Abstract
CGRP is an extensively studied neuropeptide that has been implicated in the pathophysiology of migraine. While a number of small molecule antagonists against the CGRP receptor have demonstrated that targeting this pathway is a valid and effective way of treating migraine, off-target hepatoxicity and formulation issues have hampered the development for regulatory approval of any therapeutic in this class. The development of monoclonal antibodies to CGRP or its receptor as therapeutic agents has allowed this pathway to be re-investigated. Herein we review why CGRP is an ideal target for the prevention of migraine and describe four monoclonal antibodies against either CGRP or its receptor that are in clinical development for the treatment of both episodic and chronic migraine. We describe what has been publically disclosed about their clinical trials and future clinical development plans.
Keywords: CGRP, chronic migraine, migraine, monoclonal antibodies
Introduction
Migraine is a chronic, recurrent disorder characterized by attacks of severe pain and associated symptoms, such as nausea, photophobia, phonophobia and worsening with exertion 1,2. Around 12% of adults have episodic migraine (headaches on less than 15 days per month) 3, while around 1% of the adult population suffers from migraine and headaches on more days than not 4, a condition termed chronic migraine. Although the ictal symptoms of migraine are the prominent feature of the disease, in-between attacks, individuals exhibit alterations of event-related potentials that indicate a state of altered brain activation 5,6, as well as impaired health-related quality of life and considerable disability 7,8. Additionally, migraine is considered a disease with potential for progression. Increased headache frequency or functional/anatomical sequelae of migraine attacks develop over time 9.
Attempts to alleviate the suffering caused by migraine over the centuries have been varied and encompass treatments as primitive as trepanation of the skull, to increasingly specific medications that act on receptor subtypes implicated in the pathophysiology of migraine 10. Epidemiological and clinical studies of migraine have highlighted the enormous economic and personal burden it brings to workplace productivity and quality of life 11. Increased awareness of migraine has given momentum to well-designed studies of the physiological and clinical characteristics of idiopathic primary headaches and the development of progressively better informed classification guidelines that provide a clear rationale for the selection of clinical treatment options 12. Despite all of these advances, nearly half of individuals with episodic or chronic migraine who are in need of preventive therapies do not receive them 13. Even more disturbing, none of the preventive medications currently available for the treatment of episodic (e.g., topiramate, propranolol, divalproex sodium, candesartan, etc.) or chronic migraine (onabotulinum toxinA) have been developed for the treatment of migraine. They were developed for other indications and serendipitously were found to be effective for migraine prevention.
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide that is involved in different physiological processes in humans. For a review of its role on migraine pathophysiology, readers are referred to references 14–17. Since its description as playing a key role in the pathophysiology of migraine, our knowledge on CGRP has increased substantially, leading to a robust interest in targeting CGRP to treat migraine. Clinical proof-of-efficacy for the acute treatment of migraine has been obtained with several small molecule CGRP receptor antagonists (CGRP-RAs) 18–20, but their development has been complicated by signs of liver toxicity associated with frequent use, or with formulation difficulties 21,22. Therefore, much recent attention has been given to the development of monoclonal antibodies (mAbs) targeting the CGRP pathway. Herein we review the current state of development in this area. We start by briefly discussing the pathophysiology of migraine and the relevance of CGRP. We follow by outlining the clinical development of the three anti-CGRP and one CGRP receptor antibodies currently in development.
CGRP as a migraine target and CGRP receptor antagonists
Migraine initiates in the brain 23,24, although animal studies suggest that important events happen outside of the central nervous system 25–27, which is amenable to pharmacotherapy that does not require blood–brain barrier penetration, such as mAbs 11.
Cortical 28–31 and subcortical events 32 are of importance in migraine, and recent evidence suggests that that key dysfunctions of migraine happen in the brainstem, in areas involved with the modulation of sensorial information 33,34. Migraine can develop with pathology in the region of the peri-aqueductal grey (PAG) 35,36, or with a lesion of the pons 37,38.
Following cortical changes, brainstem changes or both, activation of the trigeminal system is thought to occur 39. When this system is activated, neuropeptides such as CGRP are released from peripheral nerve endings in the cranium and face 40. These neuropeptides act at peripheral sites and, as discussed below, may play an important role in the generation and maintenance of headache pain and possibly in other migraine symptoms 41.
Following the activation of the trigeminal system, CGRP is released at trigeminal nerve endings in the meninges inducing vasodilation 42,43 and activation of sensory trigeminal pain neurons that innervate intracranial blood vessels and the dura mater. It has been suggested that local activation of these sensory nerves involves dural mast cells as one factor in local inflammation, causing sensitization of meningeal nociceptors, although expression of both CGRP receptor components was not observed in human mast cells 44. Nonetheless, CGRP release leads to sensory nerve activation 45 since peripheral CGRP-containing neurons (in the trigeminal ganglion and elsewhere) are polymodal nociceptors that innervate peripheral tissues and send primary afferent input to the dorsal horn, trigeminal nucleus caudalis (TNC), or nucleus of the solitary tract (which, in turn, project to the brainstem, amygdala, hypothalamus and thalamic nuclei) 46.
The initial approach of modulating CGRP function for migraine therapy began with small molecules targeting the CGRP receptor (CGRP-RAs). These molecules compete with CGRP for a binding pocket or cleft produced by the co-receptors, RAMP1 and CGRPR1. Reviews of CGRP-RA clinical studies are provided by Edvinsson & Linde 47, Silberstein 21 and Bigal et al. 48.
