Supplemental Digital Content is available in the text.
Keywords: acute cardiac care, biological markers, myocardial infarction, troponin
Abstract
Background—
Misdiagnosis of acute myocardial infarction (AMI) may significantly harm patients and may result from inappropriate clinical decision values (CDVs) for cardiac troponin (cTn) owing to limitations in the current regulatory process.
Methods and Results—
In an international, prospective, multicenter study, we quantified the incidence of inconsistencies in the diagnosis of AMI using fully characterized and clinically available high-sensitivity (hs) cTn assays (hs-cTnI, Abbott; hs-cTnT, Roche) among 2300 consecutive patients with suspected AMI. We hypothesized that the approved CDVs for the 2 assays are not biologically equivalent and might therefore contribute to inconsistencies in the diagnosis of AMI. Findings were validated by use of sex-specific CDVs and parallel measurements of other hs-cTnI assays. AMI was the adjudicated diagnosis in 473 patients (21%). Among these, 86 patients (18.2%) had inconsistent diagnoses when the approved uniform CDV was used. When sex-specific CDVs were used, 14.1% of female and 22.7% of male AMI patients had inconsistent diagnoses. Using biologically equivalent CDV reduced inconsistencies to 10% (P<0.001). These findings were confirmed with parallel measurements of other hs-cTn assays. The incidence of inconsistencies was only 7.0% for assays with CDVs that were nearly biologically equivalent. Patients with inconsistent AMI had long-term mortality comparable to that of patients with consistent diagnoses (P=NS) and a trend toward higher long-term mortality than patients diagnosed with unstable angina (P=0.05).
Conclusions—
Currently approved CDVs are not biologically equivalent and contribute to major inconsistencies in the diagnosis of AMI. One of 5 AMI patients will receive a diagnosis other than AMI if managed with the alternative hs-cTn assay.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00470587.
Acute myocardial infarction (AMI) is a major cause of death and disability worldwide. Patients with symptoms suggestive of AMI account for ≈10% of all emergency department (ED) consultations, even though only 10% to 20% of them are diagnosed as experiencing an AMI. Rapid identification of AMI is of paramount clinical importance for early treatment and management.1–3
Editorial see p 2029
Clinical Perspective on p 2040
Misdiagnosis of AMI and inconsistencies in the diagnosis of AMI may significantly harm patients. First and most important, withholding evidence-based therapies such rhythm monitoring for 24 to 48 hours, antiplatelet therapy, high-dose statins, intense lifestyle modifications, and early revascularization may increase morbidity and mortality in patients with AMI.2–5 Second, therapies approved for AMI may even be harmful for patients with other diagnoses such as peptic ulcer.2–5 Accordingly, misdiagnosis of AMI is a common cause for malpractice claims.4 The clinical introduction of the universal definition of AMI has led to a harmonization worldwide in the diagnosis of AMI and thereby contributed to a reduction in diagnostic inconsistencies.2,3,5,6 Cardiac troponin (cTn) I and T are 2 proteins unique to the heart that are specific and sensitive biomarkers of cardiomyocyte damage.2,3,6 According to the universal definition of AMI, a cTnI or cTnT level above the 99th percentile of a healthy reference population is a condition sine qua non for the diagnosis of AMI.6 There are limitations to the current regulatory approach to define clinical decision values (CDVs) for cTn. Manufacturers are asked to establish the 99th percentile in a healthy reference population. Because there is a lack of consensus on how to define healthy and because possible effects of age and sex on levels of cTnI and cTnT have recently been identified, the current regulatory process has come under scrutiny.7–11 Apparently, differences in these cohorts of healthy individuals are substantial and may lead to major differences in the resulting 99th percentiles and therefore the biologically nonequivalent CDV. The younger the reference population is and the more stringent the criteria to define cardiac health are, the lower the resulting 99th percentile is.7,12–14 The clinical availability of fully developed high-sensitivity assays for both cTnI and cTnT in Europe and other countries now provides for the first time the methodological requirement to quantify the clinical consequences of the limitations of the current regulatory approach to define the CDVs. The aim of this large multicenter study was to explore remaining sources for misdiagnosis of AMI after the introduction of the universal definition of AMI6 and to quantify inconsistencies in the diagnosis of AMI related to the limitations of the current regulatory process on how to define CDVs for cTn.7–9,13
Methods
Study Design and Population
The Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) is an ongoing prospective, international, multicenter study designed to advance the early diagnosis of AMI.15–19 From April 2006 to September 2012, consecutive adult patients presenting to the ED with symptoms suggestive of AMI with an onset or peak within the last 12 hours were recruited after providing written informed consent.
Patients were enrolled regardless of their renal function. Only patients with terminal kidney failure requiring regular dialysis were excluded. For this analysis, patients were also excluded if high-sensitivity (hs) cTnI (Abbott) or hs-cTnT (Roche) levels were not available or if the final diagnosis remained unclear after adjudication and at least 1 cTn level was elevated (possibly indicating the presence of AMI; Figure I in the online-only Data Supplement). The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees.
Routine Clinical Assessment
All patients underwent a clinical assessment that included medical history, physical examination, 12-lead ECG, continuous ECG monitoring, pulse oximetry, standard blood test, and chest radiography. Levels of cTn were measured at presentation and serially thereafter as long as clinically indicated. Treatment of patients was left to the discretion of the attending physician.
