Abstract
Purpose
We describe the “double zipper” mechanism of human male urethral formation, where the distal zipper opens the urethral groove through canalization of the urethral plate, and a second closing zipper follows behind and closes the urethral groove to form the tubular urethra.
Materials and Methods
Anonymous human fetal genital specimens were acquired and gender was determined by polymerase chain reaction of the Y chromosome. Specimens were processed for optical projection tomography, stained with E-cadherin, Ki67 and caspase 3, and imaged.
Results
Eight developing male fetal specimens from 6.5 to 16.5 weeks of gestation were analyzed by optical projection tomography, and an additional 5 specimens by serial sections. Phallus length ranged from 1.3 to 3.7 mm. The urethral plate canalized into a groove with 2 epithelial edges that subsequently fused. Ki67 staining was localized to the dorsal aspect of the urethral plate. In contrast, caspase 3 staining was not observed. The entire process was completed during a 10-week period.
Conclusions
The human male urethra appears to form by 2 mechanisms, an initial “opening zipper” that facilitates distal canalization of the solid urethral plate to form the urethral groove, which involves a high rate of epithelial proliferation (apoptosis not observed), and a “closing zipper” facilitating fusion of the 2 epithelial surfaces of the urethral groove, and thus extending the penile urethra distally. Improved knowledge of the molecular mechanisms of these processes is critical to understanding mechanisms of abnormal urethral development, such as hypospadias.
Keywords: growth and development, hypospadias, organogenesis, urethra
Understanding normal human urethral development is the first step is unraveling abnormal urethral development, of which the most common anomaly is hypospadias. Hypospadias is defined by abnormal location of the urethral meatus, impaired differentiation of the corpus spongiosum and failure of closure of the ventral foreskin.1 The urethral defects result from failure of fusion of 2 epithelial surfaces of the urethral groove, which forms via canalization of the solid urethral plate.2,3 Hypospadias results from events that may involve 1) formation of the urethral plate, 2) failure of canalization of the urethral plate, and thus failure of formation of the urethral groove, 3) failure of medial growth of the urethral fold (lateral edges of the urethral groove) or 4) failure of midline fusion of the urethral folds.4,5
Previous studies examining urethral development have relied on gross examination, serial sections and 3D reconstruction of images of serial sections.5–7 With the advent of OPT, whole mount 3D imaging of the developing human urethra can now be realized.8 We describe the “double zipper” hypothesis of urethral formation, where the “opening zipper” facilitates formation of the urethral groove distally through canalization of the urethral plate, and a “closing zipper” follows behind and closes the urethral groove to form the tubular urethra. Midline epithelial proliferation within the urethral plate is a key feature of development of the urethral groove, while apoptosis does not appear to be involved in canalization of the urethral plate.
METHODS
The study was performed after receiving approval from the committee on human research at the University of California, San Francisco. Anonymous human fetal genital specimens from elective terminations were acquired, and gestational age was estimated by heel-toe length.9,10 Fetal gender was determined by 2 methods, ie visual inspection of internal genitalia (gonads and wolffian and müllerian ducts) using a dissecting microscope, and polymerase chain reaction for X and Y-chromosomal genes.11 For the latter DNA was isolated from fetal tissue sample using the QIAamp® DNA Mini Kit. Isolated DNA was then amplified for specific primers of the X and Y chromosomes.
Optical Projection Tomography
OPT is a technique for volumetric visualization of transparent or slightly opaque objects on the cellular level up to small organisms.8 The principle of OPT is similar to computerized tomography, except for the use of visible light instead of x-rays.
Dissected human fetal genitals were fixed in formalin overnight, stored in 100% methanol and prepared for OPT.8 Samples were immunostained with anti- E-cadherin (diluted 1:100, BD Transduction Laboratories™) using the secondary antibody (goat anti-mouse diluted 1:100, Alexa Fluor® 488 nm).