Five distinct CGRP-RAs have demonstrated proof of efficacy for the acute treatment of migraine, but all were discontinued for a variety of reasons, including concerns of liver toxicity after frequent use 21,22,49. Nonetheless, in addition to demonstrating proof of concept for the acute treatment of migraine, the CGRP-RA clinical trials also demonstrated the extraordinary tolerability of this class.
Anti-CGRP antibodies
As previously discussed, the migraine brain operates in a delicate balance of many systems. Internal or external triggers may upset the balance. The brainstem tone is influenced by cyclical processes in central, sensory and autonomic systems 50. CGRP is released peripherally following activation of the trigeminovascular system by migraine triggers, and CGRP acts on trigeminal nerves to exacerbate peripheral sensory inputs to sensitized central trigeminal pathways. Therefore, the brainstem fails to modulate trigeminal nociceptive drive triggering a migraine attack 51–53. Anti-CGRP antibodies are thought to remove the excessive CGRP that is released at perivascular trigeminal sensory nerve fibres, while anti-CGRP receptor antibodies block the receptor from signalling 54. The expectation is that by doing so, antibodies against both the ligand and receptor would prevent CGRP-induced activation of sensitized central trigeminal pathways, therefore decreasing headache frequency over time.
mAbs have exquisite target specificity, thus avoiding off-target toxicities common to small molecules. In addition, most mAbs have reasonably long terminal half-lives. This extended pharmacokinetic profile results in less frequent dosing, which mitigates the need for frequent oral dosing 55. For example, while typical migraine preventive medications need to be dosed orally one to three times daily 56, a mAb therapeutic might be administered once a month or even less frequently.
From a safety and tolerability perspective, mAbs offer great potential benefit over small molecules, whose metabolic profile in humans is often not fully understood until clinical testing is underway. In many cases, toxicological issues with mAbs are due to exaggerated pharmacology, not to off-target effects such as the liver toxicity prevalent with the CGRP-RAs 57. Since CGRP is a potent vasodilator, four major cardiovascular effects are of potential concern with CGRP inhibition, a priori, medication-induced hypertension, counterbalancing the effect of anti-hypertensive drugs that have vasodilatory properties, inhibition of stress- (or ischaemia-) induced vasodilation, and impairment of cardioprotective mechanisms. This topic has been reviewed elsewhere 48 and will be detailed later in this manuscript.
There are some issues specific to the administration of a mAb. These include infusion reactions, site administration reactions, as well as more systemic immunological effects. It is not uncommon for patients treated with a biologic to experience an immune response against the therapeutic protein. Most monoclonal antibodies are now composed of almost or entirely human sequences, reducing the immunologic liability. Nonetheless, anti-drug antibodies (ADA) need to be screened for as their presence can neutralize the effectiveness of the mAb by either directly interfering with the ability of the antibody to engage its target or by reducing the effective concentration in the blood by increasing the clearance rate. However, unless the ADAs present a safety or efficacy risk, their presence is generally of minimal concern. Given the fact that three of the antibodies in development target a soluble ligand, their relative safety risk should be minimal. Nonetheless, the presence and characteristics of ADAs are being investigated in phase 2 studies. Should a patient experience an adverse reaction, the extended half-life of the mAb could become a liability. Discontinuation would not yield immediate clearance of the molecule.
At the time of this writing, there are three mAbs directed against CGRP in various stages of clinical development, LY2951742, developed by Eli Lilly and Co., ALD-403, developed by Alder Biopharmaceuticals and TEV-48125 (LBR-101), developed by Teva Pharmaceuticals. In addition, there is one mAb against the CGRP receptor complex in development by Amgen (AMG 334). Table1 contrasts relevant aspects of their development. All four companies have stated plans for development in episodic and chronic migraine.
Table 1.
Comparison of monoclonal antibodies targeting the CGRP pathway
| ALD403 | LY2951742 | AMG 334 | TEV-48125 | |
|---|---|---|---|---|
| Target | CGRP with a humanized antibody | CGRP with a humanized antibody | CGRP receptor with a human antibody | CGRP with a fully humanized antibody |
| t1/2 | Around 32 days | 25–30 days | Not disclosed | Around 45 days |
| Migraine state | Episodic and chronic | Episodic and chronic | Episodic and chronic | Episodic and chronic |
| Current development phase | Finalized positive POC study with a single dose level administered once (Phase 2a) Currently conducting dose-ranging (Phase 2b). | Finalized positive POC study with single dose level Currently conducting dose-ranging (Phase 2b). | Dose-ranging (Phase 2b) | Dose-ranging (Phase 2b) |
| Form of administration in phase 2 | i.v.* | s.c. | s.c. | s.c. |
| Phase 2 dosing frequency | Monthly for s.c. and every 3 months for i.v.† | Twice per month (2a) and once per month (2b) | Once per month | Once per month |
Announced plans to also develop a s.c. formulation.
Announced.
ALD-403
ALD-403 is a humanized antibody being developed by Alder Biopharmaceuticals. A unique feature of this programme is that the antibody is produced using yeast, not mammalian cells. According to the company, the process can yield faster production with subsequent economic advantages and a different immunogenicity profile 58.