Measurement of Sensitive and hs-cTn
Blood samples for the determination of hs-cTnI (Abbott) and hs-cTnT (Roche) were collected at presentation to the ED and serially at 1, 2, 3, and 6 hours. When treatment required transferring the patient to the catheter laboratory or coronary care unit, serial sampling was interrupted. After centrifugation, samples were frozen at −80°C until assayed in a blinded fashion in a dedicated core laboratory. The Abbott hs-cTnI assay used was the final precommercial release version of the ARCHITECT High Sensitive STAT Troponin I assay (Abbott Laboratories). Samples were thawed, mixed, and centrifuged before analysis according to the manufacturer’s instructions. The hs-cTnI assay has a 99th percentile concentration of 26.2 ng/L with a corresponding coefficient of variation (CV) of <5% and a limit of detection (LoD) of 1.9 ng/L.12 The sex-specific 99th percentile has been defined as 15.6 ng/L in women and 34.2 ng/L in men. Long-term stability of TnI and a very high correlation between plasma and serum have been demonstrated.12,20 The limit of blank and LoD of the Roche hs-cTnT assay were determined to be 3 and 5 ng/L, respectively. The 99th percentile of a healthy reference population was reported to be 14 ng/L with an imprecision corresponding to 10% CV at 13 ng/L.21 The sex-specific 99th percentile has been defined as 8.9 ng/L in women and 15.5 ng/L in men.22
The Siemens sensitive Ultra cTnI assay was performed with the use of the ADVIA Centaur immunoassay system (Siemens), with an LoD of 6 ng/L, a 99th percentile cutoff point of 40 ng/L, and a CV of <10% at 30 ng/L.23 The Siemens hs-cTnI assay, an experimental prototype assay, was performed with the use of the Dimension Vista 1500 immunoassay system (Siemens), with an LoD of 0.5 ng/L, a 99th percentile cutoff point of 9 ng/L, and a CV of <10% at 3 ng/L.24 The Beckman-Coulter hs-cTnI assay was measured on the Access 2 analyzer with the use of an investigational prototype assay. According to the manufacturer, the LoD is 2 ng/L and the 99th percentile of a healthy reference population is 9 ng/L with a 10% CV lower than the 99th percentile.20
Calculation of the glomerular filtration rate was performed by the abbreviated Modification of Diet in Renal Disease formula.25
Adjudicated Final Diagnosis
Two independent cardiologists reviewed all available medical records—patient history, physical examination, results of laboratory testing, radiological testing, ECG, echocardiography, cardiac exercise test, and lesion severity and morphology in coronary angiography—pertaining to the patient from the time of ED presentation to the 90-day follow-up. In situations of disagreement about the diagnosis, cases were reviewed and adjudicated with a third cardiologist. For all patients recruited from all sites, the adjudication of the final diagnosis was performed centrally in the core laboratory (University Hospital Basel) using hs-cTnT Roche levels with CDV as recommended by the manufacturer and as described in detail in the Methods section in the online-only Data Supplement.
AMI was defined and cTn levels were interpreted as recommended in current guidelines.2,6,9 In brief, AMI was diagnosed when there was evidence of myocardial necrosis in association with a clinical setting consistent with myocardial ischemia. Myocardial necrosis was diagnosed by at least 1 hs-cTnT value above the 99th percentile, together with a significant rise or fall.9 For hs-cTnT, the 99th percentile (14 ng/L) was used as the cutoff for myocardial necrosis.26,27 Absolute changes in hs-cTnT were used to determine significant changes on the basis of the diagnostic superiority of absolute over relative changes.17,28 On the basis of studies of the biological variation of cTn29,30 and data from previous chest pain cohort studies,23,31 a significant absolute change was defined as a rise or fall of at least 10 ng/L within 6 hours. In patients, in whom a 6-hour hs-cTnT level was not available, changes were assessed at earlier time points. In an assumption of linearity, an absolute change of 6 ng/L within 3 hours was considered. All other patients were classified as not having AMI for this analysis.
Follow-Up
After hospital discharge, patients were followed up by trained researchers at 3, 12, and 24 months by telephone or in written form.
Statistical Analysis
The primary outcome measure was the percentage of patients with an adjudicated diagnosis of AMI inconsistently assigned a diagnosis of AMI or non-AMI at presentation using the approved CDVs of the hs-cTnI and hs-cTnT assays. The selection of these assays for the primary analysis is further supported by their overall comparable diagnostic accuracy for the early diagnosis of AMI.32 Therefore, inconsistencies in the diagnosis of AMI cannot be attributed to different diagnostic performance of the assays. Among several criteria,2,3,6,33 the diagnosis of AMI invariably requires a cTn level above the CDV. Therefore, we quantified inconsistencies about the diagnosis of AMI by the position of level pairs (troponin values of different assays at the same time point taken) according to quadrants defined by the CDV for each assay. This analysis was done twice: once using a uniform CDV for both sexes and once using the sex-specific CDV for each assay. Because hs-cTnT was used for the adjudication, the CDV of the hs-cTnT assay (14 ng/L) was used as the reference to determine the biologically equivalent CDV for the other assays by linear regression analysis. Subgroup analysis was predefined to explore potential contributing causes for inconsistencies. To explore whether and to what extent preanalytical aspects (eg, small differences from the time the sample was frozen at −80°C until measurements were performed) could have played a role, we selected the subgroup of patients in whom the measurements of hs-cTnT and cTnI-ultra (Siemens) were performed from the same tube and on the same day. Patients with AMI were further classified as either MI type I or MI type II.6 MI type 4B (related to stent thrombosis) is rare in patients presenting to the ED and has a lot in common with other type 1 MIs. Therefore MI type 4B events were classified with MI type 1 for this analysis.
Data are expressed as median±interquartile range for continuous variables and as numbers and percentages for categorical variables. Continuous variables were compared with the Mann-Whitney U test, and categorical variables were compared by use of the Pearson χ2 test. All hypothesis testing was 2 tailed, and values of P<0.05 were considered statistically significant. All statistical analyses were performed with SPSS for Windows 22.0 (SPSS Inc) and MedCalc 9.6.4.0 (MedCalc software).
Results
Patient Characteristics
The baseline characteristics of 2300 patients with suspected AMI are shown in the Table. The adjudicated final diagnosis was AMI in 21% of patients, unstable angina in 9%, cardiac but noncoronary artery symptoms in 13%, noncardiac cause in 52%, and symptoms of unknown origin in 5%. Among the 473 AMI patients, 74 (16%) had ST-segment–elevation MI, and 399 (84%) had non–ST-segment–elevation MI. In the group of patients with non–ST-segment–elevation MI, 85% were classified as type 1 and 15% as type 2 AMI. Baseline levels of hs-cTnT Roche and hs-cTnI Abbott according to final diagnosis are shown in Figure II in the online-only Data Supplement.
