Abstract
Background
Outcomes associated with episodes of hypotension while hospitalized are not well understood.
Methods and Results
Using data from ASCEND-HF, we assessed factors associated with inhospital hypotension and subsequent 30-day outcomes. Patients were classified as having symptomatic or asymptomatic hypotension. Multivariable logistic regression was used to determine factors associated with in-hospital hypotension, and Cox proportional hazards models were used to assess the association between hypotension and 30-day outcomes. We also tested for treatment interaction with nesiritide on 30-day outcomes and the association between inhospital hypotension and renal function at hospital discharge. Overall, 1555/7141 (21.8%) patients had an episode of hypotension, of which 73.1% were asymptomatic and 26.9% were symptomatic. Factors strongly associated with in-hospital hypotension included randomization to nesiritide (odds ratio [OR] 1.98, 95% confidence interval [CI] 1.76–2.23; p<0.001), chronic metolazone therapy (OR 1.74, 95% CI 1.17–2.60; p<0.001), and baseline orthopnea (OR 1.31, 95% CI 1.13–1.52; p=0.001) or S3 gallop (OR 1.21, 95% CI 1.06–1.40; p=0.006). In-hospital hypotension was associated with increased hazards of 30-day mortality (hazard ratio [HR] 2.03, 95% CI 1.57–2.61; p<0.001), 30-day heart failure (HF) hospitalization or mortality (HR 1.58, 95% CI 1.34–1.86; p<0.001), and 30-day all-cause hospitalization or mortality (HR 1.40, 95% CI 1.22–1.61; p<0.001). Nesiritide had no interaction on the relationship between hypotension and 30-day outcomes (interaction p=0.874 for death, p=0.908 for death/HF hospitalization, p=0.238 death/all-cause hospitalization).
Conclusions
Hypotension while hospitalized for acute decompensated HF is an independent risk factor for adverse 30-day outcomes, and its occurrence highlights the need for modified treatment strategies.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00475852.
Keywords: acute decompensated heart failure, hypotension, nesiritide
Acute decompensated heart failure (ADHF) is a vulnerable physiologic state characterized by congestion, volume overload, and/or diminished end-organ perfusion. Due to the decompensated state, active diuresis, and the vasoactive therapies used to treat ADHF, these patients are at risk for developing hypotension while hospitalized. However, factors that contribute to in-hospital hypotension and its relationship with clinical outcomes are poorly understood.
Transient hypotension during hospitalization may diminish perfusion to vital organs and lead to end-organ dysfunction with unfavorable clinical outcomes. Prior studies have shown that large drops in systolic blood pressure (BP) from baseline values are associated with declines in renal function in ADHF patients.1,2 Declines in renal function have, in turn, been associated with increases in mortality and rehospitalization. However, the independent association of in-hospital hypotensive episodes and outcomes has not been well studied.
The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) was a large, multicenter trial that randomized patients with baseline systolic BP ≥ 100 mm Hg to receive nesiritide or placebo in addition to standard care. ASCEND-HF study investigators reported and characterized in-hospital episodes of hypotension as symptomatic or asymptomatic and recorded nadir BP values. Overall, nesiritide therapy led to higher rates of both symptomatic and asymptomatic hypotension compared with placebo, but nesiritide treatment did not change 30-day rehospitalization or mortality.3 However, the overall outcomes associated with episodes of in-hospital hypotension have not yet been examined. To better understand the clinical implications of in-hospital hypotension, we performed a post-hoc analysis of ASCEND-HF to examine patient factors associated with hypotension and the association between hypotension and 30-day outcomes.
Methods
Study Design
The study design and results of the ASCEND-HF study have been previously reported.3,4 IRB approval was obtained locally at all individual sites, and enrolled subjects provided informed consent. Briefly, 7141 patients were randomized to nesiritide or placebo within 24 hours of first intravenous (IV) therapy for ADHF. Study participants were required to have the following: dyspnea at rest or minimal activity, 1 or more accompanying signs (respiratory rate ≥ 20 breaths/minute or pulmonary congestion or edema with rales one-third or greater up the lung fields), and 1 or more objective measures of HF (congestion or edema on chest radiograph, B-type natriuretic peptide [BNP] ≥ 400 pg/mL or N-terminal pro-BNP ≥ 1000 pg/mL, pulmonary capillary wedge pressure >20 mm HG, or left ventricular ejection fraction <40% in previously 12 months).4 Key exclusions relevant to this study were “high risk” of hypotension (systolic pressure <100 mm Hg or 110 mm Hg with use of intravenous nitroglycerin), contraindications for vasodilators, unstable dose of IV vasoactive medications, milrinone or levosimendan therapy in the prior 30 days, and dobutamine treatment at ≥ 5 μg/mg/min.
