which served as an internal diploid (2N) standard and reference for DI calculation of the targeted cells. DI of 1.1 was assigned as an upper limit of the normal euploid range.
Results
Between April 2008 and March 2010, 11 participants were enrolled and received protocol therapy. Table 1 summarizes the participant characteristics, demographics, and treatment doses levels. Dysplasia severity represents the worst pre-treatment histology.
Table 1.
Participant pretreatment characteristics and treatment dose levels.
| Pt # | Age | Sex | Lesion location | ALA dose (mg/kg) | Fluence J/cm2 |
|---|---|---|---|---|---|
| 1 | 71 | M | Oral tongue | 0 | 4 |
| 2 | 70 | M | Oral cavity (alveolar ridge) | 0 | 4 |
| 3 | 67 | F | Oral cavity (lip) | 0 | 4 |
| 4 | 64 | M | Oral tongue | 0 | 4 |
| 5 | 49 | M | Oral tongue | 60 | 2 |
| 6 | 50 | M | Oral tongue | 30 | 2 |
| 7 | 74 | F | Oral tongue | 30 | 2 |
| 8 | 65 | F | Oral tongue | 30 | 2 |
| 9 | 66 | F | Oral tongue | 30 | 2 |
| 10 | 68 | F | Oral tongue | 30 | 4 |
| 11 | 48 | M | Oral tongue | 30 | 4 |
Table 2 summarizes adverse events and grade of toxicity for all related events for all participants. No severe (grade 3 or 4) toxicity was observed except for one participant (Pt #5), who experienced transient grade 3 transaminase elevation that resolved to baseline. Because this toxicity was a known side effect of ALA, the protocol was amended with ALA dose reduction from 60 mg to 30 mg for subsequent participants. No further events of liver dysfunction were observed in subsequent participants. The study was discontinued prior to completion due to slow accrual. DLT was not observed in the participants who completed treatment and MTD was not identified. No mucosal toxicity was observed. Participants reported neither acute nor delayed pain, sensitivity, swelling, or burning associated with photodynamic therapy.
Table 2. Adverse events and grade for possible, probable and definitely related events.
| ALA Dose (mg) | Light dose J/cm2 | Adverse Event | Grade |
|---|---|---|---|
| 60 | 2 | ALT elevation | 3 |
| 60 | 2 | AST elevation | 3 |
| 60 | 2 | Urine color change | 1 |
| 60 | 2 | Skin photosensitivity | 2 |
| 30 | 2 | Nausea | 1 |
| 30 | 2 | Pruritus | 1 |
| 30 | 2 | Palpitations | 1 |
| 30 | 4 | Nausea | 1 |
| 30 | 4 | Skin Photosensitivity | 1 |
| 30 | 4 | Vomiting | 1 |
No clinical responses (complete or partial) were observed in the treated participants; neither was there any clinical progression of lesions. However, participant #10, who had two leukoplakia lesions in the treatment field of the oral tongue, was observed to have a 50% decrease in one lesion and significant reduction in the mucosal prominence of the other lesion; by size criteria this did not reach the definition of partial response (see Figure 1). Following the completion of protocol follow-up assessments, this participant was observed clinically, off-protocol, and eventually achieved complete resolution of the treated leukoplakia lesions by 6 months. As of the last clinical assessment, 1.25 years post completion of therapy, complete clinical resolution persists in the treatment port although an out of field new leukoplakia lesion has been detected in the contralateral oral tongue.
Figure 1.
