Supplemental Digital Content is available in the text.
Keywords: high-sensitivity, kidney, myocardial infarction, renal insufficiency, troponin
Abstract
Background—
It is unknown whether more sensitive cardiac troponin (cTn) assays maintain their clinical utility in patients with renal dysfunction. Moreover, their optimal cutoff levels in this vulnerable patient population have not previously been defined.
Methods and Results—
In this multicenter study, we examined the clinical utility of 7 more sensitive cTn assays (3 sensitive and 4 high-sensitivity cTn assays) in patients presenting with symptoms suggestive of acute myocardial infarction. Among 2813 unselected patients, 447 (16%) had renal dysfunction (defined as Modification of Diet in Renal Disease–estimated glomerular filtration rate <60 mL·min−1·1.73 m−2). The final diagnosis was centrally adjudicated by 2 independent cardiologists using all available information, including coronary angiography and serial levels of high-sensitivity cTnT. Acute myocardial infarction was the final diagnosis in 36% of all patients with renal dysfunction. Among patients with renal dysfunction and elevated baseline cTn levels (≥99th percentile), acute myocardial infarction was the most common diagnosis for all assays (range, 45%–80%). In patients with renal dysfunction, diagnostic accuracy at presentation, quantified by the area under the receiver-operator characteristic curve, was 0.87 to 0.89 with no significant differences between the 7 more sensitive cTn assays and further increased to 0.91 to 0.95 at 3 hours. Overall, the area under the receiver-operator characteristic curve in patients with renal dysfunction was only slightly lower than in patients with normal renal function. The optimal receiver-operator characteristic curve–derived cTn cutoff levels in patients with renal dysfunction were significantly higher compared with those in patients with normal renal function (factor, 1.9–3.4).
Conclusions—
More sensitive cTn assays maintain high diagnostic accuracy in patients with renal dysfunction. To ensure the best possible clinical use, assay-specific optimal cutoff levels, which are higher in patients with renal dysfunction, should be considered.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00470587.
Acute myocardial infarction (AMI) is a major cause of death and disability in Europe and the United States.1 Its rapid and accurate diagnosis is critical for effective evidence-based medical management and treatment2–4 but is still an unmet clinical need. Delays in diagnosing disease (“rule in”) hold back prompt use of evidence-based therapies.5,6 Delays in excluding AMI (“rule out”) interfere with evaluation of alternative diagnoses and contribute to medical errors and costs associated with crowding in the emergency department (ED).7–9
Editorial see p 2029
Clinical Perspective on p 2050
For several reasons, patients with renal dysfunction merit particular attention. First, the incidence of AMI is increased in this vulnerable subgroup.10,11 Second, atypical clinical presentation of AMI may be more frequent.12,13 Third, left ventricular hypertrophy is common and often results in ECG changes that may mimic or obscure AMI. Fourth, patients with renal dysfunction are more prone to adverse events related to cardiovascular medication, for example, anticoagulation, as well as to cardiovascular procedures, including coronary angiography and coronary intervention.1,2
More sensitive cardiac troponin (cTn) assays with a limit of detection below the 99th percentile of a healthy reference population and improved precision have recently become available in clinical practice.14–16 While sensitive (s) assays allow the detection of cTn in 20% to 50% of healthy individuals, high-sensitivity (hs) assays allow the detection of cTn in even 50% to 90% of healthy individuals.17 These assays improved the early diagnosis of AMI in unselected patients with suspected AMI.18,19 However, their clinical utility in patients with renal dysfunction has recently been questioned.20–22 For example, elevated cTn levels above the 99th percentile were observed in up to 40% of patients with renal dysfunction and diagnoses other than AMI, potentially reducing the specificity for AMI.20–22 Although the 99th percentile is the undisputed reference value to diagnose AMI according to the universal definition of AMI, optimal clinical decision levels or cutoff levels at presentation to the ED may well differ from the 99th percentile.4
We therefore aimed to examine the diagnostic performance and to identify the optimal cutoff levels of 7 more sensitive cTn assays for the early diagnosis of AMI in patients with renal dysfunction.
Methods
Study Design and Population
The Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) is an ongoing prospective, international, multicenter study designed and coordinated by the University Hospital Basel (Basel, Switzerland).19,23,24 From April 2006 to June 2013, 3030 consecutive patients >18 years of age presenting to the ED with symptoms suggestive of AMI with an onset or peak within the last 12 hours were recruited after providing written informed consent. Although enrollment was completely independent of renal function, allowing the inclusion of a large number of patients with various degrees of renal dysfunction, patients with terminal kidney failure requiring regular long-term dialysis were excluded. For this analysis, patients were also excluded if no creatinine value at presentation to the ED was available (n=18), if none of the 7 investigational cTn assays were available at baseline (n=107), or if the final diagnosis remained unclear after adjudication (n=92; for details, see the online-only Data Supplement). Because some patients had missing data for some of the 7 investigational cTn assays, 7 assay-specific subcohorts with a large overlap but numerically not identical sizes were derived from the main cohort.
