This article considers the effects of Ebola virus disease on radiology departments and imaging equipment, with particular emphasis on currently available guidelines that may be applicable to radiology service delivery for maximizing the safety of patients and staff.
Abstract
The overlap of early Ebola virus disease (EVD) symptoms (eg, fever, headache, abdominal pain, diarrhea, emesis, and fatigue) with symptoms of other more common travel-related diseases (eg, malaria, typhoid fever, pneumonia, and meningococcemia) may result in delayed diagnosis of EVD before isolation of infected patients. Radiology departments should consider policies for and approaches to decontamination of expensive and potentially easily damaged radiology equipment. In addition, the protection of radiology personnel must be considered during the work-up phase of undiagnosed EVD patients presenting to emergency departments. The purpose of this article is to consider the effect of EVD on radiology departments and imaging equipment, with particular consideration of guidelines currently available from the Centers for Disease Control and Prevention that may be applicable to radiology.
© RSNA, 2015
Introduction
The current Ebola virus disease (EVD) outbreak has a recorded death toll of more than 8000 people and a case fatality rate of approximately 40%–50% (1). The World Health Organization estimated that 3%–4% of EVD infections have occurred in health care personnel (2). Thus, key components of EVD preparedness at U.S. health care institutions are training staff and ensuring that appropriate facilities, policies, and equipment are in place. Medical imaging does not provide diagnosis of EVD but may serve to help triage patients and assess complications of the disease. Patient assessment in the emergency department and/or treatment isolation care unit is likely to involve imaging services.
The overlap of early EVD symptoms (eg, fever, abdominal pain, diarrhea, emesis, and fatigue) with symptoms of other more common travel-related diseases (eg, malaria, typhoid fever, pneumonia, and meningococcemia) could result in delayed diagnosis of EVD before isolation of infected patients. Radiology departments should consider policies for and approaches to decontamination of expensive and potentially easily damaged radiology equipment. In addition, the protection of radiology personnel must be considered during the work-up of patients suspected of having EVD who present to emergency departments. The purpose of this article is to consider the effects of EVD on radiology departments and imaging equipment, with particular consideration of guidelines currently available from the Centers for Disease Control and Prevention that may be applicable to radiology (3,4).
EVD and Its Effects on the Radiology Department
The clinical characteristics and symptoms of patients with EVD have been previously reviewed (5). In brief, the Ebola virus spreads among humans through direct contact with blood or other body fluids from an infected individual (living or deceased) or possibly through exposure to objects that have been contaminated with such fluids or secretions from a patient with active disease. The spread of Ebola virus occurs only from patients who are symptomatic. No airborne transmission of Ebola virus has been reported. The mean incubation period in the current EVD outbreak is 9–11 days, with a historical range of 2–21 days. Patients eventually develop severe watery diarrhea, nausea, vomiting, and abdominal pain. Hemorrhage occurs in approximately 20% of patients and manifests late in the disease course with petechiae, bruising, and ecchymoses. Frank hemorrhage, such as severe gastrointestinal bleeding, is infrequent.
According to the U.S. Public Health Service, high risk of EVD transmission occurs from direct skin contact with or exposure to blood or body fluids of a patient with EVD in the absence of appropriate personal protective equipment (PPE) or from processing blood or body fluids of a patient with confirmed EVD without appropriate PPE or adherence to standard biosafety precautions. Surfaces touched by patients with EVD that are not visibly soiled have been negative for Ebola virus RNA (6). The virus lasted several days on dry surfaces in one experimental study; however, experts believe that fomites play little role in transmission (6,7).
Nonspecific symptoms before EVD diagnosis related to the abdomen or chest may prompt the use of imaging equipment in the emergency department, the radiology department, or outpatient imaging facilities (for radiography or, possibly, computed tomography [CT]) for patient evaluation. For patients suspected of having EVD, it may be more clinically prudent to defer imaging until EVD molecular test results are available, bearing in mind that false-negative results are more likely in the first 72 hours of symptoms. For patients with confirmed EVD who are in an isolation room, imaging may include portable radiography and ultrasonography (US). Advanced imaging modalities, such as CT and magnetic resonance (MR) imaging, may pose substantial risk for transmission or environmental contamination without substantial clinical benefit to the patient.