Hypotension
Because heart failure patients have different baseline blood pressures, the trial protocol specified that investigators report hypotension based on clinical judgment per routine clinical care compared with ambulatory blood pressure. If there was uncertainty about a patient’s baseline blood pressure, then the guidance was to classify hypotension based on a systolic BP of <90 mm Hg. Site investigators/clinicians determined whether an episode was clinically significant based on duration and standard definitions of symptoms, including lightheadedness, dizziness, feeling faint, blurred vision, auditory disturbances (such as tinnitus), emesis, or syncope. Clinically significant in duration was defined at the discretion of individual site investigators per standard of care. Investigators were trained to repeat blood pressure measurements if hypotension occurred and monitor repeat measurements until hypotension resolved per standard of care. For the purposes of this analysis, site investigators reported the lowest BP and time of hypotension for episodes occurring after randomization and before hospital discharge. If multiple episodes of either asymptomatic or symptomatic hypotension occurred, the investigators reported the most severe or lowest blood pressure among episodes. For patients that had both symptomatic and asymptomatic hypotension, data from the first event were utilized for further analysis. Routine BP measurements were taken at 0.5, 1, 3, 6, 24, and then every 24 hours thereafter post-randomization while on treatment, at the end of treatment, and hospital discharge. BP was otherwise monitored according to each hospital’s standard practice. We also performed an analysis using a numerical definition for hypotension by evaluating 30-day death and 30-day death or HF hospitalization among patients with SBP <90mmHg after study drug start.
Statistical Analysis
Categorical variables were reported as numbers and percentages and continuous variables were reported as medians with interquartile ranges or means ± standard deviations. Baseline characteristics were compared using the Wilcoxon Rank-Sum test for continuous variables and the chi-square test for categorical variables.
Logistic regression assessed the associations of baseline factors with risk of developing in-hospital hypotension. Baseline characteristics were selected using stepwise selection from a list of candidate variables chosen based on clinical review and prior publications in similar patient cohorts (see Supplemental methods).
Time-dependent Cox proportional hazards models were used to evaluate the association of in-hospital hypotension and 30-day clinical outcomes, including death, the composite of death or HF rehospitalization, and the composite of death or all-cause rehospitalization. The model was adjusted for variables previously found to be associated with risk of these outcomes in the ASCEND-HF cohort. These variables were selected by stepwise logistic regression from a larger list of possible clinically relevant variables on 25 imputed datasets. This analysis results in age, blood urea nitrogen (BUN), sodium, and SBP being included for all models. For 30-day mortality/HF hospitalization/all-cause hospitalization models, the following additional variables were included: creatinine, cerebrovascular disease, depression, hospitalization in the last year, elevated jugular venous pressure, and chronic respiratory disease. The 30-day mortality and 30-day mortality/HF hospitalization models additionally included dyspnea with minimal exertion, and the 30-day mortality/all-cause hospitalization model additionally included baseline weight. We further assessed whether there was a differential association of hypotension with outcomes by the randomized treatment using an interaction between hypotension and nesiritide therapy in adjusted models. The same methods were used in the sensitivity analysis, which included all patients who experienced SBP <90mmHg within 24 hours of study drug start.
Lastly, we assessed the relationship between in-hospital hypotension and renal function measured by creatinine at discharge or day 10, whichever came first. Renal function was assessed at pre-specified sampling intervals including at study enrollment, 24 hours, at end of study drug infusion, and the earlier of discharge or hospital day 10. Given that the main interest was to understand the exposure of hypotension as it related to the outcome of renal dysfunction, it was important to have a distinct period of time that did not overlap since it is difficult to assess the contribution to an outcome if there is overlap between exposure and outcome. Therefore we defined the exposure of interest, namely hypotension, within the first 48 hours since the highest frequency of hypotension occurred during this time period. To have standardized outcomes temporally from the exposure of hypotension, renal function which was the outcome of interest was assessed at discharge or day 10. This provided information on the most recent renal function before discharge. Differences in renal function from baseline among those experiencing versus not experiencing hypotension in the first 48 hours were assessed by linear regression with adjustment for previously identified variables shown to be related to baseline renal function in the ASCEND-HF cohort (namely BUN, age, SBP, creatinine, potassium, and weight gain).
To optimize utilization of the ASCEND-HF cohort for these analyses, multiple imputation was implemented. Those with missing baseline data had values imputed using Markov Chain Monte Carlo and regression methods. Most variables had less than 1% missing rate; only 4 variables had rates greater than 10% missing (namely qualifying episode x-ray, baseline EF, New York Heart Association class, and baseline QRS duration). In analyses requiring variable selection, variables were accepted into the final model if selected in at least 85% of the imputed datasets. Final estimates and associated standard errors reflect the combined analysis over 25 imputed data sets and account for the reduction in information due to missing values.
Results
Baseline Characteristics
Of the 7141 patients enrolled in the overall trial, 1555 patients (21.8%) patients had an episode of hypotension. Of these, 1136 (73.1%) had asymptomatic, 302 (19.4%) had symptomatic, and 117 (7.5%) had both symptomatic and asymptomatic hypotension. Baseline characteristics are listed in Table 1. Nearly two-thirds (62.2%) of patients experiencing an episode of hypotension were randomized to nesiritide therapy. The median systolic hypotensive BP for patients with symptomatic and asymptomatic hypotension was 80 mm Hg (IQR 70–87) and 83 mm Hg (IQR 79–88), respectively. The median time to hypotension was 17.2 hours (IQR 5.2–30.8) after randomization with a range from 0 to 394 hours (Figure 1). Compared with patients who did not have hypotension, subjects experiencing hypotension had lower baseline systolic and diastolic BPs, lower weight, higher baseline BNP but not NT-pro-BNP, more frequent history of myocardial infarction, more frequent history of atrial and ventricular arrhythmia, and less frequent history of pre-existing hypertension. Historical and physical examination findings of volume overload such as orthopnea, jugular venous distension, or S3 gallop were present in a higher percentage of persons who experienced hypotension than in those who did not (Table 1). The frequency of several baseline medications was statistically different among patients with hypotension compared with those without hypotension, and regional differences in reported incidence of hypotension were also observed (Table 1).