Renal function was quantified by estimating glomerular filtration rate (eGFR) with the use of the abbreviated Modification of Diet in Renal Disease Study equation based on plasma creatinine level, age, and sex, as described in detail elsewhere.25–27 For this analysis, renal dysfunction was defined as an eGFR of <60 mL·min−1·1.73 m−2.26 All creatinine measurements were performed on a Roche Modular P1 analyzer with the enzymatic Creatinine-PAP method for quantification (Roche Diagnostics, Switzerland). Serum creatinine can be converted from micromoles per liter to milligrams per deciliter by dividing by 88.4.
The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. The authors designed the study, gathered and analyzed the data, vouch for the data and analysis, wrote the paper, and made the decision to submit it for publication. The assays were donated by the manufacturers, which had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit for publication.
Routine Clinical Assessment
All patients underwent a clinical assessment that included medical history, physical examination, 12-lead ECG, continuous ECG monitoring, pulse oximetry, standard blood test, and chest radiography. Levels of cTn were measured at presentation and serially thereafter as long as clinically indicated. Timing and treatment of patients were left to discretion of the attending physician.
Adjudicated Final Diagnosis
Adjudication of the final diagnosis was performed centrally in a core laboratory (University Hospital Basel) and included levels of Roche hs-cTnT to take advantage of the higher sensitivity and higher overall diagnostic accuracy offered by hs-cTn assays (which allows the additional detection of small AMIs that were missed by the adjudication based on conventional cTn assays).20,21 Two independent cardiologists reviewed all available medical records—patient history, physical examination, results of laboratory testing (including hs-cTnT levels), radiological testing, ECG, echocardiography, cardiac exercise stress test, lesion severity, and morphology in coronary angiography—pertaining to the patient from the time of ED presentation to the 90-day follow up. Specifically, the patients’ description of pain (typical, atypical, nonspecific), time since onset and peak of symptoms, and new ECG findings were taken into account for the adjudication of the final diagnosis. Furthermore, in patients with renal dysfunction, cTn levels of prior admissions were considered to assess whether the cTn levels were elevated previously. If the patient was taken to the catheterization laboratory, the presence of an acute occlusion, an acute culprit lesion with less than Thrombolysis in Myocardial Infarction grade 3 flow, and new wall motion abnormalities were considered evidence of AMI if observed in combination with an acute rise or fall in hs-cTnT. In situations of disagreement about the diagnosis, cases were reviewed and adjudicated in conjunction with a third cardiologist.
AMI was defined and cTn levels were interpreted as recommended in current guidelines.1,2,28 In brief, AMI was diagnosed when there was evidence of myocardial necrosis in association with a clinical setting consistent with myocardial ischemia. Myocardial necrosis was diagnosed by at least 1 cTn value above the 99th percentile of healthy individuals, together with a significant rise or fall.14,28,29 The criteria used to define rise or fall are described in detail in the Methods section in the online-only Data Supplement.
Investigational cTn Analysis
Details on the 7 cTn assays used in this analysis are given in the Methods section in the online-only Data Supplement. All 7 more sensitive cTn assays were centrally measured in a core laboratory. As for all cTn assays, the 7 more sensitive cTn assays are not biologically equivalent.
Follow-Up and Clinical End Points
After hospital discharge, patients were contacted after 3, 12, and 24 months by telephone calls or in written form. Information on death was furthermore obtained from the national registry on mortality, the diagnosis registry of the hospitals, and the family physicians’ records. The primary prognostic end point was survival within 2 years.
Statistical Analysis
Details on statistical analysis can be found in the online-only Data Supplement.
Results
Patient Characteristics
Among the 2813 unselected patients in the total cohort, 447 (16%) had renal dysfunction (Table 1). Among the 7 assay-specific subcohorts, baseline characteristics and final diagnoses were comparable (Table I in the online-only Data Supplement). Patients with renal dysfunction differed from patients with normal renal function in multiple baseline characteristics, including higher prevalence of cardiovascular risk factors, previous myocardial infarction, stroke, and ECG abnormalities. In patients with renal dysfunction, the total rate of additional cardiac testing related to AMI diagnosis (in addition to detailed history, ECG, cTn, chest x-ray), including coronary angiography or cardiac stress testing with or without imaging, was similar to that of patients with normal renal function (52% in both groups; P=NS). Coronary angiography was performed more frequently in patients with renal dysfunction (33%) compared with patients with normal renal function (25%; P<0.001).
Table 1.
