Abstract
Aims
To compare the values regulators attach to different drug effects of oral antidiabetic drugs with those of doctors and patients.
Methods
We administered a ‘discrete choice’ survey to regulators, doctors and patients with type 2 diabetes in The Netherlands. Eighteen choice sets comparing two hypothetical oral antidiabetic drugs were constructed with varying drug effects on glycated haemoglobin, cardiovascular risk, bodyweight, duration of gastrointestinal complaints, frequency of hypoglycaemia and risk of bladder cancer. Responders were asked each time which drug they preferred.
Results
Fifty-two regulators, 175 doctors and 226 patients returned the survey. Multinomial conditional logit analyses showed that cardiovascular risk reduction was valued by regulators positively (odds ratio 1.98, 95% confidence interval 1.11–3.53), whereas drug choices were negatively affected by persistent gastrointestinal problems (odds ratio 0.24, 95% confidence interval 0.14–0.41) and cardiovascular risk increase (odds ratio 0.49, 95% confidence interval 0.27–0.87). Doctors and patients valued these effects in a similar manner to regulators. The values that doctors attached to large changes in glycated haemoglobin and that both doctors and patients attached to hypoglycaemia and weight gain also reached statistical significance. No group's drug choice was affected by a small absolute change in risk of bladder cancer when presented in the context of other drug effects. When comparing the groups, the value attached by regulators to less frequent hypoglycaemic episodes was significantly smaller than by patients (P = 0.044).
Conclusions
Regulators may value major benefits and risks of drugs for an individual diabetes patient mostly in the same way as doctors and patients, but differences may exist regarding the value of minor or short-term drug effects.
Keywords: benefit–risk assessment, drug preference, oral antidiabetes drug, survey
What is Already Known about this Subject
Oral antidiabetic drugs are approved without demonstrated long-term benefits, but some have been associated with important harms.
Drugs are being approved with limited involvement of important stakeholders (patients and doctors).
It is unknown whether regulators value benefits and risks of oral antidiabetic drugs in a similar manner to other stakeholders.
What this Study Adds
Regulators, patients and doctors have mostly similar values concerning major drug effects but may differ in the value they attach to minor or short-term drug effects.
Cardiovascular benefits of oral antidiabetic drugs are valued as the most important aspect by all three stakeholders.
Serious but rare risks (bladder cancer) did not impact the drug preference of any stakeholder group, but patients attached more value to symptomatic adverse drug events (hypoglycaemia) than regulators.
Introduction
There seems to be a gap between expectations about regulating drugs and the decisions on drug approval. For example, in the wake of pancreas-related adverse events of incretin mimetics (glucagon-like peptide-1 agonists and dipeptidylpeptidase-4 inhibitors), regulators were criticized for not making clear why they approved these drugs. Long-term benefits had not been demonstrated, but serious potential risks were identified 1. Earlier critical reports on the regulatory oversight of another oral antidiabetic drug, rosiglitazone, had also appeared 2,3.
A French review considered only four of 97 new drugs approved in 2010 a therapeutic advantage, and stated that regulators put companies' short-term financial interest above patients' wellbeing. The authors called for decision-makers to make patients' wellbeing their top priority 4.
Regulators base their decision to approve new drugs on an assessment of the balance between benefits and risks as determined in pre-approval studies at a population level. Doctors make decisions for individual patients, making use of population-level information. Patients may value benefits and risks from a different perspective and make a decision based on the information that they receive from their healthcare provider and other information sources, including the patient leaflet that contains the most relevant information approved by the regulators. Although regulators are likely to be blamed for being overly tolerant to drug risk following any high-profile drug safety issue, they have also been criticized for being excessively risk averse, preventing important new drugs from reaching the market in a timely manner 5.
An important question is whether regulatory decisions take the clinical needs, perspective and reality of doctors and patients sufficiently into account. It may be considered improper that patients, as the most relevant stakeholders, are not included in licensing decisions, while shared decision making is now common in daily clinical practice. Our ultimate goal is to gain insight into how involving patients and doctors in the regulatory process might affect the decision making. For this, it is important to evaluate similarities and differences in their evaluation of drug effects. Several studies have evaluated how patients and doctors value drug benefits and risks, for example in the field of diabetes 6–9, but not in comparison to regulator's assessments. Their views could be different as a result of making decisions at the population level instead of the individual patient level, but it could also be that regulators fundamentally value benefits and risks of drugs in a different manner to other stakeholders.
