Abstract
This study investigated the association of chronic childhood stress exposure with acute stress-related attentional alterations that have been previously linked to vulnerability to mental and physical illness in early adulthood. Participants were randomized in a crossover design to complete both a mild laboratory social stress task and a computerized task assessing attentional bias to socially threatening words. Salivary cortisol was measured throughout the study. Exposure to acute laboratory stress altered attentional processing, and this relationship was moderated by chronic childhood stress exposure. Also, a positive association between cortisol reactivity and attentional bias was observed, with cortisol reactivity negatively related to childhood chronic stress exposure. While previous work has supported a role for early chronic stress exposure in influencing acute stress reactivity, this work provides initial insight into how both prior chronic childhood stress and current acute stress together relate to the attentional gateway and may be associated with stress adaptation and psychological vulnerability into adulthood.
Keywords: Chronic stress, acute stress, attentional bias, family conflict, cortisol
Prior theory and research suggest that characteristics of the family environment during development may contribute to vulnerability to problems in adulthood in domains of both mental and physical health. Although the exact mechanisms underlying these relationships remain unclear, Luecken et al. (2006) present a combined cognitive-affective model to link adverse features of the early family environment to alterations in psychological and physiological stress reactivity processes, which may ultimately underlie illness vulnerability (see also Repetti, Robles, & Bridget, 2011; Repetti, Taylor, & Seeman, 2002). According to this model, prolonged exposure to psychosocial stressors during development, including even moderate levels of conflict in the early family environment, may be associated with alterations in information processing abilities that promote persisting changes in psychological and physiological responses to acute everyday stressors (Luecken et al., 2006; Luecken, Kraft, & Hagan, 2009, McEwen, 2000; Miller, Chen, & Parker, 2011).
One critical aspect of information processing is attentional control, a cognitive ability that serves as a gateway for emotional and physiological stress reactivity. As humans, our attention is drawn towards significant threatening stimuli, such as the sight of a snake in the wild (Ohman, Flykt & Esteves, 2001). This selective attention to threat, defined as attentional bias, represents an evolutionary adaptive response to prepare for and adapt to acute stress (Ohman et al., 2001). However, chronic exposure to stressful situations may precipitate a heightened and/or persistent bias in attention towards environmental cues that do not necessarily pose an actual threat, therefore promoting excessive emotional and physiological arousal. Exposure to family conflict during childhood has specifically been linked to increased attention towards threat in ambiguous social situations (Crick & Dodge, 1994; Grych & Fincham, 1990). Further, Luecken and Appelhans (2005) showed that exposure to early family adversity was associated with attentional bias towards social threat cues in current young adults. This pattern was independent of current life stress and interpreted as a potential marker for vulnerability to affective symptoms.
In addition to the facilitated attentional capture of evolutionary threats, concomitant psychobiological processes are also involved in the association between acute stress and increased attentional processing of these stimuli. A surge in cortisol released as part of an acute stress response binds to and stimulates receptors concentrated in the medial prefrontal cortex, a region underlying selective attention (Liston et al., 2006; Lupien & McEwen, 1997). In studies involving an experimental manipulation of glucocorticoids, cortisol modulates short-term selective attentional processing in a dose-dependent fashion. Specifically, an inverted U-shaped curve reflects a low to medium-dose enhancement of selective attention that heightens cognitive focus useful for overcoming a stressor. A high-dose dampening of selective attention functions as part of a negative feedback cycle to turn off the stress response once the threat has been addressed (Hopper et al., 2004).
Several recent studies corroborate the relationship between stress-induced variations in levels of cortisol and heightened attentional processing of threat (e.g., Applehans & Luecken, 2006; Ellenbogen et al., 2002, 2006; Ellenbogen, Carson & Pishva, 2010; McHugh et al., 2010; Pilgrim, Marin, & Lupien, 2010; van Honk et al., 2000). For example, Ellenbogen, Carson, and Pishva (2010) embedded an attentional orienting task within a laboratory social stress paradigm. Attentional shifting in response to negative stimuli was related to increased cortisol reactivity to the stress task, potentially representing glucocorticoid stimulation in medial prefrontal regions. However, repeated and prolonged activation of the HPA axis, as in cases of chronic stress exposure, is associated with a flattening of diurnal cortisol rhythms, increased ambient cortisol levels, and decreased acute stress reactivity (Miller, Chen, & Zhou, 2007). As such, individuals exposed to high levels of early family conflict may not show patterns of adaptive HPA axis reactivity and attendant downstream cognitive processes.
Responses to stress and the physical and emotional consequences of stress may be further modified by gender. For example, there are considerable gender differences in the prevalence of diagnosed mood disorders often linked to stress, with women having a significantly greater risk of affective psychopathology compared to men (Kessler et al., 2005). Additionally, research in animal models suggests that females may be more sensitive to stress-related brain changes (e.g., Sterrenberg et al., 2011), providing a psychobiological explanation for sex-specific differences in psychosocial and health outcomes associated with chronic stress. Although physiological stress reactivity patterns related to disease have been found to vary by gender (see Kudielka & Kirschbaum, 2005; Matthews & Gallo, 2011 for reviews), little research has looked specifically at the potential cognitive mechanisms linking chronic stress and women’s mental health risk.
At this time, few existing studies measure attentional bias primed with an acute stress exposure. However, no study to our knowledge has previously attempted to assess alterations in attentional processing of socially threatening information due to the experience of interpersonal social threat. Further, this study provides the first investigation of an acute stress-related manipulation of attentional bias that is examined within the context of prior chronic stress exposure. Such a combined approach uniquely sheds light on the potential long-term significance of the family environment during development as a risk factor for neurocognitive changes and psychobiological dysregulation that may underlie vulnerability to stress-related disorders.
Based on previous studies citing heightened attentional processing of threat through priming with negative stimuli (e.g., Beck et al., 2011), we first hypothesized that exposure to an interpersonal laboratory stressor would be associated with bias towards social threat stimuli. This would extend the current literature to include evidence for upregulation of attentional processing of socially threatening information due to the experience of acute interpersonal social stress.