Following recovery from EVD, other imaging modalities (eg, CT or MR imaging) may be used to assess sequelae of the infection. In the following discussion, we consider three areas of concern to radiology departments: (a) protection of radiology staff, (b) protection and decontamination of imaging equipment, and (c) imaging of recovered patients after discharge.
Protection of Radiology Staff
Protecting health care personnel from the hazard posed by a patient known to have or suspected of having EVD involves several layers of safety controls. Administrative controls are measures designed to reduce staff exposure to EVD. These controls include planning for the possibility of an EVD case, limiting the number of staff entering the patient’s room, limiting imaging to bedside modalities to avoid patient transport, careful screening to identify possible cases, and communication among hospital staff once a possible case is identified. Environmental controls, if available, attempt to minimize the hazard to health care personnel with use of facilities and equipment such as anterooms and portable isolation chambers. PPE is a central feature for protection of staff from infectious particles (8).
PPE for health care personnel who will be in the same room as a patient suspected of having or confirmed to have EVD must leave no skin or clothing uncovered (Fig 1). Although personnel in the anteroom can wear lower-level PPE, it may be advantageous for technologists in the anteroom to wear full PPE in the event that they need to troubleshoot the imaging procedure inside the patient’s room. Hospital infection control personnel should be integral to training other health staff in the use of PPE. Hospital infection control personnel will also be essential in planning, practicing, and executing any imaging procedure. Imaging procedures are ideally performed in the patient’s isolation room.
Figure 1:
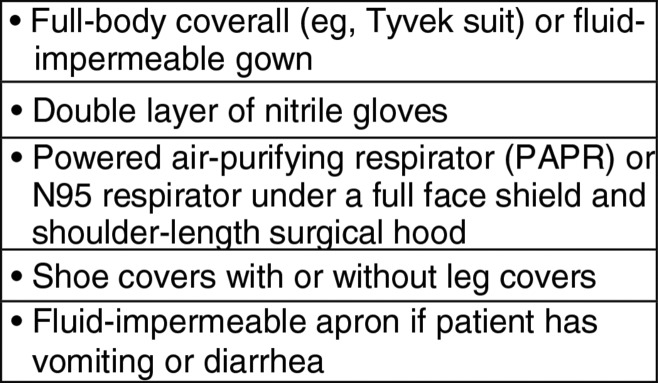
PPE for health care personnel in the same room as patient suspected of having or known to have EVD.
Preprocedural training of radiology personnel is necessary to learn procedures to don and doff the PPE. Trained observers give instructions and watch for any missteps that could lead to self-contamination while removing PPE.
Point-of-Care Imaging
Experience at several institutions in the United States during the EVD outbreak response supports the use of point-of-care imaging to avoid potential nosocomial disease spread. For radiology, a portable radiography unit was placed inside the patient room at the National Institutes of Health Clinical Center to obtain digital radiographs. Ideally a portable unit is dedicated to the isolation room to reduce cross-contamination risk, although a unit could be decontaminated as described in the procedure below (step i). A representative floor plan for the isolation unit is shown in Figure 2. A procedure for portable radiography, illustrated in Figure 3 and based on the principles outlined by Centers for Disease Control and Prevention guidelines as well as experts at biocontainment facilities (10,11), is outlined as follows: (a) The portable radiography unit is stored in a “cold” area outside the containment room(s) when not in use. (b) The x-ray detector is double-bagged by using fluid-impermeable plastic material. If the patient is actively vomiting, there is a contamination risk to the main radiography unit (normally placed at a distance of 40 inches to the patient). In that case, the radiography equipment is covered by two layers of fluid-impermeable plastic. (c) The radiologic technologist dons PPE in the designated donning area. (d) A clinician (physician or nurse) inside the containment room wearing PPE handles the double-bagged detector and is the only one to touch the patient (including for positioning). (e) Once the patient is positioned for radiography by the clinician, the technologist obtains the image. (f) The clinician wipes down the outer bag twice with disinfectant wipes, then takes the double-bagged detector to the threshold between the containment room and the anteroom and removes and discards the outside bag while the anteroom nurse pulls the single-bagged detector out of the bag. (g) The single bag covering is wiped down twice with disinfectant wipes. (h) The detector is then removed from the single bag and wiped down twice with isopropyl alcohol (other disinfectants may damage the detector). (i) The portable radiography unit is wiped down twice with disinfectant wipes and rolled over a mat saturated with disinfectant and returned to another room, where it is treated with hydrogen peroxide vapor before storage and subsequent use. (j) The detector cassette is processed normally.