To compare the drug preferences and values underlying drug choices of regulators with those of doctors and patients, we conducted a ‘discrete choice’ survey, where these groups were asked which hypothetical oral antidiabetic drugs (OADs) with varying benefits and risks they preferred for a typical patient with type 2 diabetes.
Methods
Study participants
We performed a survey to estimate the relative value that regulators, doctors and type 2 diabetes patients assign to different drug effects. All 65 clinical and pharmacovigilance assessors and 14 members of the Dutch Medicines Evaluation Board were sent an e-mail containing a link to the survey website and unique login information. After 2 and 3 months, an e-mail reminder was sent to nonresponders. Doctors included were general practitioners and internists practising in diabetes care. A randomly selected list of 593 general practitioners was obtained from the Dutch institute of research in healthcare (NIVEL). A list of 252 internists practising in diabetes care was compiled from the websites of all Dutch hospitals. These doctors were sent a paper-based questionnaire including an option to respond electronically through a secure website. Two months later, nonresponders were reminded to fill in the questionnaire. Patients between the age of 60 and 75 years, receiving at least one prescription of an OAD in the preceding 4 months, were identified from pharmacies in the northern part of The Netherlands. Pharmacy interns contacted these patients by telephone and requested permission to send a questionnaire. Two weeks after the questionnaire was sent, the interns telephoned again to follow up on nonresponders. A waiver was obtained from the medical ethical committee of the University Medical Center Groningen for this survey.
Study design
A discrete choice experimental design was used, where participants were asked to choose between several pairs of hypothetical OADs. The method incorporates a trade-off to elicit participants' willingness to accept certain risks in exchange for a certain amount of benefit 10. We followed a step-wise approach in designing and conducting our discrete choice experiments 11. We first identified the relevant drug effects to create the hypothetical OADs. To that end, we performed 22 in-depth interviews (six patients, three nurses, nine doctors, three regulators and a pharmacist). In addition, an informal review of the literature (including studies into patient preferences for certain drug effects) 6–9, regulatory requirements and product labelling of OADs was conducted. The effects selected (Table 1) were as follows.
Control of diabetes, the efficacy parameter used in trials for market authorization applications.
Impact on cardiovascular outcomes. Controlling blood glucose levels should ultimately lead to a decreased cardiovascular (CV) risk, but an increase in risk was shown with rosiglitazone 12.
Duration of gastrointestinal (GI) complaints [common adverse drug reaction (ADR) of various OADs] 8,9.
Risk of bladder cancer, a serious but rare ADR that has led to debate about the safety of pioglitazone 13–15.
Table 1.
Drug effects and levels as used for the hypothetical drugs
| Drug effect | Levels |
|---|---|
| Glycated haemoglobin | Small decrease from 8.5 to 8.0% (too small) |
| Intermediate decrease from 8.5 to 7.5% (suboptimal) | |
| Large decrease from 8.5 to 6.9% (optimal) | |
| Cardiovascular disease | An increased (4%) risk; 4 instead of 3 of 100 patients |
| Unchanged (3%) risk; 3 of 100 patients | |
| A decreased (2%) risk; 2 instead of 3 of 100 patients | |
| Effect on bodyweight | 5% (4.5 kg) weight gain |
| No influence on weight | |
| 10% (9 kg) weight loss | |
| Mild nausea, vomiting or diarrhoea | Throughout the use of the drug |
| During the first 2 weeks of use of the drug | |
| No stomach complaints | |
| Hypoglycaemia | More than 2 times per month |
| 1–2 times per month | |
| None | |
| Risk of cancer | Increased (0.06%) risk; 6 instead of 4 of 10 000 patients |
| Unchanged (0.04%) risk; 4 of 10 000 patients |
In a second step, we defined the levels of variation of these drug effects for the hypothetical drugs (Table 1). We selected levels of variation that were considered to be plausible and representative of existing OADs.
Finally, we defined the choice tasks to elicit preferences of study participants.