ALD-403 was first tested in phase 1, in a two part, placebo-controlled, single ascending dose study conducted to evaluate the safety and tolerability of two different formulations, administered subcutaneously and intravenously. In the first part of the study, healthy volunteers were enrolled and followed for 12 weeks after a single ALD-403 administration, with pharmacokinetic and pharmacodynamic assessments conducted. ALD-403 displayed a half-life of approximately 32 days for the 1000 mg dose and linear pharmacokinetics for doses ranging from 1 to 1000 mg. Dose-related inhibition of vasodilation induced by topically applied capsaicin was observed 59. In the second part of the study, ALD-403, placebo or sumatriptan were administered, and no impact on blood pressure was seen 59,60, akin to what had been seen in the development of CGRP-RAs 18. In the third part of the study, a s.c. formulation was tested against the i.v. formulation and was found to have around 70% bioequivalence 59.
Proof-of-concept was obtained in a phase 2a study, where 163 participants with episodic migraine (who had between 4–14 days of headache) received a single 1000 mg i.v. dose of ALD-403 or placebo and were followed for 6 months. Participants were not allowed any migraine preventive medication. After a 1 month run in phase, patients were randomized in a 1: 1 ratio. The primary end point was a decrease in the number of migraine days (defined as a day fulfilling criteria for migraine or probable migraine) in the second month after treatment relative to baseline (before treatment). This differs from trials conducted by other companies, where the third month was selected for analysis, and was likely driven by the fact that in this proof-of-concept study the medication was given only once.
Participants had a mean age of around 39 years and over 80% were women. They had, on average, 9–10 days of headache per month, 8–9 of them qualifying for migraine days. For the primary end point, participants receiving ALD-403 had a decrease of 5.6 headache days vs. 4.6 in the placebo group (P = 0.03). Results were also significant at the end of month 1 (5.6 vs. 3.9, P < 0.01) but not at month 3 (5.6 vs. 4.6). At any given month post-randomization, around 75% of participants in the ALD-403 group had more than 50% decrease in headache frequency. For placebo, values ranged from 50% to 67% depending on the month 61 (Table2).
Table 2.
Proof-of-concept data for two anti-CGRP monoclonal antibodies
| ALD403 | LY2951742 | |||||
|---|---|---|---|---|---|---|
| Drug | Placebo | Difference | Drug | Placebo | Difference | |
| Decrease in migraine frequency (reduction in migraine days per month from baseline) | ||||||
| 1 month | 5.6 | 3.9 | 1.7§ | 3.7 | 2.3 | 1.4* |
| 2 months | 5.6 | 4.6 | 1.0‡ | 4.3 | 2.9 | 1.4† |
| 3 months | 5.6 | 4.6 | 1.0 | 4.2 | 3.0 | 1.2† |
| Decrease in headache frequency (reduction in headache days per month from baseline) | Not reported | |||||
| 1 month | 4.3 | 2.3 | 2.0§ | |||
| 2 months | 4.6 | 3.7 | 0.9 | |||
| 3 months | 4.9 | 3.7 | 1.2* | |||
| Any adverse events | 57% | 52% | 5% | 72% | 67% | 5% |
P ≤ 0.01;
P value not calculated;
P ≤ 0.05;
P ≤ 0.001
Adverse events were experienced by 46 (57%) of 81 patients in the ALD403 group and 43 (52%) of 82 in the placebo group. The most frequent adverse events were upper respiratory tract infection (placebo six [7%] patients vs. ALD403 seven [9%] patients), urinary tract infection (four [5%] vs. one [1%]), fatigue (three [4%] vs. three [4%]), back pain (four [5%] vs. three [4%]), arthralgia (four [5%] vs. one [1%]), and nausea and vomiting (two [2%] vs. three [4%]). Six serious adverse events were reported by three patients and were judged to be unrelated to study drug 61.
Accordingly, ALD-403 successfully demonstrated efficacy in this proof-of-concept study. Of note, when focusing on the absolute efficacy of the compound, values are quite remarkable (61% of participants having more than 50% improvement in migraine frequency over the 3 month period) 62. However, the placebo rate was extremely high, and the therapeutic gain (placebo-subtracted values) was less substantial and comparable with available preventive medications. Factors contributing to the high placebo rate could be the exclusion of more severely affected patients (those on preventive medications not willing to discontinue it), the fact that it was administered as an intravenous injection given only one time, as well as the novelty of the therapeutic.
The company has announced plans to start phase 2b in 2014, and at the time of writing one phase 2 study in chronic migraine for ALD-403 is listed on clinicaltrials.gov. In this study, the primary outcome measure will be the change in migraine days from baseline to week 12. Four different doses are being tested vs. placebo, all delivered intravenously. The company also disclosed plans to proceed with the development of ALD-403 formulated for intravenous (given every 3 months) and subcutaneous (given every month) use 59.
AMG 334
Amgen's antibody, AMG 334, is the only anti-CGRP antibody that targets the CGRP receptor complex, instead of the free ligand 63. Several phase 1 studies have been completed to test the safety and tolerability of AMG 334. In one completed study, approximately 68 healthy subjects and migraine patients were administered single ascending doses of AMG 334 by i.v. or s.c. routes. Another phase 1 study tested multiple doses of AMG 334 in both healthy subjects and migraine patients. Three dose levels were examined, all administered via s.c. injection, testing the safety, pharmacokinetics and pharmacodynamics following multiple injections 64,65. Another phase 1 study was conducted in women with hot flushes associated with menopause. Data have not been disseminated for any of these trials.
