Abstract
Aim
Drug-induced liver injury (DILI) is often responsible for acute liver failure, drug withdrawal, boxed warnings or drug non-approval. Therefore, we conducted a case–control study to determine the hepatotoxic risk of a wide range of drugs.
Methods
The Berlin Case–Control Surveillance Study FAKOS included all 51 Berlin hospitals in a hospital network. Between 2002 and 2011, 198 patients with acute idiopathic hepatitis, 377 inpatient controls and 708 outpatient controls were ascertained. Case patients were thoroughly validated using anamnestic, clinical, laboratory and histological data. Drug exposure was obtained in a face-to-face interview. A possible drug aetiology was assessed in individual patients by applying the updated Council for International Organizations of Medical Sciences (CIOMS) scale. Drug risks were further quantified [odds ratios (OR) with 95% confidence intervals (CI)] in a case–control design with unconditional logistic regression analysis. Drug intake in the last 28 days before index date was considered for the analysis.
Results
The study corroborated hepatotoxic risks for a number of drugs, including phenprocoumon (OR 3.3, 95% CI 1.5, 6.7), amiodarone (OR 5.5, 95% CI 1.3, 21.2), clozapine (OR 34.6, 95% CI 2.8, 824.9) and flupirtine (OR 40.2, 95% CI 5.5, 856.9). Increased risks were also suggested for less commonly reported substances such as angiotensin II receptor blockers, atypical antipsychotics and for biperiden, a drug never before reported to be hepatotoxic.
Conclusions
Our study identified a large number of drugs as possible causes of hepatotoxicity. The observed risk for seldom reported substances highlights the need for further post-authorization safety studies not exclusively focusing on drugs already labelled as potentially hepatotoxic.
Keywords: drug safety, hepatotoxicity, pharmacovigilance
What is Already Known about this Subject
Drug-induced liver injury (DILI) accounts for almost every second acute liver failure and often leads to drug withdrawal, boxed warnings or drug non-approval.
Dose-related and thus predictable DILI is a rarity, making a quantification of the hepatotoxic risk of drugs necessary.
What this Study Adds
This study is the first to quantify the hepatotoxic risk of flupirtine, irbesartan, clozapine and olanzapine, drugs previously associated with DILI in case reports.
A novel hepatotoxic risk is suggested for biperiden, highlighting the need for post-authorization safety studies considering also older drugs not labelled as hepatotoxic.
Introduction
Drug-induced liver injury (DILI) has been attracting increased attention over the past years, since it is responsible for approximately 50% of all cases of acute liver failure (ALF) 1 and belongs to the most common causes of drug withdrawal, boxed warnings or denial of approval 2.
The symptoms of DILI range from mildly elevated liver enzymes to severe hepatic damage requiring liver transplantation with poor transplant-free survival of the respective cases 2. Its pattern is classified as hepatocellular, cholestatic or mixed based on the ratio (R) of the relative rise of alanine aminotransferase (ALT), as a multiple of its upper limit of normal (ULN), to the relative rise of alkaline phosphatase (ALP) (Table 1) 3.
Table 1.
Inclusion criteria of idiopathic hepatitis in FAKOS and patterns of DILI
| Inclusion criteria for idiopathic hepatitis in FAKOS | |
|---|---|
| ALT > 3 × ULN | |
| or AST > 3 × ULN | |
| or total bilirubin > 2 mg dl−1 |
| Patterns of DILI | R: (ALT/ULN):(ALP/ULN) |
|---|---|
| Hepatocellular (ALT > 2 ×ULN) | >5 |
| Cholestatic (ALP > 2 × ULN) | <2 |
| Mixed (ALT and ALP > 2 × ULN) | 2–5 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
To date, case reports and cohort studies have revealed numerous drugs that may cause hepatic damage. However, only a minority of these medications have a dose-related and thus predictable hepatotoxicity 4, as most of them display an idiosyncratic mode, either immune-mediated or metabolic 5. Therefore, more studies are needed in order to quantify the risk of different drugs. Although case–control studies have been described as an excellent approach for validation and assessment of adverse drug reactions 6, only a few epidemiological studies of this type have been conducted on hepatotoxicity so far, and most of them have focused on specific drugs 7,8.
Here we present the results of the Berlin Case–Control Surveillance Study (Fall-Kontroll-Studie (FAKOS)) 9,10 regarding hepatotoxicity. Two different methods of identifying drugs that may cause hepatic damage were used. Firstly, we applied a standardized causality assessment on all individual patients diagnosed with idiopathic hepatitis (IH) using defined clinical criteria to detect a possible drug-relation of the disease. Secondly, a case–control study was performed in order to quantify drug risks.
Methods
Case identification and recruitment
To study serious toxicity of drugs, the hospital based case–control surveillance study FAKOS was initiated in 2000 9. For the here reported study of hepatotoxicity, it ascertained potential cases of IH in more than 180 Departments of Internal Medicine, Neurology, Psychiatry and Anaesthesiology of all 51 Berlin hospitals from October 2002 until December 2011. The physicians in these departments were contacted regularly at 2- to 3-week intervals to identify potential cases. Cases diagnosed between these intervals were actively reported to the study centre. Presentations on the study were organized in the hospitals to increase awareness of the study. Eligible patients were contacted by a trained staff member of FAKOS in order to obtain the patients' written informed consent. Furthermore, a standardized face-to-face interview was conducted, ascertaining information on all previous drug intakes, co-morbidities, demographic data and other possible risk factors such as chemicals or solvents. Interviews began with questions on demographic data and co-morbidities, in order to improve the memory capacity of the patients interviewed regarding drug intake. Drug intake was also ascertained by reviewing medical charts. Berlin, with 2.8 million inhabitants as an adult source population, comprised the study region.
Case definition
Patients with a minimum age of 18 years and a new diagnosis of IH within the last 6 months were included in the study. Inclusion laboratory criteria were an elevation of ALT or aspartate aminotransferase (AST) threefold above ULN or an elevation of total bilirubin higher than 2 mg dl–1. An elevation of ALP was not considered in the inclusion criteria, as ALP is not routinely assessed upon suspicion of hepatotoxicity in Germany and it may be related to non-hepatic pathologies (e.g. different bone tissue diseases or cholelithiasis).
Excluded were patients with underlying liver disease (including among others acute or chronic viral or other infectious hepatitis, alcoholic fatty liver disease, autoimmune hepatitis, liver tumours or hepatic metastases, extrahepatic bile duct obstruction, ischaemic hepatitis or congestive hepatopathy). In the case of isolated bilirubin elevation, primary hyperbilirubinaemias (e.g. Gilbert–Meulengracht syndrome) and obstetric cholestasis were additional exclusion criteria. As drug use in ambulatory care differs from that in hospital (e.g. by use of intravenously applied anti-infectives in hospital patients with serious infectious diseases), a distinction was made between patients who developed IH in hospital (‘inpatient cases’) and patients who developed IH in the outpatient setting (‘outpatient cases’) and were hospitalized due to the hepatitis. The index date was defined as the date of the elevation of liver enzymes as mentioned above or the onset of clinical signs of hepatitis, whichever occurred earlier.