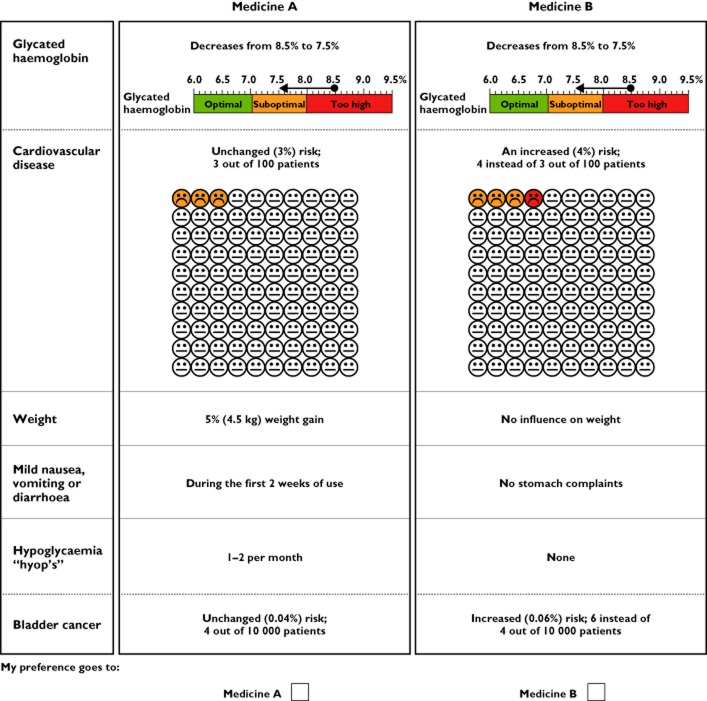
Participants were presented several sets of two hypothetical OADs and asked each time to choose between these two (Figure 1). Each set differed on at least two drug effects. An orthogonal algorithm (Orthoplan, SPSS v.19; SPSS Inc., Chicago, IL, USA) was used to select the minimal number of choice sets needed to enable the estimation of all main effects. The final selection comprised 18 choice sets. Regulators received all choice sets. To minimize the burden for patients and doctors, they received a random set of six choice sets each. This approach is considered appropriate when at least 60 responses per group (doctors and patients) are received for each scenario. An example of a choice set is shown in Figure 1.
Figure 1.
Example of a discrete choice task
Survey instructions
At the start of the survey, the participants were presented with a description of a hypothetical diabetes patient; the patient vignette. This patient was a 67-year-old male, weighing 90 kg, using metformin, antihypertensive medication and a statin. His blood glucose was poorly controlled and he was in need of a second drug in addition to the metformin. The regulators were asked to indicate which of the two OADs they felt was more appropriate for the patient. Doctors were asked to imagine that they were treating this patient and base their choices on their treatment preferences for that patient. The patients were asked to imagine being this patient and to indicate which drug they preferred based on their own preference. The scenario also read that all drugs had a similar impact on microvascular outcomes. The patient vignette was repeated above each choice question.
Additional data collection
At the end of the survey, a number of general questions were included. Regulators were asked their gender and how many years they had worked for the Dutch Medicines Evaluation Board (MEB). Doctors were asked their gender, whether they were general practitioners or specialists and how many years they had been registered as practitioners. Patients were asked their gender, age, highest completed education and the year when they were diagnosed as having diabetes.
Analyses
We present descriptive analysis of which hypothetical drug was preferred per choice set by each of the responder groups. We evaluated the choices that were made by the respondents to check whether respondents made the same choices each time. For modelling of the data from the discrete choice experiments, we used multinomial statistical models, in which one of the levels for each drug effect is set as reference level 16.
The following levels were chosen as reference: a small decrease in glycated haemoglobin (HbA1c) level, no change in CV risk, no effect on weight, no stomach complaints, no episodes of hypoglycaemia and no change in risk of bladder cancer. The main effect model included 11 dummy variables, because five of the drug effects varied on three levels and one drug effect on two levels. We performed conditional (fixed-effects) logistic regression analysis to detect which variables influenced choice for the groups of regulators, doctors and patients separately (STATA 13.1 Special Edition; Statacorp, College Station, TX, USA). We used seemingly unrelated estimation (postestimation command suest) to test (Chow test) for statistical differences between odds ratios (ORs) between regulators vs. patients and regulators vs. doctors.
Results
Respondents
The survey using hypothetical drugs to elucidate what values regulators, doctors and patients attach to OAD benefits and risks was returned by 52 (66%) regulators, 175 (21%) medical doctors and 226 (72%) patients. The regulators had a median work experience of 5 years [interquartile range (IQR) 3–11 years] and comprised a mixture of clinical and pharmacovigilance assessors as well as seven independent members of the MEB. The doctors comprised a group of 130 general practitioners and 45 internists, of whom 49% had >20 years of experience in clinical practice. The median age of the patients was 67 years (IQR 64–71 years); 48% were women, and they had median disease duration of 7.5 years (IQR 3–13 years; Table 2). The participants were most outspoken in their preferences in sets 6, 8, 9, 10 and 13 (Figure 2). We manually checked for respondents who answered only A or only B and found only two such cases, both by patients. Few respondents (two of 52 regulators, one of 175 doctors and nine of 226 patients) provided partly completed or not completed surveys. Excluding these cases, in a sensitivity analysis, did not affect our analyses (data not shown).