AMG 334 transitioned directly from phase 1 to phase 2b. This mAb is being independently tested in episodic and chronic migraine patients. In the episodic migraine trial, three dose groups plus placebo are being tested, and the medication is given subcutaneously once per month. The primary end point for this study is the change in monthly migraine days from baseline in the last 4 weeks of a 12 week, double-blind treatment phase. Eligible patients have had a history of migraine for at least 12 months prior to screening and experience from 4 to 14 migraine days a month. At clinicaltrials.gov, the final completion of the trial (efficacy and safety) was estimated to be July 2015 66.
AMG 334 is also being tested in patients with chronic migraine. After a run-in phase, patients are randomized to one of two doses of the medication or placebo, given subcutaneously monthly for 3 months, followed by a 9 month, open label long term safety extension phase. Estimated completion of this study is August 2016 67,68. In both the episodic and chronic migraine trials, patients cannot be on preventive medications.
Very little is known about this compound, as no data have been shared publically. An important discussion topic is the use of receptor vs. ligand targets for mAbs. It could be hypothesized that a mAb directed against a receptor may result in complete blockade of signalling, compared with incomplete blockade with a ligand mAb. In a recent debate at the American Headache Society safety concerns were raised about the ramifications of total signalling inhibition, while the author of the presentation reported that no safety or cardiovascular concerns have been seen with this approach using AMG 334 69.
LY2951742
LY2951742 is a humanized mAb anti-CGRP being developed by Eli Lilly and Co. Initial development of the antibody was conducted by Arteaus Therapeutics The LY2951742 phase 1 programme assessed 56 subjects. In the first phase of the programme, the safety and tolerability of LY2951742 was examined following escalating single doses. After the initial phase was completed, a repeat dose expansion phase was initiated, where subjects received repeat doses of LY2951742 every other week for 6 weeks. Safety, tolerability, PK and PD were assessed in this phase of the study. LY2951742 was reported to be well tolerated and showed linear PK with a t1/2 ranging from 25–30 days and a tmax from 7–14 days for the s.c. formulation 70. Accordingly, the half-life of this compound is in the lower range of the CGRP antibodies in development.
Proof-of-concept (phase 2a) for the compound has been concluded and data have been published in peer-review medical journals 71. The study was conducted in 35 centres in the USA. Patients aged 18–65 years with 4 to 14 migraine headache days per month were randomly assigned (1: 1) to LY2951742 or placebo. LY2951742 (150 mg) or placebo were given as a s.c. injection once every 2 weeks for 12 weeks. The primary end point was the mean change in number of migraine headache days per 28 day period assessed at 9–12 weeks after the start of therapy. Safety was assessed over 24 weeks, including the 12 week treatment period and the subsequent 12 weeks after the end of study drug administration.
For the primary end point, the mean change from baseline to week 12 in the number of migraine headache days was –4.2 days (62.5% decrease) in the LY2951742 group vs. –3·0 (42.3% decrease) in the placebo group (P = 0.003) (Table2). Secondary end points were equally positive. Compared with the placebo group, subjects receiving LY2951742 showed a greater reduction in the number of headache days and the number of migraine plus probable migraine headache days relative to placebo. A 50% response rate at the third month was achieved by 70.4% active vs. 45.2% placebo patients. Quality of life and disability measurements improved significantly more in those receiving active therapy 71.
Adverse events were reported to a similar extent in both groups: 77 (72%) of 107 patients in the LY2951742 group and 74 (67%) of 110 patients in the placebo group. The most common adverse events for both the LY2951742 and placebo groups were upper respiratory infections and viral infections. There were no serious adverse events that were considered to be treatment-related. Injection site reactions (mild pain or erythema) seemed to be more common in those receiving active treatment (20% vs. 6%).
There were no clinically important changes in laboratory parameters, ECGs or vital signs between the groups.
Anti-drug antibodies were detected in eight patients at screening, and at the end of the study they were detected in 20 patients. There are no mentions about whether the antibodies were neutralizing to LY2951742, or more likely to be associated with adverse events, relative to cases without anti-drug antibodies 71.
The company is currently conducting a large phase 2b study, where four different doses of LY2951742 are being tested against placebo. Based on what is publically disclosed, it is not clear whether the study is being conducted in episodic migraine only, or in episodic and chronic migraine. In contrast to phase 2a, where the compound was given every 2 weeks, in this study it is being given once per month. The double-blind phase lasts for 3 months 72.
TEV-48125 (LBR-101)
TEV-48125 (LBR-101, formerly known as RN-307), is a fully humanized anti-CGRP mAb, acquired by Teva from Labrys Biologics. In contrast with the other mAbs in development, TEV-48125 was developed for episodic and chronic migraine from the start.