Second, we hypothesized that a history of exposure to high levels of family conflict during childhood would moderate this relationship. That is, we predicted that exposure to higher levels of childhood family conflict would be associated with attentional bias towards social threat stimuli after exposure to an interpersonal stressor in the laboratory.
Third, in light of established links between chronic stress exposure and changes in psychobiological stress reactivity, as well as prior evidence for cortisol-related alterations in attentional biases, we explored the role of cortisol reactivity in alterations in attentional bias after exposure to an acute stress task. Specifically, we predicted that chronic stress exposure during childhood would be related to reduced cortisol reactivity to an acute laboratory stressor. Further, we predicted that cortisol reactivity would account for variability in attentional bias to threat observed after acute laboratory stress exposure.
Method
Participants
Participants for this study include 116 female undergraduate students currently enrolled at a selective university in the southeastern U.S. The mean age of the sample was 18.96 years (SD = 1.13), with a range of 18 to 22 years. The sample was 78.6% Caucasian, 8.5% African American, 6.8% Asian American, 4.3% Hispanic/Latina, and 0.9% endorsed more than one ethnicity. All participants were recruited through a university on-line participant pool management system. The only requirement for inclusion in the study was current full-time enrollment status as an undergraduate student and self-identified gender as female. The study was approved by the Institutional Review Board.
Procedure
Study participation lasted approximately two hours in duration and included exposure to an acute laboratory stress task and a computerized attentional bias task, as well as the provision of five saliva samples and completion of questionnaires. After written informed consent was obtained by a graduate research assistant, the participant sat in a quiet room where magazines were provided for 15 minutes in order to allow the participant to adjust to her surroundings. At the end of this 15-minute period, the first (baseline) saliva sample was collected, and the participant completed the PANAS. The participant then completed either the stress reactivity task or the dot-probe attentional bias task, and a second saliva sample was collected, and the participant completed the PANAS. After completing the second task, a third saliva sample was collected, and the participant completed the PANAS. The participant was then escorted to a quiet room for 30 minutes where she filled out questionnaires. The fourth and fifth saliva samples were collected at 15-minute intervals during this time.
Experimental stress manipulation (Noisy Neighbor Task)
The Noisy Neighbor Task was used instrumentally as an acute laboratory stressor and as a measure of physiological and psychological stress reactivity. During the task, each participant engaged in a conversation role-play with a gender-matched research assistant in which the participant was prompted to present an argument to convince a neighbor to lower the volume of her music. Participants were told they would be evaluated on the persuasiveness of their argument and were videotaped during the conversation. The research assistant responded to statements made by the participant with an ordered list of memorized prompts that were sufficiently generic to maintain the exchange. If the participant stopped responding in the conversation, she was prompted by the research assistant to continue until 10 minutes had elapsed.
This task has been used previously by Luecken, Kraft, and Hagan (2009) and induces a mild psychological and physiological stress response due to the social interaction and feelings of social evaluation induced during the activity. Stress responses to this task have previously been measured by Luecken et al. by self-report on questionnaires of pre/post-task levels of emotion as well as physiological (i.e., salivary cortisol and heart rate) data.
Attentional bias (dot probe detection task)
Attentional bias to socially threatening words was assessed using a modified dot-probe task (MacLeod, Mathews, & Tata, 1986; Mogg & Bradley, 2005; Cisler & Koster, 2010). For the dot-probe task, participants sat at a computer terminal. A white fixation cross was displayed in the center of a black screen. Participants were presented with two words in the center of the screen in white uppercase letters, with one word one centimeter above and one word one centimeter below the former location of the central fixation cross. A white dot then replaced one of the words. Participants were asked to indicate the location of the dot by pressing keys on a keyboard. The “C” key was relabeled with the word “UP” and was used to indicate that the dot had replaced the top word, and the “M” key was relabeled with the word “DOWN” and used to indicate that the dot had replaced the bottom word. One negatively valenced word and one neutral word (e.g., shelf, curtain) or two neutral words were used in each pair. All valenced and neutral words were matched for length and frequency of use in the English language (Harris, 2003). Valenced words included those related to social threat (e.g., criticized, loner).
Participants were presented with 6 learning trials in which the experimenter demonstrated the procedure, 12 practice trials, and 120 total test trials. Sixty trials included unmasked stimuli presented at the conscious level of awareness for 1250ms, and the remaining 60 included masked stimuli presented below the level of conscious awareness for 20ms that were then covered with a string of nonsensical letters (e.g., SPTUZKT) to prevent participants from being able to view the shadow of previously presented stimuli. These two conditions have been extensively used to tap unique phases of attention regulation (i.e., conscious and pre-conscious; Egloff and Hock, 2003). Each of the two groups of stimulus display durations included 30 word pairs reflecting a neutral word and social threat word and 30 word pairs of two neutral words. A 90 second rest break was provided midway through the task, with random selection of display time (i.e., masked vs. unmasked) and trial type (i.e., social threat/neutral, neutral/neutral) throughout.
E-Prime 2.0 experimental presentation software (Psychology Software Tools, Pittsburgh, PA) was used to conduct the dot probe tasks and record responses as well as response latencies for each trial. Incorrect responses were discarded in the analyses, and response latencies were used as a measure of bias in automatic selective attention in this study. Shorter response latencies on trials in which threat words were replaced by dot probes and longer response latencies on trials in which neutral words were replaced by dot probes indicated an attentional bias towards negative words. The reverse pattern indicated an attentional bias away from negative words.
A standard lexical decision-making task (e.g., Mogg, Bradley, & Hallowell, 1994) was subsequently conducted in order to assess whether participants were able to consciously perceive masked stimuli. This lexical decision task required participants to decide whether a real word or nonsense word was presented during a masked trial to determine whether or not participants could read the content of words at 20ms. A 50% overall accuracy rate on this validity task would indicate that participants were guessing and/or unable to read the content of words.
