Abstract
Notoamide S has been hypothesized to be a key biosynthetic intermediate for characteristic metabolites, stephacidin A, notoamide B, and versicolamide B, in Aspergillus sp., but has not yet been isolated. The isolation of notoamide S and an enantiomeric mixture of 6-epi-stephacidin A enriched with the (−)-isomer from A. amoenus is reported. The presence of (+)-versicolamide B suggests that the fungus possesses only the oxidase, which converts (+)-6-epi-stephacidin A into (+)-versicolamide B, but not for (−)-6-epi-stephacidin A.
Graphical abstract
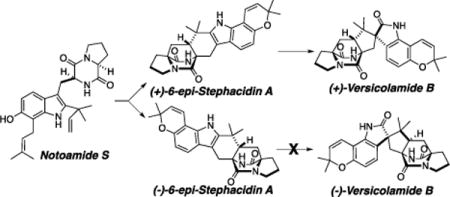
In our ongoing studies on the notoamide and stephacidin biosynthesis in two closely related fungi of the genus Aspergillus, we previously reported that the A. protuberus (formerly Aspergillus sp. MF297-2) produces (+)-stephacidin A, (−)-notoamide B, and (+)-versicolamide B1,2 and the A. amoenus (formerly A. versicolor NRRL 35600) produces the enantiomers, (−)-stephacidin A and (+)-notoamide B, but the same enantiomer of (+)-versicolamide B as produced in the A. protuberus (Figure 1).3 Stephacidin A, notoamide B, and versicolamide B are prenylated indole alkaloids containing a characteristic bicyclo[2.2.2]diazaoctane core structure, which is likely to arise from an intramolecular hetero Diels-Alder (IMDA) reaction (Scheme 1). In order to verify the molecular basis for the biogenesis of metabolites with this unique core structure, we performed the bioconversions of synthetic, isotopically labeled compounds, i.e. notoamide E,4 notoamide S,5,6 notoamide T,7 6-epi-notoamide T,2 and stephacidin A.8 Among them, notoamides S and T and 6-epi-notoamide T have not yet been isolated from the two fungal cultures, although notoamide S is strongly implicated to undergo the IMDA reaction to afford notoamide T and its 6-epi isomer through the achiral azadiene followed by cyclization and rearrangement to afford stephacidin A, notoamide B, and versicolamide B (Schemes 1 and 2). The bioconversion of notoamide S in the A. amoenus afforded notoamides C and D, (−)-stephacidin A, (+)-notoamide B, and (+)-versicolamide B.6 Notoamide T was converted into stephacidin A and notoamide B in the A. protuberus and A. amoenus7 and 6-epi-notoamide T was converted to 6-epi-stephacidin A and versicolamide B in the A. protuberus.2 These incorporation experiments of notoamide T and its 6-epi-isomer were performed with their racemic mixtures, and interestingly the two fungi converted both exogenous as well as endogenous substrates to products. In order to confirm the presence of notoamides S and T and 6-epi-notoamide T as precursors in the fungal culture, we previously carefully analyzed the time-course of the metabolic profile in the culture of the A. protuberus but could not obtain these metabolites.
Figure 1.
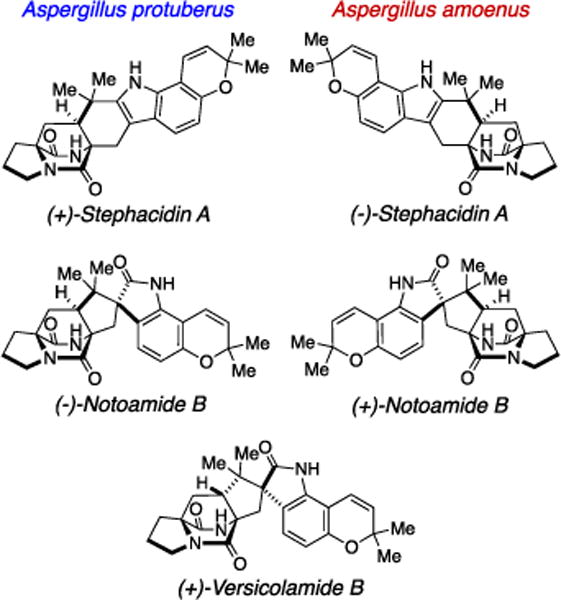
Structures of the metabolites produced by Aspergillus protuberus (formerly Aspergillus sp. MF297-2) and A. amoenus (formerly A. versicolor NRRL 35600).