Case validation and characterization
IH was validated as certain, probable, possible or unlikely based on information about laboratory, imaging or histological tests and clinical information including exclusion criteria provided on a standardized form by the treating physician to the study centre. The authors responsible for this task were Antonios Douros, Frank Andersohn, and Edeltraut Garbe. In the beginning of the study complex cases were discussed with a panel of Berlin hepatologists in order to resolve potential differences among reviewers and to clarify questions on case validation and drug causality assessment. Grading as hepatocellular, cholestatic or mixed type was based on the determination of R as stated above. In the few cases where the histological findings differed from the ones provided by calculating R, histology was decisive for the classification. When ALT or ALP was lacking and a biopsy had not been conducted, IH was graded as unclassifiable. In order to assess a possible involvement of the immune system as part of drug hypersensitivity, corresponding clinical symptoms (fever, rash, lymphadenopathy, arthralgia or myalgia) as well as laboratory findings (eosinophil counting) were documented. ALF was defined as severe coagulopathy (international normalized ratio > 1.5 or prothrombin time < 40%) and hepatic encephalopathy.
Control selection
Control selection took place from January 2002 until December 2011 at the same hospitals where cases were ascertained in order to evaluate further drug risks in a case–control approach. For the recruitment of controls, 80 Departments of Surgery and Orthopaedics were additionally included to the departments used for case and control ascertainment. Controls for ‘outpatient’ cases (‘outpatient controls’) were distinguished from those for inpatient cases (‘inpatient controls’). In ‘outpatient’ controls, the index date was defined as the date of hospitalization or the date of the diagnosis of the control disease if this preceded hospitalization, and drug use before the index date was ascertained. In inpatient controls, the index date was the date of the interview and in-hospital medication was documented. The aim was to raise overall number of controls by several fold compared with cases to increase study power. Thus, the control : case ratio was approximately 5, as higher ratios would only lead to a marginal further increase in power 11. An extensive list of possible control diseases was set up for the selection of controls to represent drug use in the population at large. Incident and not prevalent diseases were preferred to exclude control patients with chronic administration of specific drugs. Furthermore, a wide mixture of orthopaedic, neurological or internal control diseases was pursued. The prevalences of the different groups of diseases for inpatient and ‘outpatient’ controls are illustrated in Table S1 and Table S2, respectively. Informed consent from control patients was obtained using the same form as for cases. Drug intake in controls was obtained using the same face-to-face interview as for cases. Medical charts were also reviewed in controls. Patients with a diagnosis of any hepatic disease were excluded as controls.
Standardized assessment of drug causality in individual cases
A possible drug aetiology was assessed for each case by applying the updated Council for International Organizations of Medical Sciences (CIOMS) scale 12. There are two different CIOMS subscales, the first being for the hepatocellular type of liver injury and the second for the cholestatic or mixed type. The updated CIOMS scale takes into account (i) information on the time interval from the beginning of the drug or herb until the onset of symptoms or laboratory abnormalities, (ii) the time course of dechallenge, (iii) the existence of risk factors such as age ≥ 55 years or high alcohol use, (iv) concomitant medications, (v) the exclusion of alternative causes such as viral hepatitis, hepatobiliary diseases, alcoholism, ischaemia or complications of underlying diseases, (vi) previous information on hepatotoxicity of the drug or herb and (vii) a response to unintentional re-administration. According to this scale, drug causality can be ‘highly probable’ (score ≥ 9), ‘probable’ (score 6–8), ‘possible’ (score 3–5), ‘unlikely’ 1,2 or ‘excluded’ (score ≤ 0). In the case of unintentional re-administration of the drug, rechallenge was evaluated according to the criteria previously summarized by Teschke et al. 12. Rechallenge was assessed as positive if ALT (or ALP in the case of cholestatic or mixed liver injury) was below five times the ULN directly before re-exposure and at least doubled after re-exposure. Other variations were assessed as negative or uninterpretable. Suspected drugs were grouped according to the Anatomical Therapeutic Chemical Classification System 13.
Case–control analysis
The case–control analysis included all IH cases validated as certain or probable regardless of the results of drug causality assessment, as ascertainment of cases independent of exposure is a prerequisite in case–control studies 14. Separate analyses were performed to compare outpatient cases with outpatient controls and inpatient cases with inpatient controls with respect to outpatient or inpatient drug use, respectively. Cases and controls were considered as exposed to a drug, if the drug had been taken in the last 28 days before the index date. Odds ratio (OR) and 95% confidence interval (CI) of hepatitis associated with exposure to specific drugs were calculated with unconditional logistic regression analysis adjusting for age and gender (‘single drug assessment’) and for age, gender and all other drugs which were significant in the single drug assessment in the first analysis (‘joint drug assessment’). In the analyses considering inpatient drug intake, an additional adjustment for the type of clinical department was conducted.
Statistical analysis
All risk calculations were performed with SAS statistical software package (version 9.1, SAS Institute Inc, Cary, NC, USA). Comparisons between groups were conducted with Student's t-test for continuous data and chi-square test for categorical data. A two-tailed P value < 0.05 was considered significant. The Ethics Committee of the Charité-Universitätsmedizin Berlin approved the study.
Results
Overall, 514 patients with suspected possible IH were notified to the study centre by the participating hospitals during the study period. Of those, 287 patients were not included in the study for the reasons outlined in Figure 1, with the exclusion criterion most commonly met being alcoholic liver disease. In 29 cases IH was validated only as possible, so these cases were also not further considered in the analysis. In seven of them no relevant differential diagnoses had been excluded, and in five of them extrahepatic bile duct obstruction was a more probable aetiology than drug-induced hepatotoxicity. The final population for the analysis thus included 198 patients with certain or probable IH. Of those, 76 patients were inpatient cases and 122 patients were outpatient cases.
Figure 1.
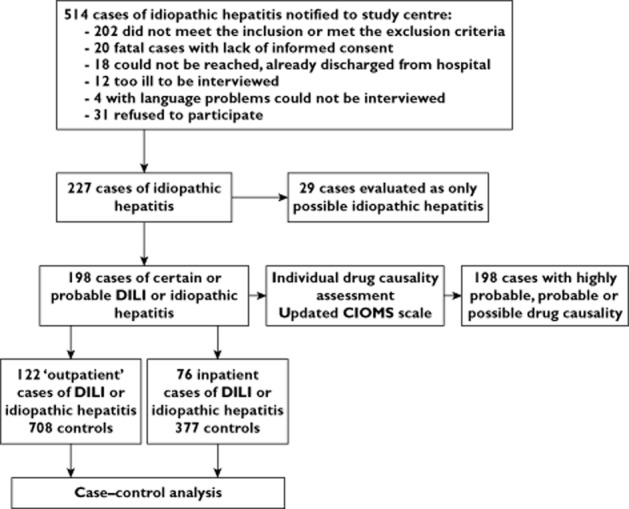
Study design. DILI, drug-induced liver injury; CIOMS, Council for International Organizations of Medical Sciences
Drug causality assessment in individual cases
In a total of 189 cases, the drug relationship according to the updated CIOMS scale was judged as at least ‘possible’, whereas in nine cases no drug relationship was observed or was judged as ‘unlikely’. A total of 177 different drugs or herbs were identified in these 189 patients as being related to liver injury. Eleven drugs were assessed as ‘highly probable’ and 74 drugs or herbs were assessed as ‘probable’, some of them more than once (Table 2). Table S4 exhibits the drug or herbs assessed as ‘possible’. Altogether, drugs of the nervous system (28%), anti-infectives (18%) and cardiovascular drugs (12%) were the groups represented the most. In nine patients there was an unintentional re-exposure to the suspected drug, which led in five of them to a positive rechallenge. The drugs showing a positive rechallenge were amiodarone, ramipril, acetylsalicylic acid, tamoxifen and mesalazine.