Images of the specimens were captured using Bioptonics OPT scanner 3001M (Medical Research Council Technology, London, United Kingdom), as described previously.8 The raw data (400 projected images) from each of the 2 channels were reconstructed into a pair of 3D voxel data sets using in-house reconstruction software. 3D OPT reconstructions were then loaded into PerkinElmer® Volocity software for visualization and analysis.
OPT samples were isolated from the agar blocks using the standard Bioptonics OPT protocol. Once isolated, the specimens were formalin fixed, paraffin embedded and serially sectioned at 7 microns. Immunohistochemical analysis was carried out as described previously,5 using antibodies to Ki67 (1:100, Leica Microsystems, Inc., Buffalo Grove, Illinois) to detect cellular proliferation and caspase 3 (1:200, Cell Signaling Technology, Inc., Danvers, Massachusetts) to detect apoptosis.
RESULTS
Optical Projection Tomography—Gross Visualization and Morphometrics
A total of 13 human male fetal external genitalia specimens were evaluated following confirmation of male gender by polymerase chain reaction. Under dissecting microscopy the entire fetal specimen appeared normal. Eight developing male fetal specimens from 6.5 to 16.5 weeks of gestation were processed for OPT, and the remaining 5 male specimens were exclusively analyzed by histology.
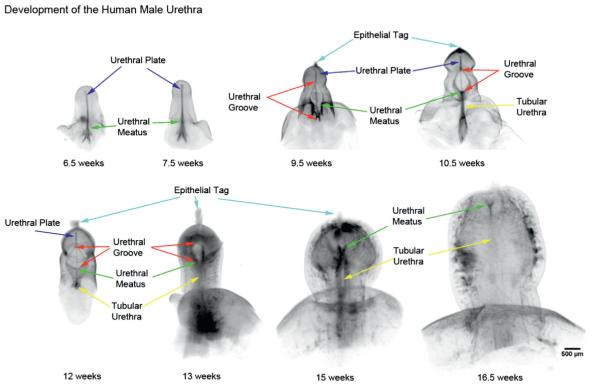
In the OPT specimens E-cadherin localized to the entire epidermis and urethral epithelial cells, including the areas of canalization and fusion. Phallus length ranged from 1.3 to 3.7 mm (fig. 1). The urethral meatus progressed from proximal to distal as gestational age increased, with progression of the urethral meatus (defined as the most proximal location where the urethra opened into the amniotic cavity) from the scrotal folds at 6.5 weeks to a terminal position on the glans at 16.5 weeks. The open urethral groove (defined by an open groove with 2 epithelial edges greater than 200 microns apart) was best seen from 9.5 to 13 weeks, with clear progression of a proximal to distal fusion of the edges of urethral groove to form the tubular penile urethra. At 13 weeks the urethral groove was located within the glans penis, with the tubular urethra completely formed within the shaft of the penis. The epithelial tag appeared at approximately 9 weeks and disappeared by 16.5 weeks. The entire process of urethral formation was completed during a 10-week period.
Figure 1.
OPT of male urethral development from 6.5 to 16.5 weeks of gestation. Note progression of urethral meatus (green arrows) from scrotal folds at 6.5 weeks to terminal position on glans at 16.5 weeks. Wide open urethral groove (red arrows) is best seen from 9.5 to 13 weeks, with clear progression of proximal to distal fusion of edges of urethral groove to form tubular urethra (yellow arrows). At 13 weeks urethral groove is within glans penis with tubular urethra completely formed within shaft of penis, consistent with endodermal theory of urethral development. No evidence of ectodermal intrusion is evident in any specimen.