Baseline Patient Characteristics
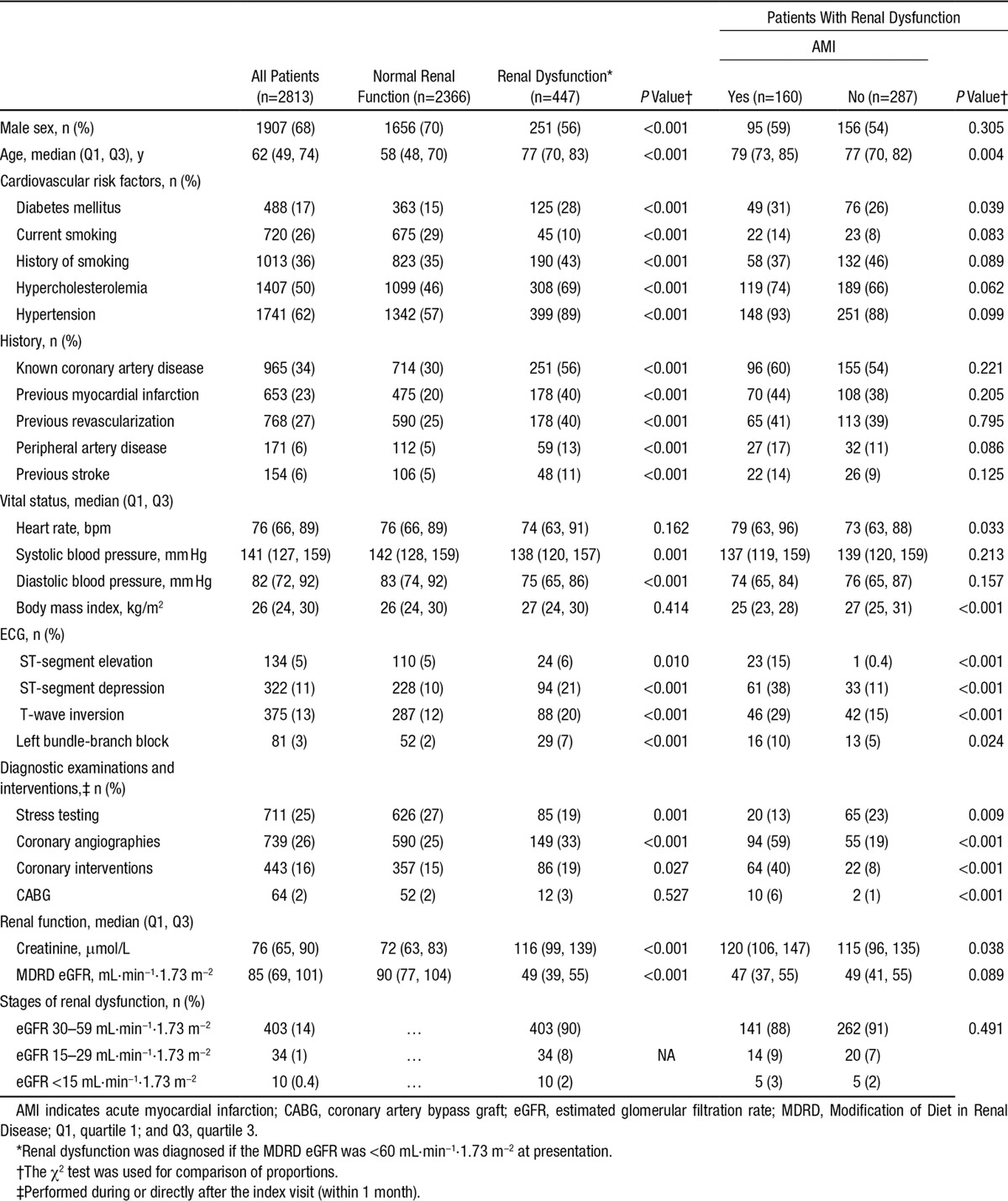
AMI was the adjudicated final diagnosis in 36% of patients with renal dysfunction compared with 18% in patients with normal renal function (P<0.001). Both type I AMI and type II AMI were more frequent in patients with renal dysfunction. Among patients with non–ST-segment–elevation myocardial infarction, type II AMI was seen in 23% of patients with renal dysfunction compared with 10% in patients with normal renal function (P<0.001; Table II in the online-only Data Supplement). Disagreement between the 2 independent cardiologists adjudicating the final diagnosis was more common in patients with renal dysfunction compared with patients with normal renal function (8.7% versus 5.9%; P=0.023) and tended to be more common in patients presenting with elevated levels of hs-cTnT compared with patients presenting with normal levels of hs-cTnT (7.4% versus 5.7%; P=0.063).