Table 1.
Baseline characteristics
| Variable | Overall Trial (N=7,141) | No Hypotension (n=5586) | Hypotension (n=1555) | P Value |
|---|---|---|---|---|
| Female Sex | 34.2 | 34.4 | 33.8 | 0.664 |
| Age | 67 (56, 76) | 67 (56, 76) | 67 (55, 77) | 0.681 |
| Race | 0.063 | |||
| White | 55.9 | 55.2 | 58.4 | |
| Black | 15.1 | 15.4 | 14.1 | |
| Asian | 24.8 | 25.3 | 22.8 | |
| Other | 4.3 | 4.2 | 4.7 | |
| Medical history | ||||
| Prior MI | 34.9 | 33.9 | 38.5 | 0.001 |
| Hypertension | 72.1 | 74.0 | 65.5 | <0.001 |
| AF | 37.4 | 36.8 | 39.7 | 0.040 |
| Ventricular tachycardia | 8.9 | 8.2 | 11.6 | <0.001 |
| PAD | 10.4 | 10.7 | 9.1 | 0.058 |
| Diabetes mellitus | 42.7 | 44.1 | 37.6 | <0.001 |
| Chronic respiratory disease | 16.5 | 16.0 | 18.4 | 0.023 |
| Measurements | ||||
| Weight, kg | 78 (64, 95) | 78.7 (65, 95.4) | 76 (62.6, 92.2) | <0.001 |
| Temperature (C) | 36.6 (36.3, 36.9) | 36.6 (36.3, 36.8) | 36.6 (36.2, 36.9) | 0.186 |
| Baseline BP, mm Hg | ||||
| Systolic | 123 (110, 140) | 126 (112, 140) | 115 (105, 130) | <0.001 |
| Diastolic | 74 (66.0, 83.0) | 75 (68, 85) | 70 (63, 80) | <0.001 |
| Heart rate, bpm | 82 (72, 95) | 82 (71, 95) | 82 (73, 95) | 0.065 |
| Respiratory rate | 23 (21, 26) | 23 (21, 26) | 23 (20, 26) | 0.777 |
| BNP (pg/mL)* | 990 (544, 1850) | 962 (530, 1820) | 1103 (597, 1968) | 0.012 |
| NT-pro-BNP (pg/mL)* | 4501 (2098, 9177) | 4461 (2110, 9229) | 4771 (2041, 9081) | 0.828 |
| Creatinine (mg/dL) | 1.2 (1.0, 1.6) | 1.2 (1, 1.6) | 1.2 (1, 1.5) | 0.598 |
| BUN (mg/dL) | 25.8 (18, 39.1) | 25.3 (18.0, 38.1) | 26.1 (18, 41.1) | 0.036 |
| Baseline sodium (mmol/L) | 139 (136, 141) | 139 (136, 141) | 138 (135, 141) | <0.001 |
| EF, % | 30 (20, 39) | 30 (22, 40) | 28 (20, 37) | <0.001 |
| EF ≤40% | 79.3 | 78.5 | 82.1 | 0.002 |
| Characteristics | ||||
| Orthopnea | 76.9 | 75.9 | 80.7 | <0.001 |
| JVD, MVR, or S3 gallop | 70.5 | 69.1 | 75.2 | <0.001 |
| X-ray showing pulmonary congestion | 80.7 | 81.5 | 77.6 | <0.001 |
| Region | <0.001 | |||
| North America | 45.4 | 43.6 | 51.8 | |
| Asia Pacific | 24.7 | 25.2 | 22.9 | |
| Latin America | 9.3 | 9.3 | 9.4 | |
| Central Europe | 13.5 | 15.2 | 7.6 | |
| Western Europe | 7.1 | 6.7 | 8.3 | |
| Baseline Medications | ||||
| ACE inhibitor or ARB | 60.8 | 60.2 | 62.9 | 0.056 |
| Beta-blocker | 58.2 | 57.3 | 61.7 | 0.002 |
| Aldosterone blocker | 27.9 | 27.0 | 30.9 | 0.003 |
| Loop diuretic | 95.1 | 95.0 | 95.4 | 0.576 |
| Calcium channel blocker | 12.9 | 14.0 | 9.2 | <0.001 |
| Chronic bumetanide | 2.5 | 2.6 | 2.2 | 0.342 |
| Chronic metolazone | 1.7 | 1.4 | 2.9 | <0.001 |
| Pre-randomization oral/topical nitrate | 23.5 | 24.1 | 21.4 | 0.021 |
| Pre-randomization IV vasodilator | 14.8 | 15.4 | 12.7 | 0.007 |
| Planned in-hospital treatment (ITT) | <0.001 | |||
| Nesiritide | 49.9 | 46.5 | 62.2 | |
| Placebo | 50.1 | 53.5 | 37.8 | |
| Bolus dose of nesiritide | 60.7 | 64.2 | 48.0 | <0.001 |
Values represent percentages or medians (interquartile range). PAD=peripheral arterial disease. BNP values were measured at individual sites, while NT-pro-BNP was measured at a core lab.