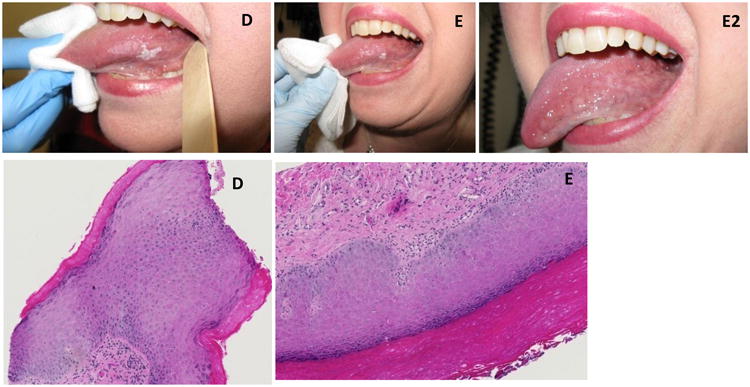
Participant #10: Clinical and histologic appearance of leukoplakia lesions at various time points: D. Baseline, E. 3 months, E2. 6 months.
Histologic response is shown in Table 3. Reduction in hyperplasia score, from moderate to mild, was observed in two participants, while increase in hyperplasia score was observed in one subject who increased from moderate to severe. Reduction in dysplasia was observed in 3 participants who received no ALA, and one participant who received 60 mg of ALA and 2 J/cm2. Reduction dysplasia from severe to none was observed in one participant (#2—no ALA) who subsequently went on to experience out of field oral cancer progression. Subject #10, who achieved late complete clinical response, had no dysplasia at baseline but mild dysplasia at 3 months.
Table 3.
Histologic assessment: hyperplasia and dysplasia severity at baseline and 3 months.
| Pt. # | Hyperplasia Baseline | Hyperplasia 3 months | Dysplasia Baseline | Dysplasia 3 months |
|---|---|---|---|---|
| 1 | Moderate | Moderate | None | None |
| 2 | Moderate | Mild | Severe | None |
| 3 | Mild | Mild | Mild | None |
| 4 | Mild | Mild | Mild | None |
| 5 | Moderate | Extensive | Moderate | None |
| 6 | Moderate | Moderate | Mild | Mild |
| 7 | Moderate | Moderate | Mild | Mild |
| 8 | Moderate | Moderate | Mild | Mild |
| 9 | Mild | Mild | None | Mild |
| 10 | Mild | Mild | None | Mild |
| 11 | Moderate | Mild | Mild | None |