cTn Levels at Presentation
In patients with renal dysfunction and in patients with normal renal function, cTn levels at presentation, as assessed by all 7 more sensitive cTn assays, were significantly higher in patients whose final diagnosis was AMI compared with those with other diagnoses (P<0.001 for comparisons). Among the patients whose final diagnosis was not AMI, patients with renal dysfunction had significantly higher baseline levels of all 7 more sensitive cTn assays compared with patients with normal renal function (P<0.001 for all comparisons with patients with normal renal function). Overall, 12% of patients with renal dysfunction and a final diagnosis other than AMI had elevated baseline levels above the 99th percentile with Abbott-Architect s-cTnI, 20% with Siemens-Ultra s-cTnI, 12% with Beckman-Coulter Accu s-cTnI, 71% with Roche hs-cTnT, 17% with Abbott hs-cTnI, 46% with Siemens hs-cTnI, and 54% with Beckman-Coulter hs-cTnI. Among patients with normal renal function, the percentages were significantly lower (7%, 7%, 7%, 15%, 6%, 23%, and 21%, respectively; P<0.001 for all comparisons; Figure 1). Among patients with renal dysfunction and elevated (≥99th percentile) baseline cTn levels, AMI was the most common diagnosis for all assays (range, 45%–80%; Figure 2). Among patients with renal dysfunction and normal baseline cTn levels, noncardiac cause of chest pain is the most common diagnosis (Figure I in the online-only Data Supplement). Details on median absolute changes of hs-cTnT during serial sampling are shown in Table IIIA and IIIB in the online-only Data Supplement.
Figure 1.
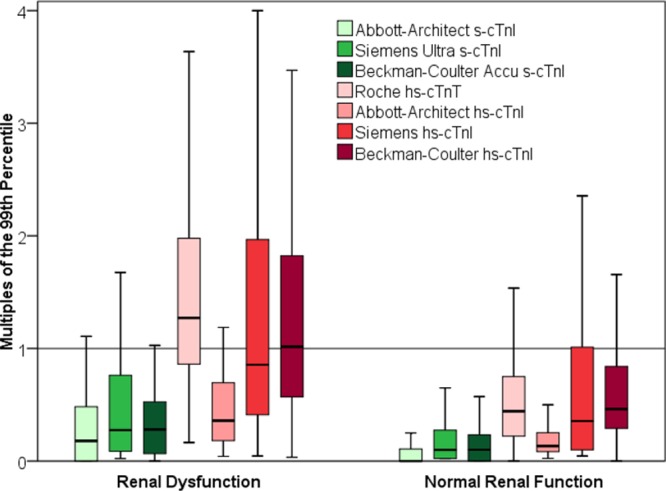
Baseline levels of more sensitive cardiac troponin (cTn) assays at presentation in patients with final diagnosis other than acute myocardial infarction. cTn levels are displayed as multiples of the 99th percentile. Boxes represent interquartile ranges; whiskers display ranges (without outliers further than 1.5 interquartile ranges). Left, In patients with renal dysfunction. Right, In patients with normal renal function. hs indicates high-sensitivity; and s, sensitive.
Figure 2.

Distribution of final diagnoses in patients with renal dysfunction and cardiac troponin (cTn) levels above the 99th percentile at presentation. hs Indicates high-sensitivity; and s, sensitive. *Coronary artery disease.
Correlations Between cTn levels and eGFR
Among patients with final diagnoses other than AMI, all 7 more sensitive cTn assays correlated significantly and inversely with renal function as quantified with the Modification of Diet in Renal Disease eGFR formula (correlation coefficient, r, ranging from −0.448 to −0.222; P<0.001 for all correlations). The correlation between eGFR and hs-cTnT was stronger compared with the correlation between eGFR and hs-cTnI as measured with all assays (Figure II in the online-only Data Supplement).
Diagnostic Accuracy of More Sensitive cTn
In patients with renal dysfunction, the diagnostic accuracy for measurements obtained at presentation, as quantified by the area under the receiver-operating characteristic curve (AUC), overall was high (AUC, 0.87–0.89) for all 7 more sensitive-cTn assays (Table 2 and Figure 3). Diagnostic accuracy further increased to 0.91 to 0.95 for samples obtained at 3 hours (Table IV in the online-only Data Supplement) and for combinations of the baseline level with early absolute changes (eg, at 1 hour: AUC, 0.90–0.93; Table V in the online-only Data Supplement). No significant differences among the 7 more sensitive cTn assays were observed (P=NS for all comparisons). Overall, the AUCs in patients with renal dysfunction were only slightly lower than in patients with normal renal function. The AUC for levels obtained at presentation in patients with normal renal function was 0.91 to 0.94 (P<0.05 for the 4 assays with the largest sample size/comparisons with patients with renal dysfunction).
Table 2.
Diagnostic Performance of cTn at Presentation in Patients With Renal Dysfunction and in Patients With Normal Renal Function

Figure 3.

Diagnostic performance of cardiac troponin (cTn) at presentation in renal dysfunction. Receiver-operating characteristic (ROC) curves are describing the diagnostic performance of the 3 sensitive (s; green) and 4 high-sensitivity (hs; red) cTn assays at presentation for the diagnosis of acute myocardial infarction in patients with renal dysfunction. The figure containing multiple curves (upper left corner) is based on the subset of patients in whom data for all 7 assays are available. The figures for the individual assays are based on all patients with available data for the respective assays to maximize precision for the determination of the respective predefined cutoff levels, which are marked as follows: 1=99th percentile, 2=optimal cutoff derived from the ROC curve, 3=sensitivity ≥90%, 4=sensitivity ≥95%, 5=specificity ≥80%, and 6=specificity ≥90%.