Indifferent Stage of Penile Development (6.5 to 7.5-Week Specimens)
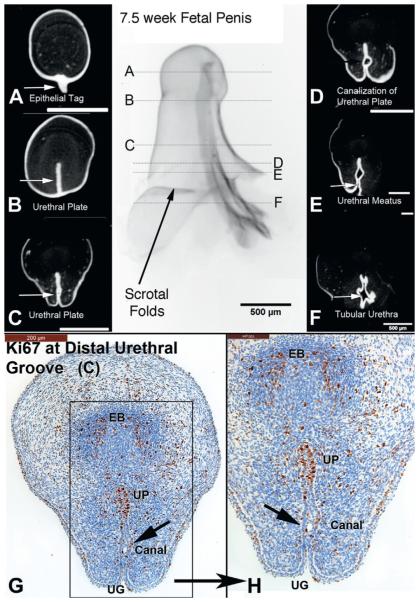
In the youngest specimen estimated at 6.5 weeks the urethral plate was not evident at the distal tip of the genital tubercle, but was for the most part solid, with evidence of canalization in the penile shaft (fig. 2). The urethral meatus was located at the scrotal folds with the tubular urethra just proximal. Staining for the proliferation marker Ki67 was localized to the nuclei predominantly within the dorsal aspect of the urethral plate and in the mesenchymal condensation representing the forming erectile body. Staining for apoptosis using caspase 3 was negative. A similar result was found in the 7.5-week specimen, with absence of the urethral plate in the distal most aspect of the genital tubercle and proximal canalization of the solid urethral plate (fig. 3). Cellular proliferation based on Ki67 staining was concentrated on the dorsal aspect of the urethral plate and in the mesenchymal condensation representing the forming erectile body. Caspase 3 was not detected in any tissues (not illustrated).
Figure 2.
A to F, OPT of 6.5-week penis at indifferent stage of development stained with E-cadherin. G to K, histological sections correspond to horizontal lines in gross OPT specimen. G and H, note extensive proliferation (Ki67) along urethral plate (UP), urethral meatus (UM) and areas of urethral plate canalization (Canal), ie “opening zipper.” I and J, high power images of G and H. K, caspase 3 was not expressed, in contrast to Ki67 in I and J, supporting role of cellular proliferation over apoptosis as means of urethral plate/groove development. EB, erectile body.
Figure 3.
A to F, OPT of 7.5-week penis at indifferent stage of development stained with E-cadherin. G and H, histological sections correspond to horizontal lines in gross specimen. D, E, G and H, note canalization (Canal) of urethral plate (UP) and cellular proliferation (Ki67) localized to dorsal urethral plate (opening zipper). EB, erectile body. UG, urethral groove.
Early Stage of Penile Development (9.5 and 10.5-Week Specimens)
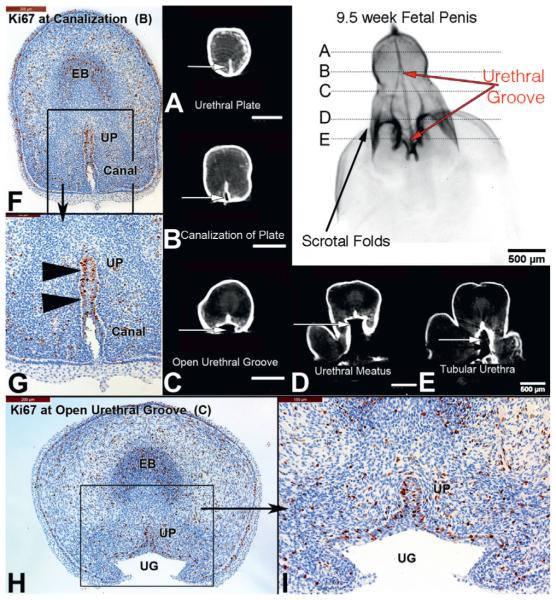
The 9.5-week penis exhibited increased growth compared to the 2 specimens in the indifferent stage (fig. 4). The urethral groove was now well developed, extending from the scrotal folds to the proximal aspect of the glans (coronal sulcus), comprising the entire length of the penile shaft. The width of the urethral groove was also quite large compared to the solid urethral plate. The solid urethral plate, now located exclusively within the glans, exhibited ventral canalization but remained solid dorsally. Cellular proliferation (Ki67) was localized to the dorsal aspect of the urethral plate. Residual urethral plate, present in the midline of the urethral groove, also remained highly proliferative.