Three study participants experienced cancer progression subsequent to completion of protocol therapy. Participant #2 (cohort 1—no ALA) developed an out of field oral squamous cell cancer 4 months after protocol PDT therapy. Participant #5 (cohort 2— 60 mg/kg ALA, 2 J/cm2) also developed an out of field oral squamous cell cancer 2 years after completion of protocol therapy. Participant #4 (cohort 1—no ALA) was diagnosed with squamous cell cancer from an area of the oral tongue consistent with the treated leukoplakia lesion 3.3 years following completion of protocol therapy. Two of these participants had either moderate or severe baseline dysplasia.
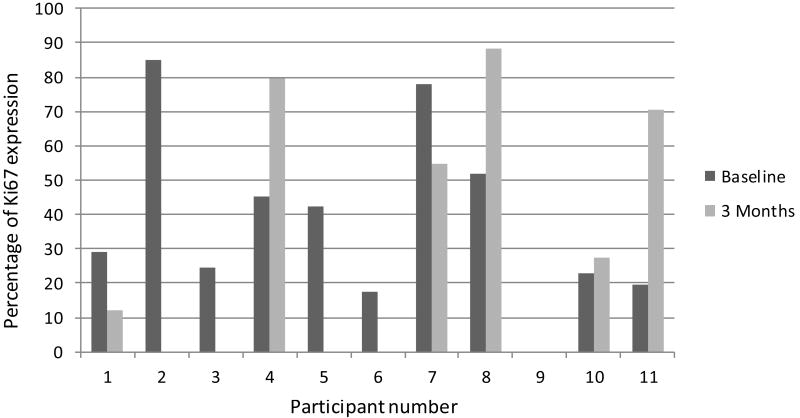
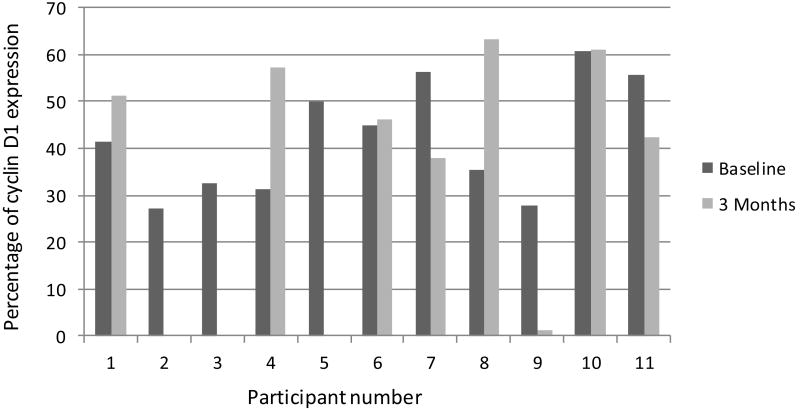
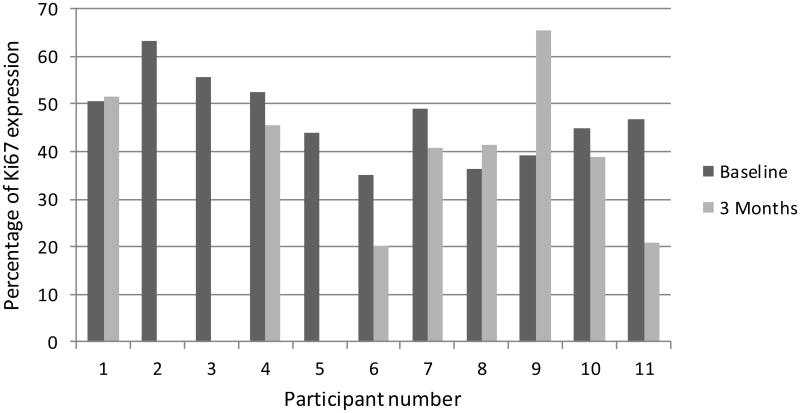
Mucosal risk markers were performed on biopsies from all treated participants. Apoptosis as evaluated by TUNEL staining and quantification revealed negligible values at baseline and at 3 months (data not shown). Expression of p53, cyclin D1, and cell proliferation (Ki-67) are shown in figures 2, 3, and 4, respectively. DNA ploidy analysis is shown in Table 4. No significant associations were seen in the comparisons between baseline and post-treatment values of the biologic mucosal risk markers. Baseline aneuploidy spanned a wide range from 35-87%. The participants who experienced off study oral cancer progression had baseline levels on the high end of this spectrum but did not have unusually high percentage of polyploid cells. Participant 10, who achieved complete clinical resolution of leukoplakia, showed no unusual histologic or mucosal marker changes.
Figure 2.
Percentage of p53 expression for each participant at baseline and 3 months.
Figure 3.
Percentage of cyclin D1 expression for each participant at baseline and 3 months.
Figure 4.
Percentage of Ki67 expression for each participant at baseline and 3 months.
Table 4.
