Abstract
Obesity is a serious chronic disease that increases the risk of numerous co-morbidities including metabolic syndrome, cardiovascular disease and cancer as well as increases risk of mortality leading some to suggest this represents accelerated aging. Obesity is associated with significant increases in oxidative stress in vivo and, despite the well-explored relationship between oxidative stress and aging, the role this plays in the increased mortality of obese subjects remains an unanswered question. Here, we addressed this by undertaking a comprehensive, longitudinal study of a group of high fat-fed obese mice and assessed both their changes in oxidative stress and in their performance in physiological assays known to decline with aging. In female C57BL/6J mice fed a high-fat diet starting in adulthood, mortality was significantly increased in high fat-fed mice as was oxidative damage in vivo. High fat-feeding significantly accelerated the decline in performance in several assays, including activity, gait, and rotarod. However, we also found that obesity had little effect on other markers and actually improved performance in grip strength, a marker of muscular function. Together, this first comprehensive assessment of longitudinal functional changes in high fat-fed mice suggests that obesity may induce segmental acceleration of some of the aging process.
Keywords: obesity, rotarod, longevity, oxidation, grip strength, respirometry, gait
Introduction
The prevalence of obesity among all age groups in the US population has risen dramatically over the last few decades due in part to increased sedentary behavior and availability of high calorie food choices (1, 2). Without intervention, a large proportion of this population will be obese (defined as BMI ≥ 30) for a significant proportion of their lives. That is, people are living longer with obesity and are thus subject to increased incidences of the numerous co-morbidities associated with this condition. Obese subjects are at an elevated risk of early mortality likely due to dramatic increases in the occurrence and severity of chronic illnesses such as cardiovascular disease, diabetes, and cancer among this group (3–5). Interestingly, the spectrum of diseases that are exacerbated by obesity are relatively similar to those that increase in prevalence with normal aging. Further, the deterioration and pathology of organs that occurs with chronic obesity is in many ways similar to that which occurs in normal aging leading some to suggest that chronic obesity accelerates the aging process.
There is substantial evidence that elevated oxidative stress mediates the progression of many of the comorbidities associated with obesity. The expansion of adipose tissue is linked with an increased production of reactive oxygen species (ROS) in both human and rodent models through various mechanisms (6, 7). Moreover, the production and secretion of inflammatory adipokines from adipose tissue under metabolic stress promotes a positive feedback loop further exacerbating oxidative stress both in this tissue and throughout the body (8, 9). The accumulation of oxidative stress is an early event in the pathology of obesity which precedes, and likely drives, the development of insulin resistance and diabetes (6, 10). This hypothesis is directly supported by studies that show reducing oxidative stress, either genetically or pharmacologically, does prevent metabolic dysfunction induced by high fat feeding in mice (11–13). This condition of high metabolic and oxidative stress is also thought to drive the prevalence and incidence of other comorbidities of obesity including cardiovascular disease and cancer (14).
Despite the well-examined relationship between aging and oxidative stress, it is not clear whether the high-levels of oxidative stress caused by obesity could contribute to an acceleration of the aging process. A limited number of studies have shown that obesity in mice, whether due to genetic mutation or high fat feeding, are relatively short-lived compared to lean mice (15–18). However, there remains a clear need to address both the causes and consequences of these outcomes. We addressed this question by undertaking a comprehensive, longitudinal study of a group of high fat-fed obese mice and assessed their performance in assays designed to measure several physiological functions known to decline with aging. By assessing a broad-range of functional assays, we can more clearly address whether caused by obesity fundamentally alters the rate of aging across a wide spectrum of tissue and organ systems or, alternatively, whether obesity shortens lifespan due to the increased incidence of a discrete spectrum of disease. In accordance with previous reports, high fat-fed female C57BL/6J mice showed a significant reduced lifespan and increased levels of oxidative stress/damage in vivo. We found that both diet and age affected metabolism, muscular function, and coordination. Further, we identified that high fat-fed mice displayed evidence for accelerated decline in function after parsing out independent effects of increased body weight in these assays. Together, our data support the notion that the increase in mortality associated with obesity may be due to an accelerated aging rate of some physiological systems.
Methods
Mice
Female C57BL/6J mice bred in-house were used for all studies. All animal studies were conducted under SPF conditions in a facility maintained at a temperature of 22–25° C under a 12 hour light cycle (06:00 on-18:00 off). From weaning until 8 months of age, mice were maintained on a standard rodent chow based on the NIH-31 open source (Harlan Teklad, Madison WI). At 8 months of age, mice were randomly assigned to new cages at a density of 5 mice/cage. Each cage was randomly assigned to be fed either the standard rodent chow or a defined, high fat diet. Mice were checked daily and provided food and water ad libitum for the remainder of their nature life. Sample sizes at the beginning of experiments were n=15 on chow and n=40 on high fat diet. All animal studies were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Health Science Center at San Antonio.
High fat diet
The high fat diet (catalog ID 58V8 or D12451) used in this study was a defined diet containing 45% total kCal from fat (or 23.6% of total weight) and was purchased from Purina/Test Diet (St. Louis MO). The primary fat sources of this diet was lard (20.7% of total weight) and soybean oil (2.9% of total weight). Monthly, average food consumption over a week period was determined per cage by measurement of remaining food and food waste.
Body composition
Body weight and composition were measured bi-weekly in non-anesthetized mice by Quantitative Magnetic Resonance imaging (QMRi) using an EchoMRI 3-in-1 composition analyzer (Echo Medical Systems, Houston TX).
Measurement of oxidative stress and inflammation
F2-isoprostane content was performed by chromatography–mass spectrometry as previously described by our group (12). Protein-bound 4-HNE was measured by immunoblot using a primary anti-4 HNE antibody (Abcam, Cambridge MA). Flash frozen liver or skeletal muscle samples were homogenized in RIPA buffer and separated by SDS-PAGE. After transfer to PVDF membrane and immunoblot, samples were visualized using ECL and quantified using ImageJ. Total superoxide and glutathione peroxidase activity were determined using colorimetric assays as per instructions of manufacturer (Cayman Chemical, Ann Arbor MI). IL-6 and TNFα were measured in plasma samples by ELISA using manufacturer’s instructions.
