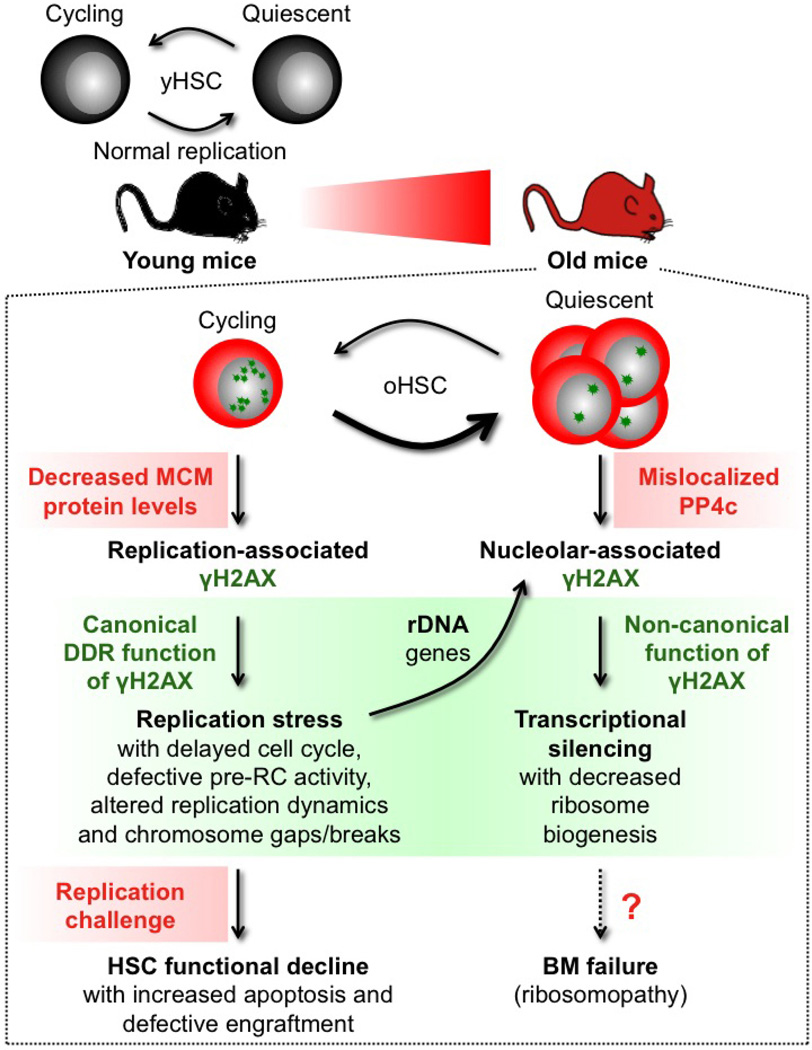
Extended Data Figure 10. Model for replication stress and γH2AX accumulation in old HSCs.
In contrast to HSCs isolated from young mice (yHSC), which replicate normally, HSCs isolated from old mice (oHSC) have severe replication defects due to decreased expression of mini-chromosome maintenance (MCM) DNA helicase components. Cycling old HSCs show heightened levels of replication-associated γH2AX foci (small green stars) and increased levels of replication stress associated with cell cycle defects, altered DNA fork replication dynamics and chromosome gaps/breaks. Nonetheless, old HSCs usually survive replication unless confronted with a strong replication challenge such as treatment with a replication stressor drug or transplantation, which preferentially kill them and lead to their defective engraftment in vivo. Cycling old HSCs are also likely to accumulate γH2AX foci on rDNA genes as a consequence of replication stress occurring at these hard-to-replicate loci during their replication in late S phase. However, stalled or collapsed replication forks appear to be repaired upon activation of the canonical DNA damage response (DDR), resulting in essentially undamaged rDNA genes in old HSCs. In contrast, the clearance of γH2AX foci is probably unfinished by the time old HSCs re-enter quiescence, thereby aggregating leftover γH2AX signals in reformed nucleoli in post-mitotic cells. We propose that ineffective dephosphorylation of γH2AX due to mislocalization of PP4c phosphatase in quiescent old HSCs contributes to the long-term persistence of nucleolar-associated γH2AX signals (large green stars) in the absence of ongoing DNA damage and DDR activation. Persistent nucleolar γH2AX also acts in a non-canonical manner as a histone modification marking the transcriptional silencing of rDNA genes and decreased ribosome biogenesis in quiescent old HSCs. Whether decreased rDNA transcription in quiescent old HSCs is involved in bone marrow (BM) failure syndromes associated with defective ribosome biogenesis (ribosomopathy) remains to be determined. However, as soon as old HSCs re-enter the cell cycle, PP4c is re-localized to the nucleus and quickly dephosphorylates nucleolar γH2AX, thereby restoring ribosome biogenesis. Our results demonstrate that accumulation of γH2AX in old HSCs marks either ongoing or residual replication stress caused by the inability of ageing HSCs to maintain normal levels of MCM proteins. They highlight the MCM DNA helicase as a potential molecular target for rejuvenation therapies.