Measures
Salivary cortisol
The five data points allow for analyses of both reactivity to stress (as reflected in increases from pre- to post-stress task) and total concentration over the assessment period (as reflected in area under the curve of the five time points). Reactivity, a measure of neuroendocrine response to a stressor, was defined as the change in salivary cortisol levels during the period of acute stress task participation and was calculated as the difference between the sample levels obtained immediately prior to commencing the task and immediately after completing the task. “Area Under the Curve,” (AUC) is a calculation of total cortisol output throughout the study period taking into account all five samples and reflects general diurnal cortisol levels and patterns of an individual. To control for diurnal fluctuations in cortisol, all appointments were scheduled for the afternoon (2–6pm). Participants were instructed to refrain from eating, alcohol use, smoking, exercise, or prescription drugs for at least two hours prior to participation. On a self-report demographics questionnaire completed by participants, 18 participants (15.5%) reported taking one prescription medication, 6 participants (4.8%) reported taking two, and one participant (<1%) reported taking 3 or more. Participants generally refrained from responding to questions about the type of medications used, with only three participants endorsing use of psychotropic/antidepressant/anxiolytic medications.
Salivary cortisol concentrations are independent of flow rate, and reflect unbound “free” levels in plasma. Saliva samples were obtained with the Salivette sampling device (Sarstedt, Rommelsdorf, Germany). Participants were instructed to place a small cotton swab in their mouths and chew on it for 2 minutes. The swabs were immediately frozen and stored at −30 °C for 1–3 months prior to analysis. Analyses of cortisol were conducted in duplicate and the mean level of the two tests was used in all analyses.
Family conflict
All participants completed the frequency (6 items) and intensity (7 items) subscales of the Children’s Perception of Interparental Conflict Scale (Grych, Seid, & Fincham, 1992). This scale is designed to measure various aspects of conflict occurring in the home from a child’s perspective. Each item is rated on a 3-point scale (true/sort of true/false). This scale has demonstrated adequate validity and internal consistency in samples of young adults (Reese-Weber & Hesson-McInnis, 2008). Internal consistency of the CPIC frequency and intensity subscales was α = .86 and .88, respectively. In order to quantify family conflict exposure prior to 16 years of age in the current study, the items from the Frequency (6 items) and Intensity (7 items) subscales of the CPIC were summed to create a CPIC total score.
Positive and Negative Affect Scale (PANAS)
All participants completed the Positive and Negative Affect Schedule (PANAS; Watson, Clark & Tellegan, 1988), a standard 20-item measure of both positive and negative emotions rated on a 1–5 scale describing current affect of the individual three times during the assessment. High internal consistency and independence for positive and negative emotions have been demonstrated for the scale (Watson et al., 1988). Internal consistency of the PANAS positive and negative affect scales was α = .83 and .81, respectively.
DSM Depression and Anxiety Symptoms
Symptoms of anxiety and depression at the time of the study were assessed by the Adult Self Report (ASR; Achenbach & Rescorla, 2003), a self-report measure assessing emotional and behavioral problems and social competence that has been normed on a nationally representative sample of adults. The ASR includes 113 items scored on a three-point scale indicating how descriptive the items are of the individual during the preceding six months and includes empirically based syndromes as well as DSM-oriented scales. The ASR has high test-retest reliability and internal consistency. The current analyses utilized the DSM Depression (items include lack of enjoyment, sleep disruption, appetite disturbance, sadness, suicidal ideation, underactivity, and feelings of worthlessness) and DSM Anxiety scales (general fears, nervousness, worries, feeling fearful, and fear of school) as covariates. Internal consistency of the DSM Depression and DSM Anxiety scales for the current sample was α = .77 and .66, respectively.
Statistical analytic approach
Descriptive statistics were first calculated for attentional bias, cortisol, and questionnaire variables for the whole sample as well as by randomization group. Because of technical difficulties in administration of the dot probe task, data for one participant was saved incorrectly and not analyzable. In addition, 13 participants provided insufficient correct responses to allow the calculation of bias scores, indicating a lack of understanding of the task or lack of motivation to perform it correctly. Thus, attentional bias scores were calculated for 102 study participants. Data from the dot probe task for these 102 participants were cleaned and bias scores calculated according to the following steps used in prior dot probe research (e.g., Glinder et al., 2007). For each participant, incorrect responses as well as responses with latencies greater than 3-seconds or greater or less than 3 SDs from both the sample mean and each participant’s mean response time were removed. This ranged from 0 to 6 trials per participant, representing 2.7% of the overall data.
In order to obtain Attentional Bias Index Scores, response latency data were initially analyzed with a 2 × 2 × 2 (dot probe position × threat word position × exposure condition) ANOVA. Response latency data were then collapsed by probe position and threat word position into a continuous bias score for analyses, following a standard procedure used extensively in the literature (e.g., Mogg, Mathews, & Eysenck, 1992; Neshat-Doost, et al., 1999; Boyer et al., 2006). This procedure is equivalent to testing the dot probe position × threat word position interaction for dot probe detection latencies and produces a score indicating the difference between trials when the dot probe succeeds threat words in a threat-neutral word pair and when the dot probe succeeds neutral words in a threat-neutral word pair. Mean reaction time data from each trial were entered into the following equation for each participant, which yielded a continuous attentional bias index score reflecting bias magnitude:
Attentional bias index score = ½[(UpLt – UpUt) + (LpUt – LpLt]
According to this equation, U = upper position, L = lower position, p = probe, t = negative word. For ease of interpretation, UpLt defines the response latency when the probe is in the upper position and the threat word is in the lower position. Positive values (attentional bias > 0) reflect a shorter reaction time when the affectively valenced word was probed compared to neutral stimuli, and negative values (attentional bias score < 0) reflect a longer reaction time when the affectively valenced word was probed compared to neutral stimuli. Two attentional bias index scores were then created for each participant reflecting: (a) attention to masked social threat words, (b) attention to unmasked social threat words (see Table 1 for descriptive statistics of attentional bias scores).
Table 1.
Descriptive Statistics for Attentional Bias Index Scores to Social Threat Stimuli
| Whole Sample | Noisy Neighbor/Dot Probe | Dot Probe/Noisy Neighbor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bias Condition | N | M (SD) | Min | Max | N | M (SD) | Min | Max | N | M (SD) | Min | Max |
| Masked Social Threat | 102 | −1.56 (38.83) | −92.88 | 115.88 | 50 | 6.26 (31.57) | −77.62 | 71.71 | 52 | −9.08 (43.72) | −92.88 | 115.88 |
| Unmasked Social Threat | 102 | −5.18 (35.47) | −80.25 | 100.63 | 50 | −6.72 (32.20) | −80.25 | 74.83 | 52 | −3.65 (38.74) | −69.13 | 100.63 |
Note. All scores reflect attentional bias scores calculated according to the explanation and equation on page 13 comparing response latencies of probed negative words to latencies of probed neutral words.