DNA Ploidy. DI= Diploidy Index, difference in DNA content expressed as the ratio of DNA fluorescence of leukoplakia sample/normal tissue sample
| Patient ID | Time of biopsy | % of Diploid Cells | Avg DI of Diploid Cells (Range) | % of Aneuploid Cells | % of Polyploid Cells | Avg DI of Polyploid Cells(Range) |
|---|---|---|---|---|---|---|
| 1 | Baseline | 65 | 0.84 (0.23-1.1) | 35.4 | 0.8 | 2.23 (2.0-2.48) |
| 3 mos. | 27 | 0.86 (0.73-1.1) | 73 | 1 | 2.31 (2.0-2.43) | |
| 2 | Baseline | 30 | 0.89 (0.73-1.1) | 70 | 0.1 | 2.57 (2.0-2.73) |
| 3 mos. | 39 | 0.93 (0.78-1.1) | 61 | 5.3 | 2.2 (2.0-2.96) | |
| 3 | Baseline | 14.2 | 0.98 (0.85-1.1) | 86 | 15.2 | 2.33 (2.0-4.52) |
| 3 mos. | 25.85 | 0.94 (0.76-1.1) | 73.95 | 0 | 2.03 (2.02-2.05) | |
| 4 | Baseline | 33 | 0.94 (0.74-1.1) | 67 | 1 | 2.46 (2.0-2.77) |
| 3 mos. | 49 | 0.99 (0.85-1.1) | 51 | 0.3 | 2.48 (2.0-3.02) | |
| 5 | Baseline | 41.7 | 0.96 (0.77-1.1) | 58.3 | 2.2 | 2.11 (2.0-2.36) |
| 3 mos. | n/a | n/a | n/a | n/a | n/a | |
| 6 | Baseline | 48.7 | 0.94 (0.75-1.1) | 51.3 | n/a | 2.31 (2.0-3.91) |
| 3 mos. | 12.78 | 0.92 (0.73-1.1) | 87.2 | 37 | 2.35 (2.0-4.33) | |
| 7 | Baseline | n/a | n/a | n/a | n/a | |
| 3 mos. | 39.5 | 0.93 (0.72-1.1) | 60.4 | 4.2 | 2.57 (2.02-3.47) | |
| 8 | Baseline | 30 | 0.93 (0.75-1.1) | 70 | 2.1 | 2.41 (2.0-2.86) |
| 3 mos. | 44.8 | 0.94 (0.72-1.1) | 55.1 | 2.2 | 2.52 (2.03-3.48) | |
| 9 | Baseline | 23 | 0.89 (0.79-1.1) | 76.8 | 2.4 | 2.36 (2.0-2.8) |
| 3 mos. | 28.1 | 0.91 (0.75-1.1) | 71.9 | 2.5 | 2.21 (2.0-2.38) | |
| 10 | Baseline | 43.8 | 0.92 (0.75-1.1) | 56.2 | 0 | 2.42 (2.0-3.24) |
| 3 mos. | 40 | 0.95 (0.78-1.1) | 59 | 1.6 | 2.34 (2.0-2.8) | |
| 11 | Baseline | 62 | 1 (0.63-1.1) | 38 | 1.3 | 2.29 (2.0-2.35) |
| 3 mos. | 53 | 0.97 (0.82-1.1) | 47 | 1.2 | 2.31 (2.0-3.6) |
Discussion
Incremental improvements have been made in the management of oral cavity HNSCC such as in post-operative combined modality treatment. 18 However, transformational breakthroughs that have significantly improved the prognosis for oral cavity HNSCC patients have not been realized. This deficiency underscores the continued need to study new cancer prevention approaches for oral pre-malignant conditions. With this goal in mind, we conducted a clinical trial to examine an ALA PDT for treating oral leukoplakia. Despite our inability to complete planned accrual, we have shown that, with light dose levels up to 4 J/cm2, no significant toxicity was encountered. One of two participants treated at this dose level achieved a persistent clinical response in the treated lesions, suggesting potential clinical efficacy that should be investigated further.