Among the studies conducted in the preclinical development of TEV-48125 are two independent monkey cardiovascular safety studies. In a single dose telemetry study, eight normotensive adult male cynomolgus monkeys were first administered vehicle only, and telemetery data were collected beginning approximately 1 h pre-dose until 22 h post-dose. Six days after vehicle administration, the same animals received a single i.v. administration of TEV-48125 (100 mg kg–1) and parameters were measured again. In a separate multiple dose safety study, 48 adult, gender-matched cynomolgus monkeys received vehicle or TEV-48125 as an intravenous injection once weekly for 14 weeks at doses of 10 mg kg–1, 100 mg kg–1 or 300 mg kg–1. In each group, two animals of each gender were allowed to recover for an additional 4 months following the end of dosing. In both of these studies, no relevant changes were noted in systolic or diastolic blood pressure relative to vehicle-treated animals. Group mean heart rates were relatively consistent across the dose groups and time points measured, with no statistical differences measured (Figure1) suggesting that at least in monkeys, cardiovascular and haemodynamic parameters do not appear to be affected by potent long term inhibition of CGRP 73.
Figure 1.
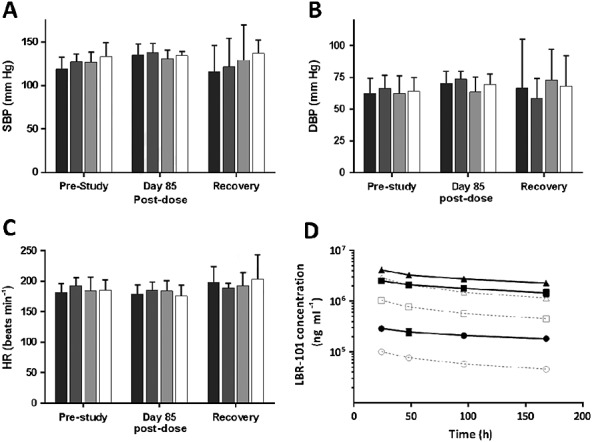
Haemodynamic data from a 14 week, repeat dose study of TEV-48125 (LBR-101) in monkeys. Data are shown with 95% confidence intervals. A: systolic blood pressure; B: diastolic blood pressure; C: heart rate; D: time–concentration profile at weeks 1 and 13. Modified from reference 73. Vehicle, 10 mg kg–1, 100 mg kg–1, 300 mg kg–1. Week 1 10 mg kg–1, 100 mg kg–1, 300 mg kg–1, Week 13 10 mg kg–1, 100 mg kg–1, 300 mg kg–1
The i.v. clinical pharmacokinetics of TEV-48125 have been studied in five different phase 1 trials with doses ranging from 10 to 2000 mg as 1 h i.v. infusions 74. Maximum plasma concentrations (Cmax) were reached shortly after the end of infusion. The terminal half-life (t½) ranged from 39.4 to 48.3 days and the increase in area under the curve appeared to be greater than dose proportional between 10 and 30 mg and to be approximately dose proportional between 30 and 1000 mg. The s.c. clinical PK were characterized in a separate phase 1 study. Accordingly, TEV-48125 has the longest half-life among the anti-CGRP antibodies 75.
As an i.v. formulation, TEV-48125 was administered to 94 subjects, while 45 received placebo. Doses ranged from 0.2 mg to 2000 mg given once (day 1) as a single i.v. infusion, or up to 300 mg given twice (day 1 and day 14) 74. Across the broad range of doses, i.v. TEV-48125 was well tolerated and overt safety findings did not emerge. Participants receiving placebo reported an average of 1.3 treatment-emergent AEs (related or not to study medication). Across all TEV-48125 doses, treatment-related adverse events (TRAEs) happened in 21.2% of subjects receiving TEV-48125, compared with 17.7% in those receiving placebo. At doses of 100 mg TEV-48125 or higher, TRAEs happened in 22.4% of participants. At doses of 1000 mg or higher, they happened in 21.7% of participants (Figure2) 74.
Figure 2.
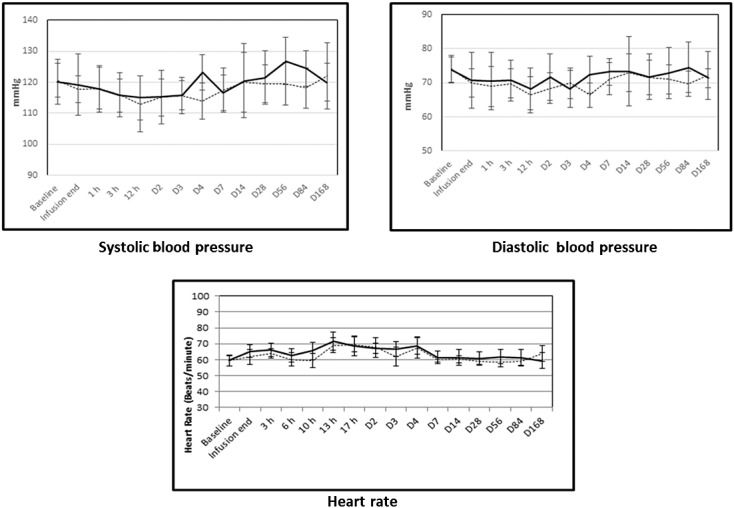
Cardiovascular parameters in subjects receiving TEV-48125 (LBR-10) or placebo. Footnote: Data from phase 1 study of TEV-48125 in normal, healthy women volunteers. TEV-48125 or placebo was delivered as a 1 h i.v. infusion. Means are shown ± SD. placebo, LBR-10
Clinical laboratory findings were similar across placebo and TEV-48125. In particular, liver function abnormalities were not observed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase or gamma-glutamyl transferase (GGT) among subjects receiving any of the studied doses of TEV-48125 74.