Table.
Baseline Characteristics
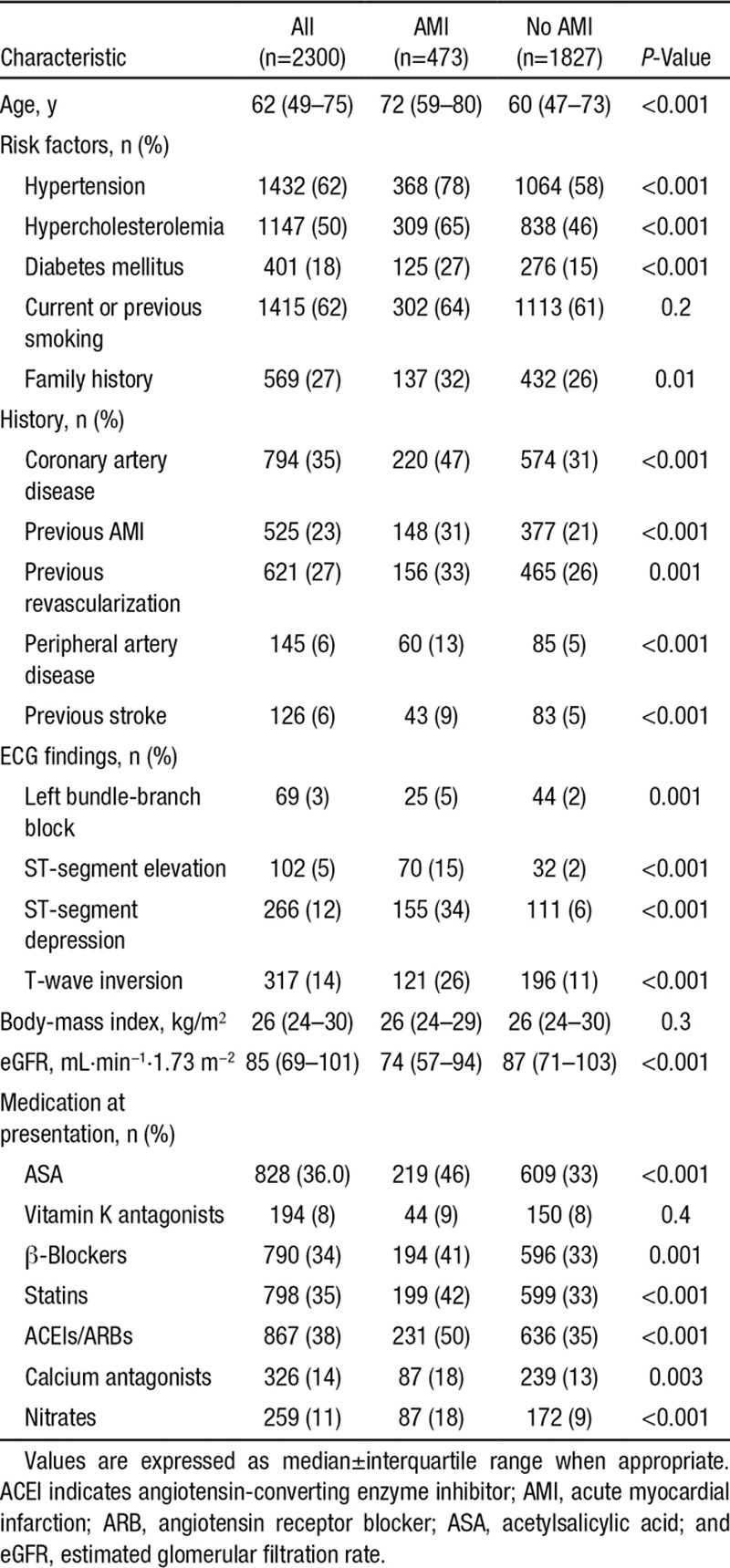
Correlation Between hs-cTnI and hs-cTnT
For hs-cTn levels measured at presentation (2300 pairs) and for all time points (6559 pairs), hs-cTnI and hs-cTnT levels correlated closely (r=0.813 and r=0.790, both P<0.001; Figure 1A). The correlation was comparable in patients with an adjudicated diagnosis of AMI (r=0.797 and r=0.770, both P<0.001; Figure 1B and 1C) and in patients with other diagnoses (r=0.799 and r=0.808, both P<0.001). Distribution of hs-cTnI and hs-cTnT values in quadrants according to the approved uniform CDVs in patients with an adjudicated diagnosis of AMI was comparable to that in the overall cohort regardless of the adjudicated diagnosis. Detectable levels (higher than or equal to the LoD) of hs-cTnI and hs-cTnT were observed in 87.7% and 75.5% of all samples (P<0.001; n=6559). The scatter between hs-cTnI and hs-cTnT seemed rather uniform throughout the entire range of hs-cTn values.
Figure 1.
Distribution of high-sensitivity cardiac troponin I (hs-TnI) and high-sensitivity cardiac troponin T (hs-TnT) values in quadrants according to the approved uniform clinical decision values (A) at any time during serial sampling in the overall cohort regardless of the adjudicated diagnosis, (B) in patients with an adjudicated diagnosis of acute myocardial infarction for levels at presentation, and (C) at any time during serial sampling.