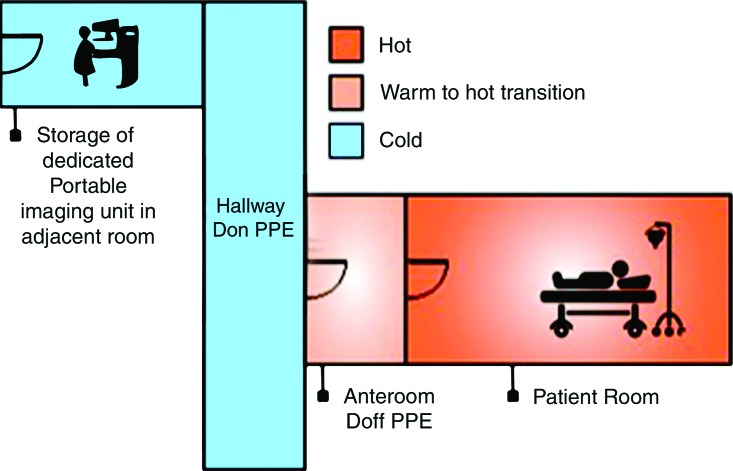
Figure 2:
Definition of hot, warm, and cold zones for isolation units. Floor plan includes a “hot room,” where the infected patient is located, a “cold area,” where no PPE is needed, and a “warm” anteroom that separates the hot and cold areas, where lower-level PPE is needed. Two doors in these compartments are never open at the same time as a means of sealing off the hot room from the cold area. (Image courtesy of Jennifer Telleria, MBA.)
Figure 3a:

Illustration of steps for portable radiography in isolation room. (a) X-ray detector is double bagged in cold zone (not shown) and handed from the radiologic technologist (right) in anteroom to the clinician (left) (physician or nurse) in hot room. The clinician in hot room positions the detector behind the patient, and the radiologic technologist operates the radiographic equipment (not shown). (b) After exposure of the x-ray detector, the outside surface of the outer bag is cleaned twice with disinfectant wipes by the clinician in the hot room, who then partially slides down outer bag without touching inner bag to prepare for the handoff. (c) At the threshold between the hot room and anteroom, the clinician in hot room (left) positions the x-ray detector so that the anteroom clinician (right) can remove the inner bag with detector from the outer bag without touching the outer bag. The outside surface of inner bag is cleaned twice with disinfectant wipes (not shown) by the anteroom clinician. The anteroom clinician removes the x-ray detector from the inner bag in the anteroom and wipes the detector twice with isopropyl alcohol along all edges (not shown). The detector is then processed in cold zone (not shown). (d) Radiologic technologist wipes portable radiography machine down twice with disinfectant wipes in hot room (not shown), pushes machine into anteroom (without stepping outside of hot room), rolling wheels over disinfectant-saturated mats, then removes outer layer of PPE in hot room (not shown). Portable radiography machine is cleaned twice with disinfectant wipes by anteroom clinician (not shown) and machine is returned to another room, where it is treated with hydrogen peroxide vapor before storage and subsequent use (not shown). A trained observer in the anteroom supervises all steps of PPE donning and doffing and equipment disinfection (not shown). Waste is handled and disposed of per Centers for Disease Control and Prevention guidelines (9). (Photos courtesy of Michael Spivey, RT, and Chelsye Nelson, BS.)