Glucose metabolism
Glucose tolerance tests were performed in mice fasted overnight and injected intraperitoneally with glucose (in saline) at a dose of 1.5 mg per kg body weight. Blood glucose levels were measured using hand-held glucometer (One Touch Ultra). Insulin levels were measured in previously-frozen plasma samples by ELISA per manufacturer’s instructions (Crystal Chem, Downer’s Grove IL).
Respirometry
Resting metabolic rate, oxygen consumption, and carbon dioxide production, were measured for a period of 24 hours using a MARS indirect calorimetry system (Sable Systems International, Las Vegas, NV). Mice were individually housed with TEK-Fresh cellulose bedding and provided food and water ad libitum during metabolic measures. Animals were habituated to the cage and respirometry system for 12 hours prior to testing.
Spontaneous activity
Mice were housed individually in clear, plexiglass (40.6 × 22.9 × 14.0 cm) cages surrounded by a 2.5-cm grid of infrared sensors to record spontaneous activity in the x, y, and z plane with normal access to food and water. Each motion detected by these sensors is recorded as a beam break. Mice are acclimated to cages for the first 12 hours, following which beam breaks are measured for 24 hours, which includes one light and one dark phase cycle.
Gait analysis
Mice were tested on the TreadScan (Clever Sys, Reston, VA) apparatus starting at a treadmill speed of 12 cm/s. Belt speed is adjusted until mice maintain a constant walking speed for 5 minutes and this speed was used for all subsequent assays. Using a high-speed digital camera to record the reflected images of the footpads at 80 frames/s, and these images are used to assess more than 40 parameters of gait using mouse-specific algorithms included in the TreadScan software program.
Grip strength
Fore- and hind-limb grip strength was measured using a Grip Strength Meter with mesh grid pull bar (Columbus Instruments 1027 CSM) designed for mice. After allowing mice to grasp this bar with both fore and hind limbs, mice are pulled horizontally across the grid until grip is lost. Without prior training, ten consecutive trials are performed and both the average and maximum grip strength are determined.
Rotarod
Mice were trained on rotarod over four sessions spanning 2 weeks. Both training and testing are performed on rotarod with an acceleration of 0.2 rotation/s starting from 4 rpm. A total of five trials are performed during the testing session with latency to fall measured as the time in seconds mice are able to remain on bar.
Statistical analysis
Differences between diet groups were analyzed by Student’s t-test. Parameters with both a diet and age component were analyzed by 2 way repeated measures ANOVA with post-hoc analysis using Holm-Sidak. Survival analyses were performed using the Log-Rank test. Correlations were determined by the methods of either Pearson product moment or Spearman rank coefficient as indicated.
Results
In this study, we began feeding female C57BL/6J mice a high fat diet at 8 months of age, or approximately ¼ of their predicted lifespan. Prior to this point, all mice were maintained on normal rodent chow based on the NIH-31 open source formula. Our rationale for this design was to test the effect of adult-onset obesity on the aging process rather than drive issues in development that may be related to consumption of a high fat diet. We defined adulthood as an approximate age at which mice have nominally reached their approximate maximum body size, length and weight. Based on The Jackson Laboratory mouse phenome database, this is between 6–12 months of age for C57BL/6J mice (19). Moreover, 8 months of age At 8 months of age, the average weight of mice in this study was 24.9 ± 0.3 g with the heaviest mouse weighing 31.9 g and the lightest weighing 18.2 g. We used female mice for this study primarily because male C57BL/6 mice often have issues with fighting that can lead to censoring and reduced animal numbers. As a consequence, we could rehouse female mice at the beginning of this high fat study so the cage density was equivalent for all animals in this study.
Changes in body composition and lifespan
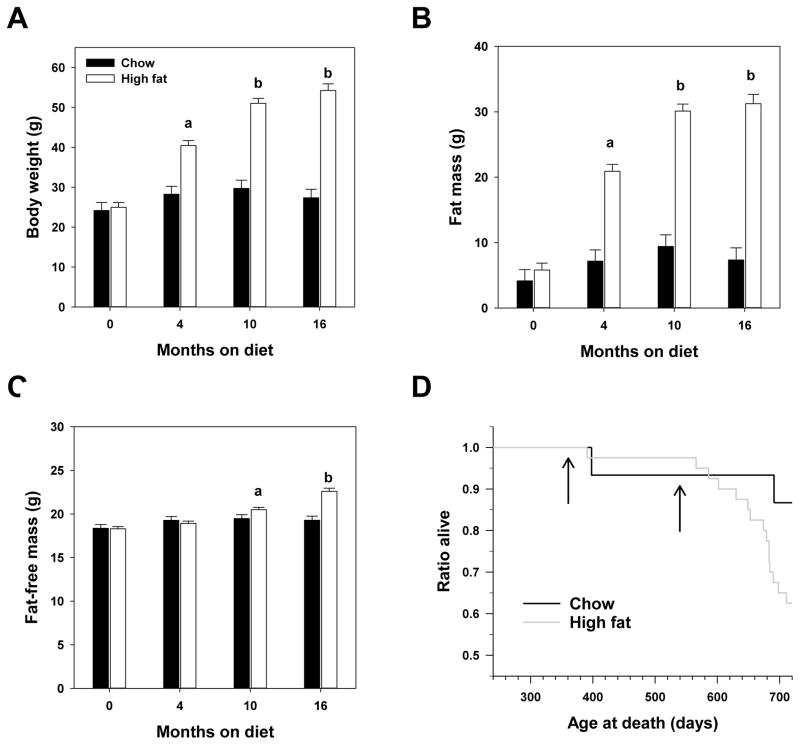
As shown in Figure 1, mice fed high fat diet become significantly heavier than their chow-fed counterparts. This difference in body weight was largely due to an approximately 6-fold increase in the average fat mass of high fat-fed mice. We found that both body weight and fat mass reached a plateau between 10 and 16 months of feeding as these two points did not differ among high fat-fed mice. Figure 1 also shows that chow-fed mice did not significantly differ in body weight, fat mass or fat-free mass throughout this study. We found only small effects of either diet or age on changes in fat-free mass suggesting that most of the changes in weight driven by either diet or age are driven largely by an increase in adipose tissue. During the course of this study, food consumption of HFD-fed mice was approximately 75% (by weight) of that of chow-fed mice (data not shown). The caloric content of the HFD was 4.6 kcal/g whereas that of the chow diet was approximately 3.0 kcal/g. Thus, even though HFD-fed mice consumed less food by weight, the diet of these mice provided both excess calories and excess fat.