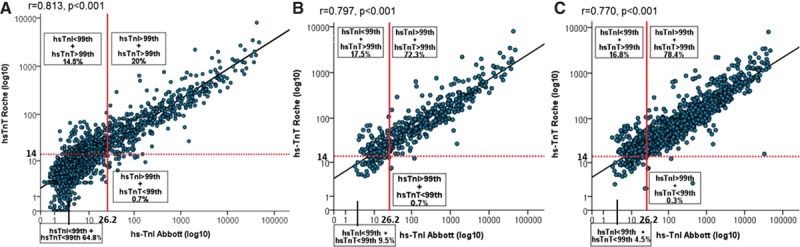
Diagnostic Inconsistencies for AMI
AMI was the adjudicated diagnosis in 473 patients (21%). Among these, 86 patients (18.2%) had inconsistent diagnoses using the uniform CDV at presentation. The incidence was 17.1% for the analysis of all level pairs obtained during serial sampling. These inconsistencies seemed to be due at least in part to the fact that the approved CDV for hs-cTnI is not biologically equivalent to the approved CDV for hs-cTnT (Figure 2). The biologically equivalent hs-cTnI value corresponding to the CDV for hs-cTnT was less than half the approved CDV for hs-cTnI. Therefore, nearly all of these inconsistencies were related to the underdiagnosis of AMI with hs-cTnI. The diagnostic accuracy for AMI of the different assays is shown in Table I in the online-only Data Supplement. At the approved CDV, hs-cTnT had higher sensitivity, whereas hs-cTnI had higher specificity and PPV.
Figure 2.
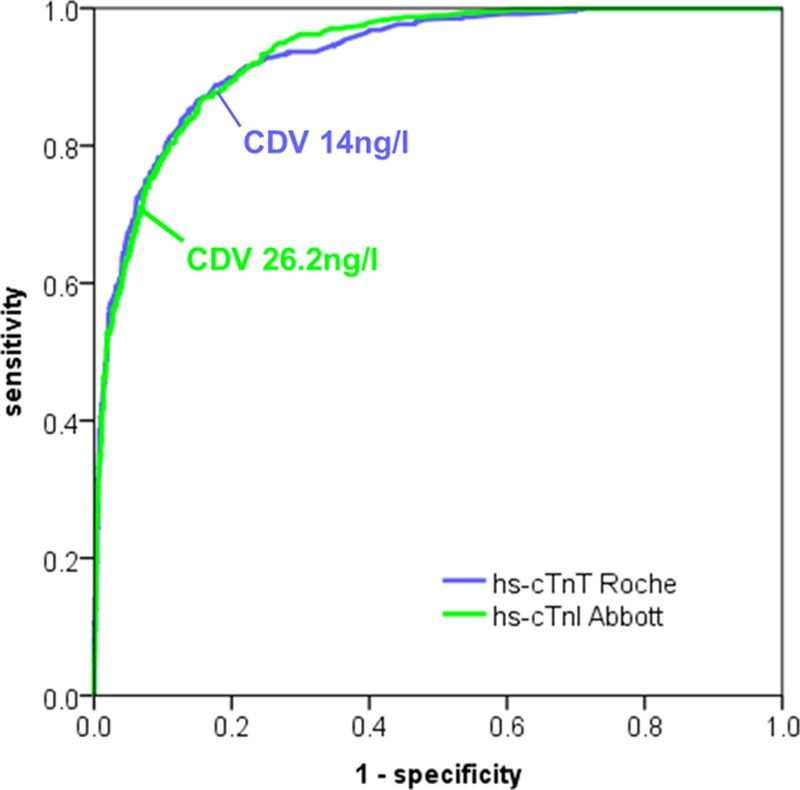
Receiver-operating characteristics curve for acute myocardial infarction (AMI). The approved clinical decision values (CDVs) for high-sensitivity cardiac troponin I (hs-TnI) and high-sensitivity cardiac troponin T (hs-TnT) are not biologically equivalent and therefore differ in their sensitivity and specificity for AMI.
With the hs-cTnI sex-specific CDV, 14.1% of women and 22.7% of men with AMI had inconsistent diagnoses at presentation, again nearly all of them related to underdiagnosis of AMI with hs-cTnI (Figure 3A and 3B). Median cTn values in patients with inconsistent diagnoses of AMI were lower than in patients with consistent diagnoses, indicating smaller infarct size (Table I in the online-only Data Supplement).
Figure 3.
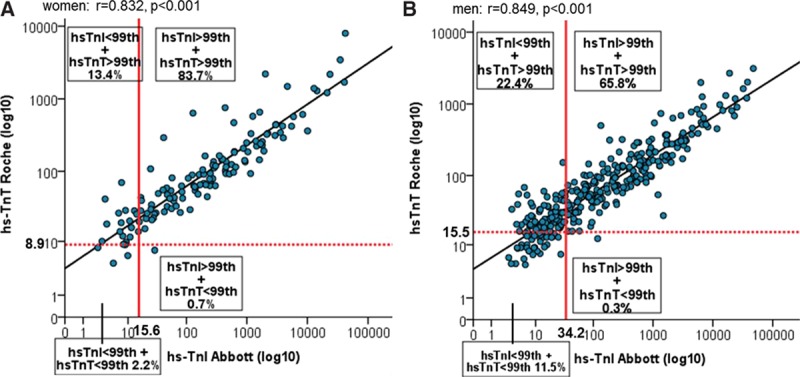
Distribution of high-sensitivity cardiac troponin I (hs-TnI) and high-sensitivity cardiac troponin T (hs-TnT) values at presentation in quadrants according to the sex-specific clinical decision values in patients with an adjudicated diagnosis of acute myocardial infarction: (A) in women; and (B) in men.
These findings were confirmed with parallel measurements using other sensitive cTn and hs-cTn assays, showing that most did not have biologically equivalent CDV (Figure 4A–4F). The incidence of inconsistencies for level pairs obtained at presentation in patients with AMI was as low as 7.0% for assays with CDVs that were nearly biologically equivalent.
Figure 4.