Figure 3b:

Illustration of steps for portable radiography in isolation room. (a) X-ray detector is double bagged in cold zone (not shown) and handed from the radiologic technologist (right) in anteroom to the clinician (left) (physician or nurse) in hot room. The clinician in hot room positions the detector behind the patient, and the radiologic technologist operates the radiographic equipment (not shown). (b) After exposure of the x-ray detector, the outside surface of the outer bag is cleaned twice with disinfectant wipes by the clinician in the hot room, who then partially slides down outer bag without touching inner bag to prepare for the handoff. (c) At the threshold between the hot room and anteroom, the clinician in hot room (left) positions the x-ray detector so that the anteroom clinician (right) can remove the inner bag with detector from the outer bag without touching the outer bag. The outside surface of inner bag is cleaned twice with disinfectant wipes (not shown) by the anteroom clinician. The anteroom clinician removes the x-ray detector from the inner bag in the anteroom and wipes the detector twice with isopropyl alcohol along all edges (not shown). The detector is then processed in cold zone (not shown). (d) Radiologic technologist wipes portable radiography machine down twice with disinfectant wipes in hot room (not shown), pushes machine into anteroom (without stepping outside of hot room), rolling wheels over disinfectant-saturated mats, then removes outer layer of PPE in hot room (not shown). Portable radiography machine is cleaned twice with disinfectant wipes by anteroom clinician (not shown) and machine is returned to another room, where it is treated with hydrogen peroxide vapor before storage and subsequent use (not shown). A trained observer in the anteroom supervises all steps of PPE donning and doffing and equipment disinfection (not shown). Waste is handled and disposed of per Centers for Disease Control and Prevention guidelines (9). (Photos courtesy of Michael Spivey, RT, and Chelsye Nelson, BS.)
Figure 3c:
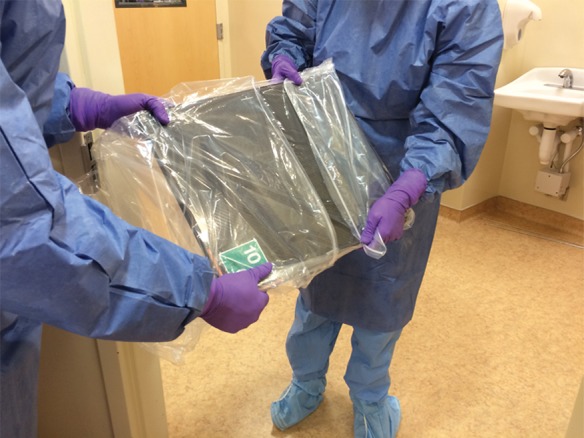
Illustration of steps for portable radiography in isolation room. (a) X-ray detector is double bagged in cold zone (not shown) and handed from the radiologic technologist (right) in anteroom to the clinician (left) (physician or nurse) in hot room. The clinician in hot room positions the detector behind the patient, and the radiologic technologist operates the radiographic equipment (not shown). (b) After exposure of the x-ray detector, the outside surface of the outer bag is cleaned twice with disinfectant wipes by the clinician in the hot room, who then partially slides down outer bag without touching inner bag to prepare for the handoff. (c) At the threshold between the hot room and anteroom, the clinician in hot room (left) positions the x-ray detector so that the anteroom clinician (right) can remove the inner bag with detector from the outer bag without touching the outer bag. The outside surface of inner bag is cleaned twice with disinfectant wipes (not shown) by the anteroom clinician. The anteroom clinician removes the x-ray detector from the inner bag in the anteroom and wipes the detector twice with isopropyl alcohol along all edges (not shown). The detector is then processed in cold zone (not shown). (d) Radiologic technologist wipes portable radiography machine down twice with disinfectant wipes in hot room (not shown), pushes machine into anteroom (without stepping outside of hot room), rolling wheels over disinfectant-saturated mats, then removes outer layer of PPE in hot room (not shown). Portable radiography machine is cleaned twice with disinfectant wipes by anteroom clinician (not shown) and machine is returned to another room, where it is treated with hydrogen peroxide vapor before storage and subsequent use (not shown). A trained observer in the anteroom supervises all steps of PPE donning and doffing and equipment disinfection (not shown). Waste is handled and disposed of per Centers for Disease Control and Prevention guidelines (9). (Photos courtesy of Michael Spivey, RT, and Chelsye Nelson, BS.)