Figure 4.
A to E, OPT of 9.5-week penis in early stages of development. B and F to I, urethral groove (UG) is now well developed comprising length of penile shaft (red arrows), and canalization (Canal) of urethral plate (UP, arrowheads) and cellular proliferation (Ki67) localized to dorsal urethral plate (opening zipper). EB, erectile body.
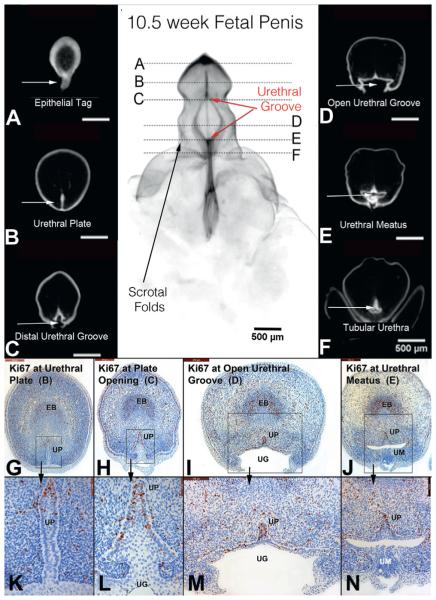
The notable feature of the 10.5-week penis was the “distal movement” of the wide urethral groove, whose proximal boundary was located at the mid shaft of the penis and whose distal boundary had extended into the glans (fig. 5). Between these 2 points a broadly opened urethral groove was evident. Proximal closure of the urethral groove created a tubular penile urethra in the penile shaft. Proliferation (Ki67) was localized to the dorsal aspect of the solid urethral plate within the glans, in the residual urethral plate in the midline of the urethral groove and in the residual urethral plate in the dorsal midline of the tubular urethra. Within the open urethral groove there was a consistent decline in proliferative activity from medial to lateral (edges of the urethral folds). Caspase 3 activity was not observed in either specimen (data not shown).
Figure 5.
A to F, OPT of 10.5-week penis in early stages of development with epithelium stained for E-cadherin. Continued proliferation (Ki67) in urethral plate (UP, opening zipper, G and H, high power k and L), open urethral groove (UG, red arrows) and fusion of urethral groove (I and M) to form tubular urethra (J and N). EB, erectile body. UM, urethral meatus.
Later Stages of Penile Development (12, 13, 15 and 16.5-Week Specimens)
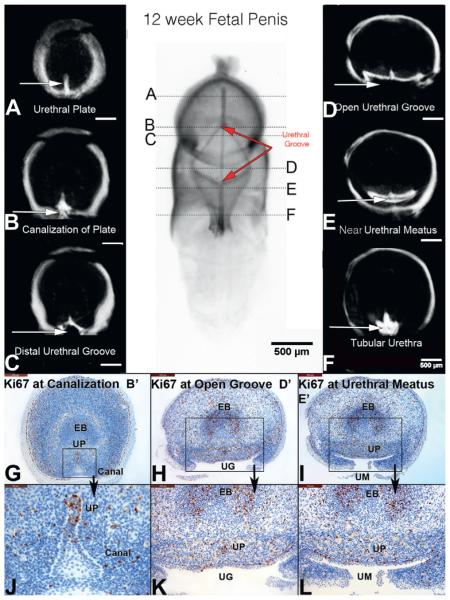
At 12 weeks the proximal aspect of the urethral groove had fused and advanced farther distally along the penile shaft, while the distal aspect of the wide urethral groove had advanced into the mid glans (fig. 6). The tubular urethra had advanced to the mid shaft of the penis. The solid urethral plate was now evident in the distal aspect of the glans, with associated canalization of the urethral plate at the mid glans. Proliferation (Ki67) within the urethral plate was again localized dorsally. In the region of the open urethral groove proliferation was greatest medially and decreased laterally to essentially zero in the edges of the urethral folds.