Among patients with different stages of renal dysfunction, AUCs for all more sensitive cTn assays were lower in the lowest tertile of renal function (eGFR ≤42 mL·min−1·1.73 m−2) compared with the intermediate tertile (eGFR, 42–53 mL·min−1·1.73 m−2). This difference was statistical significant for the 3 assays with the largest sample size. In contrast, the AUCs were comparable for all assays in patients in the highest tertile (eGFR >53 mL·min−1·1.73 m−2) and the intermediate tertile (Table VI in the online-only Data Supplement).
Diagnostic Performance in the Early Diagnosis of AMI at the 99th Percentile
Overall, at the 99th percentile, all 7 more sensitive cTn assays showed higher sensitivity (77%–98%) in patients with renal dysfunction compared with patients with normal renal function. This increase in sensitivity, however, was associated with a decrease in specificity (32%–89%; P<0.001; Table VIIA and VIIB in the online-only Data Supplement). Sensitivity and specificity at the 99th percentile differed markedly between the more sensitive cTn assays. For 3 of the 4 hs-cTn assays, the specificity and positive predictive value at the 99th percentile were <60% and 55%, respectively.
Optimal Cutoff Levels for cTn in the Early Diagnosis of AMI
The optimal cutoff levels to separate AMI from other conditions underlying acute chest pain in the ED determined by the receiver-operator characteristic curve analysis in patients with renal dysfunction were close to the 99th percentile for the 3 s-cTn assays (1.0 times the 99th percentile for Abbott-Architect s-cTnI, 1.2 times the 99th percentile for Siemens Ultra s-cTnI, and 0.9 times the 99th percentile for Beckman-Coulter Accu s-cTnI) and substantially higher for most hs-cTn assays (2.1 times the 99th percentile for Roche hs-cTnT, 1.1 times the 99th percentile for Abbott-Architect hs-cTnI, 3.6 times the 99th percentile for Siemens hs-cTnI, and 2.8 times the 99th percentile for Beckman-Coulter hs-cTnI).
Overall, all cutoff levels fulfilling a predefined criteria (derived by receiver-operator characteristic curve, optimized for sensitivity, optimized for specificity) were higher in patients with renal dysfunction compared with patients with normal renal function. The optimal receiver-operator characteristic curve–derived cutoff levels in patients with renal dysfunction were 1.9 to 3.4 times the levels in patients with normal renal function.
Prognostic Performance of More Sensitive cTn in Renal Dysfunction
Median follow-up was 759 days (first quartile, 455 days; third quartile, 895 days). Overall, 182 patients (6%) died during follow-up. Cumulative survival at 2 years was 79% in patients with renal dysfunction versus 96% in patients with normal renal function (log-rank P<0.001; Figure III in the online-only Data Supplement). Survival was 67% among patients with renal dysfunction and AMI versus 85% in patients with renal dysfunction and diagnoses other than AMI (log-rank P<0.001). Levels of cTn as measured with all 7 more sensitive cTn assays were higher in deceased patients compared with survivors and accordingly predicted long-term survival (Table VIII and Figure IV in the online-only Data Supplement).
Discussion
In this multicenter study, we examined the diagnostic performance and identified the optimal cutoff levels of 7 more sensitive cTn assays for the early diagnosis of AMI in patients with renal dysfunction. We report 7 novel findings that have important clinical implications for the early diagnosis of AMI in that they clearly highlight that more sensitive cTn assays maintain high diagnostic utility in patients with renal dysfunction as long as optimized cutoff levels are used.