Figure 3d:
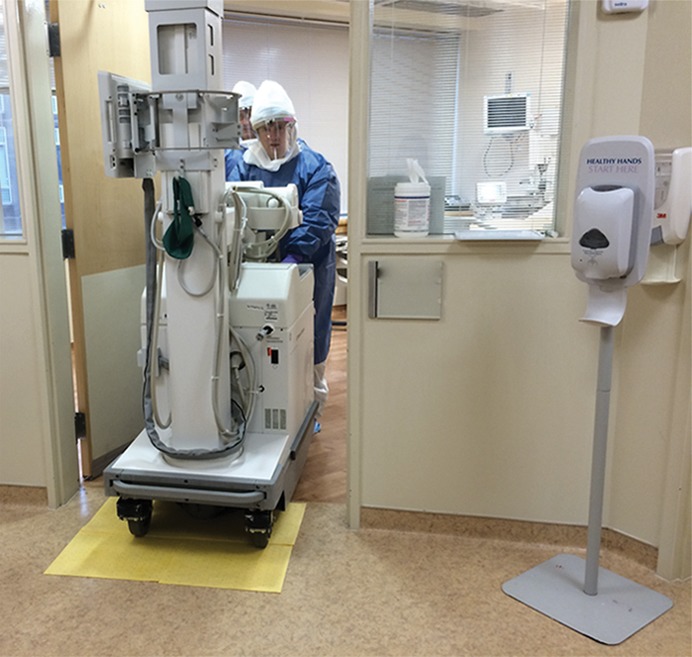
Illustration of steps for portable radiography in isolation room. (a) X-ray detector is double bagged in cold zone (not shown) and handed from the radiologic technologist (right) in anteroom to the clinician (left) (physician or nurse) in hot room. The clinician in hot room positions the detector behind the patient, and the radiologic technologist operates the radiographic equipment (not shown). (b) After exposure of the x-ray detector, the outside surface of the outer bag is cleaned twice with disinfectant wipes by the clinician in the hot room, who then partially slides down outer bag without touching inner bag to prepare for the handoff. (c) At the threshold between the hot room and anteroom, the clinician in hot room (left) positions the x-ray detector so that the anteroom clinician (right) can remove the inner bag with detector from the outer bag without touching the outer bag. The outside surface of inner bag is cleaned twice with disinfectant wipes (not shown) by the anteroom clinician. The anteroom clinician removes the x-ray detector from the inner bag in the anteroom and wipes the detector twice with isopropyl alcohol along all edges (not shown). The detector is then processed in cold zone (not shown). (d) Radiologic technologist wipes portable radiography machine down twice with disinfectant wipes in hot room (not shown), pushes machine into anteroom (without stepping outside of hot room), rolling wheels over disinfectant-saturated mats, then removes outer layer of PPE in hot room (not shown). Portable radiography machine is cleaned twice with disinfectant wipes by anteroom clinician (not shown) and machine is returned to another room, where it is treated with hydrogen peroxide vapor before storage and subsequent use (not shown). A trained observer in the anteroom supervises all steps of PPE donning and doffing and equipment disinfection (not shown). Waste is handled and disposed of per Centers for Disease Control and Prevention guidelines (9). (Photos courtesy of Michael Spivey, RT, and Chelsye Nelson, BS.)
Protection and Decontamination of Radiology Equipment
Radiology equipment has uneven edges, crevices, gaps, buttons, unsealed margins (near moving parts and gantries), and hinges that may conceal fluid leaks. Moreover, the heterogeneity of the components of the imaging devices (eg, metal, plastic, and fiberglass) may make disinfection of equipment complex. These factors make the use of disinfection techniques beyond surface cleaning, such as hydrogen peroxide vapor or ultraviolet light, appealing as adjunctive measures to decontaminate equipment. These considerations, combined with the very high cost and high patient throughput of most imaging equipment, suggest the need for careful review in the use of imaging equipment before being used for patients who have suspected or confirmed EVD.