ANCOVA procedures were then used in this analysis to test study hypotheses for differences in attentional bias index scores between groups (i.e., randomization and conflict exposure), accounting for potentially cofounding factors (i.e., DSM Depression and Anxiety scores). This procedure was first used to assess overall attentional bias to social threat words at masked and unmasked presentation levels on the dot probe task, as well as by randomization group to test Hypothesis 1, and with family conflict exposure as a moderator to test Hypothesis 2.
In the cases of statistically reliable higher-order interactions, t-tests with Bonferroni correction were used to decompose the interactions and identify the group differences that contributed to the interaction (McClelland & Judd, 1993). In order to probe the interaction of interest regarding family conflict exposure, extreme high (i.e., 1 SD above sample mean, n = 26) and low (i.e., 1 SD below sample mean, n = 24) conflict exposure groups were subsequently selected based on CPIC total score (Feldt, 1961; Holmbeck, 2001). Assumptions of each ANCOVA were tested and met; specifically, the distributions were not skewed and their variances were equivalent, Levene’s tests all non-significant. After collinearity statistics were examined, linear regression analyses were conducted to determine whether family conflict, cortisol reactivity, and their interaction accounted for significant variance in attentional bias to test Hypothesis 3.
Results
Manipulation check for laboratory stress task and current levels of distress
To test whether the Noisy Neighbor task was experienced as distressing, we evaluated cortisol reactivity as well as changes in positive and negative affect using the PANAS questionnaire from pre- to post-stress task with repeated measures 2 × 2 (time×randomization group) ANCOVA with DSM Depression and Anxiety scores as covariates. Repeated measures ANCOVA with cortisol level as the dependent variable did not yield any significant main effects or interactions, indicating no significant increase in cortisol level pre- to post-stress task (means = 7.17 and 7.30 ug/dL, respectively). Repeated measures ANCOVA with PANAS positive affect score did not yield any significant main effects or interactions, indicating no significant decrease in positive affect pre- to post-stress task (means = 20.45 and 20.10, respectively). Repeated measures ANCOVA with PANAS negative affect score yielded a significant effect of time pre-to post-stress task, means = 15.28 and 17.34, respectively, F (112) = 3.51, p < .01, d = .53, indicating a significant increase in negative affect pre- to post-stress task. The T scores for symptoms on the DSM Depression scale (mean = 56.75, SD = 8.01) and DSM Anxiety scale (mean = 56.00, SD = 7.30) on the ASR reflect a mild elevation of approximately one-half standard deviation above the normative mean for symptoms of depression and anxiety, but were not found to be significant covariates, indicating that current levels of depression and anxiety were not related to acute stress reactivity.
Attentional bias
Validation of the masked presentation rate
The average response accuracy for the trials of the lexical decision task was 52.3%, which did not significantly differ from a chance response accuracy of 50.00%, t (1223) = 1.44, p > .05. In addition, no single participant’s responses fell outside limits that could be expected by chance. Taken together, these results indicate that participants were not able to read words presented at 20ms.
Attentional bias index score
Using a 2 × 2 × 2 (dot probe position × threat word position×exposure condition) ANOVA of response latency data, a significant 2-way interaction was found for threat word position and dot probe position F (1, 101) = 4.21, p < .05, indicating that in both the masked and unmasked conditions, participants showed attentional biases away from social threat words (i.e., responding slower when the dot probes replaced the threat word of the threat-neutral word pairs and responding faster when the dot probes replaced the neutral word of the threat-neutral word pairs). No other main effects or interactions were found. Significant differences between exposure conditions (i.e., masked and unmasked presentations) would have been demonstrated by a 3-way interaction for threat word position, dot probe position, and threat word type. In examining attentional bias index scores for the sample as a whole as well as both randomization groups, scores did not differ significantly from neutral, indicating an overall absence of attentional bias with regards to masked or unmasked social threat stimuli.
Hypothesis 1: Priming of attentional bias to social threat by laboratory stress task exposure
To test whether attentional bias to social threat words was primed by exposure to a laboratory stress task, ANCOVA (randomization group × exposure condition) was conducted using the two attentional bias index scores per participant as dependent variables and the DSM Depression and DSM Anxiety scores as covariates. A significant interaction effect emerged for exposure condition×randomization group, F (1, 94) = 4.17, p < .05, η2partial = .048. Post-hoc t-tests with Bonferroni correction revealed that this interaction effect was due to significantly greater attention towards masked social threat words in the group exposed to the stress task first compared to the group exposed to the dot probe task first t (98) = −2.04, p < .05, d = .41. Comparison of attentional bias index scores for unmasked social threat words between the two randomization groups indicated no significant difference between groups, t (100) = .43, p = .67, d = .08. DSM Depression and Anxiety Scores did not differ significantly between randomization groups (DSM Depression t (100) = .82, p = .41, d = .15; DSM Anxiety t (100) = .21, p = .84, d = .04) and were not found to be significant covariates, indicating that current depression and anxiety symptoms were not significantly associated with attentional bias to social threat words. Consistent with Hypothesis 1, these findings provide support that exposure to a laboratory stress task altered attentional bias with regards to social threat.
Family conflict
Combined scores from the Frequency and Intensity subscales of the CPIC ranged from 14 to 40, with a mean of 24.22 and a standard deviation of 7.33. These results are consistent with those found by Reese-Weber and Hesson-McInnis (2008) in a community sample of late adolescents/young adults (age 17 to 21 years-old) who reported independent means of 11.23 (SD = 3.54) and 12.54 (SD = 4.24) for the Frequency and Intensity subscales of the measure, respectively. The current sample as a whole therefore did not report significantly elevated levels of family conflict compared to a normative population sample of young adults of similar age to those participating in the current study.