Table 2.
Drugs or herbs with highly probable or probable causal relationship in suspected drug-induced liver injury according to the updated Council for International Organizations of Medical Sciences (CIOMS) (12) scale categorized by main groups of the Anatomical Therapeutic Chemical (ATC) Classification System (13)
| ATC group | Highly probable | Probable |
|---|---|---|
| A/B | ||
| Omeprazole | 2 | |
| Esomeprazole | 1 | |
| Amoxicillin / Clarithromycin / Pantoprazole | 1 | |
| Mesalazine | 1 | |
| Repaglinide | 1 | |
| Phenprocoumon | 10 | |
| Acetylsalicylic acid | 1 | |
| C | ||
| Flecainide | 1 | |
| Amiodarone | 1 | 1 |
| Pentaerythrityltetranitrat | 1 | |
| Dihydralazine | 1 | |
| Hydrochlorothiazide | 1 | |
| Spironolactone | 1 | |
| Eplerenone | 1 | |
| Amlodipine | 1 | |
| Verapamil | 1 | |
| Lisinopril | 1 | |
| Ramipril | 1 | 2 |
| Irbesartan | 1 | |
| Olmesartan | 1 | |
| Telmisartan / HCT | 1 | |
| Simvastatin | 4 | |
| G/H | ||
| Dienogest / Ethinylestradiol | 1 | |
| Estradiol | 1 | |
| Methylprednisolone | 1 | |
| Thiamazole | 2 | |
| J | ||
| Doxycycline | 1 | |
| Sultamicillin | 1 | |
| Cefuroxime | 1 | |
| Ceftriaxone | 1 | |
| Imipenem / Cilastatine | 1 | |
| Sulfadiazine | 1 | |
| Clarithromycin | 2 | |
| Ciprofloxacin | 2 | |
| Norfloxacin | 1 | |
| Nitrofurantoin | 1 | |
| Fluconazole | 2 | |
| Rifampicin | 2 | |
| Isoniazid | 1 | |
| Isoniazid / Pyridoxine | 1 | |
| Pyrazinamide | 1 | 4 |
| Ethambutol | 1 | |
| L | ||
| Cyclophosphamide | 1 | |
| Erlotinib | 1 | |
| Sorafenib | 1 | |
| Pazopanib | 1 | |
| Tamoxifen | 1 | |
| Interferon β | 1 | |
| Anakinra | 1 | |
| Tocilizumab | 1 | |
| Azathioprine | 1 | 1 |
| Lenalidomide | 1 | |
| M | ||
| Diclofenac | 4 | |
| Ibuprofen | 4 | |
| Celecoxib | 1 | |
| Etoricoxib | 1 | |
| Tizanidine | 1 | |
| Tetrazepam | 1 | |
| Allopurinol | 1 | |
| Alendronic acid / Colecalciferol | 1 | |
| N | ||
| Tramadol | 1 | |
| Acetylsalicylic acid / Vitamin C | 1 | |
| Paracetamol (acetaminophen) | 3 | |
| Flupirtine | 1 | 5 |
| Carbamazepine | 2 | |
| Valproic acid | 1 | |
| Pregabalin | 2 | |
| Melperone | 1 | |
| Clozapine | 1 | |
| Olanzapine | 1 | |
| Amisulpride | 1 | |
| Risperidone | 1 | |
| Citalopram | 1 | |
| Escitalopram | 1 | |
| Mirtazapine | 1 | |
| Pyridostigmine | 1 | |
| Rest | ||
| Ayurveda | 1 | |
| Pelargonium sidoides | 1 | |
| Terbinafine | 1 | |
| Promethazine | 1 |
Drug or herb causality was assessed as ‘highly probable’ for CIOMS scores ≥ 9 and ‘probable’ for CIOMS scores 6–8.
Characteristics of cases
Table 3 illustrates clinical and laboratory characteristics of liver injury in outpatient and inpatient cases. The former group shows a predominance of female gender and of hepatocellular liver injury. Furthermore, outpatient cases had higher transaminase and bilirubin values and presented more often with symptoms like coagulopathy, fatigue, jaundice or acholic faeces/dark urine. In seven cases ALF was observed. Detailed information of these patients is given in Table S3. In nine cases there was a discrepancy regarding the pattern of liver injury between the one suggested by the R values and the one provided subsequently by liver biopsy. In all nine cases the classification of the patients was based on biopsy results. In eight of these cases the R-based pattern was hepatocellular in contrast to the mixed pattern revealed in the histological analysis. The mean time interval between bloodwork and liver biopsy in these eight cases was 7.5 days. Table S5 contains the respective data of the nine cases.
Table 3.