Scheme 1.
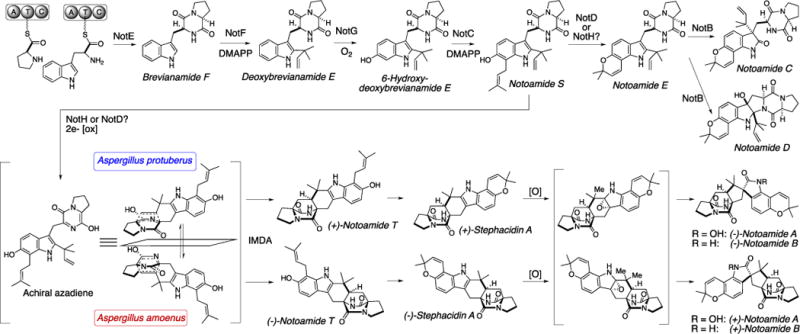
Proposed Biosynthetic Pathway of Enantiomeric Alkaloids in A. protuberus (formerly Aspergillus sp. MF297-2) and A. amoenus (formerly A. versicolor NRRL 35600).
Scheme 2.
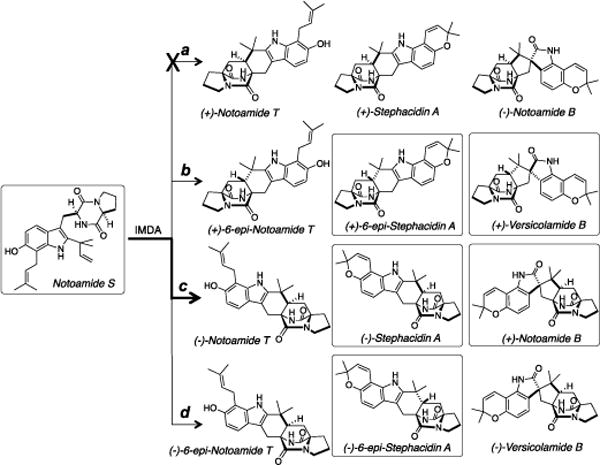
Metabolites Isolated from the Culture of A. amoenus NRRL 35600 and their Plausible Biosynthetic Pathway. The Compounds in Route c are Main Metabolites.
In the present study, we searched for the presence of these metabolites in the culture of the A. amoenus and succeeded in the isolation of notoamide S but not of notoamide T and its 6-epi-isomer. With respect to the metabolic profile by the A. protuberus and A. amoenus, production of the enantiomers of stephacidin A and notoamide B along with the presence of the same enantiomer of (+)-versicolamide B are enigmatic. 6-epi-Stephacidin A is likely the precursor of versicolamide B, and (+)-6-epi-stephacidin A was obtained from the culture of the A. protuberus.2 In the present study, we elucidated the absolute configuration of 6-epi-stephacidin A produced by the A. amoenus.
The A. amoenus was cultured on rice medium at 25 °C for one month. The culture was extracted with n-BuOH and the condensed extract was partitioned between n-hexane and 90% MeOH-H2O. The aqueous MeOH fraction was subjected to ODS column chromatography with MeOH/H2O and fractions that eluted with 75% MeOH-H2O were repeatedly purified to afford notoamide S (15.3 mg) and 6-epi-stephacidin A (1.22 mg).