Hypothesis 2: Moderation of attentional bias priming by family conflict exposure
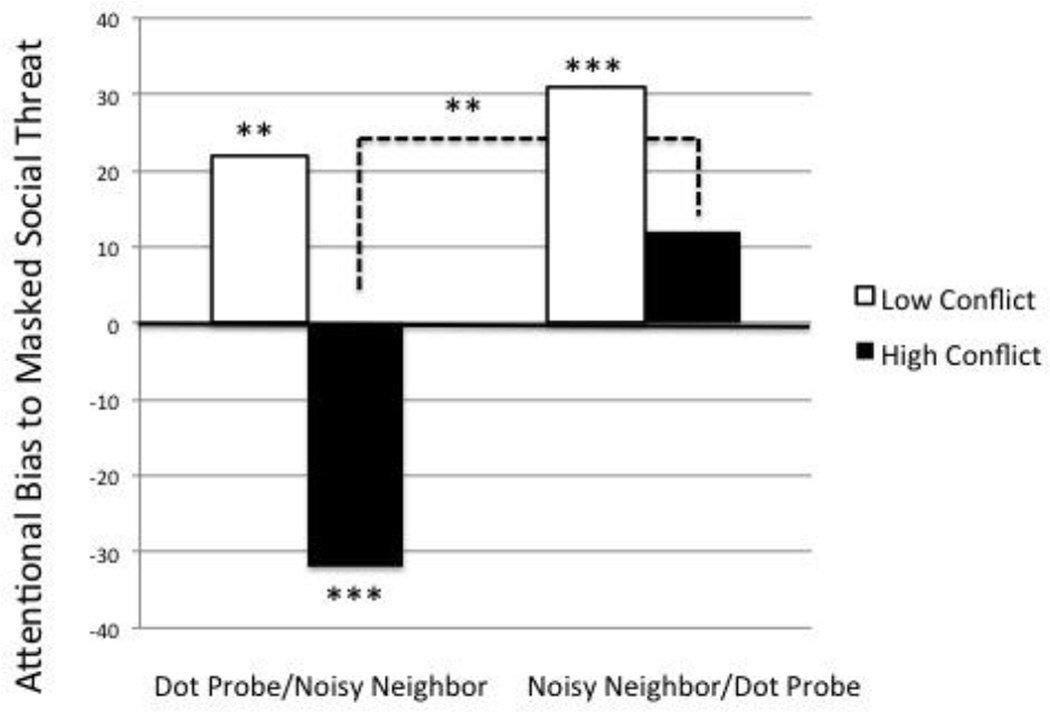
Due to the specificity of findings for priming effects on masked social threat words only, the examination of moderation of attentional bias priming by family conflict exposure was conducted only for masked stimuli. ANCOVA (randomization group × conflict exposure) was conducted using attentional bias index score for masked social threat words as the dependent variable and the DSM Depression and DSM Anxiety scores as covariates. Significant main effects of conflict, F (1, 55) = 1.71, p < .05, η2partial = .44, and task order, F (1, 55) = 4.91, p < .05, η2partial = .21, as well as an interaction, F (1, 55) = 1.74, p < .05, η2partial = .18, were found in predicting bias to masked social threat words (Figure 1). DSM Depression and Anxiety Scores were not found to be significant covariates.
Figure 1.
Interaction of conflict and randomization group on masked social threat bias for extreme high and low conflict groups, **p<.01, ***p<l.001
Drawing on approximate quintiles of the total sample, the high conflict (mean = 35.2, SD = 1.83, range = 33–40) and low conflict (mean = 15.6, SD = 1.43, range = 14–18) groups represent “high” (i.e., >/= 2 SD above population mean) and “low” (i.e., > 1 SD below population mean) levels of family conflict, respectively. As shown in Figure 1, a significant difference between randomization groups was found for attentional bias to masked social threat words, t (11) = 3.44, p < .01, d = .78. Individuals exposed to low levels of family conflict were significantly biased towards masked social threat words in both experimental conditions (dot probe first, t (11) = 3.67, p = .01, d = .89; stress task first, t (11) = 4.85, p = .001, d = 1.09). That is, they responded faster when the dot probes replaced the threat word of the threat-neutral word pairs. However, individuals exposed to high levels of family conflict during development were significantly biased away from threat stimuli when the dot probe was presented first, t (11) = −5.78, p < .001, d = 1.11. That is, they responded slower when the dot probes replaced the threat word of the threat-neutral word pairs. DSM Depression and Anxiety Scores did not differ significantly between randomization groups (DSM Depression t (48) = −.55, p = .59, d = .07; DSM Anxiety t (48) = −1.45, p = .15, d = .25) and were not found to be significant covariates.
Cortisol Levels and Reactivity
Hypothesis 3: Role of cortisol reactivity in attentional bias priming
Correlations of CPIC total score with cortisol levels for the entire sample as well as for each experimental condition are presented in Table 2. As shown in Table 2, exposure to family conflict during development as measured by the CPIC scale was significantly positively correlated with cortisol AUC in the sample as a whole (r = .26, p < .01) and in the group that performed the dot probe first (r = .28, p < .05). No significant correlations were found for cortisol reactivity.
Table 2.