Figure 1.
High fat diet started in adult female C57BL/6J increases body weight and fat mass and shortens lifespan. Average body weights (A), fat mass (B) and fat-free mass (C) of mice at indicated time points following start of feeding studies at 8 months of age. Bars indicate values ± SEM for n=15 chow (filled) or n=40 high fat (open) fed mice. Letters indicate differences among age-groups of high fat-fed mice as measured by 2 way ANOVA with Bonferroni post-hoc analyses. No differences with age were found among chow-fed mice using these tests. (D) Survival of mice from each group in this study. Arrows indicate time points (4 months and 10 months after initiation of diet studies) at which assays of health parameters were measured.
Through 24 months of age (i.e, 16 months after high fat diet was begun), we found that mortality is increased in high fat-fed mice relative to their chow-fed counterparts (Figure 1D). At this point, the survival curves for these two groups differ significantly by Log-Rank (p=0.04) with 87% of mice in the chow-fed group remaining and only 55% of the mice remaining in the high fat group, clearly showing that high fat diet shortens lifespan.
Oxidative stress and damage
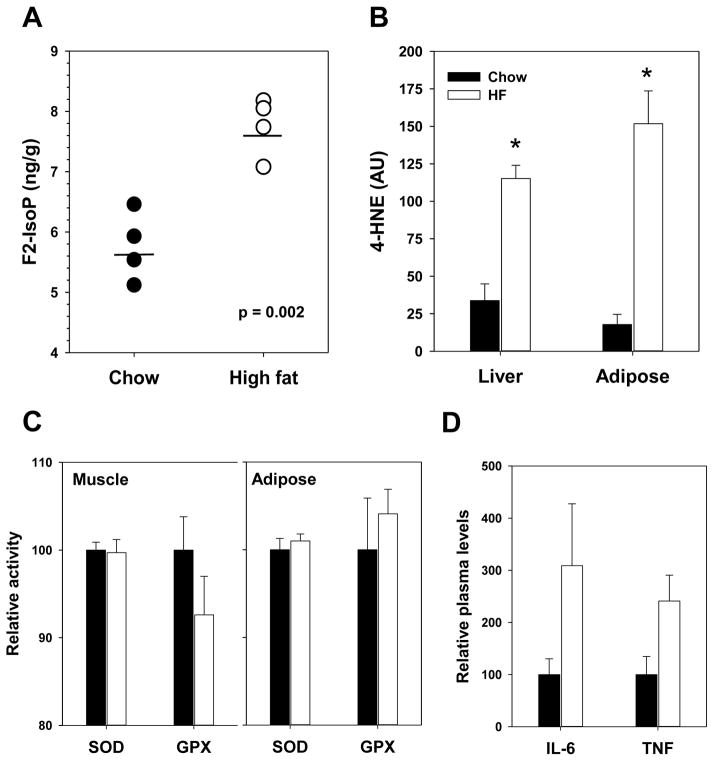
Several groups, including our own, have shown that obesity and high fat feeding are also associated with increased levels of oxidative stress and oxidative damage (6, 7, 12, 20, 21). In this study, we confirmed that high fat feeding is associated with increased levels of oxidative stress in multiple tissues. In an independent cohort of C57BL/6J female mice, we found that 4 months of high fat feeding is sufficient to increase in vivo levels of the lipid peroxidation marker F2-isoprostane in the liver (Figure 2A). Further, the protein-bound levels of a 4-hydroxynonenal (4-HNE), a reactive aldehyde generated by oxidative stress, were also increased in the liver and adipose at this time point (Figure 2B). Somewhat surprisingly, we found that high fat feeding did not alter either superoxide dismutase or glutathione peroxidase (Figure 2CD) activity in vivo suggesting that the increase in oxidative damage may be due to elevated oxidant production rather than insufficient oxidant defense. Changes in physiological function, including oxidative stress, associated with obesity have been linked to chronic stimulation of inflammatory processes. In plasma samples, we found that levels of pro-inflammatory markers IL-6 and TNFα were relatively higher in high fat-fed mice suggesting an increase in pro-inflammation (Figure 2D) although these differences did not reach statistical significance (p=0.18 and p=0.08 respectively) in the sample sizes we used.
Figure 2.
High fat feeding of adult C57BL/6J females for 4 months promotes oxidative stress. (A) F2-isoprostanes measured in liver. Circles represent values generated from individual animals, horizontal lines indicate mean values for each group and p value is given for t-test comparing diet group. (B) 4-HNE adducts measured in liver and adipose. (C) Superoxide dismutase and glutathione peroxidase activity in tissue muscle and adipose homogenates. (D) Plasma IL-6 and TNFα levels after 10 months high fat feeding. For B-D, bars indicate mean values ± SEM for mice fed chow (filled) or high fat (open) diets with asterisks representing p < 0.05 for t-test. For A-C, n=4/group; For D, n=5–18/group.
Metabolic function
We next addressed whether the shortened lifespan of high fat fed mice is associated with diminished physiological function and performance by assessing the changes in a wide range of age-related health parameters. As the aging process affects nearly all physiological functions and organ systems, we used a systematic, comprehensive approach using functional assays that require the use of different physiological inputs. Moreover, we tested mice longitudinally to monitor changes in individual mice over time and to determine whether changes in performance with age were altered by obesity. Tests were performed in mice 4 months and 10 months after diets were begun (or mice that were 12 months and 18 months of age, respectively), times at which we found no significant difference in mortality between chow and high fat-fed groups (Figure 1D). This is an important point for our analyses because it suggests that both groups were not near death and were relatively healthy during assessments.