Figure 1.
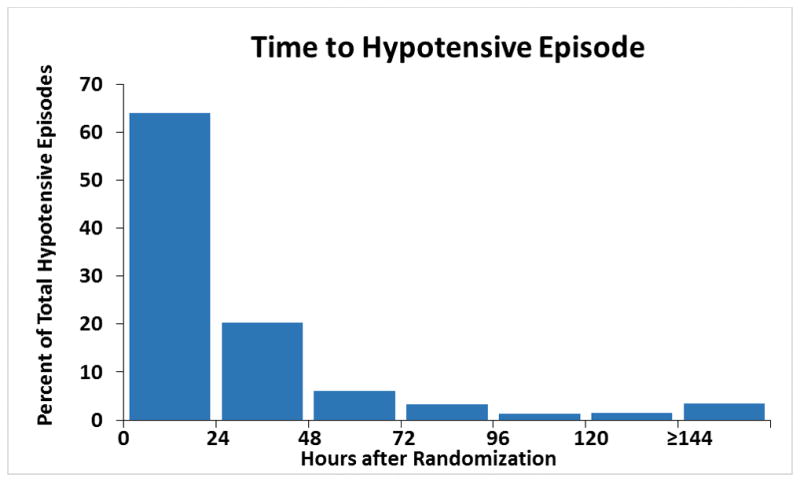
Time to first hypotensive episode from study randomization. Time from randomization in hours to any reported episode of hypotension among 1,555 patients with reported hypotension.
Variables Associated with Hypotension
Stepwise logistic regression was used to identify factors associated with a hypotensive episode. On multivariable analysis, several variables were predictive of hypotension (Table 2). The c-statistic for the final model was 0.708. Randomization to nesiritide was associated with hypotension (odds ratio [OR] 1.98, 95% confidence interval [CI] 1.76–2.23; p <0.001), along with chronic metolazone therapy (OR 1.74, 95% CI 1.17–2.60; p=0.007). However, chronic bumetanide therapy (OR 0.52, 95% CI 0.35–0.78; p=0.002) and pre-randomization calcium channel blocker therapy (OR 0.70, 95% CI 0.57–0.85; p<0.001) were less associated with hypotension. Several patient characteristics such as baseline orthopnea (OR 1.31, CI 1.13–1.52; p=0.001), baseline S3 gallop (OR 1.21, 95% CI 1.06–1.40; p=0.006), and temperature >36.4C (OR 1.38, 95% CI 1.12–1.72; p=0.003) were associated with hypotension. There was also a significant regional association for hypotension (p<0.001). Compared with North America, Central European region (OR 0.49, 95% CI 0.39–0.61), Latin American region (OR 0.78, 95% CI 0.63–0.97), and Asia Pacific region (OR 0.63, 95% CI 0.54–0.74), had less association with hypotension, while Western European region had no significant difference (OR 1.07, 95% CI 0.85–1.35). Other variables, such as BNP, NT-pro-BNP, and pre-randomization vasodilator therapy were tested but were not found to be significant after multivariable adjustment. Because of concerns regarding colinearity, both race and region were tested independently in our regression model, and region was a slightly stronger predictor of hypotension than race (c-statistic 0.708 vs. 0.702).
Table 2.
Multivariable associations with an episode of in-hospital hypotension
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Age (per 10 years over age >60) | 1.15 | 1.07, 1.22 | <0.001 |
| Measurements | |||
| Temperature >36.4C (for each degree >36.4C) | 1.38 | 1.12, 1.72 | 0.003 |
| SBP≤120 mm Hg (per each 5 mm Hg increase) | 0.79 | 0.76, 0.82 | <0.001 |
| SBP >120 mm Hg (per each 5 mm Hg increase) | 0.91 | 0.88, 0.93 | <0.001 |
| Heart rate≤75 bpm (per 5 beats) | 1.10 | 1.04, 1.17 | 0.001 |
| Baseline sodium≤145 mmol/L (per each 5 mmol/L increase) | 0.87 | 0.82, 0.93 | <0.001 |
| Characteristics | |||
| Baseline orthopnea | 1.31 | 1.13, 1.52 | <0.001 |
| Baseline S3 gallop | 1.21 | 1.06, 1.40 | 0.006 |
| Region (compared with North America) | <0. 001 | ||
| Asia Pacific | 0.63 | 0.54, 0.74 | |
| Central Europe | 0.49 | 0.39, 0.61 | |
| Latin America | 0.78 | 0.63, 0.97 | |
| Western Europe | 1.07 | 0.85, 1.35 | |
| Medications | |||
| Randomization to nesiritide | 1.98 | 1.76, 2.23 | <0.001 |
| Chronic bumetanide | 0.52 | 0.35, 0.78 | 0.002 |
| Pre-randomization calcium channel blocker | 0.70 | 0.57, 0.85 | <0.001 |
| Chronic metolazone | 1.74 | 1.17, 2.60 | 0.007 |
Association with Outcomes
Patients who experienced an episode of hypotension during hospitalization had a higher 30-day mortality risk (7.1 vs. 2.9% for no hypotension; p<0.001), risk of 30-day all-cause mortality or HF hospitalization (15.2 vs. 8.4%; p<0.001), and risk of 30-day mortality or all-cause hospitalization (21.1 vs. 13.8%; p <0.001) (Table 3). Patients with symptomatic hypotension had a higher risk of 30-day mortality than those with asymptomatic hypotension (11.6% vs. 5.6%), though there was no major difference for the composite of 30-day mortality or HF hospitalization (15.4% vs. 14.4%) or 30-day mortality or all-cause hospitalization (20.5% vs. 20.3%). Cause of death among those with symptomatic and asymptomatic hypotension was primarily due to worsening heart failure (67.2% among those with asymptomatic hypotension and 80.8% among those with symptomatic hypotension).