The sustained haemodynamic effects of CGRP inhibition following TEV-48125 were assessed via a double-blind, placebo controlled, single dose, dose escalation study, where 31 women (mean age = 56 years) were randomized to receive placebo or TEV-48125 (1 h i.v. infusion) at doses ranging from 300 to 2000 mg. The latter represents a supra-therapeutic exposure. Participants were confined for 7 days and followed after discharge for 168 days. Continuous cardiac telemetry was initiated 2 h before infusion and continued until 8 h after completion of infusion. All physical and haemodynamic assessments and ECGs were conducted six times during day 1, daily during the first 3 days of confinement, 1 week after discharge (day 14), and then 1, 2 and 3 months after infusion. No clinically relevant changes in systolic or diastolic blood pressure, heart rate, or ECG parameters (RR, PR, QRS intervals or QTcF ) were observed when comparing baseline vs. post-dose time points, or between groups for any parameter or time-point tmax (Figure2) 76.
TEV-48125 is currently being investigated in two dedicated phase 2b studies, one in high frequency episodic migraine and one in chronic migraine. Four dosing paradigms are being investigated. Both studies have a parallel design, a 1 month run-in phase followed by randomization and monthly s.c. injections for 3 months. Estimated primary completion is in the first quarter of 2015. Of notice, the episodic migraine study testing TEV-48125 included a population with a higher frequency of attacks 8–14 relative to the other CGRP mAb trials. The trials have not excluded participants on preventive medication, in an attempt to capture a population as close as possible to real life paradigms. Of course, a potential risk to this strategy is that these patients may be more severely affected than those not using preventive medications. However, we expect many patients in this category are in great need of an effective migraine preventive medication.
Conclusion
CGRP is a well-studied, ubiquitous neuropeptide found both centrally and peripherally at the very centres of the migraine process. The development of mAbs to CGRP and its receptor represents an incredible example of translational research. Over 20 years of intense research on the target translated into what may be the first class of medications being developed specifically for the prevention of migraine in over 50 years 77. Four monoclonal antibodies are currently being developed for episodic and chronic migraine. Proof-of-efficacy has been obtained and so far no safety concerns have emerged. Relevant cardiovascular effects have not been reported. Based upon the emerging data, mAbs targeting the CGRP pathway are a promising novel drug class that may provide a valuable new option for clinicians aiming to relieve the burden of individuals with episodic or chronic migraine.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare AMR had specified relationship with Labrys Biologics and Teva Pharmaceuticals in the previous 3 years. He reports no other relationships or activities that could appear to have influenced the submitted work. MEB and SW had specified relationships or activities of this type. MEB is a full time employee of Teva Pharmaceuticals. He owns stocks from Teva. He was the chief medical officer of Labrys Biologics. SW was the Head of Preclinical Research and a full time employee of Labrys Biologics. They were both involved with the development of TEV-48125 (LBR-101)
References
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Scher AI, Kolodner K, Liberman J, Steiner TJ, Stewart WF. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885–94. doi: 10.1212/wnl.58.6.885. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–66. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- Di Clemente L, Coppola G, Magis D, Fumal A, De Pasqua V, Di Piero V, Schoenen J. Interictal habituation deficit of the nociceptive blink reflex: an endophenotypic marker for presymptomatic migraine? Brain. 2007;130:765–70. doi: 10.1093/brain/awl351. [DOI] [PubMed] [Google Scholar]
- Coppola G, Vandenheede M, Di Clemente L, Ambrosini A, Fumal A, De Pasqua V, Schoenen J. Somatosensory evoked high-frequency oscillations reflecting thalamo-cortical activity are decreased in migraine patients between attacks. Brain. 2005;128:98–103. doi: 10.1093/brain/awh334. [DOI] [PubMed] [Google Scholar]
- Terwindt GM, Ferrari MD, Tijhuis M, Groenen SMA, Picavet HSJ, Launer LJ. The impact of migraine on quality of life in the general population. The GEM Study. Neurology. 2000;55:624–9. doi: 10.1212/wnl.55.5.624. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Hemelsky SW, Kolodner KN, Steiner TJ, Stewart WF. Migraine, quality of life and depression. A population-based case-control study. Neurology. 2000;55:629–35. doi: 10.1212/wnl.55.5.629. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71:848–55. doi: 10.1212/01.wnl.0000325565.63526.d2. [DOI] [PubMed] [Google Scholar]
- Lance JW, Goadsby PJ. Mechanism and Management of Headache. 7th ed. New York: Elsevier; 2005. [Google Scholar]