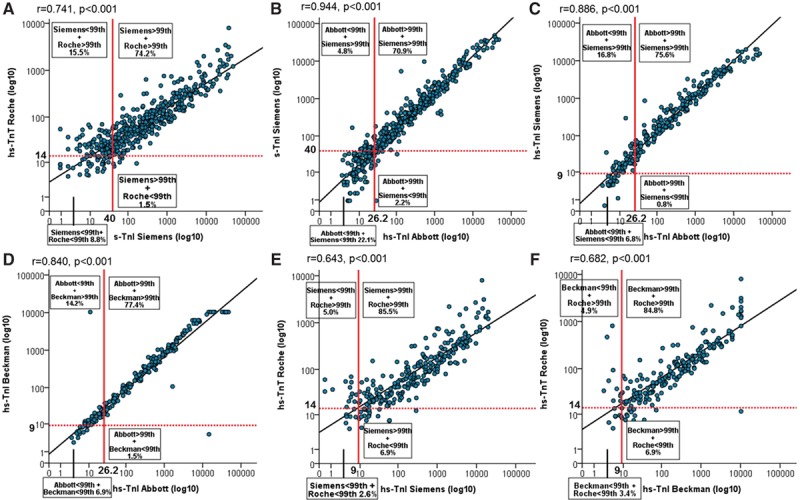
Distribution of sensitive cardiac troponin (s-Tn) and high-sensitivity cardiac troponin (hs-Tn) I and T values at presentation in quadrants according to the approved uniform clinical decision values in patients with an adjudicated diagnosis of acute myocardial infarction. A, Pairs of hs-TnT and s-TnI Ultra (Siemens); (B) pairs of hs-TnI (Abbott) and s-TnI Ultra (Siemens); (C) pairs of hs-TnI (Abbott) and hs-TnI (Siemens); (D) pairs of hs-TnI (Abbott) and hs-TnI (Beckman Coulter); (E) pairs of hs-TnT and hs-TnI (Siemens); and (F) pairs of hs-TnT and hs-TnI (Beckman Coulter).
Diagnostic Inconsistency With Biologically Equivalent CDVs
Using the hs-cTnI (Abbott) biologically equivalent CDV for hs-cTnT (8.7 instead of 26.2 ng/L) reduced inconsistencies in the diagnosis of AMI to 86 of 473 AMIs (18.2%; 83 underdiagnoses with hs-cTnI, 3 underdiagnoses with hs-cTnT) to 47 of 473 (22 underdiagnoses with Abbott, 25 underdiagnoses with Roche; 9.9%; P<0.001).
These findings were replicated with the use of other hs-cTn assays; for example, using the sensitive Ultra cTnI (Siemens) biologically equivalent CDV for hs-cTnT (9.4 instead of 40 ng/L) reduced inconsistencies from 17% to 10.3% (P<0.001).
Subgroup Analysis Concerning Potential Preanalytical Contributors
Preanalytical aspects did not seem to be relevant contributors to the observed inconsistencies because findings in the subgroup with identical preanalytical conditions and in fresh samples were similar to those in the overall cohort (Figure III and Methods section in the online-only Data Supplement).
Long-Term Mortality in Patients With Inconsistent AMI Diagnoses
Patients with inconsistent AMI diagnoses (eg, Roche+/Abbott− or Roche−/Abbott+) resulting from biologically nonequivalent CDVs for different pairs of cTn assays had long-term mortality (12 deaths among 86 patients) comparable to that of patients with consistent AMI diagnoses (Roche+/Abbott+; 66 deaths among 387 patients; P=NS for all comparisons; Figure 5A and 5B). Patients with inconsistent AMI diagnoses for Roche/Abbott had higher mortality than patients consistently diagnosed as unstable angina (15 deaths in 216 patients; P=0.05).
Figure 5.
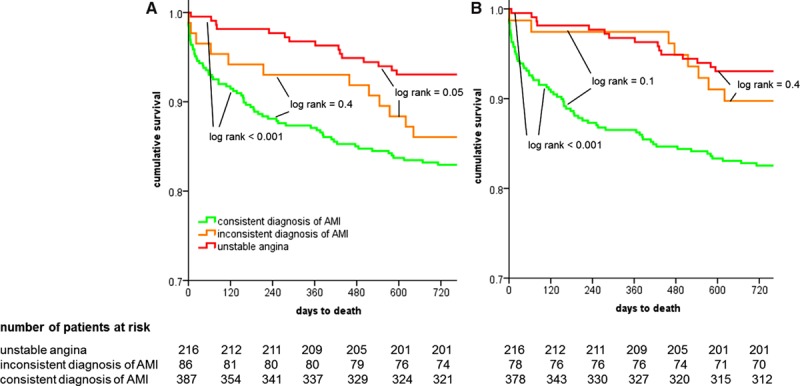
Kaplan–Meier survival curves in patients with consistent diagnoses of acute myocardial infarction (AMI), patients with inconsistent diagnoses resulting from biologically nonequivalent clinical decision values for the different pairs of cardiac troponin assays, and patients with diagnoses of unstable angina. A, Pairs of high-sensitivity cardiac troponin (hs-cTn) T and hs-cTnI (Abbott); and (B) pairs of hs-cTnT and sensitive cTnI Ultra (Siemens).