Notoamide S and 6-epi-stephacidin A were identified by 1H NMR spectra and ESIMS and the structures were corroborated by comparison to known, synthetic samples. The CD spectrum suggested that the isolated 6-epi-stephacidin A was the (−)-enantiomer. However, from the biosynthetic point of view, the precursor of (+)-versicolamide B should be (+)-6-epi-stephacidin A (Scheme 2). Thus, the isolation of the (−)-enantiomer was inconsistent with the biogenetic relationship and the small molar ellipticity of the CD spectrum suggested the possibility of an enantiomeric mixture. The 6-epi-stephacidin A we isolated was analyzed by HPLC with a chiral column and turned out to be an enantiomeric mixture enriched with the (−)-isomer. Purification of the mixture by chiral HPLC afforded (+)- and (−)-6-epi-stephacidin A in a ratio of 1:2.3 and the enantiomers showed the opposite CD spectra (Figure 2). This result suggested that notoamide S was converted to both (+)- and (−)-6-epi-stephacidin A through (+)- and (−)-6-epi-notoamide T, respectively, and subsequently only (+)-6-epi-stephacidin A was converted into (+)-versicolamide B (Scheme 2). These observations clearly indicate that the A. amoenus contains an indole oxidase that transforms (+)-6-epi-stephacidin A to (+)-versicolamide B but does not contain a suitable indole oxidase for (−)-6-epi-stephacidin A. Consequently, (−)-6-epi-stephacidin A becomes a shunt metabolite, and the fungus does not produce (−)-versicolamide B.
Figure 2.
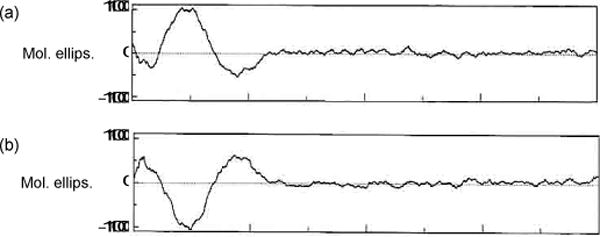
CD spectra of (+)- (a) and (−)-6-epi-stephacidin A (b) isolated from the culture of A. amoenus in MeOH.
Stephacidin A, notoamide B, and versicolamide B are all putatively biosynthesized from notoamide S by two-electron oxidation, tautomerization and IMDA reaction (Scheme 3). In the A. protuberus, (+)-stephacidin A/(−)-notoamide B and (+)-6-epi-stephacidin A/(+)-versicolamide B are exo- and endo- products, respectively, which are caused by the different orientation of the dienophile to the diene in the pathways a and b, respectively (Scheme 3 (A)). On the other hand, in the A. amoenus, (−)-stephacidin A/(+)-notoamide B and (−)-6-epi-stephacidin A are similarly produced in the pathways c and d, respectively (Scheme 3 (B)). In addition, the different orientation of the diene to the dienophile in the pathway b’ leads to the production of (+)-6-epi-stephacidin A/(+)-versicolamide B as produced in the pathway b. In the A. amoenus, the pathway b’ is more likely than the pathway b, since the positions of the diene and dienophile in the pathway b’ are the same as those in the pathways c and d. In both fungi, exo-metabolites, stephacidin A and notoamide B, are produced as major metabolites compared to endo-metabolites, 6-epi-stephacidin A and versicolamide B.
Scheme 3.
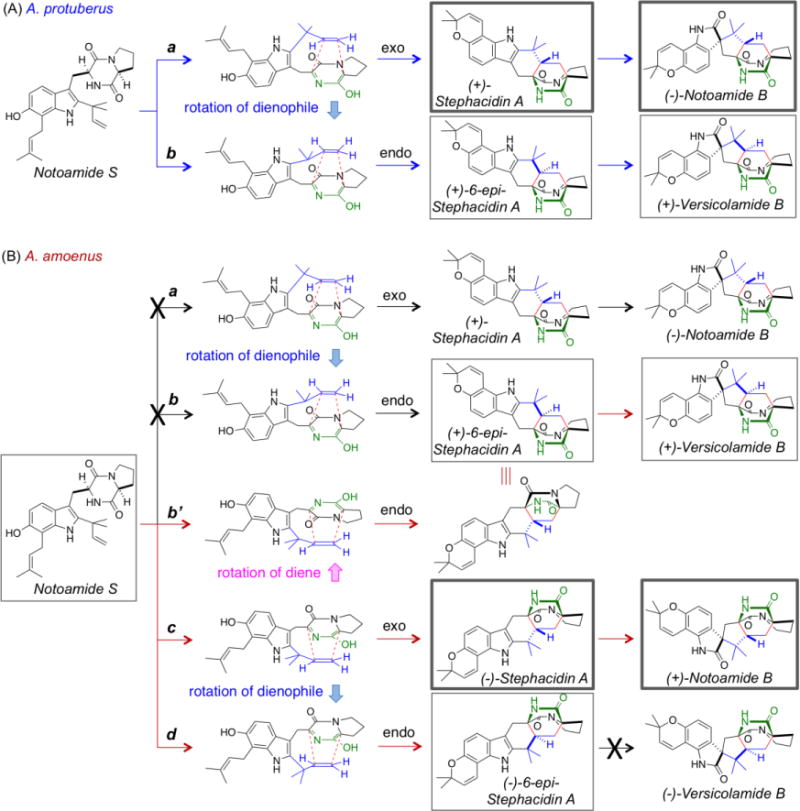
Proposed Mechanisms of IMDA Reactions for Metabolites in A. protuberus (A) and A. amoenus (B). The Compounds in Bold Squares are Main Metabolites and those in Plain Squares are Minor Metabolites.