- Bigal ME, Ferrari M, Silberstein SD, Lipton RB, Goadsby PJ. Migraine in the triptan era: lessons from epidemiology, pathophysiology, and clinical science. Headache. 2009;49(Suppl 1):S21–33. doi: 10.1111/j.1526-4610.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ. 10 years of rizatriptan. Headache. 2009;49(Suppl 1):S1–2. doi: 10.1111/j.1526-4610.2008.01334.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld AM, Bloudek LM, Becker WJ, Buse DC, Varon SF, Maglinte GA, Wilcox TK, Kawata AK, Lipton RB. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II) Headache. 2013;53:644–55. doi: 10.1111/head.12055. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Chan KY, Eftekhari S, Nilsson E, de Vries R, Saveland H, Dirven CM, Danser AH, MaassenVanDenBrink A. Effect of the calcitonin gene-related peptide (CGRP) receptor antagonist telcagepant in human cranial arteries. Cephalalgia. 2010;30:1233–40. doi: 10.1177/0333102410362122. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Petersen KA. CGRP-receptor antagonism in migraine treatment. CNS Neurol Disord Drug Targets. 2007;6:240–6. doi: 10.2174/187152707781387314. [DOI] [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142:1171–81. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–23. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–22. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM Group BBCPoCS. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Emerging target-based paradigms to prevent and treat migraine. Clin Pharmacol Ther. 2013;93:78–85. doi: 10.1038/clpt.2012.198. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Goadsby PJ. New agents for acute treatment of migraine: CGRP receptor antagonists, iNOS inhibitors. Curr Treat Options Neurol. 2012;14:50–9. doi: 10.1007/s11940-011-0155-4. [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–84. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain J Neurology. 2005;128:932–9. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43(Suppl 3):S16–20. [PubMed] [Google Scholar]
- Moskowitz MA, Cutrer FM. CGRP: blood flow and more? Cephalalgia. 1996;16:287. doi: 10.1046/j.1468-2982.1996.1605287.x. [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–7. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Edmeads J. What is migraine? Controversy and stalemate in migraine pathophysiology. J Neurol. 1991;238(Suppl 1):S2–5. doi: 10.1007/BF01642898. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- Leao AA. The slow voltage variation of cortical spreading depression of activity. Electroencephalogr Clin Neurophysiol. 1951;3:315–21. doi: 10.1016/0013-4694(51)90079-x. [DOI] [PubMed] [Google Scholar]
- Leao AA. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol. 1947;10:409–14. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–7. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–60. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Afridi S, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RSJ, Goadsby PJ. A PET study in spontaneous migraine. Arch Neurol. 2005;62:1270–5. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- Haas DC, Kent PF, Friedman DI. Headache caused by a single lesion of multiple sclerosis in the periaqueductal gray area. Headache. 1993;33:452–5. doi: 10.1111/j.1526-4610.1993.hed3308452.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Neurovascular headache and a midbrain vascular malformation- evidence for a role of the brainstem in chronic migraine. Cephalalgia. 2002;22:107–11. doi: 10.1046/j.1468-2982.2002.00323.x. [DOI] [PubMed] [Google Scholar]
- Afridi S, Goadsby PJ. New onset migraine with a brainstem cavernous angioma. J Neurol Neurosurg Psychiatry. 2003;74:680–2. doi: 10.1136/jnnp.74.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M, Gizewski ER, Limmroth V, Diener H-C, Katsarava Z. Symptomatic migraine and pontine vascular malformation: evidence for a key role of the brainstem in the pathophysiology of chronic migraine. Cephalalgia. 2006;26:763–6. doi: 10.1111/j.1468-2982.2006.01102.x. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–77. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–6. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Calcitonin gene-related peptide antagonists as treatments of migraine and other primary headaches. Drugs. 2005;65:2557–67. doi: 10.2165/00003495-200565180-00002. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Jansen Olesen I, Kingman TA, McCulloch J, Uddman R. Modification of vasoconstrictor responses in cerebral blood vessels by lesioning of the trigeminal nerve: possible involvement of CGRP. Cephalalgia Int J headache. 1995;15:373–83. doi: 10.1046/j.1468-2982.1995.1505373.x. [DOI] [PubMed] [Google Scholar]
- Troltzsch M, Denekas T, Messlinger K. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS reduces neurogenic increases in dural blood flow. Eur J Pharmacol. 2007;562:103–10. doi: 10.1016/j.ejphar.2007.01.058. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain Official J Am Pain Soc. 2013;14:1289–303. doi: 10.1016/j.jpain.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Buzzi MG, Moskowitz MA. The development of neurogenic plasma extravasation in the rat dura mater does not depend upon the degranulation of mast cells. Brain Res. 1989;477:157–65. doi: 10.1016/0006-8993(89)91403-0. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. CGRP: sensory neuropeptide with multiple neurologic implications. Neurology. 2011;77:281–7. doi: 10.1212/WNL.0b013e31822550e2. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376:645–55. doi: 10.1016/S0140-6736(10)60323-6. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53:1230–44. doi: 10.1111/head.12179. [DOI] [PubMed] [Google Scholar]
- Pettypiece S. 2009. Merck halts testing of migraine drug on liver safety (Update2) [04/04/2013]. Available from: http://www.bloomberg.com/apps/news?pid=newsarchive&sid=aKxzpNBZ2cbA.