Current guidelines for disinfection of Ebola virus–contaminated equipment recommend the use of hospital disinfectants that are registered by the U.S. Environmental Protection Agency for nonenveloped viruses such as norovirus, poliovirus, adenovirus, and rotavirus (11). Although no products currently make specific label claims about disinfection potency against the Ebola virus, enveloped viruses such as Ebola virus are more susceptible to hospital disinfectants than are nonenveloped viruses. Our institution uses wipes saturated with bleach, hydrogen peroxide, or quaternary ammonium compounds, depending on manufacturer guidelines, to clean surfaces and equipment used in the care of Ebola virus–infected patients.
The patient care and treatment rooms should have no carpet, textile curtains, or upholstered furniture. If such items, which have porous surfaces, are used in the care of a patient with EVD, they should be discarded after use. Pads and pillows should have impermeable coverings or be discarded after use. To reduce staff and nosocomial exposures, fluid-permeable items, such as linens, should also be discarded. Ebola virus is classified as a category A infectious substance, which is regulated by the U.S. Department of Transportation Hazardous Materials Regulations Code of Federal Regulations (49 CFR, Parts 171–180). Any item intended for disposal that is potentially contaminated with Ebola virus or category A infectious substance must be handled in accordance with the Hazardous Materials Regulations. Items that have been autoclaved are no longer considered infectious and are not covered by the Hazardous Materials Regulations.
When imaging is needed, point-of-care imaging in the isolation room is used as indicated earlier. If imaging within the radiology department is deemed to be crucial, planning considerations for the procedure may include clinical criteria, issues of patient transportation, isolation of the patient within the imaging suite, and decontamination of the imaging equipment and suite.
Disinfection of a contaminated procedure room would include cleaning of soiled surfaces as discussed earlier in addition to hydrogen peroxide vapor decontamination. Although there are no published studies of its use in EVD, hydrogen peroxide vapor, which is available as a contractible service to hospitals, has been used in health care settings in several countries to decontaminate rooms and equipment used for care of patients with EVD. Hydrogen peroxide vapor is a sterilant produced through a mixture of water and liquid hydrogen, which is then passed through a generator system to emit gaseous hydrogen peroxide at a target ambient concentration in a closed space. The gaseous hydrogen peroxide is circulated for a defined duration and then reabsorbed and detoxified by the system until ambient concentrations are safe for human habitation (12). Although hydrogen peroxide vapor is very effective for the disinfection of a wide range of hospital equipment and environments, its use must be preceded by cleaning to remove proteinaceous material that may not be fully penetrated by the vapor.
Planning procedures for the use and decontamination of US equipment are shown in Figure 4; decontamination procedures for CT scanners are shown in Figure 5.
Figure 4:
Planning procedures for the use and decontamination of US machines. This is a proposed method and was not tested in patients with EVD.
Figure 5:
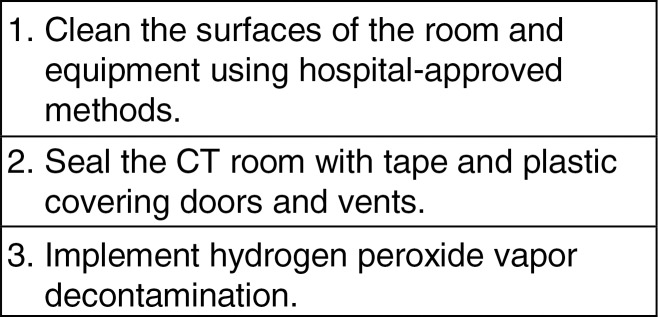
Planning procedures for decontamination of CT scanners. This is a proposed method and was not tested in patients with EVD. Fluid-impermeable barriers to cover the patient or CT beds have not been tested for impact on image quality and effectiveness at preventing contamination, particularly given the high variability of tubes and/or lines that may interfere with these barriers. Currently available hydrogen peroxide vapor systems are not compatible with MR imaging. A preliminary study shows that at least one commercial system can be operated by using the 5-gauss line as a positioning reference to ensure a safe distance from the magnetic field and maximize decontamination performance (13). It is not known if magnetic fields stronger than 1.5 T may affect the safety of this reported approach. Although there are no published studies of its use in EVD, hydrogen peroxide vapor, which is available as a contractible service to hospitals, has been used in health care settings in several countries to decontaminate rooms and equipment used for care of patients with EVD.
