Abstract
To evaluate eyes with abnormal visual fields and multifocal electroretinograms (mfERGs) but normal-appearing frequency-domain optical coherence tomography (fdOCT) scans, the thicknesses of the outer retinal layers were measured. A total of 25 eyes from 17 patients, including 15 eyes previously tested (Dale et al. in Doc Ophthalmol 120(2):175–186, 2009) were examined. All patients were evaluated with standard automated perimetry (SAP) using the 24-2 and/or 10-2 program (Zeiss Meditec), mfERG with 103 hexagons (Veris, EDI), and fdOCT imaging (3DOCT-2000, Topcon) with scans of the macula. All patients had reliable visual fields showing macular defects and good quality mfERG and fdOCT results. The mfERG results were classified as abnormal based on decreased amplitudes and/or increased latencies corresponding to the abnormal visual field. Based on visual inspection, three experienced observers classified the fdOCT scans as normal or inconclusive, as opposed to clearly abnormal. Retinal layers of the fdOCT scans were manually segmented with the aid of a computer program and compared to mean thicknesses from 20 controls. The thicknesses of the outer segment plus retinal pigment epithelium, total receptor, and inner nuclear layers were measured. Quantitative analysis of fdOCT scans demonstrated thinning of the outer retina in some scans that was not readily apparent on visual inspection. One or more of the outer retinal layers was significantly thinner in 15 of the 25 eyes. The absence of significant thinning in the other 10 eyes represents instances in which functional loss measured by visual fields and mfERGs can precede clear structural changes on fdOCT.
Keywords: Electroretinography, Optical coherence tomography, Retina
Introduction
Frequency-domain optical coherence tomography (fdOCT), also known as spectral-domain OCT, has emerged in recent years as a powerful noninvasive technique that can capture high-resolution, cross-sectional images of the retina in vivo. Compared to time-domain OCT, fdOCT technology results in faster imaging speeds and higher axial resolutions [1]. As a result, fdOCT has become an important tool in the study of retinal morphology in a variety of diseases where subtle changes in the individual retinal layers can be seen. Several studies have demonstrated a correlation between outer retinal structure as seen on fdOCT and retinal function as measured with visual fields in outer retinal pathologies such as retinitis pigmentosa [2–4], chloroquine toxicity [5, 6], Leber congenital amaurosis [7], and acute zonal occult outer retinopathy (AZOOR) [8].
While the fdOCT is a structural test, the multifocal electroretinogram (mfERG) assesses local retinal function [9, 10]. Using a stimulus display of typically 61 or 103 hexagons, local cone-driven responses are recorded under light-adapted conditions. Changes in waveform amplitude and timing reflect damage at or before the level of the bipolar cells [11]. These local changes can then be topographically compared to local regions of visual field sensitivity to confirm retinal disease.
Although there is often agreement between fdOCT and mfERG results, disagreements do occur. Dale et al. [4] showed that in some cases, the fdOCT was better able to detect small, focal retinal abnormalities, and attributed this difference to its higher spatial resolution. In other cases, retinal disease was detected on mfERG testing, but the fdOCT results appeared normal. It was less clear why this mismatch occurred. A better understanding of the factors contributing to these disagreements is important, especially as fdOCT is becoming a more accessible clinical tool.
Dale et al. [4] suggested that quantification might better identify subtle structural changes in the outer retina than the qualitative analysis they used. For example, measurements of the outer retinal layers can reveal structural damage that is not obvious on visual inspection of fdOCT scans in conditions such as retinitis pigmentosa [12, 13]. Here, we use these techniques to ask whether outer retinal changes can be measured in eyes with normal-appearing or inconclusive fdOCT scans but with abnormal visual field and mfERG results.
Methods
Subjects
Twenty-five eyes from 17 patients (54.3 ± 20.7 years) with abnormal visual field and mfERG results, but normal-appearing or inconclusive fdOCT scans were examined. Of the 25 eyes, 15 were tested between Oct 2007 and June 2009, as part of a previous study [4]; 10 eyes were tested from June 2009 to April 2011. Patients more than 80 years of age, with ocular comorbidities affecting the mfERG signal, with poor quality mfERG or fdOCT results, or with focal retinal lesions outside the central macula (i.e., outside the region captured by the fdOCT scan) were excluded. All patients were examined by a board certified ophthalmologist and underwent evaluation including medical history review, best corrected visual acuity, slit lamp biomicroscopy, dilated funduscopic examination, and standard automated perimetry (SAP). Diagnoses for the 25 eyes were also sought from the referring ophthalmologists.