Table 3.
Adjusted outcomes in patients with and without hypotension*
| Outcome | Total | No Hypotension | Hypotension | Adjusted HR | 95% CI | Cox P Value |
|---|---|---|---|---|---|---|
| 30-day mortality | 273/7118 (3.8) | 162/5565 (2.9) | 111/1553 (7.1) | 2.03 | 1.57, 2.61 | <0.001 |
| 30-day mortality or HF rehospitalization | 686/6938 (9.9) | 455/5422 (8.4) | 231/1516 (15.2) | 1.58 | 1.34, 1.86 | <0.001 |
| 30-day mortality or all-cause hospitalization | 1067/6942 (15.4) | 747/5424 (13.8) | 320/1518 (21.1) | 1.40 | 1.22, 1.61 | <0.001 |
Values presented as n/N (%), unless otherwise indicated. Please see methods section for adjustment variables.
Test for interaction of nesiritide on relationship of in-hospital hypotension and 30-day outcomes: 30-day mortality, p=0.874; 30-day mortality/HF hospitalization, p=0.908; 30-day mortality/all-cause hospitalization, p=0.238.
After multivariable adjustment, investigator reported in-hospital hypotension was still associated with an increased hazard of 30-day mortality (HR 2.03, 95% CI 1.57–2.61; p<0.001), 30-day mortality or HF hospitalization (HR 1.58, 95% CI 1.34–1.86; p<0.001), and 30-day mortality or all-cause hospitalization (HR 1.40, 95% CI 1.22–1.61; p<0.001) (Table 3). To confirm these results using a numeric definition of hypotension, we performed an analysis to assess adjusted outcomes among patients with SBP <90mmHg within 24 hours of study drug start. The adjusted outcomes using a numeric definition for hypotension (30-day mortality HR 2.01, 95% CI 1.45–2.78 and 30-day mortality or HF hospitalization HR 1.49, 95% CI 1.19–1.87) were similar to those reported for investigator reported hypotension.
To test whether nesiritide therapy affected the relationship between hypotension and 30-day outcomes, we tested for the interaction between assignment to nesiritide and hypotension for 30-day outcomes. No statistical interaction was found for the 3 30-day outcomes (interaction p=0.874, p=0.908, p=0.238 for death, death/HF hospitalization, and death/all-cause hospitalization), indicating that nesiritide did not alter the relationship between hypotension and 30-day outcomes.
Relationship between Hypotension and Renal Function
Of 1555 patients experiencing hypotension, 1303 (83.8%) experienced it within the first 48 hours of randomization. Among patients experiencing hypotension within the first 48 hours compared with those not experiencing hypotension while hospitalized, creatinine values were similar at baseline (1.20 [IQR 1.00–1.50] vs. 1.24 [IQR 1.00–1.60]) and discharge/10 days (1.25 [IQR 1.00–1.60] vs. 1.29 [IQR 1.01–1.64]) (Figure 2). There was no relationship between hypotension in the first 48 hours of randomization and creatinine value at discharge/day 10 after multivariable adjustment (p=0.366).
Figure 2.
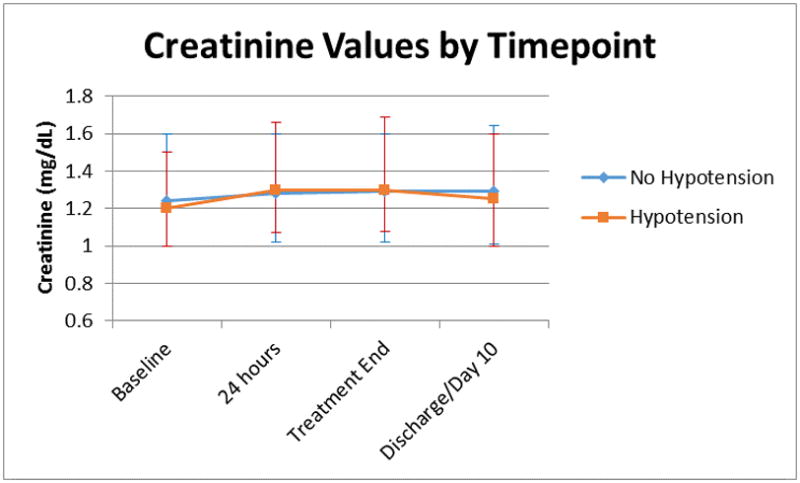
Median creatinine values among patients with versus without hypotension. Values represent the median creatinine values at various timepoints among the 1303 patients experiencing hypotension within 48 hours of randomization. Error bars represent interquartile range.