Table 2.
Demographic characteristics of responders
| Demographics | Number (%) |
|---|---|
| Median (IQR) | |
| Regulators (n = 52) | |
| Gender [female; n (%)] | 24 (48%) |
| Experience at the MEB [years; median (IQR)] | 5 (3–11) |
| Doctors (n = 175) | |
| Gender [female, n (%)] | 69 (39%) |
| General practitioners [n (%)] | 130 (73%) |
| Years of experience [n (%)] | |
| <1 year | 3 (2%) |
| 1–5 years | 12 (7%) |
| 5–10 years | 24 (14%) |
| 10–20 years | 50 (28%) |
| >20 years | 87 (49%) |
| Patients (n = 226) | |
| Gender [female; n (%)] | 108 (47.8%) |
| Age [years; median (IQR)] | 67 (64–71) |
| Duration of diabetes [years; median (IQR)] | 7.5 (3–13) |
Abbreviations are as follows: IQR, interquartile range; MEB, Dutch Medicines Evaluation Board.
Figure 2.
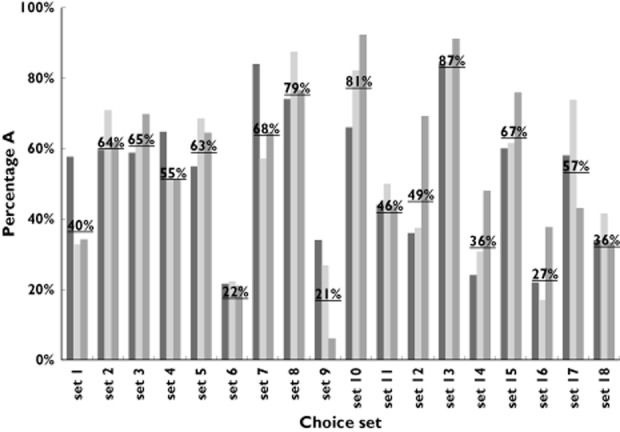
Percentage of respondents preferring the hypothetical drug A for each choice set per group of respondents. The underlined percentage is the average of all responders preferring the hypothetical drug A in a given choice set. ▪, regulators;  , doctors;
, doctors;  , patients
, patients
Values attached to drug effects
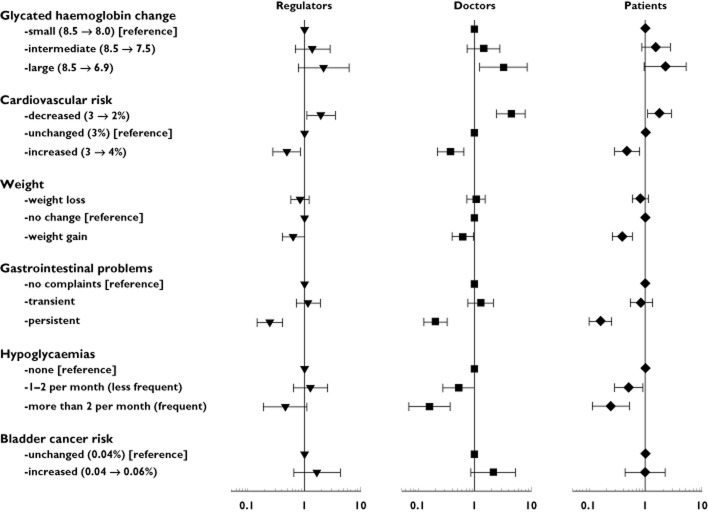
A reduction in CV risk was the drug effect that positively affected the choice of the regulators [OR 1.98, 95% confidence interval (CI) 1.11–3.53], whereas persistent GI problems (OR 0.24, 95% CI 0.14–0.41) and CV risk increase (OR 0.49, 95% CI 0.27–0.87) were considered as significant negative drug effects. Also, doctors and patients valued CV risk decrease positively [OR 4.40 (95% CI 2.44–7.94) and OR 1.74 (95% CI 1.06–2.86), respectively]; and CV risk increase negatively [OR 0.37 (95% CI 0.22–0.65) and OR 0.47 (95% CI 0.28–0.78), respectively]. Likewise, persistent GI problems affected drug choice negatively for both doctors (OR 0.20, 95% CI 0.12–0.32) and patients (OR 0.16, 95% CI 0.10–0.25), whereas temporary GI effects did not affect drug choice for any responder group (Figure 3). While weight gain as a negative drug effect was only marginally significant for the regulators' choices (OR 0.64, 95% CI 0.41–1.00) and doctors (OR 0.62, 95% CI 0.41–0.94), it had a more pronounced significant value for patients (OR 0.39, 95% CI 0.26–0.59). The occurrence of episodes of hypoglycaemia did not significantly affect the drug choice of regulators, whereas it did affect the choices of doctors and patients. Frequent hypoglycaemias (OR 0.16, 95% CI 0.07–0.37) affected the choice of doctors, and both frequent hypoglycaemias (OR 0.24, 95% CI 0.11–0.51) and less frequent hypoglycaemias (OR 0.50, 95% CI 0.28–0.90) affected the choice of the patients. Regarding HbA1c, there was no significant impact of either intermediate or large decreases in HbA1c for the regulators' or patients' choices, but doctors preferred OADs with a large decrease in HbA1c levels (OR 3.24, 95% CI 1.25–8.43). Regulators were in agreement with doctors and patients regarding the lack of importance of changes in risk of bladder cancer (Figure 3).