We observed no adverse toxicity related to mucosal damage, in contrast to other ALA PDT studies that examined 628-635 nm light at much higher light intensity (100-200 J/cm2) and observed significant pain in 30-44% of participants. 12, 13 Our observation of tolerability of low light intensity is in agreement with the phase I/II study by Shafirstein et al that examined 585 nm laser doses from 6 to 8 J/cm2 with topical or intralesional injected ALA. In this study only minor pain effects were observed at the 6 and 7 J/cm2 dose levels. 19 The MTD was 8 J/cm2—dose limiting pain, requiring analgesia, was observed at this level. This study also demonstrated therapeutic efficacy with 7/17 patients (41%) achieving significant response—defined as at least 75% resolution of the lesion. Other oral cavity ALA PDT clinical studies examined higher light intensity (> 100 J/cm2) but did not rigorously examine report toxicity and tolerability.23-27 It is also not clear whether higher light intensity is associated with increased efficacy, however it does appear that increased mucosal damage and pain may be closely correlated. Similarly, no conclusions can be drawn from this limited data set regarding optimal light wavelength, so long as it is at one of the absorption peaks for protoporphyrin IX. In the context of this disease in which re-treatment may be required due to its propensity for recurrence and multifocal behavior, the importance of future studies to determine treatment parameters that optimize the therapeutic ratio of ALA PDT cannot be overstated.
Several limitations were encountered in the conduct of our study that reflect common issues encountered in chemoprevention clinical trials. The design of the light dose cohorts was planned to carefully delineate toxicity—in particular, purpura which can be caused by laser light damage rather than PDT effects from ALA. However, these rigorous design requirements also increase the sample size and duration of the study. Compared to therapeutic trials, chemoprevention clinical trials can pose unique challenges that can dramatically affect accrual. 20 For instance, accrual of participants with a pre-exisiting potentially malignant disorders, such as leukoplakia, to a chemoprevention study may rely upon casting a much wider geographic net. This, in turn, can be complicated by the fact that expanded population pools may also have alternative choices for experimental therapy. Two national studies overlapped with our study—Pioglitazone for Oral Premalignant Lesion Study (NCT009513790), and the Erlotinib Prevention of Oral Cancer Study (NCT00402779).
While we observed no significant toxicity related to the light alone or ALA PDT, we did observe one participant who experienced transient grade 3 transaminase elevation. Infrequent occurrences of transient transaminase elevation from ALA have been well described in the literature. 8 A study amendment to modify the dose of ALA was instituted following this event. Based upon the data from Barrett's esophagus studies that routinely use 60mg/kg, it could be argued that use of the standard ALA dose of 60mg/kg is reasonable for use in future ALA PDT studies for oral leukoplakia as well. In contrast to chemoprevention studies with long term interventions where moderate to severe toxicities cannot be tolerated over long periods of time, such toxicities (as long as they are brief and rapidly reversible) may be acceptable in the setting of single, time-limited intervention such as ALA-PDT. Given that, some efficacy was seen in one participant treated with 30 mg ALA and a light dose of 4 J/cm2, this lower dose of ALA should be investigated first.
The absence of treatment-related pain is likely attributable to the relatively low light intensity that was used. In contrast, other reported studies used much higher light intensity with commensurate higher incidence of pain.
Of note, three of the treated study participants later developed squamous cell carcinoma of the oral cavity, well after the completion protocol therapy. In two cases the tumors occurred outside of the laser treatment port. Given the estimate that 30% of patients with oral leukoplakia later develop SCCHN within an 8 year period, this observation is not unusual. 21 However, these data do raise a potential shortcoming of ALA PDT. While ALA PDT could be effective in ablating potentially malignant lesions, this therapy is not likely to reduce this incidence of subsequent cancers due to field cancerization effect. Lee and colleagues have shown in patients with leukoplakia, 59% of subsequent cancers occurred at the site of the previous leukoplakia, while 41% occurred elsewhere. 21 Thus systemic therapy, alone or as a complement to PDT, may be a more desirable approach. Systemic therapy is not without challenges as it would likely need to be administered for a lengthy period of time and thus its effectiveness is likely to be more influenced by toxicity and compliance issues. Perhaps a local treatment approach to potentially malignant lesions, such as PDT, coupled with oral screening, offers another potential approach to cancer risk reduction in this high risk population. The results of our trial show that future clinical studies that seek to definitively examine the efficacy of ALA PDT must take into consideration out of field cancer progression as a study endpoint and that long term follow-up is important for all leukoplakia studies.