Discussion
In a large randomized, placebo-controlled trial in patients with ADHF, we examined the occurrence and consequences of episodic hypotension during hospitalization. Hypotensive episodes were common, occurring in >20% of patients despite trial exclusion of patients who were felt to be at high risk for hypotension. While the majority of these episodes were asymptomatic, hypotension whether asymptomatic or symptomatic, was associated with worse 30-day outcomes. Therefore, any episode of hypotension in the setting of ADHF should be treated as a significant event with subsequent strategies of care aimed at mitigating the risk for future clinical events.
In patients with ADHF, BP varies considerably during the course of hospitalization, and it is established that hypotension at hospital presentation is associated with poor outcomes.5–10 However, 75% of patients with ADHF do not have low BP at the time of hospitalization, yet they remain at risk for hypotension while hospitalized. Partly, this may reflect hemodynamic instability from the decompensated state and the loss of adrenergic tone, contributed in part by neurohormonal blocking agents and vasodilators which are the mainstays of evidence-based HF therapy and can lead to substantial BP reductions.5,8,9,11 In such cases, physicians often tolerate transient hypotension in the hope of improving symptoms and longer-term outcomes. Prior randomized trials have not investigated the impact of post-randomization hypotension on subsequent clinical outcomes, and our analysis of ASCEND-HF demonstrates that post-enrollment, in-hospital hypotension is significantly associated with adverse 30-day outcomes.
Even though randomization to nesiritide therapy was strongly associated with in-hospital hypotension, nesiritide treatment had no effect on the association between hypotension and 30-day outcomes. This suggests that the relationship between hypotension and outcomes is independent of treatment. In a broader context, this study demonstrates the importance of large clinical trials for examining outcomes by highlighting that one cannot assume that a linear pathway exists from treatment to a surrogate endpoint such as hypotension or worsening renal function to an outcome, and that we must fully test these relationships to prevent making premature assumptions.
For example, in ADHF patients, hypotension and passive congestion, neurohormonal activation, oxidative stress, and inflammation all combine to promote kidney injury. Hypotension may contribute to worsening renal function and adverse longitudinal outcomes, as suggested by data from EVEREST showing that renal function declined in proportion to the magnitude of SBP decrease and data from Pre-RELAX-AHF showing that worsening renal function is an independent predictor of 60- and 90-day mortality.1,2 However, this analysis of ASCEND-HF failed to show differences in renal function among patients with and without episodic hypotension at any time point even after adjustment for variables known to affect renal function. Though we did not assess the magnitude of SBP change, the lengths of hospitalizations among trial participants varied, and therapeutic decisions may have corrected transient renal dysfunction, our data show that in-hospital episodes of hypotension had no clinically meaningful impact on renal function at hospital discharge. It is possible that more frequent serial measurements of renal function or use of highly sensitive biomarkers such as cystatin c might have identified subtle differences in renal function. While hypoperfusion injury to other organs might explain the association with poor outcomes, our data suggest that in-hospital hypotension is a clinical marker for HF severity and that other unknown mechanisms may have contributed to the poorer outcomes among those with hypotension.
Factors associated with hypotension in ADHF have not been well-studied and have shown inconsistent results.8,12 In this study, nesiritide therapy, age, baseline orthopnea and S3 gallop, and chronic metolazone therapy were found to contribute to the risk of hypotension. Orthopnea and S3 gallop are symptoms and signs of congestion and volume overload, and may identify persons that have a poor prognosis.7,13 Moreover, chronic metolazone therapy is usually reserved for patients with refractory volume overload who may have more advanced HF. Hence, patients that have more severe illness may be at higher risk for hypotension. In addition, univariate descriptive statistics also indicated that there was less use of nesiritide bolus and pre-randomization IV vasodilators among those experiencing hypotension, while pre-randomization calcium channel blocker therapy was among variables associated with less hypotension. This could reflect confounding by indication, whereby patients with pre-existing hypertension or higher baseline blood pressure may be more likely to receive afterload reducing therapies and possibly less susceptible to hypotension. Taken in concert, we suspect that patients that appear to have unfavorable clinical indicators may be less likely to receive therapies such as calcium channel blockers or IV vasodilators due to concerns for adverse effects such as hypotension. Such treatment bias agrees with our hypothesis and study findings that patients with prominent physical findings of volume overload are more likely to have hypotension.