Selected demographic, clinical, and laboratory features of subjects with drug-induced liver injury
| Characteristics | ‘Outpatient’ cases | Inpatient cases | P value |
|---|---|---|---|
| n = 122 (%) | n = 76 (%) | ||
| Gender, n (%) | |||
| - Male | 44 (36) | 40 (53) | |
| - Female | 78 (64) | 36 (47) | 0.03 |
| Age (years) mean ± standard deviation | 55.6 ± 17.2 | 55.0 ± 17.5 | NS |
| Grading, n (%) | |||
| - hepatocellular | 84 (68.9) | 35 (46.1) | <0.01 |
| - cholestatic | 11 (9) | 15 (19.7) | 0.0497 |
| - mixed | 24 (19.7) | 15 (19.7) | >0.05 |
| - unclassifiable | 3 (2.5) | 11 (14.5) | <0.01 |
| Signs of hypersensitivity†, n (%) | 27 (22.1) | 13 (17.1) | NS |
| ALT/ULN, mean ± standard deviation | 31.1 ± 28.2 | 15.8 ± 20.5 | <0.001 |
| AST/ULN, mean ± standard deviation | 24.7 ± 30.9 | 13.6 ± 29.4 | 0.01 |
| ALP/ULN, mean ± standard deviation | 1.9 ± 1.4 | 2.6 ± 3.8 | NS |
| Bilirubin total/ULN, mean ± standard deviation | 7.4 ± 8.6 | 3.3 ± 5.1 | <0.001 |
| Hyperbilirubinaemia‡, n (%) | 86 (70.5) | 30 (39.5) | <0.001 |
| Coagulopathy§, n (%) | 39 (32) | 9 (11.8) | <0.01 |
| Serology testing | |||
| - Hepatitis A virus | 104 (85.2) | 55 (72.4) | |
| - Hepatitis B virus | 116 (95.1) | 63 (82.9) | |
| - Hepatitis C virus | 113 (92.6) | 59 (77.6) | |
| - Cytomegalovirus | 40 (32.8) | 10 (13.2) | |
| - Epstein–Barr virus | 43 (35.2) | 14 (18.4) | |
| - Hepatitis E virus | 17 (13.9) | 1 (1.3) | |
| - Herpes simplex virus | 17 (13.9) | 3 (3.9) | |
| - Varicella zoster virus | 12 (9.8) | 3 (3.9) | |
| Autoimmune antibodies testing | 88 (72.1) | 27 (35.5) | |
| Abdominal sonography | 121 (99.2) | 70 (92.1) | |
| Histology | 62 (50.8) | 13 (17.1) | |
| Fatigue, n (%) | 81 (66.4) | 38 (50) | 0.03 |
| Jaundice, n (%) | 54 (44.3) | 19 (25) | <0.01 |
| Acholic faeces/dark urine, n (%) | 41 (33.6) | 12 (15.8) | <0.01 |
| Abdominal pain, n (%) | 44 (36.1) | 19 (25) | NS |
| ALF¶, n (%) | 6 (4.9) | 1 (1.3) | NS |
| - ALF requiring liver transplantation, n (%) | 1 (0.8) | 0 (0) | |
| Hepatic encephalopathy, n (%) | 6 (4.9) | 3 (3.9) | NS |
| Death, n (%) | 4 (3.3) | 4 (5.3) | NS |
| - liver injury as certain cause of death, n (%) | 1 (0.8) | 1 (1.3) | |
| Drug causality assessment | |||
| - ≥ 1 medication assessed as highly probable (CIOMS > 8) | 7 (5.7) | 4 (5.3) | |
| - ≥ 1 medication assessed as probable (CIOMS 6–8) | 55 (45.1) | 25 (32.9) | |
| - ≥ 1 medication assessed as possible (CIOMS 3–5 | 66 (54.1) | 54 (71.1) | |
| Challenge (time to onset) | |||
| - From the beginning of the drug: 5 days – 90 days | 46 (40.0)** | 52 (70.3)* | |
| - From the beginning of the drug: < 5 days – > 90 days | 69 (60.0)** | 27 (36.5)* | |
| - From drug cessation: < 15 days (<30 days in cholestatic / mixed cases) | 16 (13.9)** | 16 (21.6)* | |
| Dechallenge | |||
| - Strongly positive dechallenge*** | 49 (42.6)** | 36 (48.6)* | |
| - Slightly positive dechallenge**** | 38 (33.0)** | 27 (36.5)* | |
| - No information / negative dechallenge***** | 28 (24.3)** | 11 (14.9)* | |
| Comedication ascertainment | |||
| - No / no information | 5 (4.1) | 1 (1.3) | |
| - Concomitant / incompatible time course | 56 (45.9) | 22 (28.9) | |
| - Concomitant / compatible time course | 10 (8.2) | 7 (9.2) | |
| - Concomitant / compatible time course / hepatotoxic | 60 (49.2) | 50 (65.8) |
Based on n = 74 due to two cases without drug causality and thus without challenge or dechallenge.
Based on n = 115 due to seven cases without drug causality and thus without challenge or dechallenge.
ALT decrease ≥ 50% within 8 days (cases of hepatocellular DILI) or ALP decrease ≥ 50% within 180 days (cases of cholestatic/mixed DILI).
ALT decrease ≥ 50% within 9–30 days (cases of hepatocellular DILI) or ALP decrease ≤ 50% within 180 days (cases of cholestatic/mixed DILI).
All other items from the updated CIOMS scale regarding dechallenge.
Fever, rash, lymphadenopathy, arthralgia, myalgia and/or eosinophilia.
Bilirubin > 18.8 μmol l−1.
International normalized ratio > 1.2 or prothrombin time < 70%. ¶Severe coagulopathy (international normalized ratio > 1.5 or prothrombin time < 40%) and hepatic encephalopathy. ALF, acute liver failure; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NS, non-significant; ULN, upper limit of normal.
Case–control analysis
For the case–control analyses we used the final population of 198 cases. One hundred and twenty-two outpatient cases were compared with 708 outpatient controls and 76 inpatient cases were compared with 377 inpatient controls. The median age of outpatient and inpatient controls was 56 and 55 years, respectively. Concerning gender, 49% of outpatient controls and 48% of inpatient controls were male. Risk estimates for drugs administered in outpatient and inpatient cases are shown in Table 4 and Table 5, respectively. The highest adjusted risk estimates were calculated for flupirtine (OR 40.2; 95% CI 5.5, 856.9), clozapine (OR 34.6; 95% CI 2.8, 824.9), clarithromycin (OR 30.2; 95% CI 1.2, > 999.9) and irbesartan (OR 25.0; 95% CI 1.6, 701.5).
Table 4.