Figure 3.
Perceived value of drug effects by regulators, doctors and patients (odds ratios and 95% confidence intervals). An odds ratio >1 indicates that responders are more inclined to choose a drug with a specific drug effect and level. Vice versa, responders are less inclined to choose drug effects and levels with an odds ratio <1
In the analyses in which regulators were compared with doctors or with patients, we observed that the value attached by regulators to less frequent hypoglycaemic episodes was smaller than by patients (P = 0.044). The value attached to individual drug effects did not differ significantly between regulators and doctors.
Discussion
To our knowledge, our study is the first to compare the value that regulators, doctors and patients attribute to drug benefits and risks. The results indicate that regulators value drug effects of OADs mostly in the same way as doctors and patients when a benefit–risk assessment is made for a patient with type 2 diabetes. Regulators, as well as both doctors and patients, valued the long-term cardiovascular benefits and symptomatic ADRs as more important for the drug choice than effects on bodyweight or an increase in bladder cancer risk. However, less frequent episodes of hypoglycaemia were considered less important by regulators than patients.
Our findings do not support the assumption that regulators have fundamentally different views from other stakeholders when valuing individual drug benefits and risks but illustrate that differences may exist regarding the relative value of specific, minor drug effects. Trade-offs between long-term benefits and symptomatic ADRs are expected to be context and disease dependent. Parents of children with cancer were more likely to accept serious ADRs than parents of children with less serious diseases 17. Input of the most relevant stakeholders, patients and their healthcare providers could offer valuable support for regulatory authorities in decision making. Some argue that patients may push for earlier availability of drugs for life-threatening diseases; however, research by the European Medicines Agency (EMA) does not support this 5,18; here, it was found that patient organizations such as EURORDIS (representing patient organizations for more than 4000 different rare diseases) stress the need for well-conducted clinical studies rather than opt for unproven off-label drug use 5,18. Our data complement findings of a small study by Britten et al. that showed patients can interpret a US Food and Drug Administration (FDA) summary of drug benefits and risks 19. This pilot study suggested that a more direct involvement of patients in regulatory decisions is feasible. The level of agreement on the benefit–risk evaluation of hypothetical drugs observed in our study indicates that involvement of patients in regulatory decisions does not necessarily result in different decisions. It could, however, result in a better understanding and acceptance of the decisions made. Still, when making specific choices between two hypothetical drug options, patients may have different preferences. In terms of transparency, European regulators have taken the view to disclose full clinical trial data supporting drug applications in the near future 20. Maybe now is also the time to expand the involvement of patients in regulatory decision making, by routinely presenting them with a summary of key benefit–risk information as available at the time of the final decision making by the US FDA or EMA and ask whether a drug should be approved. Patient involvement and participation in the postapproval process has already been implemented in the pharmacovigilance risk assessment committee 21.