Correlations of CPIC Score (Combined Frequency and Intensity Subscales) and Measures of Cortisol (ug/dL)
| Reactivity | AUC | |
|---|---|---|
| Whole Sample | −.10 | .26* |
| Noisy Neighbor/Dot Probe | −.14 | .24 |
| Dot Probe/Noisy Neighbor | −.01 | .28* |
Note. Reactivity: Change in cortisol level (ug/dL) pre- to post-Noisy Neighbor task, positive value reflects increase in cortisol level; AUC, Area under the curve, total cortisol output over study duration, calculated from five sample values
p<.05
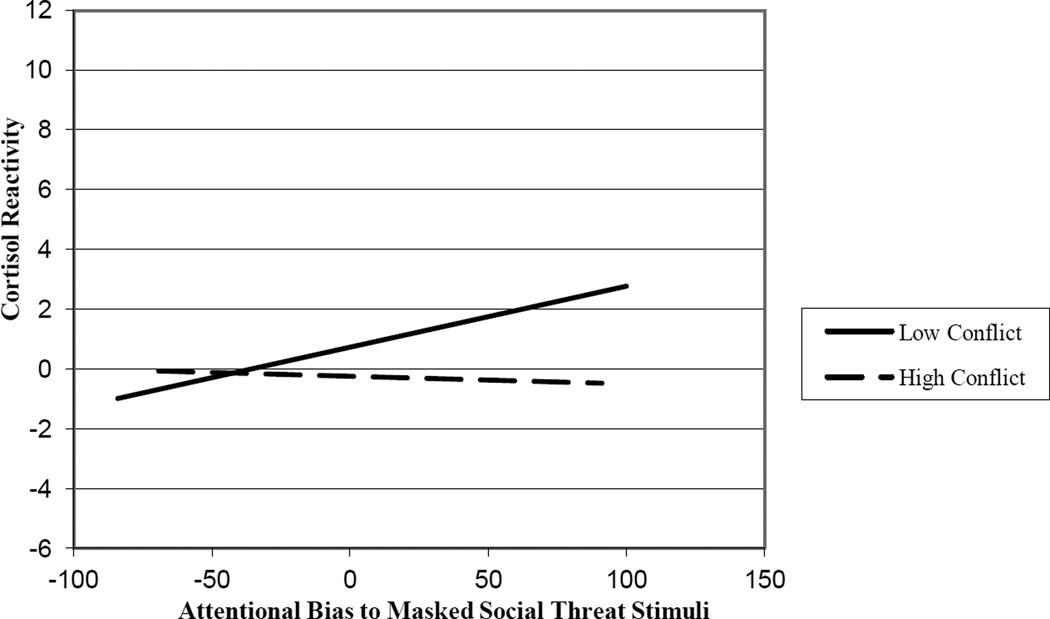
Moderation was tested by constructing a regression equation predicting attentional bias to masked social threat from CPIC total score, cortisol reactivity, and a multiplicative term representing the interaction between the two predictors. Predictor variables were centered to maximize interpretability and minimize multicollinearity, by subtracting the sample mean from individual scores (Aiken & West, 1997). The full model was significant, F (3, 47) = 5.47, p < .01, with the combined variables accounting for 21.0% of the variance in attentional bias to masked social threat words. A significant negative main effect for CPIC total score, β = −.36, sr2 = .18, p < .01 and a significant interaction of CPIC total score and cortisol reactivity, β = −.25, sr2 = .078, p < .05, were detected in predicting masked bias to social threat stimuli after exposure to a laboratory stress task. Cortisol reactivity was not a significant predictor, β = .15, ns. As shown in Figure 2, cortisol reactivity to an acute stressor was positively related to attentional bias to masked social threat stimuli primed by exposure to an acute stress task for individuals exposed to lower levels of family conflict during development.
Discussion
The current study experimentally examined the relation between acute stress reactivity and attentional bias to environmental threat. By examining these constructs within the broader context of past exposure to family conflict during development, we aimed to assess the extent to which alterations in stress reactivity processes may underlie psychological and physiological outcomes in individuals exposed to chronic stress. This would provide further information regarding the long-term significance of the family environment during development in vulnerability to stress-related disorders.
In examining the relation between acute stress and attentional bias to stimuli related to social threat, exposure to an analogue of acute interpersonal stress in the laboratory heightened attentional processing of socially threatening stimuli presented below the level of conscious awareness. Participants exposed to an acute laboratory stress task prior to completing the dot probe showed significantly greater attentional bias specifically towards masked social threat words, compared to participants who had not been exposed to the stress task. That is, participants responded faster when the dot probe replaced the threat word of the threat-neutral word pairs and responded slower when the dot probe replaced the neutral word of the threat-neutral word pairs. No significant change in bias was observed for consciously-presented threat stimuli. This finding extends the current literature on priming of attentional bias to include evidence for upregulation of attentional processing of socially threatening information due to the experience of interpersonal social threat. The pattern of increased bias towards threatening stimuli was consistent with our initial hypothesis and with previously observed adaptive increases in automatic selective attention following identification of an environmental threat (e.g., Chajut & Algom, 2003). The acute laboratory stressor, designed to be socially threatening, likely heightened emotional sensitivity to words related to social threat, resulting in a priming effect for socially threatening stimuli. In addition, results suggest that surges in glucocorticoid production and circulation stimulating medial prefrontal regions that play a role in selective attention abilities may have facilitated an upregulation in attention at low to medium levels (Liston et al., 2006; Lupien & McEwen, 1997). In the current sample, cortisol reactivity to an acute stressor was positively related to attentional bias to masked social threat stimuli after exposure to an acute stress task.
The priming of an attentional bias under conditions of stress is consistent with past studies linking the experience or perception of stress to heightened attentional processing of threat. For example, Mogg and colleagues (1994) found anxious subjects exposed to a life stressor showed an attentional bias towards threat. The deployment of attention towards threat under conditions of high stress was also found to be independent of trait anxiety level (Mogg, Mathews, Bird, MacGregor-Morris, 1990). The specificity of findings for masked stimuli indicates that participants may have been able to employ volitional coping strategies to adapt to conscious threat. Results from a dot probe study by Luecken and colleagues (2004) suggest that implementation of adaptive coping may impact attentional bias after threat cues are presented. Replication of this study in clinical, high-stress samples will be necessary in ascertaining whether current clinical characteristics or exposure to greater childhood stress may also attenuate conscious adaptive responding to acute stress.
In examining the association between acute laboratory stress exposure and automatic attentional processing within the context of self-reported exposure to earlier family conflict, a significant moderating effect of family conflict in childhood was confirmed (Figure 1). Individuals reporting high levels of family conflict were biased away from social threat stimuli presented below the level of conscious awareness when the dot probe task was presented prior to the acute stress task. However, when the stress task was presented first, individuals reporting high levels of family conflict showed a positive trending bias with regards to these stimuli, representing a significant difference in attentional bias between the two conditions. In other words, higher levels of childhood family conflict were related to greater priming of attentional bias towards to masked social threat words by exposure to a stress task. These results may signify enhanced malleability of automatic attentional processing under conditions of acute stress resulting from potential chronic stress-related changes in neurocognitive function and control. Such changes in attentional processing may represent more proximal contributors to mental and physical health disorders in chronically stressed populations.