Imaging Patients after Recovery
A patient with EVD is considered contagious when symptoms are present and results of the blood polymerase chain reaction test are positive for virus. When patients have recovered and are considered virus free and safe for discharge from isolation, it is considered safe to image them without barrier protection.
If a patient undergoes imaging in the radiology department without isolation precautions and is subsequently diagnosed with EVD, (a) the exposed sections of the radiology department should be fully closed off (including prohibition of entry, closed vents, and closed doors), (b) the equipment should be cleaned and disinfected as described below, and (c) the staff who cared for the patient as well as the patients who subsequently underwent imaging with the exposed equipment should be considered potentially exposed and managed by public health authorities.
Hospital Policies for Equipment Decontamination and the Radiology Equipment Vendor
It is the responsibility of radiology departments, in collaboration with hospital infection control specialists, to establish policies for disinfecting radiology equipment to manage infection risk. In addition, radiology departments should check with their equipment vendors to determine which disinfectants are safe for radiology equipment. Hospital procedures for infection control must allow correct operation of equipment after the disinfection procedure without damage to sensitive electronics or surfaces. These hospital-based policies should address the following: (a) communication (between ordering clinicians and radiology personnel) and oversight measures for safety and appropriateness (eg, clinical indications and timing) of radiologic procedures (14); (b) the degree of contact that defines equipment contamination; (c) the specific cleaning agents that are to be used, including the potency, the locations on equipment where the disinfectant should be applied, and how the disinfectant should be applied; (d) personnel who are authorized to perform the disinfection procedures both safely and effectively; (e) how and when the disinfection procedure is performed; (f) procedures and the frequency of infection surveillance; and (g) waste disposal and procedure for disposal and/or avoidance of equipment in the event that effective decontamination cannot be achieved.
Attention to decontamination policies is necessary to determine whether the procedures will void or limit insurance, warranties, and service contracts.
In conclusion, preparation for the possibility of imaging of patients with EVD begins with the development of standard operating procedures that are developed in conjunction with hospital epidemiologists, clinicians, ethics specialists, and infection control professionals. Judicious use of radiology studies for a patient who has suspected or confirmed EVD entails an emphasis on point-of-care imaging to minimize transmission risk and appropriate indications for radiology outside the patient room. Decontamination procedures that are safe for the patients and that will not damage radiologic equipment should be agreed upon before patient imaging. Preparedness will also include appropriate referral policies for provision of imaging services to high-risk patients, policies governing communication (between ordering clinicians and radiology personnel), oversight measures for safety and appropriateness of imaging, safety training for radiology professionals, effective surveillance, and detection of cross-contamination. With a comprehensive approach to radiology preparedness, medical imaging can effectively join the battle against the current and future outbreaks of EVD.
Advances in Knowledge
■ Radiography equipment can be safely operated in the infectious disease containment unit.
■ Decontamination methods should be implemented for radiography, US, CT, and MR imaging equipment in the setting of suspected or confirmed Ebola virus infection.
■ Standardization of hospital procedures and policies for infection control are warranted in radiology departments in preparation for patients confirmed to have or suspected of having Ebola virus disease (EVD).
Implications for Patient Care
■ The current EVD outbreak has a recorded death toll of more than 8000 people and a case fatality rate of approximately 40%–50%, with health personnel particularly at risk; therefore, key components of EVD preparedness at U.S. health care institutions are training of staff and ensuring that appropriate facilities, policies, and equipment are in place.