Regional differences in this global trial deserve comment, with Central European, Latin American, and Asia Pacific regions showing less association with hypotension. In our regression models, we did assess for colinearity between race and region; region was more strongly associated with reporting of hypotensive episodes than race. Regional variations in practice patterns and patient demographics have previously been observed. For example, the multinational EVEREST trial found that there was significant variation in medical, interventional, and device therapies across different global regions.14 In addition, South America and Eastern Europe both tended to enroll younger patients, and Eastern Europe reported the highest baseline SBP, highest baseline EF, and better baseline renal function values than other regions, suggesting that their overall population in the EVEREST trial was healthier at study enrollment. As such, Eastern Europe had the lowest adjusted rates of cardiovascular death and HF hospitalization.14 Also, data from the ADHERE-International Asia-Pacific registry showed that the Asia-Pacific region tended to have higher use of inotropes and a potentially less comorbid population of patients than other parts of the world, with less reported prevalence of coronary disease, chronic kidney disease, and atrial fibrillation than similar observational cohorts in North America and Europe.15 These differences in patient populations and treatment patterns could have had an impact on real or reported episodes of hypotension and highlight the importance of considering regional differences in the interpretation of clinical trial data.
Implications
Our results show that in-hospital hypotension is associated with increased 30-day hospitalization and mortality, regardless of study drug assignment. Further, these observations suggest that individuals who have hemodynamic instability are at high risk for subsequent adverse outcomes. This is important because many of the therapies that are currently used or are being studied for ADHF affect systemic vascular resistance and can induce hypotension. Many trials of vasodilator therapies and inotropes for ADHF have shown neutral or negative results,3,16,17 perhaps at least partially due to the association between in-hospital hypotension and adverse outcomes. Taking a broad interpretation, we believe physicians should use caution when using or studying vasoactive therapies that can lower BP, with a focus on avoidance, early detection, and correction of hypotension. While it is easy to ignore an asymptomatic episode of hypotension, the strong association of hypotension with adverse 30-day outcomes should give pause and compel a thoughtful, tailored approach to risk stratify and mitigate every patients’ risk for negative outcomes, such as optimization of neurohormonal therapies and device therapies prior to hospital discharge and early post-hospital follow-up. Future clinical trials and clinical registries should incorporate data collection for inpatient hypotensive episodes to further clarify the association between episodic hypotension and unfavorable outcomes.
Study Limitations
Several limitations for this study must be acknowledged. This is a secondary analysis of the ASCEND-HF study, which was not specifically designed to study the effects of hypotension on outcomes. Therefore, this study is observational and describes associations between treatment, hypotension, and outcomes. No causality can be proven, so the results are hypothesis-generating. Nevertheless, since hypotension is associated with adverse outcomes and nesiritide is strongly associated with hypotension, residual concern for an association between nesiritide-induced hypotension and adverse outcomes remains.18–22 While patients with symptomatic hypotension had a higher risk of 30-day mortality than those with asymptomatic hypotension, there was no major difference in the composite of 30-day mortality/all-cause or HF hospitalization, suggesting that there was perhaps a higher rate of re-hospitalization among those with asymptomatic than symptomatic hypotension. The reasons for similar risk for the composite may have been due to differences in mortality and length of stay, or from play of chance for this subgroup analysis. Patients with symptomatic hypotension had greater 30-day mortality (11.6% vs. 5.6%) and longer lengths of stay (median 7 days, IQR 4–13 vs. median 6 days, IQR 4–10) than those with asymptomatic hypotension. Since length of stay was shorter and mortality was greater among those with symptomatic hypotension, the exposure period for risk of readmission was shorter, possibly leading to similar rates for the composite of death/readmission at 30-days. Also, hypotension was defined by site investigators relative to baseline clinical status, which may have affected reporting of events. Nevertheless, our sensitivity analysis showed that patients with a SBP <90mmHg within 24 hours of study drug start have similar adjusted outcomes as patients with investigator reported hypotension, which further supports that low blood pressure while hospitalized with ADHF is associated with poor outcomes. In addition, even though adjusted analysis was performed to minimize the possibility of confounders, all confounding factors may not have been identified. Finally, since ASCEND-HF was a large study, baseline differences in patients with and without hypotension may have been statistically different without being clinically meaningful, so caution must be used when interpreting the results.
Summary
Hypotension is common during hospitalization for ADHF and is an important, independent predictor of adverse 30-day outcomes. Several patient factors such as concurrent medication use and severity of HF are associated with the risk of hypotension. In-hospital hypotension should be more widely recognized as an unfavorable prognostic factor, regardless of the cause for hypotension.
Supplementary Material
Acknowledgments
The co-authors thank Elizabeth Cook for her editorial assistance in the preparation of this manuscript.
Sources of Funding
ASCEND-HF was supported by Johnson & Johnson. PA Patel was supported by grants from the NIH T32-HL007101 and by internal funding from Duke Clinical Research Institute.