- Cholerton M. The hypothalamus in migraine. Optician. 1948;116:275–7. [PubMed] [Google Scholar]
- Durham PL. Inhibition of calcitonin gene-related peptide function: a promising strategy for treating migraine. Headache. 2008;48:1269–75. doi: 10.1111/j.1526-4610.2008.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL, Masterson CG. Two mechanisms involved in trigeminal CGRP release: implications for migraine treatment. Headache. 2013;53:67–80. doi: 10.1111/j.1526-4610.2012.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Walter S. Monoclonal antibodies for migraine: Preventing calcitonin gene-related peptide Activity. CNS Drugs. 2014;28:389–99. doi: 10.1007/s40263-014-0156-4. [DOI] [PubMed] [Google Scholar]
- Baumann A. Early development of therapeutic biologics--pharmacokinetics. Curr Drug Metab. 2006;7:15–21. doi: 10.2174/138920006774832604. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen PC. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2013;80:869–70. doi: 10.1212/01.wnl.0000427909.23467.39. [DOI] [PubMed] [Google Scholar]
- Berton E. Safety pharmacology: similarities and differences between small molecules and novel biotherapeutics. In: Cavagnaro J, editor. Preclinical safety evaluation of biopharmaceuticals. New York, NY: Wiley; 2008. [Google Scholar]
- Young S. 2013. Preventing migraines with a new kind of antibody http://medcitynews.com/2013/05/preventing-migraines-with-a-new-kind-of-antibody/2013 [cited 10/08/2013]. Available from: http://medcitynews.com/2013/05/preventing-migraines-with-a-new-kind-of-antibody/
- Alder BioPharmaceuticals I. 2014. Form S-1 Registration statement, under the Securities Act of 1933 http://www.sec.gov/Archives/edgar/data/1423824/000119312514104868/d657876ds1.htm2014 [cited 09/01/2014]. Available from: http://www.sec.gov/Archives/edgar/data/1423824/000119312514104868/d657876ds1.htm.
- Alder Biopharmaceuticals I. Safety Tolerability and Pharmacokinetics of ALD403: Alder Biopharmaceuticals, Inc. Available from: http://clinicaltrial.gov/ct2/show/NCT01579383?term=ALD403&rank=1.
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–7. doi: 10.1016/S1474-4422(14)70209-1. investigators ALDs. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E Quality Standards Subcommittee of the American Academy of N; the American Headache S. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–45. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amgen - Science - Pipeline. 2013. cited 2013 04/10. Available from: http://www.amgen.com/science/pipe.html.
- Ascending Single Doses of AMG 334 in healthy subjects and migraine patients. 2013. cited 2013 04/10. Available from: http://clinicaltrial.gov/ct2/show/NCT01688739?term=AMG334&rank=1.
- Ascending multiple-doses of AMG 334 in healthy subjects and in migraine patients. 2013. cited 2013 04/10. Available from: http://clinicaltrial.gov/ct2/show/NCT01723514?term=AMG334&rank=2.
- Amgen. 2013. A phase 2 study to evaluate the efficacy and safety of AMG 334 in migraine prevention: clinicaltrial.gov [cited 2014 01/18/2014]. Available from: http://clinicaltrial.gov/ct2/show/NCT01952574?term=AMG+334&rank=4.
- Amgen. 2014. A study to evaluate the efficacy and safety of AMG 334 in chronic migraine prevention [cited 2014 09/01/2014]. Available from: http://clinicaltrials.gov/ct2/show/NCT02066415?term=AMG334&rank=6.
- Amgen. 2014. A study to assess the long-term safety and efficacy of AMG 334 in chronic migraine prevention [cited 2014 09/01/2014]. Available from: http://clinicaltrials.gov/ct2/show/NCT02174861?term=AMG334&rank=5.
- Anderson P. 2014. Monoclonal antibodies promising for migraine prevention: Medscape [cited 2014 09/01/2014]. Available from: http://www.medscape.com/viewarticle/827838.
- de Hoon J, Montieth D, Vermeersch S, Van Hecken A, Abu-Raddad E, Collins E, Schuetz T, Scherer J, Grayzel D. Safety, pharmacokinetics, and pharmacodynamics of LY2951742: A monoclonal antibody targeting CGRP. Cephalalgia. 2013 . Available from: http://cep.sagepub.com/content/33/8_suppl/1.full.pdf+html. [Google Scholar]
- Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurology. 2014;13:885–92. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- Company ELa. 2014. A study of LY2951742 in participants with migraine headache [cited 2014 09/01/2014]. Available from: http://clinicaltrials.gov/ct2/show/NCT02163993?term=LY2951742&rank=1.
- Walter S, Alibhoy A, Escandon R, Bigal ME. Evaluation of cardiovascular parameters in cynomolgus monkeys following IV administration of LBR-101, a monoclonal antibody against calcitonin gene-related peptide. MAbs. 2014;6:871–8. doi: 10.4161/mabs.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Escandon R, Bronson M, Walter S, Sudworth M, Huggins JP, Garzone P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: Results of the Phase 1 program. Cephalalgia. 2013;34:483–92. doi: 10.1177/0333102413517775. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S. Monoclonal antibodies for migraine: preventing calcitonin gene-related peptide activity. CNS Drugs. 2014;28:389–99. doi: 10.1007/s40263-014-0156-4. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Bronson M, Alibhoy A, Sager P, Escandon R. Cardiovascular and Hemodynamic parameters in women following prolonged CGRP inhibition using LBR-101, a monoclonal antibody against CGRP. Cephalalgia. 2014;34:968–76. doi: 10.1177/0333102414527646. [DOI] [PubMed] [Google Scholar]
- Koehler PJ, Tfelt-Hansen PC. History of methysergide in migraine. Cephalalgia. 2008;28:1126–35. doi: 10.1111/j.1468-2982.2008.01648.x. [DOI] [PubMed] [Google Scholar]