Discussion
Misdiagnosis of AMI may occur in patients with or without ST-segment elevation and may significantly harm patients.5 This large, multicenter study reports 8 major findings about the misdiagnosis of AMI related to the limitations of the current regulatory process on how to define CDVs for cTn. First, among level pairs of hs-cTnI and hs-cTnT, the correlation was high and similar for levels obtained at presentation and during serial sampling, as well as in the overall diagnostic cohort and in patients adjudicated to have AMI. Second and most important, with the use of the approved uniform CDV, the 99th percentile of healthy individuals, almost 1 of 5 AMI patients had inconsistent diagnoses using hs-cTnI versus hs-cTnT. This means that of 100 patients diagnosed as having AMI in institution A with hs-cTnT, 20 patients would receive a diagnosis other than AMI if treated in institution B using hs-cTnI. It is important to highlight that these assays overall have comparable diagnostic accuracy for the early diagnosis of AMI.32 Therefore, the observed inconsistencies cannot be attributed to differences in diagnostic accuracy. Inconsistencies seemed to be attributable to a large extent to the fact that the approved CDV for hs-cTnI is not biologically equivalent to the approved CDV for hs-cTnT. In fact, the biologically equivalent hs-cTnI value corresponding to the CDV for hs-cTnT was less than half the approved CDV for hs-cTnI. This finding is supported by a recent observation that the 99th percentile of hs-cTnI in an Australian cohort of healthy individuals was also less than half the approved CDV for this assay.12 The observation that most of the consistencies were related to underdiagnosis of AMI with hs-cTnI does not indicate that the hs-cTnI assay would have lower sensitivity. In fact, our data confirmed previous investigations documenting even higher analytical sensitivity for hs-cTnI versus hs-cTnT with a substantially higher percentage of patients with detectable hs-cTnI versus hs-cTnT levels.13 Therefore, among 2 excellent hs-cTn assays that overall have very high and comparable diagnostic accuracy in the early diagnosis of AMI,32 the test with even higher analytical sensitivity applied clinically as approved in many countries worldwide results in a substantial underdiagnosis of AMI because the approved CDV is much higher than the biologically equivalent CDV of hs-cTnT. It is important to highlight that the use of hs-cTnT as reference and for adjudication does not affect the main findings of the study. If we would have used hs-cTnI as the reference, the percentage of inconsistencies would have been identical, merely with the majority of inconsistencies resulting from overdiagnosis of AMI with hs-cTnT. In addition, it is important to emphasize that our findings do not and should not be used to criticize manufacturers or regulators but rather to quantify the limitations of the current process and to encourage them to pursue new roads for the definition of CDV. To put the implications of these inconsistencies into perspective, it might help to highlight that the change in AMI incidence resulting from these inconsistencies is of a magnitude similar to that of the change in AMI incidence occurring when switching clinically from a conventional cTn assay to an hs-cTn assay.34 Third, the discrepancies resulting from biologically nonequivalent CDVs affected all patients regardless of their final diagnosis and therefore are also independent of the details of the adjudication. For example, they result in discrepancies of similar magnitude between the diagnoses of pericarditis and perimyocarditis, with the respective differences in patient management. Fourth, the incidence of inconsistencies could not be significantly reduced with the use of sex-specific CDVs because the sex-specific CDV for hs-cTnI is not biologically equivalent to the sex-specific CDV for hs-cTnT in both women and men. Fifth, these findings were confirmed in parallel measurements with several other hs-cTnI assays. Sixth, the incidence of inconsistencies was as low as 7.0% for level pairs of hs-cTnI assays with CDVs that were nearly biologically equivalent, further supporting the hypothesis that more than half of the observed inconsistencies in the diagnosis of AMI could be avoided by a new regulatory process that ensures that CDVs for cTn are biologically equivalent. Seventh, preanalytical issues did not seem to be relevant contributors to our findings. Among 1355 patients with parallel measurements of hs-cTnT and sensitive Ultra cTnI performed from the same tube and on the same day, as well as hs-cTnI and hs-cTnT in fresh samples, findings were similar to those of the overall cohort. Eighth, patients with inconsistent AMI diagnoses had long-term mortality comparable to that of patients with consistent AMI diagnosis. This observation supports the conclusion that these events are clinically meaningful and require detection and appropriate treatment.
The findings of this large multicenter study corroborate and extend other recent observations that had begun to challenge the current regulatory process to define CDVs for cTn, the 99th percentile of healthy individuals, by documenting the effect of population selection.7,9,12,13,35 In the current regulatory process, each manufacturer defines the 99th percentile in a separate cohort of healthy individuals. Apparently, differences in these cohorts of healthy individuals are substantial and lead to major differences in the resulting 99th percentiles (the CDV). The younger the reference population is and the more stringent the criteria to define cardiac health are, the lower the resulting 99th percentile is.7,12–14
Our findings also have an impact on clinical trials that use AMI as the primary end point and highlight the potential underreporting or overreporting of AMI events, depending on the assay used. That could substantially affect the reporting of AMI rates that test the efficacy or safety of drug compounds being considered for approval in case of imbalance in the assay type. In some trials, the ratio of the peak cTn to the 99th percentile is expressed as a multiple to provide a gross estimate of AMI size. This measure is based on the assumption that the 99th percentiles of different cTn assays are biologically identical. The findings of this study clearly highlight that this assumption seems to be incorrect and accordingly raise serious concerns about the use of this measure in clinical trials.
How could a new regulatory process be designed to provide biologically equivalent CDVs for cTn? It is necessary to examine the problem from several perspectives to find a cut point or cut points that have high sensitivity and specificity in all circumstances. One might consider 2 novel approaches. First, all manufacturers could use the same cohort of healthy individuals for the derivation of the respective 99th percentile of all clinical assays. Although this solution seems rather obvious and has been discussed by laboratory experts, regulators, and diagnostic companies for many years, the lack of data quantifying the limitations of the current regulatory process might well have delayed implementation. Second, if this collaborative effort among different manufacturers fails, an alternative would be to appropriately define the CDV (99th percentile) of 1 hs-cTn assay in a very large, well-characterized cohort of healthy individuals and to perform parallel measurements with all other clinical hs-cTn assays in a disease cohort such as APACE to establish biologically equivalent CDVs, for example, via linear regression or receiver-operating characteristics curve analysis. Our findings are supported by inconsistencies about AMI diagnosis observed with the use of 2 conventional cTn assays applying the 10% CV level as CDVs, which resulted in 11.1% inconsistencies.36
One cannot reiterate often enough the clinical rule that levels of cTn must always be used and interpreted in conjunction with all other clinical information.9 On the other hand, regardless of the clinical presentation, regardless of the ECG findings, and regardless of the findings during coronary angiography, unless there is a rise in cTn above the 99th percentile indicating relevant cardiomyocyte damage, a nonfatal clinical event cannot be classified as AMI. Therefore, despite the clinical rule mentioned above, cTn has become such a crucial diagnostic tool that its regulatory aspects should fulfill the highest standards. Unfortunately, our data clearly show that currently this does not seem to be the case.