The procedures adhered to the tenets of the Declaration of Helsinki, and the protocol was approved by the Committee of the Institutional Board of Research Associates of Columbia University. Written informed consent was obtained from all subjects prior to their participation.
Standard automated perimetry
Standard automated perimetry was performed using the SITA Standard 24-2 and/or 10-2 visual field program in the Humphrey Visual Field Analyzer (Carl Zeiss Meditec Inc, Dublin, CA). Refractive errors were corrected with appropriate trial lenses. All patients had reliable visual fields (global indices <33%) showing central macular defects.
The mfERG recording technique and analysis
The mfERG testing was performed according to ISCEV guidelines [14]. As previously described, 8-min long recordings were obtained with a Burian-Allen corneal electrode and a forehead ground electrode, with signal cutoffs set at 10 and 100 Hz. A 103 scaled hexagon display (Veris, EDI, Redwood City, CA), with a mean luminance of 100 cd/m2 and a contrast of ≥90%, was used. Patients were instructed to look at a central fixation target, either an “X” or a central circle if the “X” could not be visualized. Using commercial software, the mfERG responses were then analyzed with artifact rejection and with and without spatial averaging of 1/6 of the surrounding responses.
The mfERG results were reviewed for changes in waveform amplitude and first-order latency. An mfERG response was classified as abnormal if decreased amplitudes and/or increased latencies were observed in regions corresponding to visual field sensitivity loss on SAP.
The fdOCT scan acquisition and analysis
The fdOCT imaging (3D OCT-2000, Topcon Inc, Paramus, NJ) was acquired on the same day as the mfERG and included 6 mm by 6 mm cube and 6 mm line scans of the macula (central 10°). Based on visual inspection, three experienced individuals classified the fdOCT scans as normal or inconclusive, as opposed to clearly abnormal. FdOCT scans were classified as inconclusive when the observed changes were too slight or subtle for the experienced individual to judge confidently as abnormal, given the variability among controls.
Normal or inconclusive scans were analyzed with computer-aided manual segmentation [12, 13, 15]. Typically, a 6-mm horizontal line scan was used. If unavailable, the centermost scan from the cube scan was selected. The borders determined are shown in Fig. 1. Thicknesses of the retinal layers of the fdOCT scans were compared to mean thicknesses from 20 controls. The thicknesses of the outer segment plus retinal pigment epithelium layer (OS+: Bruch’s membrane/choroid border to inner–outer segment line), total receptor (REC+: Bruch’s membrane/choroid to outer plexiform–inner nuclear layer (INL) border), and the INL (outer plexiform/INL border to INL/inner plexiform border) were measured.
Fig. 1.
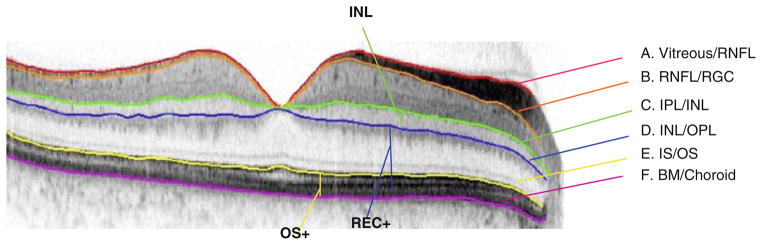
The highlighted borders show the boundaries drawn using a computer-aided manual segmentation technique. Thicknesses between two boundaries can then be measured. The OS+ thickness (yellow vertical line), REC+ thickness (blue vertical line), and INL thickness (green vertical line) were measured
Results
Of the 25 patient eyes with abnormal mfERG and visual field tests, but normal or inconclusive fdOCT scans, a total of 15 eyes (10 patients) showed thinning of one or more retinal layers. The visual acuity of these 15 eyes ranged from 20/20 to 20/200. A layer was classified as abnormally thin if the thickness fell below the minimum thickness measured in controls (blue dashed line, Fig. 2) and/or below the control mean minus two standard deviations (red dashed line, Fig. 2). Eight eyes were found to have a thinner OS+ layer, 10 eyes a thinner REC+ layer, and five eyes a thinner INL layer (see red rectangles in Fig. 2). One eye showed thinning of all three layers. Six eyes showed thinning in the OS+ and REC+ layers. Below, we illustrate the basic findings with two examples.