Figure 6.
A to F, OPT of 12-week penis in mid stages of development. Note that proximal urethral groove (UG) has fused (closing zipper) and advanced to distal aspect of shaft of penis, and distal groove (opening zipper) has advanced into glans (red arrows). Canal, canalization. EB, erectile body. UM, urethral meatus. UP, urethral plate.
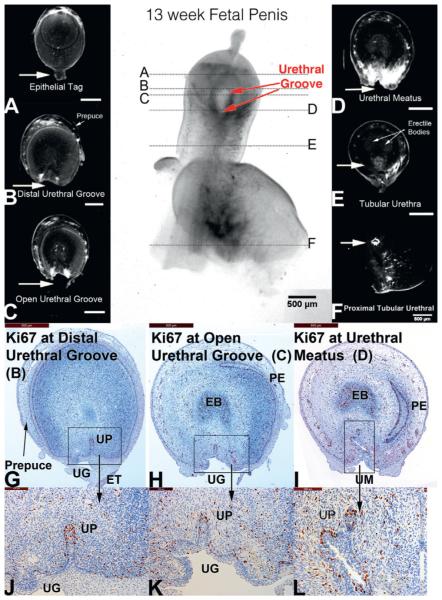
At 13 weeks the urethral groove was decreased in length and entirely within the glans (fig. 7). The epithelial tag was still visible on the glans. The tubular urethra was completely formed throughout the entire penile shaft. Within the glans cellular proliferation (Ki67) remained active and localized to the dorsal aspect of the urethral plate. More proximally within the urethral groove and urethral meatus the dorsal aspect of the residual urethral plate remained active for Ki67 staining but qualitatively less than in the solid urethral plate distally.
Figure 7.
A to F, OPT of 13-week penis in late stages of development. Urethral groove (UG) is contained within proximal glans and is shorter compared to earlier specimens (red arrows). EB, erectile body. ET, epithelial tag. PE, preputial epithelium. UM, urethral meatus. UP, urethral plate.
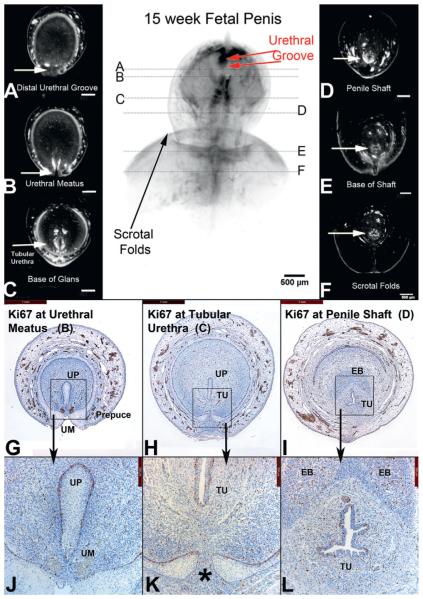
At 15 weeks the penis was almost completely formed (fig. 8). The urethral groove was shorter compared to earlier stages of development. The location of the groove was in the distal glans close to the final terminal position of the normal urethral meatus. Fusion of the ventral foreskin was almost complete. Where urethral formation was still active in the distal glans, cellular proliferation remained active in the dorsal aspect of the urethral plate. In the completely formed tubular urethra proliferation was equally distributed around the entire urethral epithelium. Caspase 3 was rarely detected in these specimens (data not shown). In the 16.5-week specimen the urethra had reached its normal position on the glans, the foreskin had fused ventrally and the epithelial tag was no longer present (fig. 1).
Figure 8.