Several limitations of this study should be considered in the interpretation of our findings. First, we quantified inconsistencies exclusively in the diagnosis of AMI. It is important to highlight that patients with other diagnoses possibly underlying acute chest pain (eg, pericarditis versus perimyocarditis) also will receive inconsistent diagnoses as a result of the biologically nonequivalent CDVs. However, we are unable to quantify their incidence with sufficient precision. Therefore, this analysis tends to underestimate the clinical implications of biologically nonequivalent CDVs of cTn. Second, our main findings were obtained by measuring in parallel the 2 hs-cTn assays that have been approved for clinical use in Europe and many other countries. Accordingly, the CDVs of these assays are well established. The CDVs of some of the precommercial hs-cTnI assays used for the validation of our findings may be considered less well established. Third, although this study included patients with various types of chronic kidney disease and various degrees of renal dysfunction, patients with terminal kidney disease on chronic hemodialysis were excluded. Therefore, we cannot comment on CDVs of cTnI or cTnT in those patients. Fourth, this study cannot quantify the increase in morbidity or mortality possibly associated with missing these 18% of AMI patients with discordant CDVs, who usually have small AMIs. Given the substantial reduction in morbidity and mortality shown by currently available treatments for AMI, including rhythm monitoring, antiplatelet therapy, high-dose statins, intense lifestyle modifications, and early revascularization,2,23 we assume that overall the harm could be substantial.
Conclusions
Currently approved CDVs for cTn are not biologically equivalent and therefore contribute to major inconsistencies in the diagnosis of AMI. One of 5 AMI patients will receive a diagnosis other than AMI if managed with the alternative hs-cTn assay.
Acknowledgments
We thank the patients who participated in the study, the staff of the EDs, the research coordinators, and the laboratory technicians (particularly Esther Garrido, Irina Klimmeck, Kathrin Meissner, and Fausta Chiaverio) for their most valuable efforts.
Sources of Funding
This study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Roche, Nanosphere, Siemens, 8sense, Bühlmann, and BRAHMS.
Disclosures
Dr Mueller has received research grants from the Swiss National Science Foundation and the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, 8sense, Abbott, ALERE, Brahms, Critical Diagnostics, Nanosphere, Roche, Siemens, and the University Hospital Basel, as well as speaker or consulting honoraria from Abbott, ALERE, Brahms, Cardiorentis, Novartis, Roche, and Siemens. Dr Reichlin has received research grants from the Swiss National Science Foundation (PASMP3-136995), the Swiss Heart Foundation, the University of Basel, the Professor Max Cloetta Foundation, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria from Brahms and Roche. The other authors report no conflicts. The sponsors had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication. Drs Wildi, Gimenez, and Müller had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Drs Wildi and Gimenez contributed equally and should be considered the first author.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.114.014129/-/DC1.
CLINICAL PERSPECTIVE
In an international, prospective, multicenter study, we quantified the incidence of inconsistencies in the diagnosis of acute myocardial infarction (AMI) using fully characterized and clinically available high-sensitivity cardiac troponin (hs-cTn) assays (hs-cTnI, Abbott; hs-cTnT, Roche) among 2300 consecutive patients with suspected AMI. Currently approved clinical decision values are not biologically equivalent and contribute to major inconsistencies in the diagnosis of AMI: Almost 1 of 5 AMI patients had inconsistent diagnoses with the use of hs-cTnI versus hs-cTnT. The incidence of inconsistencies was significantly reduced with clinical decision values that were nearly biologically equivalent, supporting the assumption that half of the observed inconsistencies in the diagnosis of AMI could be avoided by a new regulatory process that ensures that clinical decision values for cTn are biologically equivalent. Patients with inconsistent AMI diagnoses had long-term mortality comparable to that of patients with consistent diagnoses of AMI. This finding suggests that these events require detection and appropriate treatment.
References
- 1.Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;29:1–32. [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e663–e828. doi: 10.1161/CIR.0b013e31828478ac. [DOI] [PubMed] [Google Scholar]
- 3.Hamm CW, Bassand JP, Agewall S, Bax JJ, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D, ESC Committee for Practice Guidelines, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Document R, Achenbach S, Badimon L, Bertrand M, Botker HE, Collet JP, Crea F, Danchin N, Falk E, Goudevenos J, Gulba D, Hambrecht R, Herrmann J, Kastrati A, Kjeldsen K, Kristensen SD, Lancellotti P, Mehilli J, Merkely B, Montalescot G, Neumann FJ, Neyses L, Perk J, Roffi M, Romeo F, Ruda M, Swahn E, Valgimigli M, Vrints CJ, Widimsky P. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation. Eur Hear J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 4.Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med. 2010;17:553–560. doi: 10.1111/j.1553-2712.2010.00729.x. doi: 10.1111/j.1553-2712.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 5.McCabe JM, Armstrong EJ, Kulkarni A, Hoffmayer KS, Bhave PD, Garg S, Patel A, MacGregor JS, Hsue P, Stein JC, Kinlay S, Ganz P. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate-SF registry. Arch Intern Med. 2012;172:864–871. doi: 10.1001/archinternmed.2012.945. doi: 10.1001/archinternmed.2012.945. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 7.Reiter M, Twerenbold R, Reichlin T, Haaf P, Peter F, Meissner J, Hochholzer W, Stelzig C, Freese M, Heinisch C, Breidthardt T, Freidank H, Winkler K, Campodarve I, Gea J, Mueller C. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32:1379–1389. doi: 10.1093/eurheartj/ehr033. doi: 10.1093/eurheartj/ehr033. [DOI] [PubMed] [Google Scholar]