Fig. 2.
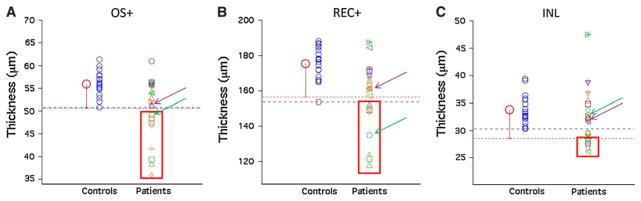
a Measurement of the OS+ thicknesses in individual patients (symbols, right) compared to individual controls (blue circles, left). The mean thickness of the controls is represented by the larger red circle. The measured thickness in a patient eye was considered thin if it was less than the minimum thickness in controls (blue dashed line) and/or below the control mean minus two standard deviations (red dashed line). Thinning was observed in some patient eyes (red rectangle). Purple arrows point to thicknesses measured in Patient 1 and green arrows point to thicknesses measured in Patient 2 as described in the text. b Measurement of the REC+ thicknesses in individual patients and individual controls. c Measurement of the INL thicknesses in individual patients and individual controls
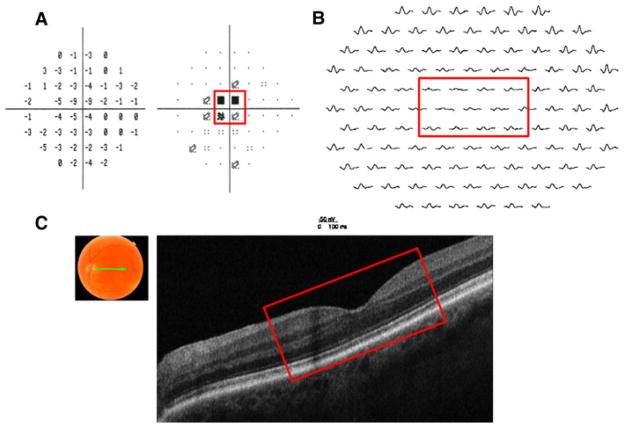
First, patient 1 is a 75-year-old man complaining of a sudden loss of his central vision in the left eye following cataract surgery. Visual acuity in the left eye decreased from 20/30 1 day postsurgery to 20/80 1 week later. Visual acuity in the right eye was counting fingers since childhood secondary to infantile glaucoma. Funduscopic examination revealed a normal-appearing fundus. Visual field testing revealed a dense scotoma within the central 6–12° (see Fig. 3a). The mfERG from the left eye showed decreased response amplitudes and delayed timing in the region corresponding to the scotoma seen on the visual field (see Fig. 3b). However, the fdOCT scan in the same region of the retina, shown as the red rectangle in Fig. 3c, did not show a clear structural abnormality that could account for the functional loss. Quantitatively, the outer retinal layer thicknesses (purple arrows, Fig. 2) fall within the normal range of controls. This patient was eventually found to have antibodies that react with the inner and outer nuclear layers of the retina, the inherent retinal vasculature, and the sheath of the optic nerve. Thus, in this case, a patient with a clear retinal problem and an abnormal visual field and mfERG had a normal fdOCT even after careful measurement.
Fig. 3.