Footnotes
Disclosures
PA Patel, G Heizer, PJ Schulte, K Dickstein, V Hasselblad, and JJV McMurray report no relevant disclosures. CM O’Connor reports consulting fees from Novella and Amgen, ownership/partnership/principal in Biscardia, LLC, and research support from Otsuka, Roche Diagnostics, BG Medicine, Critical Diagnostics, Astellas, Gilead, GE Healthcare, and ResMed. RM Mills is a full-time employee of Janssen Research & Development, LLC. JA Ezekowitz reports consulting fees from Pfizer, Abbott Labs, Servier, and research support from Amgen and Johnson and Johnson. PW Armstrong reports research support from Johnson & Johnson. RC Starling reports consulting fees from Novartis, BioControl, and Medtronic, ownership/partnership/principal in Cardiomems, research support from the National Institutes of Health, Medtronic, Biotronik, Novartis, and Thoratec, and receipt of benefits from the American Board of Internal Medicine. WHW Tang receives research support from the US National Institutes of Health. RM Califf reports consulting fees from KOWA, Eli Lilly, Glaxo Smith-Kline, WebMD, Bristol-Myers-Squibb, Nitrox LLC, Bayer, Orexigen Therapeutics, Sanofi-Aventis, Medtronic, Boehringer Ingelheim, and Gilead and research support from BMS, Roche, Merck, Novartis, Scios/Johnson and Johnson, Amilyn, Bristol-Myers-Squibb, and Bayer. AF Hernandez reports consulting fees from Sanofi, Johnson and Johnson, AstraZeneca, and Corthera and research support from Amylin and Scios/Johnson and Johnson.
References
- 1.Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G, Teerlink JR, Greenberg BH, Filippatos G, Teichman SL, Metra M. Early drop in systolic blood pressure and worsening renal function in acute heart failure: Renal results of pre-relax-ahf. Eur J Heart Fail. 2011;13:961–967. doi: 10.1093/eurjhf/hfr060. [DOI] [PubMed] [Google Scholar]
- 2.Blair JE, Pang PS, Schrier RW, Metra M, Traver B, Cook T, Campia U, Ambrosy A, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Konstam MA, Gheorghiade M. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the everest trial. Eur Heart J. 2011;32:2563–2572. doi: 10.1093/eurheartj/ehr238. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez AF, O’Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale and design of the acute study of clinical effectiveness of nesiritide in decompensated heart failure trial (ASCEND-HF) Am Heart J. 2009;157:271–277. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, Fonarow GC, Masoudi FA. A validated risk score for in-hospital mortality in patients with heart failure from the american heart association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3:25–32. doi: 10.1161/CIRCOUTCOMES.109.854877. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O’Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Pang PS, Ambrosy AP, Lan G, Schmidt P, Filippatos G, Konstam M, Swedberg K, Cook T, Traver B, Maggioni A, Burnett J, Grinfeld L, Udelson J, Zannad F. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: Analysis from the everest trial. Heart Fail Rev. 2012;17:485–509. doi: 10.1007/s10741-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 9.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 10.Oliva F, Mortara A, Cacciatore G, Chinaglia A, Di Lenarda A, Gorini M, Metra M, Senni M, Maggioni AP, Tavazzi L. Acute heart failure patient profiles, management and inhospital outcome: Results of the italian registry on heart failure outcome. Eur J Heart Fail. 2012;14:1208–1217. doi: 10.1093/eurjhf/hfs117. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Vaduganathan M, Ambrosy A, Bohm M, Campia U, Cleland JG, Fedele F, Fonarow GC, Maggioni AP, Mebazaa A, Mehra M, Metra M, Nodari S, Pang PS, Ponikowski P, Sabbah HN, Komajda M, Butler J. Current management and future directions for the treatment of patients hospitalized for heart failure with low blood pressure. Heart Fail Rev. 2013;18:107–122. doi: 10.1007/s10741-012-9315-1. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RJ, Spencer FA, Szklo-Coxe M, Tisminetzky M, Yarzebski J, Lessard D, Gore JM, Gaasch W. Symptom presentation in patients hospitalized with acute heart failure. Clin Cardiol. 2010;33:E73–80. doi: 10.1002/clc.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC, Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the everest (efficacy of vasopressin antagonism in heart failure: Outcome study with tolvaptan) program. J Am Coll Cardiol. 2008;52:1640–1648. doi: 10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 15.Atherton JJ, Hayward CS, Wan Ahmad WA, Kwok B, Jorge J, Hernandez AF, Liang L, Kociol RD, Krum H. Patient characteristics from a regional multicenter database of acute decompensated heart failure in asia pacific (adhere international-asia pacific) J Card Fail. 2012;18:82–88. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 17.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE, Jr, Wheeler W, Soler-Soler J, Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The flolan international randomized survival trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 18.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide study group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 19.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39:798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 20.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 21.Peacock WF, Emerman CL, Silver MA. Nesiritide added to standard care favorably reduces systolic blood pressure compared with standard care alone in patients with acute decompensated heart failure. Am J Emerg Med. 2005;23:327–331. doi: 10.1016/j.ajem.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Yancy CW, Krum H, Massie BM, Silver MA, Stevenson LW, Cheng M, Kim SS, Evans R. Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: Results of the second follow-up serial infusions of nesiritide (FUSION II) trial. Circ Heart Fail. 2008;1:9–16. doi: 10.1161/CIRCHEARTFAILURE.108.767483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