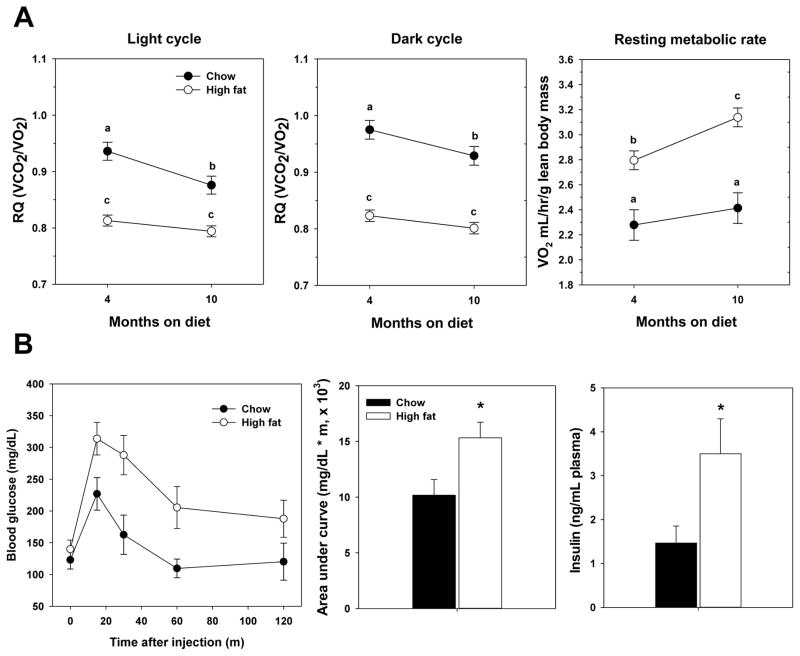
Because of the difference in fat intake between the two diets, we predicted dramatic differences in metabolism between groups of mice. Indeed, the respiratory quotient (RQ) of high fat-fed mice was significantly lower than the RQ of chow-fed mice in both light cycle and dark cycle, suggesting preferential utilization of fat energy sources over carbohydrate (Figure 3). In terms of aging effects, we found that RQ in mice fed the chow diet became significantly lower between both time points suggesting a metabolic alteration from high carbohydrate utilization to more utilization of fat sources with advanced age. However, we found no significant change in this group over time in high fat-fed mice even though RQ for this group was lower at both time points relative to chow-fed mice. In contrast to these data, resting metabolic rate (RMR) was significantly higher in mice fed the high fat diet compared to chow-fed mice (Figure 3A). As recently reported, indirect calorimetry may significantly underestimate RMR in chow fed, though not high fat-fed, mice suggesting that the relative diet-difference in this parameter may not be as large as we report here (22). However, we found no change in RMR in chow-fed mice over this period, though this lack of change may be largely due to the relatively young age of mice (18 months) at the second time period. In contrast, high fat fed mice showed a further increase in RMR with advancing age. This is in contrast to the generally held notion that metabolic rate declines with age (23) and thus may suggest an adaptation to chronic high fat diet. In an independent cohort of mice, we confirmed that high fat feeding promoted metabolic dysfunction consistent with the development of a diabetes-like condition. In chronically high-fed mice (10 months), glucose tolerance was significantly impaired and circulating insulin levels were significantly elevated (Figure 3B).
Figure 3.
Metabolic function is altered by both diet and age. A. Respiratory quotients during light and dark cycles and resting metabolic rate are given for mice 4 months and 10 months after starting high fat diet. Circles represent mean values ± SEM for n=15 chow (filled) or n=40 high fat (open) fed mice. Letters indicate differences among groups as measured by 2 way ANOVA with Bonferroni post-hoc analyses. B. Glucose tolerance tests and insulin levels in chow and high fat fed mice after 10 months of high fat diet. Circles or bars represent mean values ± SEM for n=5 chow (filled) or n=5 high fat (open) fed mice.
Activity and strength parameters
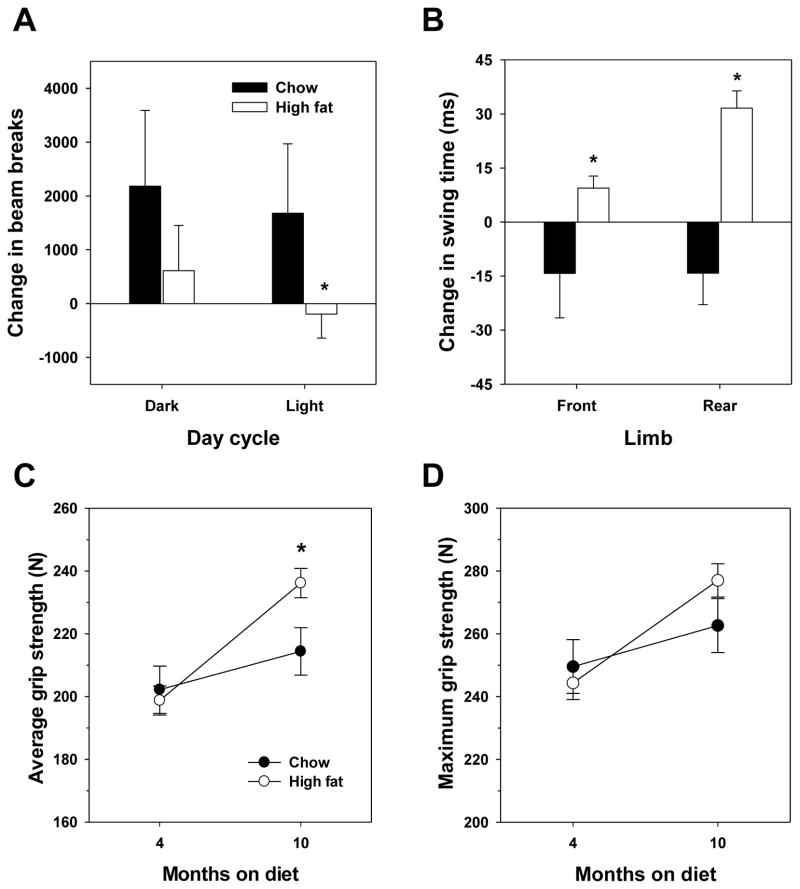
At the initial time point in this study, we found that spontaneous activity was not altered by feeding high fat diet for 4 months. However, we did find that the change in spontaneous activity from 4 months to 10 months was altered in a diet-specific manner. Chow-fed mice showed a modest increase in activity as measured by beam breaks between 4 and 10 months of feeding (12 and 18 months of age). In contrast, high fat-fed mice showed no change in activity over this time period with a slight trend toward reduced activity (Figure 4A). This difference between groups was significant during the light cycle of testing.