Patient 1. a 24-2 SAP demonstrates a central scotoma in the left eye (red box). b mfERG with reduced amplitude and increased latency in the left eye in the retinal region corresponding to the SAP defect (red box). c fdOCT demonstrates a reasonably normal-appearing retina in the corresponding region of VF and mfERG abnormalities (red box)
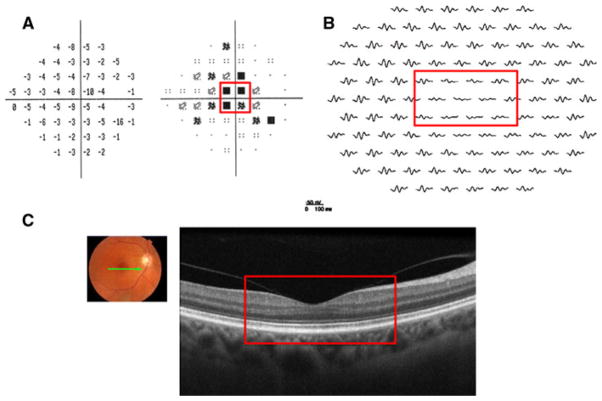
Patient 2 is a 57-year-old woman who presented with a complaint of a “dark spot” in her central vision and photopsia in her right eye of 5 months duration. Her visual acuity was 20/200 in the right eye and 20/20 in the left. Funduscopic exam revealed an abnormal foveal reflex and disc pallor in the right eye. As shown in Fig. 4a, a dense central scotoma was found on visual field testing. This was consistent with decreased mfERG response amplitudes (see Fig. 4b). FdOCT imaging, on the other hand, demonstrated a normal-appearing macula based on visual inspection (see Fig. 4c). Quantitative analysis with segmentation revealed thinning of the outer retina, particularly the OS+ and REC+ layers (green arrows, Fig. 2). A final diagnosis for the underlying cause for the retinal defect has yet to be made.
Fig. 4.
Patient 2. a 24-2 SAP demonstrates a central scotoma in the right eye (red box). b mfERG with reduced amplitude in the right eye in the retinal region corresponding to the SAP defect (red box). c fdOCT demonstrates a normal-appearing retina in the region corresponding to the VF and mfERG abnormalities (red box)
To better understand the structure–function dissociation, we sought diagnoses for the eyes. The diagnoses for the 15 eyes with outer retinal thinning cone or cone-rod dystrophy (8), and unknown (7). The remaining 10 eyes carried the following diagnoses: cone dystrophy (1), atypical sectoral retinitis pigmentosa (2), and unknown (7). An “unknown” designation here refers to cases in which a final consensus regarding the diagnosis has not yet been reached.
Discussion
A normal-appearing fdOCT scan, but an abnormal visual field and mfERG result implies that early functional loss can precede obvious structural changes seen on fdOCT. One might predict that early changes affecting the retina at the cellular level, for example, would escape the resolution of a structural exam such as the fdOCT while functional changes would be evident. In fact, studies have shown that the IS/OS junction can appear reasonably intact qualitatively in outer retinal diseases even when functional deficits are present [2, 16]. For example, patients with retinitis pigmentosa [2] have well preserved IS/OS lines in regions of the visual field with sensitivity losses less than about −9 dB. Further, quantification of the transition zone from normal to abnormal regions in patients with RP reveals subtle changes in the thickness of the outer nuclear and OS layers [13]. These findings prompted us to carefully quantify the thickness of these layers in patients with normal-appearing fdOCT scans, but clearly abnormal retina function based upon visual field and mfERG findings.
Of the 25 eyes studied, 15 eyes were found to have abnormal thinning of one or more outer retinal regions when quantitative techniques were applied. In particular, measured thicknesses for the REC+ layer demonstrated the most thinning, suggesting that this particular layer may be the most sensitive thickness in detecting retinal disease. The group of the remaining 10 eyes with normal range outer retinal thicknesses also demonstrates that in certain diseases, the functional loss measured by visual fields and mfERG testing precedes clear evidence of anatomical thinning. For example, as illustrated by patient 1, diseases with an antibody-mediated mechanism can cause severe functional loss, while the signal produced by outer retinal layers remains reasonably normal on OCT.
This study shows the importance of carefully measuring the outer retina, which can in some cases detect thinning of the outer retina even when the anatomy appears normal based on visual inspection alone. It also underscores the importance of continuing to use functional testing together with fdOCT. With current fdOCT scanning and analysis technology, we are still not detecting structural damage in eyes with clear functional abnormalities.