Odds ratios (OR) of drug-induced liver injury for different drugs based on outpatient cases and controls (numbers in bold indicate significant results)
| ATC group | OR* (95% CI) | 122 cases n (%) | 708 controls n (%) |
|---|---|---|---|
| Alimentary tract and metabolism (A) | |||
| Pantoprazole | 1.04 (0.4, 2.3) | 18 (14.8) | 47 (6.6) |
| Metoclopramide | 2.4 (0.5, 9.1) | 6 (4.9) | 10 (1.4) |
| Magnesium | 0.3 (0.1, 0.9) | 4 (3.3) | 60 (8.5) |
| Blood and blood forming organs (B) | |||
| Phenprocoumon | 3.3 (1.5, 6.7) | 16 (13.1) | 39 (5.5) |
| Enoxaparin | 4.5 (0.5, 46.7) | 3 (2.5) | 2 (0.3) |
| Acetylsalicylic acid | 0.3 (0.1, 0.7) | 9 (7.4) | 133 (18.8) |
| Cardiovascular system (C) | |||
| Bisoprolol + hydrochlorothiazide | 13.1 (1.0, 149.3) | 4 (3.3) | 2 (0.3) |
| Lisinopril | 3.9 (0.8, 15.6) | 5 (4.1) | 7 (1.0) |
| Irbesartan | 25.0 (1.6, 701.5) | 3 (2.5) | 1 (0.1) |
| Systemic hormonal preparations (G/H) | |||
| Combined oral contraceptives | 1.1 (0.1, 5.2) | 11 (9.0) | 19 (2.7) |
| Oestrogen + levonogestrel | 2.8 (0.3, 36.0) | 5 (4.1) | 6 (0.8) |
| Oestrogen + dienogest | 13.2 (1.2, 218.6) | 4 (3.3) | 2 (0.3) |
| Glucocorticoids | 1.8 (0.7, 4.1) | 17 (13.9) | 46 (6.5) |
| Dexamethasone | 9.3 (0.8, 225.6) | 4 (3.3) | 1 (0.1) |
| Antiinfectives (J) | |||
| Antibiotics | 1.3 (0.2, 5.2) | 17 (13.9) | 26 (3.7) |
| Macrolides | 0.3 (0.01, 7.3) | 6 (4.9) | 3 (0.4) |
| Clarithromycin | 30.2 (1.2, >999.9) | 4 (3.3) | 1 (0.1) |
| Beta-lactam antibiotics | 2.4 (0.3, 20.9) | 9 (7.4) | 12 (1.7) |
| Fluoroquinolones | 21.0 (1.2, 634.9) | 5 (4.1) | 3 (0.4) |
| Ciprofloxacin | 0.3 (0.01, 10.4) | 3 (2.5) | 2 (0.3) |
| Rifampicin | 6.7 (0.1, 350.3) | 2 (1.6) | 1 (0.1) |
| Ethambutol + pyrazinamide | 14.1 (0.8, 392.0) | 3 (2.5) | 1 (0.1) |
| Aciclovir | 3.3 (0.2, 40.4) | 3 (2.5) | 2 (0.3) |
| Musculo-skeletal and nervous system (M/N) | |||
| Ibuprofen | 1.3 (0.5, 2.9) | 15 (12.3) | 43 (6.1) |
| Metamizole | 5.2 (2.0, 13.4) | 13 (10.7) | 14 (2.0) |
| Flupirtine | 40.2 (5.5, 856.9) | 8 (6.6) | 1 (0.1) |
| Valerian radix | 5.3 (0.98, 27.3) | 5 (4.1) | 4 (0.6) |
| Antidepressants | 0.9 (0.3, 2.5) | 15 (12.3) | 40 (5.6) |
| Amitriptyline | 0.9 (0.1, 9.0) | 4 (3.3) | 5 (0.7) |
| Citalopram | 7.1 (1.2, 44.1) | 6 (4.9) | 7 (1.0) |
| Various | |||
| Terbinafine | 20.1 (1.9, 440.4) | 2 (1.6) | 1 (0.1) |
| Cough and cold preparations | 1.8 (0.2, 20.4) | 4 (3.3) | 4 (0.6) |
| Mucolytics | 2.3 (0.4, 8.9) | 7 (5.7) | 15 (2.1) |
| Hypromellose | 8.9 (0.9, 92.7) | 3 (2.5) | 2 (0.3) |
Joint drug assessment (adjusted for age, gender and all drugs with a significant OR in single drug assessment, i.e. all drugs illustrated in Table 4).
Table 5.
Odds ratios (OR) of drug-induced liver injury for different drugs based on inpatient cases and controls (numbers in bold indicate significant results)
| ATC group | OR* (95% CI) | 76 cases n (%) | 377 controls n (%) |
|---|---|---|---|
| Alimentary tract and metabolism (A) | |||
| Pantoprazole | 1.6 (0.8, 3.2) | 33 (43.4) | 133 (35.3) |
| Metoclopramide | 1.1 (0.4, 2.9) | 16 (21.1) | 38 (10.1) |
| Dimetindene | 3.9 (0.6, 20.4) | 5 (6.6) | 6 (1.6) |
| Lactulose | 3.0 (0.9, 9.1) | 10 (13.2) | 17 (4.5) |
| Insulin | 0.7 (0.2, 2.5) | 11 (14.5) | 32 (8.5) |
| Multivitamins | 1.9 (0.2, 12.6) | 5 (6.6) | 7 (1.9) |
| Potassium | 0.8 (0.3, 2.0) | 22 (28.9) | 72 (19.1) |
| Blood and blood forming organs (B) | |||
| Heparin | 2.2 (0.8, 5.9) | 17 (22.4) | 38 (10.1) |
| Enoxaparin | 1.6 (0.7, 3.6) | 20 (26.3) | 67 (17.8) |
| Certoparin | 4.9 (0.8, 24.1) | 6 (7.9) | 6 (1.6) |
| Phytomenadione | 2.0 (0.4, 8.5) | 7 (9.2) | 11 (2.9) |
| Erythrocytes | 0.5 (0.1, 3.7) | 6 (7.9) | 10 (2.7) |
| Cardiovascular system (C) | |||
| Amiodarone | 5.5 (1.3, 21.2) | 7 (9.2) | 10 (2.7) |
| Norepinephrine | 0.3 (0.01, 8.4) | 3 (3.9) | 3 (0.8) |
| Dobutamine | 11.8 (0.5, 831.8) | 4 (5.3) | 1 (0.3) |
| Olmesartan | 7.0 (0.4, 88.4) | 3 (3.9) | 2 (0.5) |
| Antiinfectives (J) | |||
| Antibiotics | 0.6 (0.2, 1.6) | 30 (39.5) | 134 (35.5) |
| Macrolides | 1.4 (0.3, 6.3) | 9 (11.8) | 22 (5.8) |
| Fluoroquinolones | 1.8 (0.5, 6.3) | 14 (18.4) | 40 (10.6) |
| Moxifloxacin | 3.0 (0.2, 32.4) | 3 (3.9) | 3 (0.8) |
| Rifampicin | 6.7 (0.2, 337.0) | 4 (5.3) | 1 (0.3) |
| Ethambutol + pyrazinamide | 12.8 (0.6, 521.2) | 4 (5.3) | 1 (0.3) |
| Musculo-skeletal and nervous system (M/N) | |||
| Methocarbamol | 4.2 (0.1, 164.7) | 3 (3.9) | 1 (0.3) |
| Etomidate | 0.6 (0.1, 5.8) | 5 (6.6) | 7 (1.9) |
| Bupivacaine | 4.2 (0.2, 148.3) | 3 (3.9) | 1 (0.3) |
| Pethidine | 4.3 (0.6, 25.8) | 3 (3.9) | 5 (1.3) |
| Fentanyl | 0.6 (0.1, 2.8) | 8 (10.5) | 20 (5.3) |
| Metamizole | 1.0 (0.4, 2.2) | 26 (34.2) | 101 (26.8) |
| Paracetamol | 1.5 (0.6, 3.5) | 21 (27.6) | 52 (13.8) |
| Carbamazepine | 2.8 (0.4, 15.1) | 4 (5.3) | 5 (1.3) |
| Biperiden | 21.5 (1.03, 759.5) | 5 (6.6) | 1 (0.3) |
| Haloperidol | 1.7 (0.1, 17.5) | 6 (7.9) | 4 (1.1) |
| Clozapine | 34.6 (2.8, 824.9) | 5 (6.6) | 1 (0.3) |
| Olanzapine | 18.6 (2.8, 68.4) | 6 (7.9) | 2 (0.5) |
| Diazepam | 1.5 (0.4, 5.5) | 14 (18.4) | 14 (3.7) |
| Lorazepam | 2.2 (0.8, 6.3) | 16 (21.1) | 26 (6.9) |
| Midazolam | 2.0 (0.5, 7.0) | 14 (18.4) | 23 (6.1) |
| Zolpidem | 2.4 (0.7, 7.7) | 9 (11.8) | 15 (4.0) |
| Hypericum perforatum | 2.9 (0.2, 35.3) | 3 (3.9) | 2 (0.5) |
| Various | |||
| Cough and cold preparations | 0.5 (0.04, 5.7) | 5 (6.6) | 8 (2.1) |
| Mucolytics | 3.0 (0.5, 13.0) | 8 (10.5) | 18 (4.8) |
| Hydrocortisone | 5.4 (0.3, 80.9) | 3 (3.9) | 2 (0.5) |
Joint drug assessment (adjusted for age, gender, type of clinical department and all drugs with a significant OR in single drug assessment, i.e. all drugs illustrated in Table 5).