Figure 4.
Age-related changes in activity and gait are diet-dependent. (A) Difference in activity (beam breaks) and (B) Difference in swing time of front and rear feet (treadscan analysis) between 4 month and 10 month time-points after starting high fat diet. (C) Average and (D) maximum grip strength measured 4 and 10 months after starting high fat diet. For A and B, bars indicate mean values ± SEM for n=15 chow (filled) or n=40 high fat (open) fed mice. For C and D, mean values ± SEM for n=15 chow (filled) or n=40 high fat (open) fed mice. For all, asterisks represent p < 0.05 for t-test.
We predicted the difference in activity might be partially related to altered physics of walking due to the expansion of fat mass in high fat fed mice. This was assessed by gait analysis of these mice; to simplify analyses, we focused on swing time, or the amount of time during each stride that the measured foot is not in contact with the tread. Aging has been reported to alter gait of C57BL/6 mice potentially indicative of longer and less frequent steps to maintain the same walking rate (24). At our initial time point, we observed that swing time reduced by 4 months of high fat feeding (135 ± 11 ms vs. 117 ± 2 ms for the front limb, 126 ± 7 ms vs. 108 ± 3 ms for the rear limb); i.e., the duration of stride was less in fat mice, indicative of shorter, more frequent steps. We also found that high fat-fed mice have a significantly wider stance than chow-fed mice (29.0 ± 0.4 mm vs. 30.1 ± 3 mm) suggesting the reduced swing time could be compensation for the increased bulk of these mice. When changes with age were assessed, we found a significant difference between diet groups with high fat fed mice tending to increase swing time and chow fed groups showing a moderate decrease in this parameter (Figure 4B). In line with the findings of Fahlstrom et al. (24), we also found the change in gait width significantly increased between these two time points in high fat-fed mice but not in chow-fed mice (1.25 mm vs. −0.42 mm). We could not exclude the possibility that these differences could be due to the bulk of accumulating fat mass in high fat-fed mice, so we also calculated the regression between change in body weight and each parameter. There was no significant correlation between change in front limb swing time (R2 = 0.035) or track width (R2= 0.094), though there was a significant correlation between body weight and rear limb swing time (R2= 0.141).
Differences in diet did not affect grip strength, a measurement of muscle function, at the initial point of assessment. However, both average (Figure 4C) and maximum (Figure 4D) grip strength were increased with age in the time frame of this study. Surprisingly, high fat diet appeared to have a beneficial effect on average grip strength in mice tested at 18 months of age.
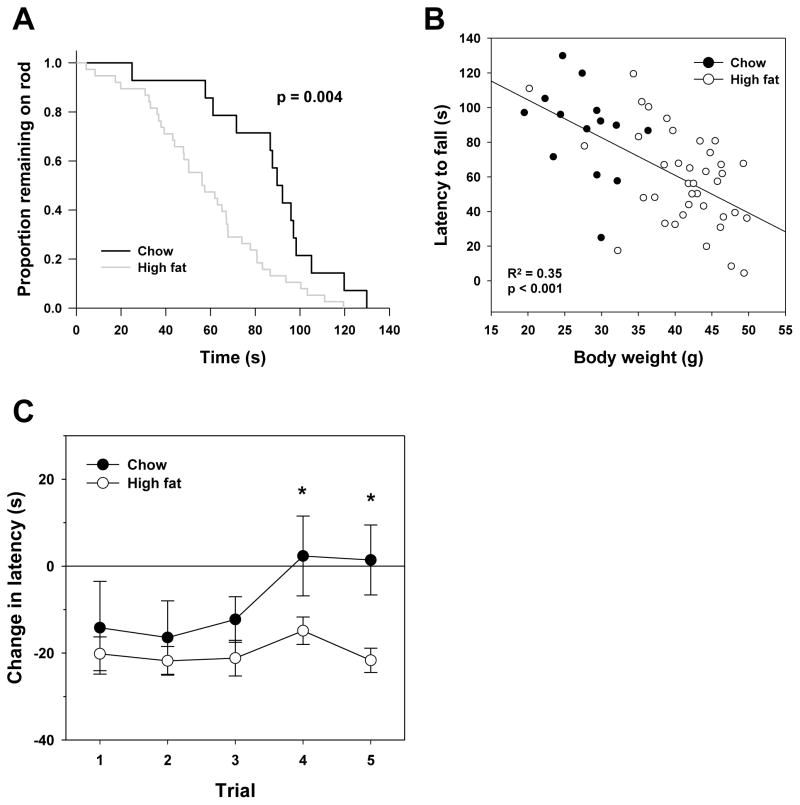
We also measured rotarod performance as a general assessment of changes in neuromuscular function. During the initial measurement following 4 months of feeding studies, it was clear that high fat-fed mice performed significantly worse on this assay than did chow-fed mice (Figure 5A). However, others have noted that body size directly affects performance in this assay (25). Here, we found a clear correlation between body size and ability to stay on the rod (Figure 5B) suggesting that poor performance of high fat-fed mice in this assay was likely due to their increase in size. We attempted to normalize for this difference by comparing only the change in rotarod performance of mice that were tested at both time points in this study. Both groups performed more poorly during their initial reintroduction to the rotarod with a significant reduction in time that mice remained on the rod (trials 1–3). However, once acclimated to the rotarod (trials 4 and 5), chow-fed mice showed no decline in performance compared to their earlier tests whereas high fat-fed mice performed more poorly in all trials tested following 10 months of feeding compared to those at 4 months of feeding (Figure 5C). Importantly, we found no significant correlation in each trial between the change in rotarod performance between tests and change in body weight (Pearson correlation p>0.1 for all trials) suggesting these results are not driven by differences in body size.