A to F, OPT of 15-week penis in late stages of development. Note urethral groove is contained within distal glans and is shorter compared to earlier specimens (red arrows). In A prepuce has fused ventrally (G, H, J, K). Asterisk indicates fusion of preputial folds. EB, erectile body. TU, tubular urethra. UM, urethral meatus. UP, urethral plate.
DISCUSSION
Our observations regarding the developing human fetal urethra are in agreement with previous series.3,6,7 OPT confirms a solid urethral plate that canalizes, forming the urethral groove that progressively advances and fuses to form the tubular penile urethra. The process starts proximally at the scrotal folds and progresses along the penile shaft to the glans to form the terminal urethral meatus. There is no evidence of ectodermal intrusion of epidermal cells that meet the solid and/or canalized urethral plate or urethral groove, consistent with the endodermal theory of urethral development.2,3
The mechanism of penile urethral formation appears to simulate a “double zipper.” The first or opening zipper involves the canalization process within the urethral plate to create the urethral groove. This process is seen in the OPT images initially starting at the scrotal fold/penile junction (fig. 2, E, H and K) and is visualized in the cross-sectional OPT and histological sections (fig. 3, C, G and H). During these early stages of development at 6.5 and 7.5 weeks of gestation there is already evidence of canalization of the urethral plate (fig. 2, C, G and I), consistent with subsequent development of the urethral groove between 7.5 and 9.5 weeks of gestation (fig. 4). The “opening zipper,” starting at the urethral meatus, opens the urethral groove through canalization of the urethral plate and lateral expansion of the urethral groove, a process that may be facilitated by exceptionally high proliferative activity in the dorsal aspect of the urethral plate and the residual urethral plate in the midline of the urethral groove.
Between 9.5 and 15 weeks of gestation the “opening zipper” constitutes the distal leading edge of the urethral groove, which moves toward and then into the glans to its final terminal position. The initial zipper leaves behind a wide urethral groove with lateral epithelial edges (urethral folds) that ultimately fuse together to form the tubular urethra as the “closing zipper” fuses the edges of the urethral groove and distally extends the tubular urethra. Between 9.5 and 10.5 weeks the “closing zipper” extends the tubular urethra well into the penile shaft, with the midline fusion of the epithelial edges of the urethral groove (urethral folds) well visualized in the OPT images of the developing human penis (fig. 5, D, I, J, M and N, and fig. 6, E, F, I and L). The process continues through the glans penis (figs. 6 to 8) in similar fashion, with ultimate fusion of the foreskin ventrally (fig. 8, G to L).
The mechanism of action of the 2 zippers remains unknown. Our data reveal that cellular proliferation based on Ki67 immunostaining is an important component of formation and lateral expansion of the urethral groove (figs. 2 to 8). Pechriggl et al also observed Ki67 staining in the developing human male urethra.12 Proliferation was consistently greatest in the dorsal aspect of the urethral plate, as well as in the residual urethral plate in the midline of the urethral groove, with a substantial decline in proliferative activity laterally within the epithelium of the urethral groove. This pattern is suggestive of a centrally placed proliferative zone generating post-mitotic epithelial cells that migrate laterally to expand the width of the urethral groove in a process somewhat akin to that occurring in intestinal crypts/villi.
In the opening zipper, which extends the urethral groove distally, cellular proliferation appears in conjunction with epithelial de-adhesion events that facilitate canalization of the urethral plate. The process of canalization of the urethral plate does not appear to involve apoptosis since caspase 3 positive cells were rarely observed throughout penile development (fig. 2, K). The proximal or closing zipper, in turn, requires adhesion of the epithelial surfaces of the urethral folds to form the tubular penile urethra. Using a mouse model of urethral seam fusion, we have demonstrated that the distal aspect of the mouse urethra, and especially the urethral meatus, forms by fusion of the edges of the urethral folds, resulting in a midline epithelial seam.5,13
OPT is an innovative technique for selective staining and visualization of the developing penile urethra. Our analysis confirms and refines the elegant work of Altemus and Hutchins, who described development of the penile urethra using gross examination, serial sections and artistic rendering of human fetal specimens.6 We and others have performed molecular analysis of human urethral development, showing differential expression of cytokeratins, uroplakins, E-cadherin and mesenchymal markers.3,4,12,14 Mammalian animal studies have also defined critical genes, such as Shh, FGF10 and FGFr2-111b, that are required for urethral development.15,16
Our study has a number of limitations. We assumed based on microscopic examination of the fetal tissue that the specimens we were analyzing were normal. We were also limited by the relatively small sample of specimens available for examination.