Frequent hypoglycaemic events significantly affected drug choice by doctors and patients, but not by regulators. The difference between patients and regulators reached statistical significance for the less frequent episodes, although this may be a chance finding due to multiple testing. It seems, however, that the regulators may be more willing to accept hypoglycaemia as a pharmacologically inevitable ADR of some OADs. Doctors and patients may have real experience with hypoglycaemia and its effects on quality of life and put more value on this ADR when choosing a drug for a specific patient 8,9,22. Nevertheless, it remains surprising that regulators did not attach much value to drugs causing hypoglycaemic events, because the EMA diabetes guideline even specifies detailed analyses of occurrence and severity of hypoglycaemic events 23. All groups valued changes in CV risk as important, considering this effect as one of the principal aims of OAD treatment 24. Whether this also implies that CV benefits need to be demonstrated before approval in view of the respondents cannot be answered with our survey. It is, however, of note to learn that two recently approved second-line OADs, which were approved based on their glucose-lowering effects, were not able to show cardiovascular benefit in their dedicated outcome studies 25,26 Neither regulators nor patients placed a significant weight on changes in HbA1c in relation to the other included drug effects, whereas doctors did. The regulators' choice may seem surprising, because HbA1c is the efficacy parameter based on which new OADs are approved. However, this finding may be explained by recent evidence that more aggressive glucose-controlling strategies do not always translate into better long-term patient outcome; i.e. micro- and/or macrovascular benefit 27,28. While the scenario read that all drugs had a similar impact on microvascular outcomes, the simultaneously presented effects of our hypothetical drugs on CV risk may have influenced this finding. These effects are usually not known when the drug receives market approval 29,30. A previous study that showed patients did consider HbA1c an important drug effect did not test the effect of changes in HbA1c in the context of a beneficial effect on CV risk 9.
Regulators placed similar values on persistent GI complications to doctors and patients, acknowledging the disruptive effect that persistent symptomatic ADRs can have on the patients' quality of life and, most probably, treatment compliance. Our finding that persistent ADRs are seen as more important than transient ADRs is in line with other studies 8,9. Regulators, doctors and patients also agreed in the limited value they attached to changes in bodyweight, although patients were less inclined than others to choose drugs that resulted in weight gain. This may be explained by the fact that regulators look at bodyweight as an indicator of metabolic control, while patients may already have struggled with losing weight as a means to control their diabetes or because of social pressure to be thin 31. Finally, an interesting observation was that regulators, doctors and patients agreed in the lack of value they attached to an increased risk of bladder cancer. It could be that they interpreted that risk as less relevant in view of the low absolute risk. We displayed the risk in absolute terms, and not as a relative risk, giving a more accurate picture of the clinical relevance of this drug effect, which may have contributed to its interpretation 32. The duration of use of the OAD was not defined, although from the context it was clear that it was intended for long-term use. Thus, the increased risk presented in our choice sets would also be in line with current knowledge that bladder cancer is related to the cumulative pioglitazone dose received 33. We cannot but speculate whether a general risk of cancer, or a more publicized risk, such as pancreatic cancer that is linked to glucagon-like peptide-1 agonists, would have resulted in a different effect 1. It is known, however, that media attention may amplify perceived risks 34.
Limitations
The results of a discrete choice model are inherently subjective to the drug effects and levels chosen. When larger differences in levels are presented, different effects on preferences may result 35. We selected levels that are plausible and representative of what is known about real OADs to minimize this effect. By focusing on OADs, we could include a range of different drug effects. However, for other drug classes with other drug effect profiles the results might have been different. Nonetheless, similar results could be expected for other preventive treatments, such as for antihypertensive drugs, for which short-term risk reduction, long-term benefits and symptomatic ADRs also need to be considered when making drug choices. Drug scenarios were presented in various ways. Some of the effects were depicted only with text and numbers, others with coloured pictures, hence being more noticeable. The design of the scale figure used to describe changes in HbA1c is based on a previous discrete choice study 9. The design of the ‘smiley-face’ matrix used to describe changes in risk of CV events was intended to make the proportions presented understandable to all groups of respondents 36. The results show that most value was attached to a drug effect (persistent GI problems) that were presented with text only, indicating that this difference in presentation did not lead respondents to disregard those drug effects.
We used a typical 67-year-old diabetes patient, for whom a drug choice had to be made. A different patient vignette, e.g. an elderly patient, might have changed the responses. Gastroenterologists valued ADRs as less important when presented with a vignette of an elderly patient than of a younger patient 37. However, the aim of the present study was to assess differences between groups when presented with the same patient. The patient population was selected from pharmacies in the northern part of The Netherlands and might not necessarily represent patients from outside The Netherlands. The patients selected for the study were 60–75 years of age and might differ in their preferences or values from those older or younger. However, this criterion should also facilitate the patient imagining himself or herself as the ‘typical’ patient for whom a drug choice had to be made. Likewise, the doctors and regulators selected for this study were working in The Netherlands and could have different values from those working in other countries.