Figure 5.
Rotarod performance is altered by diet and age. (A) Average rotarod performance for chow (dark) and high fat fed (light) mice 4 months after diet started. p-value given is the result of Log-Rank test comparing the length of time each group remained on rod. (B) Correlation between body weight and time maintained on rotarod. Open (high fat) and closed (chow) circles indicate individual animals with trend-line given. R2 and p value are calculated for Spearman’s correlation. (C) Difference in latency to fall from rod between 4 month and 10 month time-points after starting high fat diet. Circles represent mean values ± SEM for n=15 chow (filled) or n=40 high fat (open) fed mice with asterisks representing p < 0.05 for t-test.
Correlation between health and lifespan
Lastly, we asked whether the changes over time in any of these parameters were correlated with the lifespan of mice on either diet. At the time of analysis, a total of 22 mice had died, of which 20 were in the high fat fed group and 2 in the chow group and we used the data collected on all of these mice regardless of diet for subsequent analyses. Within this group, lifespan was significantly correlated with an age-related change in RQ in both the light and dark cycle (and subsequently RMR because these parameters are auto-correlated), total and dark cycle beam breaks, and swing time of the front foot (Table 1). While a small data set, it seemed reasonable to propose that these correlations could be driven largely by differences in weight. However, only markers of changes in mass-specific metabolism (RQ and RMR) were negatively correlated with maximum weight (Table 2), suggesting that body weight is independent of the correlations between longevity and changes in either activity or gait.
Table 1.
Correlation between lifespan and age-related change in parameters of healthspan.
| Δ LCRQ | Δ DCRQ | Δ RMR | Δ LC beam breaks | Δ DC beam breaks | Δ total beam breaks | |
|---|---|---|---|---|---|---|
| Age at death | −0.77 (<0.001) | −0.75 (<0.001) | −0.66 (p<0.001) | 0.16 (0.51) | 0.49 (0.03) | 0.49 (0.03) |
| Δ swing time FF | Δ swing time RF | Δ avg grip | Δ max grip | Δ rotarod tr4 | Δ rotarod tr5 | |
| Age at death | 0.49 (0.04) | 0.44 (0.06) | 0.26 (0.99) | 0.06 (0.80) | 0.34 (0.17) | 0.04 (0.86) |
Data presented are Pearson correlation coefficients between indicated variables with p values given in parentheses. Values that reach statistical significance are highlighted in bold. Abbreviations: LCRQ – light cycle respiratory quotient, DCRQ – dark cycle respiratory quotient, RMR – resting metabolic rate, LC – light cycle, DC – dark cycle, FF – front foot, RF – right foot, avg grip – average grip strength, max grip – maximum grip strength, rotarod tr4 – rotarod trial 4, rotarod tr5 – rotarod trial 5.
Table 2.
Correlation between body weight and significant associations with lifespan.
| Δ LCRQ | Δ DCRQ | Δ RMR | Δ DC beam breaks | Δ total beam breaks | Δ swing time FF | |
|---|---|---|---|---|---|---|
| Max weight | −0.49 (0.02) | −0.45 (0.04) | −0.58 (0.004) | 0.13 (0.60) | 0.12 (0.61) | 0.06 (0.81) |
Data presented are Pearson correlation coefficients between indicated variables with p values given in parentheses. Values that reach statistical significance are given in bold. Abbreviations: LCRQ – light cycle respiratory quotient, DCRQ – dark cycle respiratory quotient, RMR – resting metabolic rate, DC – dark cycle, FF – front foot.
Discussion
Our study shows evidence for accelerated loss of some, but not all, physiological functions with age in female C57BL/6J fed a high fat diet started in adulthood. The data presented here are a part of an ongoing study addressing the relationship between oxidative stress, obesity and aging. Even as an incomplete data set, our findings clearly show that the consumption of high fat diet both increases oxidative stress and damage among multiple tissues and markedly shortens lifespan. A cautious interpretation of these findings is that obesity in mice has both acute effects and chronic effects on performance in these assays designed to measure change in age-related physiology. While this might suggest an increased propensity towards chronic conditions that lead to mortality, a greater dissection of the progression and development of pathologies caused by obesity will help determine whether this truly is an acceleration of aging. It is of interest to note that the fat content of the high fat diet used in this study is not markedly different from that of average diet in the US population (26). Our data suggest that the growing population defined as obese are likely at greater risk for developing more general age-related dysfunction in addition to diseases attributed directly to fat accumulation.
Metabolic regulation in response to nutrient availability is a significant factor in the control of aging. Dietary, or caloric restriction, represents the most significant example of this relationship with reduced nutrient intake leading to extension of lifespan and promotion of healthy aging. Maintaining, or improving, mitochondrial function, and thus the control of cellular energy metabolism, plays a key role in the prevention of several age-related diseases by dietary restriction (27). On the other hand, obesity and nutrient excess drives mitochondrial energetic dysfunction including, but not limited to, increased production of reactive oxygen species (ROS), reduced ATP production, and dysregulation of mitochondrial signaling (28). Here, we found that chronic obesity promoted declines in functional performance that were suggestive of reduced muscle and nerve function (i.e., rotarod, reduced front/hind limb swing-time, etc.). Several lines of study have demonstrated that reduced mitochondrial function, and in particular increased ROS production, are associated with age-related decline in these systems (29, 30). In at least two different studies, obesity-induced mitochondrial dysfunction was mitigated with the administration of mitochondrial antioxidants (11, 13). Moreover, reducing mitochondrial ROS production has been shown to extend mouse lifespan under normal aging conditions (31). Whereas others have suggested that obese rodents perform poorly in measures of muscle, neurological and cognitive function in rodents (32–34), our study suggests that these may largely be caused by gradual declines with age not merely as a consequence of being obese. One potential interpretation of this result is that some accumulating factor, like oxidative damage, could ultimately be responsible. It would be of great interest to determine if the functional declines with age or even the shortening of lifespan in high fat-fed mice we report here could be mitigated through the reduction of oxidative stress and/or damage.