First, cTn levels at presentation, as assessed by all 7 more sensitive cTn assays, were significantly higher in patients whose final diagnosis was AMI compared with those with other final diagnoses. The prevalence of elevated cTn levels above the 99th percentile in patients with renal dysfunction and a final diagnosis other than AMI differed substantially among the 7 more sensitive cTn assays, ranging from 12% to 71%. Second, despite this, AMI remained the most common final diagnosis among patients with elevated cTn levels for all assays (range, 45%–80%). Third and perhaps most important, for all 7 more sensitive cTn assays, the diagnostic accuracy at presentation was high in patients with renal dysfunction with an AUC ranging from 0.87 to 0.89 and further increased for later sampling points and for combinations of the baseline level with early absolute changes. The diagnostic accuracy of the more sensitive cTn assays at presentation was only slightly lower compared with that in patients with normal renal function. Fourth, diagnostic accuracies were comparable among the 7 more sensitive cTn assays in patients with renal dysfunction with no systematic superiority of hs-cTn assays over sensitive assays. Fifth, at the 99th percentile, all cTn assays showed higher sensitivity but lower specificity in patients with renal dysfunction compared with patients with normal renal function, reflecting the higher baseline levels observed in patients with renal dysfunction even in the absence of AMI. Sixth, the receiver-operator characteristic curve–derived optimal cutoff levels in patients with renal dysfunction were 2- to 3-times higher in patients with renal dysfunction compared with patients with normal renal function. Seventh, cTn as measured with all 7 more sensitive cTn assays also retained prognostic value and predicted 2-year survival in patients with renal dysfunction. These findings extend the observations made in previous studies investigating the prognostic value of cTn in various other settings.30–33
Although the 99th percentile of healthy individuals is the undisputed reference value to diagnose AMI according to the universal definition of AMI,4 optimal clinical decision levels or cutoff levels at presentation to the ED may well differ from the 99th percentile of healthy individuals. For example, if we aim to rule out AMI at presentation to the ED, the cutoff level achieving high sensitivity and negative predictive value will likely be lower than the 99th percentile to allow for a further increase in cTn during serial sampling. Alternatively, if we aim to rule in AMI at presentation to the ED, the cutoff level achieving high specificity and positive predictive value will likely be higher than the 99th percentile because mild elevations in cTn can often be caused by conditions other than AMI. The fine-tuning of clinical decision levels for specific clinical settings (eg, ED) and patient populations (eg, renal dysfunction) is a key step in the clinical implementation of novel diagnostic tools such as biomarkers and has recently been done successfully for other biomarkers such as B-type natriuretic peptide and procalcitonin.34–36
Our findings highlight that these clinical decision levels are assay specific and need to be determined for each assay individually. For example, the clinical decision level for cTn assay A achieving a specificity of 90% in patients with renal dysfunction cannot be reliably extrapolated from observations made with cTn assay B. To some extent, this requirement is explained by biochemical differences among the cTn assays and the challenges to define a healthy reference population to determine the 99th percentile.4,14 The 99th percentile is currently derived for each assay individually in unstandardized, healthy cohorts that differ from community-based cohorts.37 In addition, as shown, for example, by Gore et al,37 the 99th percentile of community-based cohorts also differs largely and will depend on the cohort’s mean age and the prevalence of cardiovascular comorbidities and renal dysfunction. Some of the differences observed for the performance of the more sensitive cTn assays at the respective 99th percentile of healthy individuals may be associated at least in part with differences between the cohorts of healthy individuals chosen for the determination of the 99th percentile. Of note, the 99th percentile of the Roche hs-cTnT, the assay used for the adjudication of the final diagnosis in the present analysis, has rather consistently been reported to be ≈14 ng/L, whereas the findings for other hs-cTn assays have been more variable.38
Our data also confirm previous observations that the diagnostic challenge in patients with renal dysfunction appears to be largely confined to patients presenting without persistent ST-segment elevation and that ST-segment depression or T-wave inversion is much more common in patients with renal dysfunction, even in the absence of AMI.2–4
This study is the first analysis that specifically examined diagnostic performance of more sensitive cTn assays in patients presenting to the ED with renal dysfunction and symptoms suggestive of AMI. Our findings may also help to better put into perspective a contradictory conclusion derived from a recent retrospective single-center study analyzing all ED patients with renal dysfunction regardless of symptoms, clinical gestalt, and clinical pretest probability for AMI, which reported lower-than-expected diagnostic accuracy of hs-cTnT for AMI.22 In that cohort, only 37% of patients had a clinical suspicion of AMI, and trauma, stroke, epileptic seizures, and acute heart failure accounted for the majority of patients. In those patients, the clinical role of measuring cTn is controversial and not at all comparable to the measurement in patients presenting with suspected AMI. In addition, that population of patients provides important methodological challenges for the adjudication of AMI based on the information obtained during routine clinical care, which might have further contributed to those findings. The findings from this prospective multicenter study using a gold standard diagnosis centrally adjudicated by 2 independent cardiologists should help to avoid possible misunderstandings related to the diagnostic utility of more sensitive cTn assays in patients with suspected AMI and renal dysfunctions.
The following limitations of the present study merit consideration. First, we evaluated 7 more sensitive-cTn assays. We hypothesize that our findings can be generalized to other cTn assays with similar sensitivity and precision. However, additional studies need to confirm this hypothesis. Second, in this ongoing prospective study, the subgroup analysis of patients with renal dysfunction was not predefined at the time of the writing of the first protocol but was added as an amendment in 2009, when we were still blinded to the results. Third, we cannot comment on the clinical utility of more sensitive cTn assays in patients undergoing dialysis because such patients were excluded from our study.39 Fourth, to reflect the clinical information available to the ED physician when interpreting cTn levels, we classified renal dysfunction according to eGFR on the basis of the serum creatinine level obtained in the ED. Accordingly, this classification differs from the definition of chronic kidney disease, which would require renal dysfunction to be present for 3 months.25–27
Conclusions
More sensitive cTn assays maintain high diagnostic accuracy in patients with suspected AMI and renal dysfunction. To ensure the best possible clinical use, assay-specific optimal cutoff levels, which are higher in patients with renal dysfunction, should be considered.