Discussion
DILI is responsible for nearly half of ALF cases in the US and often leads to drug withdrawal or drug non-approval 2,4. Due to the idiosyncratic, non-dose-related mode of action that most of the causative medications have, more studies are needed in order to quantify the hepatotoxic risk of different drugs.
Based on the case–control analyses or the individual causality assessment, our results corroborate risks for a number of drugs, such as phenprocoumon 15, amiodarone 16, terbinafine 17, clarithromycin 18, antituberculotic agents 19, fluorquinolones 20 or oral contraceptives 21. They also indicate a hepatotoxic risk for less commonly reported substances, such as mesalazine 22, ramipril 23, angiotensin II receptor blockers 24 or cefuroxime 25, and for compounds considered possessing a low hepatotoxic potential such as citalopram 26. Concerning the respective hazard of atypical antipsychotics, our results suggest increased risks for agents described as potentially hepatotoxic in the medical literature such as clozapine, olanzapine and risperidone 27–29, but also for amisulpride, a drug so far associated only with asymptomatic elevation of liver enzymes 30.
To the best of our knowledge, this study is the first to quantify the hepatotoxic risk of the non-opioid analgesic flupirtine. Recently, the European Medicines Agency recommended specific risk minimization measures regarding its use after reviewing spontaneous reports on flupirtine-associated hepatotoxicity 31. Our cases with flupirtine-induced liver injury have been demonstrated in detail elsewhere 32.
Interestingly, the muscarinic receptor antagonist and antiparkinson drug biperiden, which was so far never reported to be hepatotoxic, showed an increased risk. In the English medical literature, there is only one case report of hepatotoxicity induced by an antimuscarinic agent, i.e. the overactive bladder medication tolterodine 33. However, muscarinic activation has been shown to attenuate liver injury in mice 34.
Paracetamol (acetaminophen), one of the few drugs associated with dose-dependent hepatotoxicity 5, failed to reach a significant risk estimate in the case–control analysis, and was validated as ‘probable’ in only three cases in the individual causality assessment. These findings contradict data derived from the US or the UK showing that paracetamol is the commonest cause of ALF accounting for up to 50% of the respective cases 35,36. On the other hand, a recent German study on ALF found that paracetamol was responsible for only 9% of the cases 37. A lower incidence in Germany could be the result of restrictive measures taken by the authorities, as packs containing more than 10 g are available only on prescription, and paracetamol is only available in pharmacies 38.
Herbs or dietary supplements accounted for 3.5% of all substances assessed as at least ‘possible’ in the causality assessment. Moreover, valerian radix showed an increased OR in the case–control analysis, although it marginally missed statistical significance. This heterogenic group of substances has attracted increased attention in the past years, as the incidence of herb-induced liver injury seems to increase 39. The fact that FAKOS cases were partially assessed during the first half of the last decade could account for the lower frequency of herb-induced hepatotoxicity in our data compared with recent publications 39.
Our results show that drugs of the nervous system were the ones most commonly associated with hepatotoxicity, followed by anti-infectives. The antimicrobial combination of amoxicillin and clavulanic acid (AC) was not involved in any case with drug-induced aetiology. This is not in line with previous studies on hepatotoxicity which have shown a predominance of anti-infectives and of AC in particular 40–43. Taking into consideration that AC-induced liver injury is often cholestatic with a predominant increase of ALP, we cannot exclude an underascertainment of such cases, as our inclusion criteria did not include ALP values.
Almost one fifth of our patients with drug-induced disease (19%) presented clinical or laboratory signs of drug hypersensitivity, with these results being in agreement with previously published data 40. Regarding disease symptoms, our data revealed more severe courses in the outpatient setting as compared with the inpatient setting. This could be a result of the different settings, with inpatients getting daily examined by physicians and liver enzymes being regularly controlled, thereby reducing the chances of an advanced hepatic damage.
All seven patients who developed ALF were female and showed a hepatocellular pattern of liver injury (Table S3), supporting previous findings suggesting female gender and hepatocellular pattern among risk factors associated with severe drug-induced hepatic damage 40. One ALF patient presented without jaundice, underlining the possibility of anicteric severe hepatic damage 44,45. Finally, six ALF patients showed improvement after drug cessation with two of them achieving complete recovery, although idiosyncratic drug-induced ALF usually has a poor prognosis 2. However, the low number of respective cases in our study makes further analysis of these data challenging.
Some limitations and strengths of our study should be mentioned. Due to the naturalistic setting of this study, with investigators having no influence on the collection of laboratory data, only a fraction of the patients were tested for hepatitis E virus or for the hepatotropic viruses cytomegalovirus and Epstein-Barr virus, respectively (Table 3; no tests revealed signs of acute infection). The fact that reports of alleged hepatotoxicity may sometimes conceal cases of infectious or other non-drug-induced hepatitis has been previously discussed 46,47. Furthermore, as case validation was performed not blinded to drug exposure, selection bias cannot be excluded. Precision of the risk estimate for some drugs is affected by the low number of cases, this being indicated by the wide CI. Therefore, such results should be interpreted cautiously. Finally, follow-up data were not systematically ascertained, therefore not allowing an evaluation of the long term prognosis of drug-induced hepatotoxicity and the development of chronic hepatopathy.
To our knowledge, FAKOS represents the second 7 case–control study on hepatotoxicity which did not restrict its focus to a specific set of drugs previously reported as hepatotoxic in the literature. This allowed risk quantification also for medications which have not or very rarely been reported as hepatotoxic in the past. Through the surveillance of a large number of mostly general hospitals with various medical departments, a wide range of drugs could be assessed. For the causality assessment we used the updated CIOMS scale 12. CIOMS was specifically developed for DILI and was evaluated based on cases with positive rechallenge, the gold standard for validating drug causality assessment methods, thereby showing high sensitivity, specificity and predictive validity 12,48. It can be also used by non-experts and exhibits a clear improvement over scores that are not liver specific and do not consider hepatotoxicity characteristics 40,49–56. By including IH patients we also considered cases independent of exposure, which is a prerequisite in case–control studies in order to avoid selection bias 14. Furthermore, although no individual matching was conducted during selection of controls, due to frequency matching and adjustment for age and gender in the case–control analysis we did not expect any significant confounding resulting from these variables. Excessive drinking as defined in the updated CIOMS scale 12 did not statistically differ between cases and controls (data not shown), and the respective cases were only included after eliminating alcohol-related hepatotoxicity via bloodwork, imaging or histological analysis. However, selection bias cannot be completely excluded as recruitment of controls was conducted in more departments than recruitment of cases 57.