Observed of changes in histologic grade of dysplasia or hyperplasia were inconclusive in this study. This may be primarily due to insufficient sampling. However, it is important to note that histologic endpoints have certain inherent uncertainties that complicate their utility in assessing response. 22 Variability of histology within a single leukoplakia lesion or between different lesions in the same patient may be highly divergent. Thus a baseline biopsy may not accurately reflect the overall severity of disease. Likewise, a post therapy biopsy may not accurately reflect true overall response to an intervention. Two of the participants with the worst baseline dysplasia score did subsequently go on to have oral cancer progression. We observed no clear association between baseline and post therapy measurements of mucosal risk markers. However, analogous to histologic grading, mucosal risk markers have inherent uncertainties that limit interpretation.
In summary, this study did not reach its primary endpoint regarding the optimal dosing or toxicity of ALA PDT for oral leukoplakia. We observed no significant toxicity and one participant treated at the 4 J/cm2 dose level achieved a delayed but durable complete clinical response. Our experience points out the necessity for future studies, potential benefit of this approach, and highlights potential pitfalls that should be considered in the design of future phase I studies of photodynamic therapy for leukoplakia.
Acknowledgments
Supported by N01CN35157
Stuart Marcus, M.D., Ph.D. (DUSA Pharmaceuticals, Wilmingham, MA) for supplying agent and device, and Judy Smith (DCP) for study support.
Footnotes
Data not presented elsewhere
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37(3):127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelty CJ, Marcus SL, Ackroyd R. Photodynamic therapy for Barrett's esophagus: a review. Dis Esophagus. 2002;15:137–144. doi: 10.1046/j.1442-2050.2002.00243.x. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W, Soo KC, Sivanandan R, Olivo M. Detection of squamous cell carcinomas and pre-cancerous lesions in the oral cavity by quantification of 5-aminolevulinic acid induced fluorescence endoscopic images. Lasers Surg Med. 2002;31:151–157. doi: 10.1002/lsm.10105. [DOI] [PubMed] [Google Scholar]
- 5.Ebihara A, Krasieva TB, Liaw LH, et al. Detection and diagnosis of oral cancer by light-induced fluorescence. Lasers Surg Med. 2003;32:17–24. doi: 10.1002/lsm.10137. [DOI] [PubMed] [Google Scholar]
- 6.Ackroyd R, Brown N, Vernon D, et al. 5-Aminolevulinic acid photosensitization of dysplastic Barrett's esophagus: a pharmacokinetic study. Photochem Photobiol. 1999;70:656–662. [PubMed] [Google Scholar]
- 7.Ackroyd R, Brown NJ, Davis MF, et al. Photodynamic therapy for dysplastic Barrett's oesophagus: a prospective, double blind, randomised, placebo controlled trial. Gut. 2000;47:612–617. doi: 10.1136/gut.47.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelty CJ, Ackroyd R, Brown NJ, Brown SB, Reed MW. Comparison of high- vs low-dose 5-aminolevulinic acid for photodynamic therapy of Barrett's esophagus. Surg Endosc. 2004;18:452–458. doi: 10.1007/s00464-003-9062-4. [DOI] [PubMed] [Google Scholar]
- 9.Gossner L, Stolte M, Sroka R, et al. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett's esophagus by means of 5-aminolevulinic acid. Gastroenterology. 1998;114:448–455. doi: 10.1016/s0016-5085(98)70527-x. [DOI] [PubMed] [Google Scholar]