Sources of Funding
The study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Roche, Nanosphere, Siemens, 8sense, Bühlmann, and B.R.A.H.M.S.
Disclosures
Dr Mueller has received research grants from the Swiss National Science Foundation and the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, 8sense, Abbott, ALERE, Brahms, Critical Diagnostics, Nanosphere, Roche, Siemens, and the University Hospital Basel, as well as speaker honoraria from Abbott, ALERE, Brahms, Novartis, Roche, and Siemens. Dr Reichlin has received research grants from the Swiss National Science Foundation (PASMP3-136995), the Swiss Heart Foundation, the University of Basel, the Professor Max Cloetta Foundation, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria from Brahms and Roche. The other authors report no conflicts. The sponsors had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.114.014245/-/DC1.
CLINICAL PERSPECTIVE
In this multicenter study, we examined the diagnostic performance and identified the optimal cutoff levels of 7 more sensitive cardiac troponin (cTn) assays for the early diagnosis of acute myocardial infarction (AMI) in patients with renal dysfunction. We report 7 novel findings that have important clinical implications for the early diagnosis of AMI in that they clearly highlight that more sensitive cTn assays maintain high diagnostic utility in patients with renal dysfunction as long as optimized cutoff levels are used. First, cTn levels at presentations, as assessed by all the more sensitive cTn assays, were significantly higher in patients whose final diagnosis was AMI compared with those with other final diagnoses. The prevalence of elevated cTn levels above the 99th percentile in patients with renal dysfunction and a final diagnosis other than AMI differed substantially among the 7 more sensitive cTn assays, ranging from 12% to 71%. Second, despite this, AMI remained the most common final diagnosis among patients with elevated cTn levels for all assays (range, 45%–80%). Third and perhaps most important, for all 7 more sensitive cTn assays, the diagnostic accuracy at presentation was high in patients with renal dysfunction and further increased for later sampling points. Diagnostic accuracy of the more sensitive cTn assays at presentation was only slightly lower compared with that in patients with normal renal function. Fourth, diagnostic accuracies were comparable among the 7 more sensitive cTn assays in patients with renal dysfunction with no systematic superiority of high-sensitivity cTn assays over sensitive assays. Fifth, at the 99th percentile, all cTn assays showed higher sensitivity but lower specificity in patients with renal dysfunction compared with patients with normal renal function, reflecting the higher baseline levels observed in patients with renal dysfunction even in the absence of AMI. Sixth, the receiver-operating characteristics curve–derived optimal cutoff levels in patients with renal dysfunction were 2- to 3-times higher in patients with renal dysfunction compared with patients with normal renal function. Seventh, cTn as measured with all 7 more sensitive cTn assays also retained prognostic value and predicted 2-year survival in patients with renal dysfunction.
References
- 1.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL 2012 Writing Committee Members; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 2.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D ESC Committee for Practice Guidelines. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX Force CAT. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary : a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr 2011 Writing Group Members; ACCF/AHA Task Force Members. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 6.Deleted in proof. [Google Scholar]
- 7.Forberg JL, Henriksen LS, Edenbrandt L, Ekelund U. Direct hospital costs of chest pain patients attending the emergency department: a retrospective study. BMC Emerg Med. 2006;6:6. doi: 10.1186/1471-227X-6-6. doi: 10.1186/1471-227X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiemann O. Variations in hospitalisation costs for acute myocardial infarction: a comparison across Europe. Health Econ. 2008;17(suppl):S33–S45. doi: 10.1002/hec.1322. doi: 10.1002/hec.1322. [DOI] [PubMed] [Google Scholar]
- 9.Pines JM, Pollack CV, Jr, Diercks DB, Chang AM, Shofer FS, Hollander JE. The association between emergency department crowding and adverse cardiovascular outcomes in patients with chest pain. Acad Emerg Med. 2009;16:617–625. doi: 10.1111/j.1553-2712.2009.00456.x. doi: 10.1111/j.1553-2712.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 12.Aronow WS, Ahn C, Mercando AD, Epstein S. Prevalence of coronary artery disease, complex ventricular arrhythmias, and silent myocardial ischemia and incidence of new coronary events in older persons with chronic renal insufficiency and with normal renal function. Am J Cardiol. 2000;86:1142–1143, A9. doi: 10.1016/s0002-9149(00)01176-0. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S, Uzu T, Inenaga T, Kimura G. Prediction of coronary artery disease and cardiac events using electrocardiographic changes during hemodialysis. Am J Kidney Dis. 2000;36:592–599. doi: 10.1053/ajkd.2000.16198. doi: 10.1053/ajkd.2000.16198. [DOI] [PubMed] [Google Scholar]