In summary, our study identified a large number of drugs as possible causes of DILI. Through case–control analysis we found an increased risk not only for medications previously reported as hepatotoxic but also for medications seldom associated with liver injury highlighting the need for further post-authorization safety studies on this matter. In all patients with newly developed jaundice or abnormal laboratory results of liver tests a drug aetiology must be included as a differential diagnosis and a precise medication history has to be taken.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that this study was funded fully by the Federal Institute for Drugs and Medical Devices in Germany (BfArM), grant number: V-5238/68605/2012. The BfArM played no role in the study design, collection, analysis and interpretation of the data and or the writing of the report.
AD, EB, AK, MT, GS had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. FA acted as a consultant for Abbott, Astra Zeneca, and Bayer Health Care. RK has consultant/advisory arrangements or received honoraria for lectures from Bayer Health Care, Berlin-Chemie, Bristol-Myers Squibb, Daiichi Sankyo, Merck, Recordati Pharma, Servier and Trommsdorff. EG had consultant arrangements with Bayer AG, Novartis, GSK, Teva, Nycomed, Takeda and Schwabe and has received grant/research support from Bayer AG, Novartis, Celgene, Sanofi-Aventis, Sanofi-Pasteur, Mundipharma, GSK, Takeda and Stada.
Supporting Information
Table S1
Classification of the inpatient control diseases (n = 377) based on the International Statistical Classification of Diseases and Related Health Problems (ICD 10) with prevalences and most frequent diagnoses of each group
Table S2
Classification of the ‘outpatient’ control diseases (n = 708) based on the International Statistical Classification of Diseases and Related Health Problems (ICD 10) with prevalences and most frequent diagnoses of each group
Table S3
Characteristics of the seven cases with drug-induced acute liver failure
Table S4
Drugs or herbs with possible causal relationship in suspected drug-induced liver injury according to the updated Council for International Organizations of Medical Sciences (CIOMS) scale (1) categorized by main groups of the Anatomical Therapeutic Chemical (ATC) Classification System (2)
Table S5
Data on the nine cases with discrepancies between the pattern of liver injury suggested by the R values and the one revealed after histological testing
References
- Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134–144. doi: 10.1055/s-0034-1375955. [DOI] [PubMed] [Google Scholar]
- Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou C. Criteria of drug-induced liver disorders – report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6:673–684. doi: 10.1517/14740338.6.6.673. [DOI] [PubMed] [Google Scholar]
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- de Abajo FJ, Montero D, Madurga M, Garcia Rodriguez LA. Acute and clinically relevant drug-induced liver injury: a population based case–control study. Br J Clin Pharmacol. 2004;58:71–80. doi: 10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Wallander MA, Stricker BH. The risk of acute liver injury associated with cimetidine and other acid-suppressing anti-ulcer drugs. Br J Clin Pharmacol. 1997;43:183–188. doi: 10.1046/j.1365-2125.1997.05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe E, Andersohn F, Bronder E, Klimpel A, Thomae M, Schrezenmeier H, Hildebrandt M, Spath-Schwalbe E, Gruneisen A, Mayer B, Salama A, Kurtal H. Drug induced immune haemolytic anaemia in the Berlin Case-Control Surveillance Study. Br J Haematol. 2011;154:644–653. doi: 10.1111/j.1365-2141.2011.08784.x. [DOI] [PubMed] [Google Scholar]
- Garbe E, Andersohn F, Bronder E, Salama A, Klimpel A, Thomae M, Schrezenmeier H, Hildebrandt M, Spath-Schwalbe E, Gruneisen A, Meyer O, Kurtal H. Drug-induced immune thrombocytopaenia: results from the Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2012;68:821–832. doi: 10.1007/s00228-011-1184-3. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet. 2005;365:1429–1433. doi: 10.1016/S0140-6736(05)66379-9. [DOI] [PubMed] [Google Scholar]
- Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6:17–32. doi: 10.4254/wjh.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD) Available at http://www.who.int/classifications/atcddd/en (last accessed 1 January 2003). 24 February 2012.
- Dicker RC. Designing studies in the field. In: Gregg MB, editor. Field Epidemiology. 2nd edn. New York: Oxford University Press, Inc; 2002. p. 122. [Google Scholar]
- Schimanski CC, Burg J, Mohler M, Hohler T, Kanzler S, Otto G, Galle PR, Lohse AW. Phenprocoumon-induced liver disease ranges from mild acute hepatitis to (sub-) acute liver failure. J Hepatol. 2004;41:67–74. doi: 10.1016/j.jhep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Lewis JH, Ranard RC, Caruso A, Jackson LK, Mullick F, Ishak KG, Seeff LB, Zimmerman HJ. Amiodarone hepatotoxicity – prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9:679–685. doi: 10.1002/hep.1840090504. [DOI] [PubMed] [Google Scholar]
- Kao WY, Su CW, Huang YS, Chou YC, Chen YC, Chung WH, Hou MC, Lin HC, Lee FY, Wu JC. Risk of oral antifungal agent-induced liver injury in Taiwanese. Br J Clin Pharmacol. 2014;77:180–189. doi: 10.1111/bcp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz A, Heim MH, Eriksson U, Marsch S, Terracciano L, Krahenbuhl S. Fulminant liver failure associated with clarithromycin. Ann Pharmacother. 2003;37:57–60. doi: 10.1345/aph.1C171. [DOI] [PubMed] [Google Scholar]
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WCM, van der Ven AJAM, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9:517–523. doi: 10.1016/j.cgh.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AJ, Simon FR. Estrogen-induced cholestasis: clues to pathogenesis and treatment. Hepatology. 1983;3:607–613. doi: 10.1002/hep.1840030422. [DOI] [PubMed] [Google Scholar]
- Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010;28:508–518. doi: 10.1159/000320410. [DOI] [PubMed] [Google Scholar]
- Yeung E, Wong FS, Wanless IR, Shiota K, Guindi M, Joshi S, Gardiner G. Ramipril-associated hepatotoxicity. Arch Pathol Lab Med. 2003;127:1493–1497. doi: 10.5858/2003-127-1493-RH. [DOI] [PubMed] [Google Scholar]
- Andrade RJ, Lucena MI, Fernandez MC, Vega JL, Garcia-Cortes M, Casado M, Guerrero-Sanchez E, Pulido-Fernandez F. Cholestatic hepatitis related to use of irbesartan: a case report and a literature review of angiotensin II antagonist-associated hepatotoxicity. Eur J Gastroenterol Hepatol. 2002;14:887–890. doi: 10.1097/00042737-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Koklu S, Yuksel O, Yolcu OF, Arhan M, Altiparmak E. Cholestatic attack due to ampicillin and cross-reactivity to cefuroxime. Ann Pharmacother. 2004;38:1539–1540. doi: 10.1345/aph.1E025. [DOI] [PubMed] [Google Scholar]
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–415. doi: 10.1176/appi.ajp.2013.13050709. [DOI] [PubMed] [Google Scholar]
- Brown CA, Telio S, Warnock CA, Wong AH. Clozapine toxicity and hepatitis. J Clin Psychopharmacol. 2013;33:570–571. doi: 10.1097/JCP.0b013e3182946586. [DOI] [PubMed] [Google Scholar]
- Ozcanli T, Erdogan A, Ozdemir S, Onen B, Ozmen M, Doksat K, Sonsuz A. Severe liver enzyme elevations after three years of olanzapine treatment: a case report and review of olanzapine associated hepatotoxicity. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1163–1166. doi: 10.1016/j.pnpbp.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Paulzen M, Orfanos S, Grunder G. Remission of drug-induced hepatitis after switching from risperidone to paliperidone. Am J Psychiatry. 2010;167:351–352. doi: 10.1176/appi.ajp.2009.09081243. [DOI] [PubMed] [Google Scholar]
- Mouradian-Stamatiadis L, Dumortier G, Januel D, Delmas BA, Cabaret W. Liver function tests during treatment with antipsychotic drugs: a case series of 23 patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1409–1411. doi: 10.1016/s0278-5846(02)00263-4. [DOI] [PubMed] [Google Scholar]
- PRAC recommends restricting the use of flupirtine-containing medicines. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Flupirtine-containing_medicines/human_referral_prac_000019.jsp&mid=WC0b01ac05805c516f (last accessed 14 June 2013). 18 June 2013.
- Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Orzechowski HD, Kreutz R, Garbe E. Flupirtine-induced liver injury-Seven cases from the Berlin Case-Control Surveillance Study and review of the German spontaneous adverse drug reaction reporting database. Eur J Clin Pharmacol. 2014;70:453–459. doi: 10.1007/s00228-013-1631-4. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Keller MJ, Krahenbuhl S. Tolterodine-associated acute mixed liver injury. Ann Pharmacother. 2002;36:817–819. doi: 10.1345/aph.1A418. [DOI] [PubMed] [Google Scholar]
- Khurana S, Jadeja R, Twaddell W, Cheng K, Rachakonda V, Saxena N, Raufman JP. Effects of modulating M3 muscarinic receptor activity on azoxymethane-induced liver injury in mice. Biochem Pharmacol. 2013;86:329–338. doi: 10.1016/j.bcp.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig DG, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 2012;73:285–294. doi: 10.1111/j.1365-2125.2011.04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Hadem J, Tacke F, Bruns T, Langgartner J, Strnad P, Denk GU, Fikatas P, Manns MP, Hofmann WP, Gerken G, Grunhage F, Umgelter A, Trautwein C, Canbay A. Etiologies and outcomes of acute liver failure in Germany. Clin Gastroenterol Hepatol. 2012;10:664–669. doi: 10.1016/j.cgh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Protocol of the 60th Meeting of the Expert Advisory Committee for Prescription-Only Issues. Available at http://www.bfarm.de/DE/Pharmakovigilanz/Verschreibungspflicht/Protokolle/60Sitzung/top6.html?nn=1011772 (last accessed 15 January 2008). 9 May 2012.
- Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, Gonzalez-Grande R, Pizarro A, Duran JA, Jimenez M, Rodrigo L, Romero-Gomez M, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmeron J, Martin-Vivaldi R. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valle MB, Av KV, Alem N, Olsson R, Bjornsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–1195. doi: 10.1111/j.1365-2036.2006.03117.x. [DOI] [PubMed] [Google Scholar]
- Al-Homaidhi H, Abdel-Haq NM, El-Baba M, Asmar BI. Severe hepatitis associated with oxacillin therapy. South Med J. 2002;95:650–652. [PubMed] [Google Scholar]
- Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aithal GP, Rawlins MD, Day CP. Accuracy of hepatic adverse drug reaction reporting in one English health region. BMJ. 1999;319:1541. doi: 10.1136/bmj.319.7224.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J. Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol. 2014;13:248–255. [PubMed] [Google Scholar]
- Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs – II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- Garcia-Cortes M, Stephens C, Lucena MI, Fernandez-Castaner A, Andrade RJ. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol. 2011;55:683–691. doi: 10.1016/j.jhep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Schulze J, Glass X. Statin hepatotoxicity and the dilemma of causality in rare hepatic adverse drug reactions. J Hepatol. 2012;57:702–703. doi: 10.1016/j.jhep.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Stammschulte T, Gundert-Remy U. Spontaneous reports of primarily suspected herbal hepatotoxicity by Pelargonium sidoides: was causality adequately ascertained? Regul Toxicol Pharmacol. 2012;64:342–344. doi: 10.1016/j.yrtph.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Teschke R, Frenzel C, Schulze J, Eickhoff A. Suspected herbal hepatotoxicity: the pharmacovigilance dilemma with disputed and obsolete evaluation methods. Regul Toxicol Pharmacol. 2012;64:343–344. [Google Scholar]
- Teschke R, Frenzel C, Wolff A, Herzog J, Glass X, Schulze J, Eickhoff A. Initially purported hepatotoxicity by Pelargonium sidoides: the dilemma of pharmacovigilance and proposals for improvements. Ann Hepatol. 2012;11:500–512. [PubMed] [Google Scholar]
- Teschke R, Eickhoff A, Schulze J. Drug- and herb-induced liver injury in clinical and translational hepatology: causality assessment methods, quo vadis? J Clin Translat Hepatol. 2013;1:59–74. doi: 10.14218/JCTH.2013.D002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R, Frenzel C, Schulze J, Eickhoff A. Spontaneous reports of primarily suspected herbal hepatotoxicity by Pelargonium sidoides: was causality adequately ascertained? Regul Toxicol Pharmacol. 2012;63:1–9. doi: 10.1016/j.yrtph.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. Am J Epidemiol. 1992;135:1019–1028. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Classification of the inpatient control diseases (n = 377) based on the International Statistical Classification of Diseases and Related Health Problems (ICD 10) with prevalences and most frequent diagnoses of each group
Table S2
Classification of the ‘outpatient’ control diseases (n = 708) based on the International Statistical Classification of Diseases and Related Health Problems (ICD 10) with prevalences and most frequent diagnoses of each group
Table S3
Characteristics of the seven cases with drug-induced acute liver failure
Table S4
Drugs or herbs with possible causal relationship in suspected drug-induced liver injury according to the updated Council for International Organizations of Medical Sciences (CIOMS) scale (1) categorized by main groups of the Anatomical Therapeutic Chemical (ATC) Classification System (2)
Table S5
Data on the nine cases with discrepancies between the pattern of liver injury suggested by the R values and the one revealed after histological testing