As expected, the body composition changes caused by high fat diet in this study were largely driven by an increase in fat mass. There is growing recognition for the role of adipose tissue in the aging process. Largely thought of as an inert, energy storing tissue, white adipose tissue, particularly visceral fat, is often highly pro-inflammatory and is dramatically responsive to dietary inputs (8). Moreover, obesity and conditions of over nutrition promote cellular dysfunction of the adipose cells that contribute to further inflammation in this tissue (9). The reduction of adipose tissue by calorie restriction has been proposed to play a key role in this treatment’s pro-longevity effects (35). More directly, removal of visceral adipose tissue alone is sufficient to extend the lifespan of rats (36). It seems likely that the exacerbation of inflammation following the expansion of adipose tissue in obesity is a likely contributor to the increase in local and circulating oxidative stress found in obese subjects (6). Indeed, rats fed a high fat diet, but restricted to 60% of the caloric intake of rats fed high fat diet ad libitum, had reduced fat mass, inflammation and oxidative stress showing a dissociation of the effects of a high fat diet and effects of obesity (37). This would seem to suggest that excess caloric intake may be a more important “pro-longevity” factor than the increased intake of fat per se. Further addressing of this important question is needed. As mentioned above, excess fat tissue appears to accelerate the onset of many age-related diseases (3–5). In the clinically obese, the mass of adipose tissue can often reach greater than 50% of total mass (8) and it is easy to envision the increased inflammation and oxidative stress driving the higher incidence of disease and mortality in these patients. Anti-inflammatories have been used as therapeutics against obesity-induced metabolic disease, though the potential for more broad range benefit against mortality with obesity has not been addressed (38).
An interesting finding from our study is that the changes in some parameters of health between 12 and 18 months of age, including activity, front/hind limb swing time, and rotarod performance, seem to be predictive of remaining lifespan in mice fed high fat diets (Tables 1 and 2). Kiepert et al. addressed a similar analysis in a cohort of chow and high fat fed mice and found that lifespan negatively correlated with maximum body weight, rate of fat gain and energy expenditure (39). They found no correlation with activity, though this discrepancy with our study could be explained by the fact that activity was only measured at one time point by Kiepert et al. rather than as a change in activity. The relative decline in activity with age in high fat-fed mice that we report could also have broader implications on the subsequent health of the animal. In such a “vicious-cycle”, obese mice might physically move less thereby compounding the effects of metabolic dysfunction and potentially contributing to increased mortality. Exercise has the potential to improve pathological outcomes independently of its effects on weight (40). Moreover exercise could conceivable act to reduce some of the pro-inflammatory effects of obesity that have been proposed to mitigate the detrimental effects of this condition. Further tests of the effects of exercise on healthy aging in this model would be of interest.
While our study hints that obesity fundamentally alters the rate of aging, there is also evidence that the detrimental effects of obesity can be reversed through weight loss. List et al. found that weight cycling in mice, i.e., alternating between high fat and low fat diets every 4 weeks, did not significantly shorten lifespan relative to mice kept on low fat diet for life (41). Despite being larger and fatter than low fat-fed mice, weight cycled mice also showed a significant reduction in the pro-inflammatory cytokine IL-6. It could be that reducing inflammation, or improving glucose metabolism or some other individual physiological marker could be key to improving age-related health in the obese. Several candidate biomarkers for longevity or healthy aging have been proposed, but these have largely been generated in lean, “normally” aging rodents or healthy populations. However, it isn’t clear whether these biomarkers maintain the same validity in obese or metabolically stressed populations raising question about their usefulness in general.
By taking a comprehensive approach to assessing function, we were able to discover that some longitudinal performance results showed an improvement in age, some no difference, and some a decline with age. The design of our study somewhat limits the interpretation of these effects in normal, chow-fed mice as our latest time point at which we assessed mice was 18 months of age; the median lifespan of C57BL/6J mice is approximately one year greater than this so control mice were still relatively young when tested (42, 43). However, we were still able to discern some functional changes with age using these assays. Our findings that high fat-feeding promoted significant declines in some assays support the hypothesis that obesity accelerates aging, but it also of interest that obesity seemed to improve some functions relative to control mice including grip strength and perhaps RMR. While it’s possible these results could simply be driven by differences in size or body composition that confound these assays, it is interesting to propose that chronic obesity may actually confer some physiological benefits with age (44, 45).
In conclusion, the study highlights the risk that life-long obesity has on both longevity and physiological function. Furthermore, we find evidence that some tissues may undergo an acceleration of the aging process with obesity that is associated with increased levels oxidative stress. Until recently, medicine has largely addressed obesity’s direct effects on metabolic dysfunctions and has not clearly understood the broader physiological implications of this condition. It is now clear that obesity increases the incidence of many diseases beyond diabetes and metabolic syndrome, including those generally thought of as age-related (3–5). As others have pointed out, finding ways to slow aging may have more far-reaching impact on treating these patients than individual disease treatment (46). As our results suggest, understanding these treatments could also benefit obese patients by extending their period of healthy aging.
Acknowledgments
Animal studies were performed in the Healthspan and Functional Assessment Core of the San Antonio Nathan Shock Center for the basic biology of aging. This research was supported in part by funding from the American Federation for Aging Research (Y.Z. and A.B.S.), the Biomedical Laboratory Research & Development Service of the Veteran’s Affairs Office of Research and Development (1I01BX000547 to A.R.) and Geriatric Research Education and Clinical Center of the South Texas Veterans Healthcare System (A.B.S.).