Cortisol patterns as measured over the course of the study (reactivity, AUC) were examined within the context of the acute laboratory stressor and self-reported history of family conflict (Table 2). Research on chronic stress exposure predicts that repeated and prolonged activation of the HPA axis promotes a flattening of diurnal cortisol rhythms, increased ambient cortisol levels, and decreased acute stress reactivity (Miller, Chen, & Zhou, 2007). As hypothesized, individuals in the current study reporting a history of higher levels of family conflict tended to have increased AUC.
Although a significant increase in cortisol resulting from acute laboratory stress exposure was not found for the sample as a whole, a significant interaction was observed between cortisol reactivity and family conflict exposure in predicting attentional bias to masked social threat stimuli. Cortisol reactivity was positively related to attentional bias to social threat stimuli presented below the level of conscious awareness after exposure to an acute stress task for individuals exposed to lower levels of chronic stress during development. For individuals exposed to higher levels, cortisol reactivity to an acute stressor did not predict altered attentional processing of social threat. The specificity of findings for lower stress individuals may represent persisting effects of chronic stress-related changes in HPA axis functioning (Juster, McEwen, & Lupien, 2010; McEwen, 2008; Repetti et al., 2011).
If the HPA axis does in fact become dysregulated due to prolonged activation by psychosocial stress during early developmental periods, the resulting higher levels of ambient circulating cortisol may prevent typical and adaptive HPA axis reactivity. Such a state has been shown to contribute to poor long-term health (Lovallo, 2011). Taken together, our results suggest differential contributions of cognitive and psychobiological factors related to an individual’s history of stress exposure in attentional alterations measured under conditions of acute stress. These outcomes may shed light on underlying mechanisms of stress-related pathology. That is, while an adaptive surge in cortisol may typically underlie upregulated attentional processing of threat after acute stress exposure, this mechanism may not be available in individuals with a history of childhood chronic stress exposure due to dysregulation of the system. As such, dysfunction in cognitive-affective mechanisms may play a larger role in attentional changes under acute stress in these individuals, which may serve as a basis for future research into cognitive vulnerabilities to deleterious stress-related outcomes.
The current study has several strengths related to the use of a novel multi-method approach in order to further examine the interrelationships among the key constructs. First, an experimental behavioral paradigm (the dot probe) was utilized to measure automatic aspects of attention that have been previously linked to the processes of coping and emotion regulation (see Compas & Boyer, 2001 for review) and symptoms of psychopathology (see Bar-Heim et al., 2007 for review). Second, the laboratory stress task, used to learn about how individuals react and adapt to stress, has been found to be an ecologically valid example of acute stress in this population (Luecken, Kraft, & Hagan, 2009). The task also satisfies the criteria put forth by Dickerson and Kemeny (2004) that define successful laboratory stress induction paradigms by including social-evaluative, motivated performance, interpersonal aspects. Third, cortisol was collected throughout the crossover experimental design as a measure of both acute stress reactivity as well as the effects of chronic stress to further elucidate the psychological and biological processes that may impact automatic attentional processing of environmental threat. Although no significant change in cortisol level was noticed pre- to post-stress task, the typical diurnal pattern of cortisol fluctuation that peaks in the early morning and declines steadily over the course of the day may also have concealed any mild to moderate surges that occurred as a result of the task. Other psychobiological and neuroendocrine sequelae related to acute stress exposure and HPA axis alteration, including markers related to autonomic nervous system activation (e.g., adrenaline, heart rate, blood glucose) and inflammation (e.g., cytokines), may have provided more sensitive indicators of stress responsivity in this study. Further, the significant increase in negative affect as a result of the stress task supports its efficacy in inducing a mild level of stress in this sample. This crossover, multi-method approach sheds further light on the psychobiology of both acute and chronic stress and how individual differences in reactivity may influence psychological outcomes uniquely and through alterations in attentional processing.
The main limitations of the current study can be addressed in future research. Chiefly, this study relied on analyses conducted over the course of a single laboratory session with a fairly homogeneous sample of college students from a selective university. Further, the measure of chronic developmental stress was a retrospective questionnaire of interparental conflict prior to age 16 years. It is of note, however, that this and similar retrospective questionnaires asking about conflict and disturbances in the early home environment have been used successfully in studies of older adolescents and young adults and are associated with persisting psychobiological sequelae related to chronic stress into adulthood. For example, results of a study by Miller and Chen (2010) indicated that older adolescent females who retrospectively endorsed a “harsh family climate” during childhood showed increased inflammation and impaired stress reactivity.
As such, this study crucially provides novel insights into mechanisms that may underlie the development of psychopathology in young women at high risk due to early family environmental factors. Examining these processes in young women from clinical high-stress samples and those in exceptional circumstances may aid in further understanding the development of these basic human processes. Such work would provide insight into endogenous factors that may affect development of the prefrontal cortex and how subsequent cognitive factors may affect long-term outcomes in stress reactivity that contribute to mental and physical health.
Figure 2.
Interaction of conflict exposure and cortisol reactivity in predicting attentional bias to masked social threat after acute stress exposure. Low Conflict = −1 SD on CPIC; High Conflict = +1 SD on CPIC.
Acknowledgements
This work was supported by a National Science Foundation Graduate Research Fellowship and a gift by Patricia and Rodes Hart.