- 8.Koerbin G, Abhayaratna WP, Potter JM, Apple FS, Jaffe AS, Ravalico TH, Hickman PE. Effect of population selection on 99th percentile values for a high sensitivity cardiac troponin I and T assays. Clin Biochem. 2013;46:1636–1643. doi: 10.1016/j.clinbiochem.2013.08.004. doi: 10.1016/j.clinbiochem.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 10.Morrow DA, Antman EM. Evaluation of high-sensitivity assays for cardiac troponin. Clin Chem. 2009;55:5–8. doi: 10.1373/clinchem.2008.117218. doi: 10.1373/clinchem.2008.117218. [DOI] [PubMed] [Google Scholar]
- 11.Paul M. McKie, Heublein DM, Scott CG, Gantzer M Lou, Ramila A. Mehta, Redfield RJRMM, Burnett JC, Jr, Jaffe AS. Defining high-sensitivity cardiac troponin concentrations in the community. Clin Chem. 2013;59:1099–1107. doi: 10.1373/clinchem.2012.198614. [DOI] [PubMed] [Google Scholar]
- 12.Koerbin G, Tate J, Potter JM, Cavanaugh J, Glasgow N, Hickman PE. Characterisation of a highly sensitive troponin I assay and its application to a cardio-healthy population. Clin Chem Lab Med. 2012;50:871–878. doi: 10.1515/cclm-2011-0540. doi: 10.1515/cclm-2011-0540. [DOI] [PubMed] [Google Scholar]
- 13.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–1581. doi: 10.1373/clinchem.2012.192716. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 14.Collinson PO, Heung YM, Gaze D, Boa F, Senior R, Christenson R, Apple FS. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem. 2012;58:219–225. doi: 10.1373/clinchem.2011.171082. doi: 10.1373/clinchem.2011.171082. [DOI] [PubMed] [Google Scholar]
- 15.Reiter M, Twerenbold R, Reichlin T, Haaf P, Peter F, Meissner J, Hochholzer W, Stelzig C, Freese M, Heinisch C, Breidthardt T, Freidank H, Winkler K, Campodarve I, Gea J, Mueller C. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32:1379–1389. doi: 10.1093/eurheartj/ehr033. doi: 10.1093/eurheartj/ehr033. [DOI] [PubMed] [Google Scholar]
- 16.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 17.Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, Bassetti S, Steuer S, Winkler K, Peter F, Meissner J, Haaf P, Potocki M, Drexler B, Osswald S, Mueller C. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 18.Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Gimenez M, Haaf P, Potocki M, Wildi K, Balmelli C, Freese M, Stelzig C, Freidank H, Osswald S, Mueller C. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]
- 19.Cullen L, Mueller C, Parsonage WA, Wildi K, Greenslade JH, Twerenbold R, Aldous S, Meller B, Tate JR, Reichlin T, Hammett CJ, Zellweger C, Ungerer JP, Rubini Gimenez M, Troughton R, Murray K, Brown AF, Mueller M, George P, Mosimann T, Flaws DF, Reiter M, Lamanna A, Haaf P, Pemberton CJ, Richards AM, Chu K, Reid CM, Peacock WF, Jaffe AS, Florkowski C, Deely JM, Than M. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol. 2013;62:1242–1249. doi: 10.1016/j.jacc.2013.02.078. doi: 10.1016/j.jacc.2013.02.078. [DOI] [PubMed] [Google Scholar]
- 20.Kavsak PA, MacRae AR, Yerna MJ, Jaffe AS. Analytic and clinical utility of a next-generation, highly sensitive cardiac troponin I assay for early detection of myocardial injury. Clin Chem. 2009;55:573–577. doi: 10.1373/clinchem.2008.116020. doi: 10.1373/clinchem.2008.116020. [DOI] [PubMed] [Google Scholar]
- 21.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 22.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H, Melanson SE, Panteghini M, Venge P, Zorn M, Jarolim P, Bruton D, Jarausch J, Jaffe AS. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011;412:748–754. doi: 10.1016/j.cca.2010.12.034. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Fröhlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 24.Apple FS, Collinson PO IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–986. doi: 10.1067/mhj.2002.124048. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]
- 27.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH National Academy of Clinical Biochemistry; IFCC Committee for Standardization of Markers of Cardiac Damage. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007;115:e352–e355. doi: 10.1161/CIRCULATIONAHA.107.182881. doi: 10.1161/CIRCULATIONAHA.107.182881. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 29.Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:1086–1090. doi: 10.1373/clinchem.2009.140616. doi: 10.1373/clinchem.2009.140616. [DOI] [PubMed] [Google Scholar]
- 30.Wu AH, Lu QA, Todd J, Moecks J, Wians F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55:52–58. doi: 10.1373/clinchem.2008.107391. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 31.Apple FS, Pearce LA, Smith SW, Kaczmarek JM, Murakami MM. Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem. 2009;55:930–937. doi: 10.1373/clinchem.2008.114728. doi: 10.1373/clinchem.2008.114728. [DOI] [PubMed] [Google Scholar]
- 32.Rubini Gimenez M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Schaefer M, Zellweger C, Moehring B, Stallone F, Sou S, Mueller M, Denhaerynck K, Mosimann T, Reiter M, Freese M, Stelzig C, I K, Voegele J, Hartmann B, Rentsch K, Osswald S, Mueller C. Direct comparison of high-sensitivity cardiac troponin I versus T for the early diagnosis of acute myocardial infarction. Eur Hear J. 2014;35:2303–2311. doi: 10.1093/eurheartj/ehu188. [DOI] [PubMed] [Google Scholar]
- 33.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [Google Scholar]
- 34.Reichlin T, Twerenbold R, Reiter M, Steuer S, Bassetti S, Balmelli C, Winkler K, Kurz S, Stelzig C, Freese M, Drexler B, Haaf P, Zellweger C, Osswald S, Mueller C. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med. 2012;125:1205–1213.e1. doi: 10.1016/j.amjmed.2012.07.015. doi: 10.1016/j.amjmed.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Keller T, Ojeda F, Zeller T, Wild PS, Tzikas S, Sinning CR, Peetz D, Münzel T, Blankenberg S, Lackner KJ. Defining a reference population to determine the 99th percentile of a contemporary sensitive cardiac troponin I assay. Int J Cardiol. 2013;167:1423–1429. doi: 10.1016/j.ijcard.2012.04.063. doi: 10.1016/j.ijcard.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 36.Muhlestein JB, Anderson J, May H, Ilstrup SJ, Bach PR, Lappe D. A real world comparison between the Abbott troponin-I and the Roche troponin-T assays in the assessment of acute myocardial injury. J Am Coll Cardiol. 2013;61:E1155. [Google Scholar]