The low response rate of doctors is a limitation, but respondents were representative in terms of gender and location in The Netherlands 38. A low response by doctors has been more often reported and we cannot exclude the possibility that it may have biased our results in the sense that doctors who are especially interested in diabetes treatment may be overrepresented. There is no indication, however, that more interested doctors would value drug effects differently. The relatively high response rate among patients, in contrast, suggests that they found the task interesting. This is encouraging because it suggests a certain willingness to participate in the drug decision-making process.
Implications for policy and practice
In conclusion, our results show that regulators may value major long-term benefits and risks of drugs in the same way as doctors and patients but may differ on the value of other drug effects. As such, the input of these other stakeholders could support regulators in making decisions that sufficiently reflect the clinical needs of doctors and patients. So far, to a limited extent they are involved in regulatory activities in Europe, such as by participation in scientific advisory groups, but it has been suggested that their role needs further strengthening 39. Britten et al. showed that patients can interpret information about drug benefits and risks at a population level as provided in the regulatory process 19. The input of doctors and patients may be helpful especially for decisions that are not that straightforward and may provoke debate, such as regarding the impact of small risks for serious adverse effects or of uncertain benefits.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: this study was supported in part by an unconditional grant in the context of the Escher project (T6-202), a project of the Dutch Top Institute Pharma; the funder did not have any role in the design or execution of the study; the authors report no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. The authors who are employed by the Dutch Medicines Evaluation Board (PAdG, SMJS and PGMM) state that the opinions in this paper are their own and do not necessarily reflect those of the agency.
Contributors
PGMM designed the study, monitored data collection for the whole trial, wrote the statistical analysis plan, analysed the data and drafted and revised the paper. PGMM is guarantor. AHA designed the study, monitored data collection for the whole trial, wrote the statistical analysis plan, cleaned and analysed the data and drafted and revised the paper. PD, FMH-R, PAdG, PFMK and SMJS designed the study, monitored data collection, interpreted the data and drafted and revised the paper. EQ wrote the statistical analysis plan, analysed the data and revised the paper. PGMM affirms that the manuscript is an honest, accurate and transparent account of the study being reported and that no important aspects of the study have been omitted.
The authors thank all respondents (regulators, doctors and patients) for their willingness to participate in this survey.
References
- Gale EA. GLP-1 based agents and acute pancreatitis: drug safety falls victim to the three monkey paradigm. BMJ. 2013;346:f1263. doi: 10.1136/bmj.f1263. [DOI] [PubMed] [Google Scholar]
- Godlee F. Rosiglitazone: a cautionary tale. BMJ. 2010;341:c4896. [Google Scholar]
- Cohen D. Rosiglitazone: what went wrong? BMJ. 2010;341:c4848. doi: 10.1136/bmj.c4848. [DOI] [PubMed] [Google Scholar]
- Prescrire International. New drugs and indications in 2010: inadequate assessment; patients at risk. Rev Prescrire. 2011;31:134–141. [PubMed] [Google Scholar]
- Eichler HG, Bloechl-Daum B, Brasseur D, Breckenridge A, Leufkens H, Raine J, Salmonson T, Schneider CK, Rasi G. The risks of risk aversion in drug regulation. Nat Rev Drug Discov. 2013;12:907–916. doi: 10.1038/nrd4129. [DOI] [PubMed] [Google Scholar]
- Porzsolt F, Clouth J, Deutschmann M, Hippler HJ. Preferences of diabetes patients and physicians: a feasibility study to identify the key indicators for appraisal of health care values. Health Qual Life Outcomes. 2010;8:125. doi: 10.1186/1477-7525-8-125. . doi: 10.1186/1477-7525-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products – liraglutide and exenatide – for the treatment of type 2 diabetes. J Med Econ. 2010;13:655–661. doi: 10.3111/13696998.2010.529377. [DOI] [PubMed] [Google Scholar]
- Bogelund M, Vilsboll T, Faber J, Henriksen JE, Gjesing RP, Lammert M. Patient preferences for diabetes management among people with type 2 diabetes in Denmark – a discrete choice experiment. Curr Med Res Opin. 2011;27:2175–2183. doi: 10.1185/03007995.2011.625404. [DOI] [PubMed] [Google Scholar]
- Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with type 2 diabetes using oral glucose-lowering agents. Diabet Med. 2009;26:416–424. doi: 10.1111/j.1464-5491.2009.02696.x. [DOI] [PubMed] [Google Scholar]
- Johnson FR, Hauber AB, Poulos CM. 2009. A brief introduction to the use of stated-choice methods to measure preferences for treatment benefits and risks. Research Triangle Institute;. Report no. RR-0009-0909.
- Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Hillaire-Buys D, Faillie J, Montastruc J. Pioglitazone and bladder cancer. Lancet. 2011;378:1543–1544. doi: 10.1016/S0140-6736(11)61662-0. [DOI] [PubMed] [Google Scholar]
- Faillie JL, Petit P, Montastruc JL, Hillaire-Buys D. Scientific evidence and controversies about pioglitazone and bladder cancer: which lessons can be drawn? Drug Saf. 2013;36:693–707. doi: 10.1007/s40264-013-0086-y. [DOI] [PubMed] [Google Scholar]
- Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184:E675–683. doi: 10.1503/cmaj.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arons AM, Krabbe PF. Probabilistic choice models in health-state valuation research: background, theories, assumptions and applications. Expert Rev Pharmacoecon Outcomes Res. 2013;13:93–108. doi: 10.1586/erp.12.85. [DOI] [PubMed] [Google Scholar]
- Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, Turner MA, Young B. Enhancing communication about paediatric medicines: lessons from a qualitative study of parents' experiences of their child's suspected adverse drug reaction. PLoS One. 2012;7:e46022. doi: 10.1371/journal.pone.0046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicines for children: better, more and faster. EURORDIS position paper on the proposal for a regulation on medicinal products for paediatric use [Internet]. Available at http://www.eurordis.org/IMG/pdf/eurordis__position__medicines_children_31jan05.pdf (last accessed 16 June 2014)
- Britten N, Denford S, Stein K. Involving patients in drug licensing decisions. BMJ. 2013;347:f4329. doi: 10.1136/bmj.f4329. [DOI] [PubMed] [Google Scholar]
- Hawkes N. Industry and drug regulators disagree on which data should remain confidential. BMJ. 2013;347:f5390. doi: 10.1136/bmj.f5390. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Pharmacovigilance Risk Assessment Committee, composition [Internet]. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/general/general_content_000537.jsp&mid=WC0b01ac058058cb18 (last accessed 16 June 2014)
- Buusman A, Andersen M, Merrild C, Elverdam B. Factors influencing GPs' choice between drugs in a therapeutic drug group. A qualitative study. Scand J Prim Health Care. 2007;25:208–213. doi: 10.1080/02813430701652036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. 2012. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. European Medicines Agency;. Report no. CPMP/EWP/1080/00 Rev. 1.
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. 2012. Guideline on clinical evaluation of medicinal products in the treatment or prevention of diabetes mellitus. Report nr CPMP/EWP/1080/00 Rev.1.
- Lehman R, Yudkin JS, Krumholz H. Licensing drugs for diabetes. BMJ. 2010;341:c4805. doi: 10.1136/bmj.c4805. [DOI] [PubMed] [Google Scholar]
- Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health. 2008;11:478–486. doi: 10.1111/j.1524-4733.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- Malenka DJ, Baron JA, Johansen S, Wahrenberger JW, Ross JM. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;8:543–548. doi: 10.1007/BF02599636. [DOI] [PubMed] [Google Scholar]
- Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke UK. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:258–273. doi: 10.1111/bcp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovic P. The Perception of Risk. London: Earthscan; 2000. [Google Scholar]
- Mangham LJ, Hanson K, McPake B. How to do (or not to do) … designing a discrete choice experiment for application in a low-income country. Health Policy Plan. 2009;24:151–158. doi: 10.1093/heapol/czn047. [DOI] [PubMed] [Google Scholar]
- Hildon Z, Allwood D, Black N. Impact of format and content of visual display of data on comprehension, choice and preference: a systematic review. Int J Qual Health Care. 2012;24:55–64. doi: 10.1093/intqhc/mzr072. [DOI] [PubMed] [Google Scholar]
- Johnson FR, Hauber B, Özdemir S, Siegel CA, Hass S, Sands BE. Are gastroenterologists less tolerant of treatment risks than patients? Benefit–risk preferences in Crohn's disease management. J Manag Care Pharm. 2010;16:616–628. doi: 10.18553/jmcp.2010.16.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Professions in Healthcare. Database. 2011. . [ Beroepen in de Gezondheidszorg. Databank ] [Internet]. Available at http://www.nivel.nl/databank. (last accessed 16 June 2014)
- Ebbers HC, Pieters T, Leufkens HG, Schellekens H. Effective pharmaceutical regulation needs alignment with doctors. Drug Discov Today. 2012;17:100–103. doi: 10.1016/j.drudis.2011.09.018. [DOI] [PubMed] [Google Scholar]