■ The overlap of early EVD symptoms (eg, fever, abdominal pain, diarrhea, emesis, and fatigue) with symptoms of other more common travel-related diseases (eg, malaria, typhoid fever, pneumonia, and meningococcemia) could result in delayed diagnosis of EVD before isolation of infected patients, and radiology departments should consider policies and approaches to decontamination of expensive and potentially easily damaged radiology equipment with complex and sometimes exposed circuits and electronics.
■ To perform radiologic examinations in Ebola virus isolation units, radiology workers must don personal protective equipment that fully covers all body surfaces and follow detailed protocols for operating the imaging equipment and dectector processing to prevent infection transmission.
Received November 17, 2014; revision requested December 30; revision received January 13, 2015; accepted January 15; final version accepted January 22.
The views expressed are those of the authors and do not necessarily represent the opinion of the National Institutes of Health, Department of Health and Human Services, or the Federal Government.
Funding: This research was funded by the intramural research programs of the National Instititue of Allergy and Infectious Diseases, National Institutes of Health Clinical Center, and NIH Center for Infectious Disease Imaging.
Abbreviations:
- EVD
- Ebola virus disease
- PPE
- personal protective equipment
Disclosures of Conflicts of Interest: D.J.M. disclosed no relevant relationships. T.N.P. disclosed no relevant relationships. L.R.F. disclosed no relevant relationships. D.A.B. disclosed no relevant relationships.
References
- 1.World Health Organization . Ebola data and statistics. http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-20150106?lang=en. Accessed January 6, 2015.
- 2.World Health Organization . Ebola response roadmap: situation report. http://www.who.int/csr/disease/ebola/situation-reports/en/. Accessed January 6, 2015.
- 3.Center for Disease Control . Detailed hospital checklist for Ebola preparedness. http://www.cdc.gov/vhf/ebola/pdf/hospital-checklist-ebola-preparedness.pdf. Accessed October 28, 2014.
- 4.Center for Disease Control . Ebola virus disease information for healthcare workers and settings. http://www.cdc.gov/vhf/ebola/hcp/index.html. Accessed October 28, 2014.
- 5.Bluemke DA, Meltzer CC. Ebola virus disease: radiology preparedness. Radiology 2014142502. [DOI] [PubMed]
- 6.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007;196(Suppl 2):S142–S147. [DOI] [PubMed] [Google Scholar]
- 7.Center for Disease Control . Review of human-to-human transmission of Ebola virus. http://www.cdc.gov/vhf/ebola/transmission/human-transmission.html. Accessed January 6, 2015.
- 8.Center for Disease Control Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola Virus Disease in U.S . hospitals, including procedures for putting on (donning) and removing (doffing). http://www.cdc.gov/vhf/ebola/hcp/procedures-for-ppe.html. Accessed January 6, 2015.
- 9.Centers for Disease Control and Prevention . Ebola-associated waste management. http://www.cdc.gov/vhf/ebola/hcp/medical-waste-management.html. Accessed January 8, 2015.
- 10.Smith PW, Anderson AO, Christopher GW, et al. Designing a biocontainment unit to care for patients with serious communicable diseases: a consensus statement. Biosecur Bioterror 2006;4(4):351–365. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention . Interim guidance for environmental infection control in hospitals for Ebola virus. http://www.cdc.gov/vhf/ebola/hcp/environmental-infection-control-in-hospitals.html. Accessed January 6, 2015.
- 12.Heckert RA, Best M, Jordan LT, Dulac GC, Eddington DL, Sterritt WG. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl Environ Microbiol 1997;63(10):3916–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams B, Dunn RS. Hydrogen peroxide apor decontamination in the MRI environment [abstr]. In: Proceedings of the Twenty-Second Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2014. [Google Scholar]
- 14.Auffermann WF, Kraft CS, Vanairsdale S, Lyon GM, III, Tridandapani S. Radiographic imaging for patients with contagious infectious diseases: how to acquire chest radiographs of patients infected with the Ebola virus. AJR Am J Roentgenol 2015;204(1):44–48. [DOI] [PubMed] [Google Scholar]