- 10.Tan WC, Fulljames C, Stone N, et al. Photodynamic therapy using 5-aminolaevulinic acid for oesophageal adenocarcinoma associated with Barrett's metaplasia. J Photochem Photobiol B. 1999;53:75–80. doi: 10.1016/s1011-1344(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JC, Chiang CP, Chen HM, et al. Photodynamic Therapy of oral dysplasia with topical 5-aminolevulinic acid and light-emitting diode array. Lasers Surg Med. 2004;34:18–24. doi: 10.1002/lsm.10250. [DOI] [PubMed] [Google Scholar]
- 12.Sieron A, Adamek M, Kawczyk-Krupka A, Mazur S, Ilewicz L. Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of oral leukoplakia. J Oral Pathol Med. 2003;32:330–336. doi: 10.1034/j.1600-0714.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 13.Fan KF, Hopper C, Speight PM, Buonaccorsi G, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer. 1996;78:1374–1383. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1374::AID-CNCR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Brothwell DJ, Lewis DW, Bradley G, et al. Observer agreement in the grading of oral epithelial dysplasia. Community Dent Oral Epidemiol. 2003;31:300–305. doi: 10.1034/j.1600-0528.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 15.Ecker RC, Steiner GE. Microscopy-based multicolor tissue cytometry at the single-cell level. Cytometry A. 2004;59:182–190. doi: 10.1002/cyto.a.20052. [DOI] [PubMed] [Google Scholar]
- 16.Bouquot JE, Speight PM, Farthing PM. Epithelial dysplasia of the oral mucosa -diagnostic problems and prognostic features. Current Diagnostic Pathology. 2006;12:11–21. [Google Scholar]
- 17.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 18.Shafirstein G, Friedman A, Siegel E, et al. Using 5-aminolevulinic Acid and pulsed dye laser for photodynamic treatment of oral leukoplakia. Arch Otolaryngol Head Neck Surg. 2011;137:1117–1123. doi: 10.1001/archoto.2011.178. [DOI] [PubMed] [Google Scholar]
- 19.Richmond E, O'Mara A. Conducting chemoprevention clinical trials: challenges and solutions. Semin Oncol. 2010;37:402–406. doi: 10.1053/j.seminoncol.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–1710. [PubMed] [Google Scholar]
- 21.Szabo E. Assessing efficacy in early-phase cancer prevention trials: the case of oral premalignancy. Cancer Prev Res (Phila) 2008;1:312–315. doi: 10.1158/1940-6207.CAPR-08-0171. [DOI] [PubMed] [Google Scholar]
- 22.Chen HM, Yu CH, Tu PC, Yeh CY, Tsai T, Chiang CP. Successful treatment of oral verrucous hyperplasia and oral leukoplakia with topical 5-aminolevulinic acid-mediated photodynamic therapy. Lasers Surg Med. 2005;37(2):114–122. doi: 10.1002/lsm.20214. [DOI] [PubMed] [Google Scholar]
- 23.Jerjes W, Upile T, Hamdoon Z, Mosse CA, Akram S, Hopper C. Photodynamic therapy outcome for oral dysplasia. Lasers Surg Med. 2011;43(3):192–199. doi: 10.1002/lsm.21036. [DOI] [PubMed] [Google Scholar]
- 24.Lin HP, Chen HM, Yu CH, Yang H, Wang YP, Chiang CP. Topical photodynamic therapy is very effective for oral verrucous hyperplasia and oral erythroleukoplakia. J Oral Pathol Med. 2010;39(8):624–630. doi: 10.1111/j.1600-0714.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 25.Leunig A, Betz CS, Baumgartner R, Grevers G, Issing WJ. Initial experience in the treatment of oral leukoplakia with high-dose vitamin A and follow-up 5-aminolevulinic acid induced protoporphyrin IX fluorescence. Eur Arch Otorhinolaryngol. 2000;257(6):327–331. doi: 10.1007/s004059900222. [DOI] [PubMed] [Google Scholar]
- 26.Yu CH, Lin HP, Chen HM, Yang H, Wang YP, Chiang CP. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg Med. 2009;41(9):628–633. doi: 10.1002/lsm.20841. [DOI] [PubMed] [Google Scholar]