CONCLUSIONS
We define the ontogeny of human male urethral development by OPT and document 2 mechanisms for further study. The first is an opening zipper that facilitates distal canalization of the solid urethral plate to form the urethral groove, which involves a high rate of epithelial proliferation dorsally within the urethral plate. The process of canalization of the urethral plate apparently does not involve apoptosis. The other mechanism is the closing zipper that facilitates fusion of the 2 epithelial surfaces of the urethral groove and thus extends the penile urethra distally. Improved knowledge of the molecular mechanisms of these processes is critical to understanding mechanisms of abnormal urethral development, such as hypospadias.
Acknowledgments
Supported by National Institutes of Health Grant DK058105.
Abbreviations and Acronyms
- 3D
3-dimensional
- OPT
optical projection tomography
Footnotes
Study received approval of University of California, San Francisco committee on human research.
REFERENCES
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg. 2006;41:463. doi: 10.1016/j.jpedsurg.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Glenister TW. A correlation of the normal and abnormal development of the penile urethra and of the infra-umbilical abdominal wall. Br J Urol. 1958;30:117. doi: 10.1111/j.1464-410x.1958.tb06224.x. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation. 1999;64:115. doi: 10.1046/j.1432-0436.1999.6420115.x. [DOI] [PubMed] [Google Scholar]
- Baskin LS. Hypospadias and urethral development. J Urol. 2000;163:951. [PubMed] [Google Scholar]
- Baskin LS, Erol A, Jegatheesan P, et al. Urethral seam formation and hypospadias. Cell Tissue Res. 2001;305:379. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- Altemus AR, Hutchins GM. Development of the human anterior urethra. J Urol. 1991;146:1085. doi: 10.1016/s0022-5347(17)38008-4. [DOI] [PubMed] [Google Scholar]
- van der Werff JF, Nievelstein RA, Brands E, et al. Normal development of the male anterior urethra. Teratology. 2000;61:172. doi: 10.1002/(SICI)1096-9926(200003)61:3<172::AID-TERA4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Sharpe J, Ahlgren U, Perry P, et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science. 2002;296:541. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, et al. Improving the accuracy of fetal foot length to confirm gestational duration. Obstet Gynecol. 2005;105:773. doi: 10.1097/01.AOG.0000154159.75022.11. [DOI] [PubMed] [Google Scholar]
- Mercer BM, Sklar S, Shariatmadar A, et al. Fetal foot length as a predictor of gestational age. Am J Obstet Gynecol. 1987;156:350. doi: 10.1016/0002-9378(87)90282-1. [DOI] [PubMed] [Google Scholar]
- Cui KH, Warnes GM, Jeffrey R, et al. Sex determination of preimplantation embryos by human testis-determining-gene amplification. Lancet. 1994;343:79. doi: 10.1016/s0140-6736(94)90815-x. [DOI] [PubMed] [Google Scholar]
- Pechriggl EJ, Bitsche M, Blumer MJ, et al. The male urethra: spatiotemporal distribution of molecular markers during early development. Ann Anat. 2013;195:260. doi: 10.1016/j.aanat.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Kim KS, Liu W, Cunha GR, et al. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307:145. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Li YW, et al. Anatomical studies of hypospadias. J Urol. 1998;160:1108. doi: 10.1097/00005392-199809020-00039. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, et al. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. 127. Development. 2000:2471. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]