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. PRevalence of overweight and obesity among us children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among us adults: The national health and nutrition examination surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The Impact of Obesity on US Mortality Levels: The Importance of Age and Cohort Factors in Population Estimates. American Journal of Public Health. 2013;103:1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, Moore SC, Robien K, Rosenberg PS, Singh PN, Weiderpass E, Adami HO, Anton-Culver H, Ballard-Barbash R, Buring JE, Freedman DM, Fraser GE, Beane Freeman LE, Gapstur SM, Gaziano JM, Giles GG, Håkansson N, Hoppin JA, Hu FB, Koenig K, Linet MS, Park Y, Patel AV, Purdue MP, Schairer C, Sesso HD, Visvanathan K, White E, Wolk A, Zeleniuch-Jacquotte A, Hartge P. Association between Class III Obesity (BMI of 40–59 kg/m2) and Mortality: A Pooled Analysis of 20 Prospective Studies. PLoS Med. 2014;11:e1001673. doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Mamun AA, Bonneux L. Obesity in Adulthood and Its Consequences for Life Expectancy: A Life-Table Analysis. Annals of Internal Medicine. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keaney JF, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in The Framingham Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 8.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: Which role for oxidative stress? Metabolism. 1995;44:363–368. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proceedings of the National Academy of Sciences. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Qi W, Richardson A, Van Remmen H, Ikeno Y, Salmon AB. Oxidative damage associated with obesity is prevented by overexpression of CuZn- or Mn-superoxide dismutase. Biochemical and biophysical research communications. 2013;438:78–83. doi: 10.1016/j.bbrc.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of Clinical Investigation. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda M, Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obesity Research & Clinical Practice. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Rame JE, Barouch LA, Sack MN, Lynn EG, Abu-Asab M, Tsokos M, Kern SJ, Barb JJ, Munson PJ, Halushka MK, Miller KL, Fox-Talbot K, Zhang J, Hare JM, Solomon MA, Danner RL. Caloric restriction in leptin deficiency does not correct myocardial steatosis: failure to normalize PPARα/PGC1α and thermogenic glycerolipid/fatty acid cycling. 2011;43 doi: 10.1152/physiolgenomics.00088.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proceedings of the National Academy of Sciences. 1984;81:1835–1838. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silberberg M, Silberberg R. Factors modifying the lifespan of mice. The American journal of physiology. 1954;177:23–26. doi: 10.1152/ajplegacy.1954.177.1.23. [DOI] [PubMed] [Google Scholar]
- 18.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouse Phenome Database web site. The Jackson Laboratory; Bar Harbor, ME: 2014. Growth curve analysis of 7 inbred strains of mice. www.phenome.jax.org. [Google Scholar]
- 20.Muscogiuri G, Salmon AB, Aguayo-Mazzucato C, Li M, Balas B, Guardado-Mendoza R, Giaccari A, Reddick RL, Reyna SM, Weir G, DeFronzo RA, Van Remmen H, Musi N. Genetic Disruption of SOD1 Gene Causes Glucose Intolerance and Impairs β-cell Function. Diabetes. 2013 doi: 10.2337/db13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Styskal J, Nwagwu FA, Watkins YN, Liang H, Richardson A, Musi N, Salmon AB. Methionine sulfoxide reductase A affects insulin resistance by protecting insulin receptorfunction. Free Radical Biology and Medicine. 2013;56:123–132. doi: 10.1016/j.freeradbiomed.2012.10.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett CML, Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Molecular Metabolism. 3:460–464. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piers LS, Soares MJ, McCormack LM, O’Dea K. Is there evidence for an age-related reduction in metabolic rate? 1998;85 doi: 10.1152/jappl.1998.85.6.2196. [DOI] [PubMed] [Google Scholar]
- 24.Fahlstrom A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32:1868–1880. doi: 10.1016/j.neurobiolaging.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Réndon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin Extends Life and Health in C57BL/6 Mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014;69A:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.service, U. S. D. o. A. A. R, editor Nutrient Intakes from Food: Mean Amounts of Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2009–2010. 2012. [Google Scholar]
- 27.López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Current opinion in endocrinology, diabetes, and obesity. 2010;17:446–452. doi: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. The FASEB Journal. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brierley EJ, Johnson MA, Lightowlers RN, James OFW, Turnbull DM. Role of mitochondrial DNA mutations in human aging: Implications for the central nervous system and muscle. Annals of Neurology. 1998;43:217–223. doi: 10.1002/ana.410430212. [DOI] [PubMed] [Google Scholar]
- 31.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of Murine Life Span by Overexpression of Catalase Targeted to Mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 32.Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menshikova EV, Ritov VB, Toledo FGS, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. 2005;288 doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 34.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. Journal of Neurochemistry. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huffman DM, Barzilai N. Contribution of adipose tissue to health span and longevity. Interdisciplinary topics in gerontology. 2010;37:1–19. doi: 10.1159/000319991. [DOI] [PubMed] [Google Scholar]
- 36.Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, Barzilai N. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators of inflammation. 2012;2012:984643. doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The Effects of Salsalate on Glycemic Control in Patients With Type 2 DiabetesA Randomized Trial. Annals of Internal Medicine. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keipert S, Voigt A, Klaus S. Dietary effects on body composition, glucose metabolism, and longevity are modulated by skeletal muscle mitochondrial uncoupling in mice. Aging Cell. 2011;10:122–136. doi: 10.1111/j.1474-9726.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koga S, Kojima A, Ishikawa C, Kuwabara S, Arai K, Yoshiyama Y. Effects of diet-induced obesity and voluntary exercise in a tauopathy mouse model: Implications of persistent hyperleptinemia and enhanced astrocytic leptin receptor expression. Neurobiology of Disease. 2014;71:180–192. doi: 10.1016/j.nbd.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 41.List EO, Berryman DE, Wright-Piekarski J, Jara A, Funk K, Kopchick JJ. The effects of weight cycling on lifespan in male C57BL/6J mice. Int J Obes. 2013;37:1088–1094. doi: 10.1038/ijo.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmon AB, Pérez VI, Bokov A, Jernigan A, Kim G, Zhao H, Levine RL, Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. The FASEB Journal. 2009;23:3601–3608. doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prescott HC, Chang VW, O’Brien JMJ, Langa KM, Iwashyna TJ. Obesity and 1-Year Outcomes in Older Americans With Severe Sepsis*. Critical Care Medicine. 2014;42:1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaberlin JR, Ma Y, Zhang J, Ahuja SS, Lindsey ML, Halade GV. Obese and diabetic KKAy mice show increased mortality but improved cardiac function following myocardial infarction. Cardiovascular Pathology. 2013;22:481–487. doi: 10.1016/j.carpath.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D, Olshansky SJ. Substantial Health And Economic Returns From Delayed Aging May Warrant A New Focus For Medical Research. Health Affairs. 2013;32:1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]