In conclusion, we have successfully isolated natural notoamide S from the A. amoenus NRRL 35600 (formerly A. versicolor NRRL 35600), which was previously bioconverted into the products containing a bicyclo[2.2.2]diazaoctane core structure, (−)-stephacidin A, (+)-notoamide B, and (+)-versicolamide B.6 The finding of notoamide S in the culture further confirms that it is a key biosynthetic precursor of these natural products. In this study we isolated 6-epi-stephacidin A from the A. amoenus as non-racemic mixture enriched with the (−)-isomer. With the presence of the (+)-enantiomer of versicolamide B in the culture of A. amoenus, the result strongly suggests that the fungus possesses a highly enantio-discriminating oxidase, which selectively converts (+)-6-epi-stephacidin A into (+)-versicolamide B, but is unreactive toward (−)-6-epi-stephacidin A present (Scheme 3 (B)). We have previously reported that the biosynthetic gene clusters of A. protuberus and A. amoenus are orthologous with an overall nucleotide identity of 71%.9 These phylogenetically closely related species in Aspergillus section Versicolores10 have curiously evolved enantiodivergent biosynthetic pathways to the stephacidins and notoamides but converge on the production of (+)-versicolamide B. Efforts to clarify the underlying genetic and biochemical basis for the biogenesis of these structurally complex alkaloids are under investigation in our laboratories.
Supplementary Material
Acknowledgments
This work was financially supported in part by Grants-in-Aid for Scientific Research (Nos. 23108518 and 25108719 to S.T. and 24710252 to H.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from the Nagase Science and Technology Foundation (to S.T.). Financial support from the National Institutes of Health (R01 CA070375 to R.M.W. & D.H.S.) is gratefully acknowledged.
Footnotes
Supporting Information
Fungal culture procedures, isolation, and spectra. This material is free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
References
- 1.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem, Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Nakahara T, Yamaguchi M, Kagiyama I, Finefield JM, Sunderhaus JD, Sherman DH, Williams RM, Tsukamoto S. Tetrahedron Lett. 2015;56:247–251. doi: 10.1016/j.tetlet.2014.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem, Int Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J Am Chem Soc. 2009;131:3834–3835. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAfoos TJ, Li S, Tsukamoto S, Sherman DH, Williams RM. Heterocycles. 2010;82:461–472. doi: 10.3987/COM-10-S(E)19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Finefield JM, Sunderhaus JD, McAfoos TJ, Williams RM, Sherman DH. J Am Chem Soc. 2012;134:788–791. doi: 10.1021/ja2093212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunderhaus JD, McAfoos TJ, Finefield JM, Kato H, Li S, Tsukamoto S, Sherman DH, Williams RM. Org Lett. 2013;15:22–25. doi: 10.1021/ol302901p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finefield JM, Kato H, Greshock TJ, Sherman DH, Tsukamoto S, Williams RM. Org Lett. 2011;13:3802–3805. doi: 10.1021/ol201284y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Anand K, Tran H, Yu F, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. MedChemComm. 2012;3:987–996. doi: 10.1039/C2MD20029E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurjevi Z, Peterson SW, Horn BW. IMA Fungus. 2012;3:59–79. doi: 10.5598/imafungus.2012.03.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