Footnotes
Conflict of Interest: Authors C.A., P.G., S.V., and B.C. declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: SAGE Publications, Inc; 1991. [Google Scholar]
- Applehans BM, Luecken LJ. Attentional processes, anxiety, and the regulation of cortisol reactivity. Anxiety, Stress & Coping: An International Journal. 2006;19:81–92. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck JE, Lipani TA, Baber KF, Dufton L, Garber J, Smith CA, Walker LS. Attentional bias to pain and social threat in pediatric patients with functional abdominal pain and pain-free youth before and after performance evaluation. Pain. 2011;152:1061–1067. doi: 10.1016/j.pain.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer MC, Compas BE, Stanger C, Colletti RB, Konik BS, Morrow SB, Thomsen AH. Attentional biases to pain and social threat in children with recurrent abdominal pain. Journal of Pediatric Psychology. 2006;31:209–220. doi: 10.1093/jpepsy/jsj015. [DOI] [PubMed] [Google Scholar]
- Chajut E, Algom D. Selective attention improves under stress: Implications for theories of social cognition. Journal of Personality and Social Psychology. 2003;85:231–248. doi: 10.1037/0022-3514.85.2.231. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Reviews. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Boyer MC. Coping and attention: implications for child health and pediatric conditions. Journal of Developmental and Behavioral Pediatrics. 2001;22:323–333. doi: 10.1097/00004703-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychological Bulletin. 1994;115:74–101. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Egloff B, Hock M. Assessing attentional bias toward threat-related stimuli: A comparison of the emotional Stroop task and the attentional probe task. Personality and Individual Differences. 2003;35:475–483. [Google Scholar]
- Ellenbogen MA, Carson RJ, Pishva R. Automatic emotional information processing and the cortisol response to acute psychosocial stress. Cognitive, Affective & Behavioral Neuroscience. 2010;10:71–82. doi: 10.3758/CABN.10.1.71. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker C-D. Stress and selective attention: The interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39:723–732. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker C-D. Automatic and effortful emotional information processing regulates different aspects of the stress response. Psychoneuroendocrinology. 2006;31:373–387. doi: 10.1016/j.psyneuen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Feldt LS. The use of extreme groups to test for the presence of a relationship. Psychometrika. 1961;26:307–316. [Google Scholar]
- Glinder J, Beckjord E, Kaiser C, Compas BE. Psychological adjustment to breast cancer: Automatic and controlled responses to stress. Psychology and Health. 2007;22:337–359. [Google Scholar]
- Grych JH, Fincham FD. Marital conflict and children’s adjustment; A cognitive-contextual framework. Psychological Bulletin. 1990;108:267–290. doi: 10.1037/0033-2909.108.2.267. [DOI] [PubMed] [Google Scholar]
- Grych JH, Seid M, Fincham FD. Assessing marital conflict from the child’s perspective: The Children’s Perception of Interparental Conflict Scale. Child Development. 1992;63:558–572. doi: 10.1111/j.1467-8624.1992.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Harris J. Wordcount. 2003 Retrieved from http://www.wordcount.org. [Google Scholar]
- Holmbeck GN, Shapera WE, Hommeyer JS. Observed and perceived parenting behaviors and psychosocial adjustment in preadolescents with spina bifida. In: Barber BK, editor. Intrusive Parenting: How psychological control affects children and adolescents. Washington DC: American Psychological Association; 2001. pp. 191–234. [Google Scholar]
- van Honk J, Tuiten A, van den Hout M, Kopη2partialchaar H, Thijssen J, de Haan E, Verbaten R. Conscious and preconscious selective attention to social threat: different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25:577–591. doi: 10.1016/s0306-4530(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Karlsgodt KH, Adler CM, Macklin EA, Lukas SE, Elman I. Effects of acute cortisol and cocaine administration on attention, recall and recognition task performance in individuals with cocaine dependence. Human Psychopharmacology. 2004;19:511–516. doi: 10.1002/hup.616. [DOI] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR. Do low levels of stress reactivity signal poor states of health? Biological Psychology. 2011;86:121–128. doi: 10.1016/j.biopsycho.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Appelhans B. Information-processing biases in young adults from bereaved and divorced families. Journal of Abnormal Psychology. 2005;114:309–313. doi: 10.1037/0021-843X.114.2.309. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Appelhans BM, Kraft A, Brown A. Never far from home: A cognitive-affective model of the impact of early-life family relationships on physiological stress responses in adulthood. Journal of Social and Personal Relationships. 2006;23:189–203. [Google Scholar]
- Luecken LJ, Kraft A, Hagan MJ. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Hormones and Behavior. 2009;55:412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Reviews. 2004;24:171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Research Reviews. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological Perspectives on Pathways Linking Socioeconomic Status and Physical Health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114:376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis, allostatic load, and the aging nervous system: Role of excitatory amino acids and excitotoxicity. Neurochemical Research. 2000;25:1219–1231. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Behar E, Gutner CA, Geem D, Otto MW. Cortisol, stress, and attentional bias toward threat. Anxiety, Stress, and Coping. 2010;23:529–545. doi: 10.1080/10615801003596969. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Eysenck M. Attentional bias to threat in clinical anxiety states. Cognition and Emotion. 1992;6:149–159. [Google Scholar]
- Mogg K, Bradley B. Attentional Bias in Generalized Anxiety Disorder Versus Depressive Disorder. Cognitive Therapy and Research. 2005;29:29–45. [Google Scholar]
- Mogg K, Bradley BP, Hallowell N. Attentional bias to threat: Roles of trait anxiety, stressful events, and awareness. The Quarterly Journal of Experimental Psychology. 1994;47A:841–864. doi: 10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Bird C, Macgregor-Morris R. Effects of stress and anxiety on the processing of threat stimuli. Journal of Personality and Social Psychology. 1990;59:1230–1237. doi: 10.1037//0022-3514.59.6.1230. [DOI] [PubMed] [Google Scholar]
- Neshat-Doost H, Moradi A, Taghavi R, Yule W, Dalgleish T. Lack of attentional bias for emotional information in clinically depressed children and adolescents on the dot probe task. Journal of Child Psychology and Psychiatry. 1999;41:363–368. [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology. General. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Pilgrim K, Marin M-F, Lupien SJ. Attentional orienting toward social stress stimuli predicts increased cortisol responsivity to psychosocial stress irrespective of the early socioeconomic status. Psychoneuroendocrinology. 2010;35:588–595. doi: 10.1016/j.psyneuen.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Reese-Weber M, Hesson-McInnis The Children’s Perception of Interparental Conflict Scale: Comparing factor structure between developmental periods. Educational and Psychological Measurement. 2008;68:1008–1023. [Google Scholar]
- Repetti RS, Robles TF, Reynolds BR. Allostatic processes in the family. Development and Psychopathology. 2011;23:921–938. doi: 10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6:e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]