Acknowledgments
We thank Drs. M. Behrens, S. Kane, J. Bortz, S. Forman, and R. Lesser for referring patients. This work was supported by a Doris Duke Charitable Foundation grant to CLT and a NIH grant R01-09076 to DCH.
Abbreviations
- mfERG
Multifocal electroretinogram
- fdOCT
Frequency-domain optical coherence tomography
- SAP
Standard automated perimetry
Footnotes
Conflict of interest: None.
Contributor Information
Christine L. Talamini, Department of Psychology, Columbia University, 406 Schermerhorn Hall, 1190 Amsterdam Avenue, New York, NY 10027, USA
Ali S. Raza, Department of Psychology, Columbia University, 406 Schermerhorn Hall, 1190 Amsterdam Avenue, New York, NY 10027, USA
Elizabeth A. Dale, Department of Ophthalmology, Columbia University, New York, NY, USA
Vivienne C. Greenstein, Department of Ophthalmology, Columbia University, New York, NY, USA
Jeffrey G. Odel, Department of Ophthalmology, Columbia University, New York, NY, USA
Donald C. Hood, Email: dch3@columbia.edu, Department of Psychology, Columbia University, 406 Schermerhorn Hall, 1190 Amsterdam Avenue, New York, NY 10027, USA. Department of Ophthalmology, Columbia University, New York, NY, USA
References
- 1.Ko TH, Fujimoto JG, Schuman JS, Paunescu LA, Kowalevicz AM, Hartl I, Drexler W, Wollstein G, Ishikawa H, Duker JS. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology. 2005;112(11):1922.e1–1922.e15. doi: 10.1016/j.ophtha.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rangaswamy NV, Patel HM, Locke KG, Hood DC, Birch DG. A comparison of visual field sensitivity to photoreceptor thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010;51(8):4213–4219. doi: 10.1167/iovs.09-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood DC, Ramachandran R, Holopigian K, Lazow M, Birch DG, Greenstein VC. Method for deriving visual field boundaries from OCT scans of patients with retinitis pigmentosa. Biomed Opt Express. 2011;2(5):1106–1114. doi: 10.1364/BOE.2.001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dale EA, Hood DC, Greenstein VC, Odel JG. A comparison of multifocal ERG and frequency domain OCT changes in patients with abnormalities of the retina. Doc Ophthalmol. 2009;120(2):175–186. doi: 10.1007/s10633-009-9210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009;93(11):1444–1447. doi: 10.1136/bjo.2008.157198. [DOI] [PubMed] [Google Scholar]
- 6.Kellner U, Kellner S, Weinitz S. Chloroquine retinopathy: lipofuscin- and melanin-related fundus autofluorescence, optical coherence tomography and multifocal electroretinography. Doc Ophthalmol. 2008;116(2):119–127. doi: 10.1007/s10633-007-9105-6. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson SG, Aleman TS, Cideciyan AV, Roman AJ, Sumaroka A, Windsor EA, Schwartz SB, Heon E, Stone EM. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2009;50(5):2368–2375. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta K, Sato A, Fukui E. Spectral domain optical coherence tomographic findings at convalescent stage of acute zonal occult outer retinopathy. Clin Ophthalmol. 2009;3:423–428. doi: 10.2147/opth.s6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutter EE, Tran D. The field topography of ERG components in man—I. The photopic luminance response. Vis Res. 1992;32(3):433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- 10.Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19(5):607–646. doi: 10.1016/s1350-9462(00)00013-6. [DOI] [PubMed] [Google Scholar]
- 11.Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43(5):1673–1685. [PubMed] [Google Scholar]
- 12.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(5):2328–2336. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood DC, Lazow MA, Locke KG, Greenstein VC, Birch DG. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52(1):101–108. doi: 10.1167/iovs.10-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Palmowski-Wolfe AM. ISCEV guidelines for clinical multifocal electroretinography (2007 edition) Doc Ophthalmol. 2008;116(1):1–11. doi: 10.1007/s10633-007-9089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88(1):113–123. doi: 10.1097/OPX.0b013e3181fc3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birch DG, Wen Y, Locke KG, Hood DC. Rod sensitivity, cone sensitivity and photoreceptor layer thickness in retinal degenerative disease. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]