- 14.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH National Academy of Clinical Biochemistry; IFCC Committee for Standardization of Markers of Cardiac Damage. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage laboratory medicine practice guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007;115:e352–e355. doi: 10.1161/CIRCULATIONAHA.107.182881. doi: 10.1161/CIRCULATIONAHA.107.182881. [DOI] [PubMed] [Google Scholar]
- 15.Apple FS, Smith SW, Pearce LA, Ler R, Murakami MM. Use of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2008;54:723–728. doi: 10.1373/clinchem.2007.097162. doi: 10.1373/clinchem.2007.097162. [DOI] [PubMed] [Google Scholar]
- 16.Melanson SE, Morrow DA, Jarolim P. Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol. 2007;128:282–286. doi: 10.1309/Q9W5HJTT24GQCXXX. doi: 10.1309/Q9W5HJTT24GQCXXX. [DOI] [PubMed] [Google Scholar]
- 17.Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009;55:1303–1306. doi: 10.1373/clinchem.2009.128363. doi: 10.1373/clinchem.2009.128363. [DOI] [PubMed] [Google Scholar]
- 18.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 19.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 20.Flores-Solís LM, Hernández-Domínguez JL. Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60:281–290. doi: 10.7754/clin.lab.2013.121103. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Huang C, Wu B, Lian X, Mei X, Wan J. Cardiac troponin I in non-acute coronary syndrome patients with chronic kidney disease. PLoS One. 2013;8:e82752. doi: 10.1371/journal.pone.0082752. doi: 10.1371/journal.pone.0082752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfortmueller CA, Funk GC, Marti G, Leichtle AB, Fiedler GM, Schwarz C, Exadaktylos AK, Lindner G. Diagnostic performance of high-sensitive troponin T in patients with renal insufficiency. Am J Cardiol. 2013;112:1968–1972. doi: 10.1016/j.amjcard.2013.08.028. doi: 10.1016/j.amjcard.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, Bassetti S, Steuer S, Winkler K, Peter F, Meissner J, Haaf P, Potocki M, Drexler B, Osswald S, Mueller C. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 24.Reiter M, Twerenbold R, Reichlin T, Haaf P, Peter F, Meissner J, Hochholzer W, Stelzig C, Freese M, Heinisch C, Breidthardt T, Freidank H, Winkler K, Campodarve I, Gea J, Mueller C. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32:1379–1389. doi: 10.1093/eurheartj/ehr033. doi: 10.1093/eurheartj/ehr033. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS. Clinical practice: nondiabetic kidney disease. N Engl J Med. 2002;347:1505–1511. doi: 10.1056/NEJMcp013462. doi: 10.1056/NEJMcp013462. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 29.Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–986. doi: 10.1067/mhj.2002.124048. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]
- 30.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long-term mortality. Circulation. 2007;116:1907–1914. doi: 10.1161/CIRCULATIONAHA.107.708529. doi: 10.1161/CIRCULATIONAHA.107.708529. [DOI] [PubMed] [Google Scholar]
- 32.Michos ED, Wilson LM, Yeh HC, Berger Z, Suarez-Cuervo C, Stacy SR, Bass EB. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med. 2014;161:491–501. doi: 10.7326/M14-0743. doi: 10.7326/M14-0743. [DOI] [PubMed] [Google Scholar]
- 33.Stacy SR, Suarez-Cuervo C, Berger Z, Wilson LM, Yeh HC, Bass EB, Michos ED. Role of troponin in patients with chronic kidney disease and suspected acute coronary syndrome: a systematic review. Ann Intern Med. 2014;161:502–512. doi: 10.7326/M14-0746. doi: 10.7326/M14-0746. [DOI] [PubMed] [Google Scholar]
- 34.Maisel AS, Clopton P, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Steg G, Westheim A, Knudsen CW, Perez A, Kazanegra R, Bhalla V, Herrmann HC, Aumont MC, McCullough PA BNP Multinational Study Investigators. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J. 2004;147:1078–1084. doi: 10.1016/j.ahj.2004.01.013. doi: 10.1016/j.ahj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–654. doi: 10.1056/NEJMoa031681. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
- 36.Christ-Crain M, Stolz D, Bingisser R, Müller C, Miedinger D, Huber PR, Zimmerli W, Harbarth S, Tamm M, Müller B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 37.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, Ballantyne CM, de Lemos JA. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63:1441–1448. doi: 10.1016/j.jacc.2013.12.032. doi: 10.1016/j.jacc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandoval Y, Apple FS. The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem. 2014;60:455–462. doi: 10.1373/clinchem.2013.211706. doi: 10.1373/clinchem.2013.211706. [DOI] [PubMed] [Google Scholar]
- 39.Artunc F, Mueller C, Breidthardt T, Twerenbold R, Peter A, Thamer C, Weyrich P, Haering HU, Friedrich B. Sensitive troponins: which suits better for hemodialysis patients? Associated factors and prediction of mortality. PLoS One. 2012;7:e47610. doi: 10.1371/journal.pone.0047610. doi: 10.1371/journal.pone.0047610. [DOI] [PMC free article] [PubMed] [Google Scholar]


