Abstract
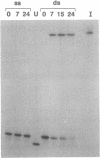
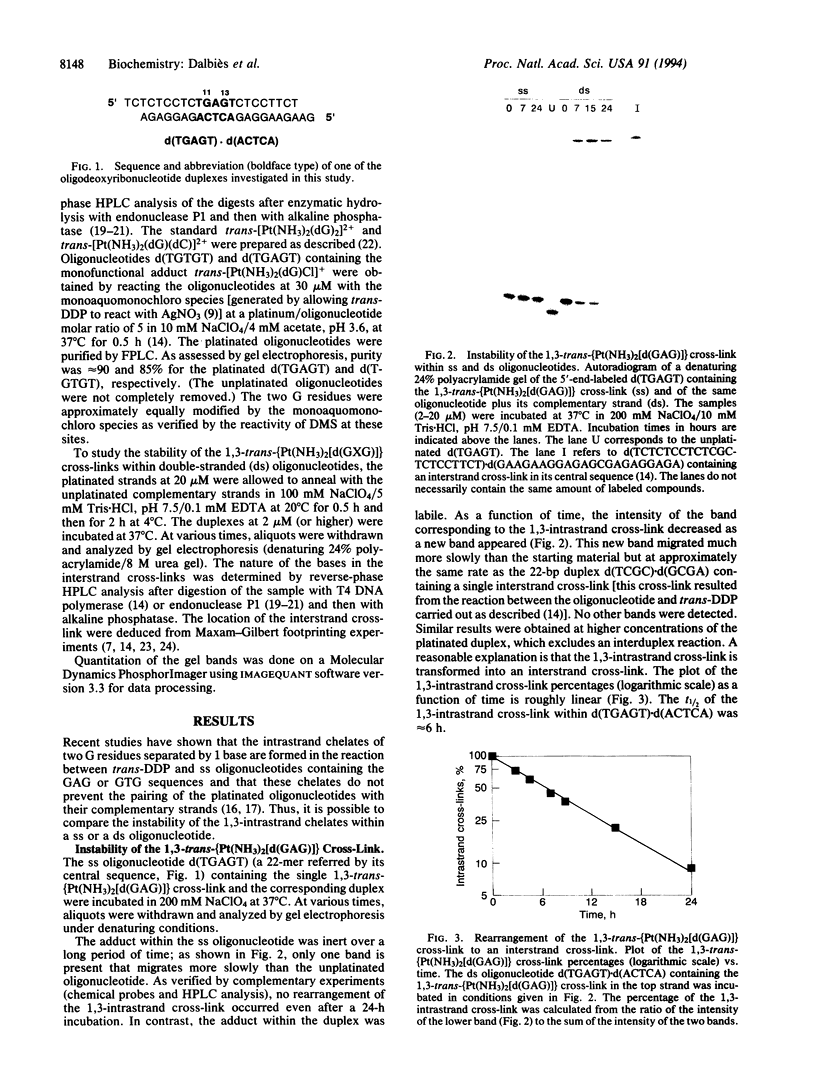
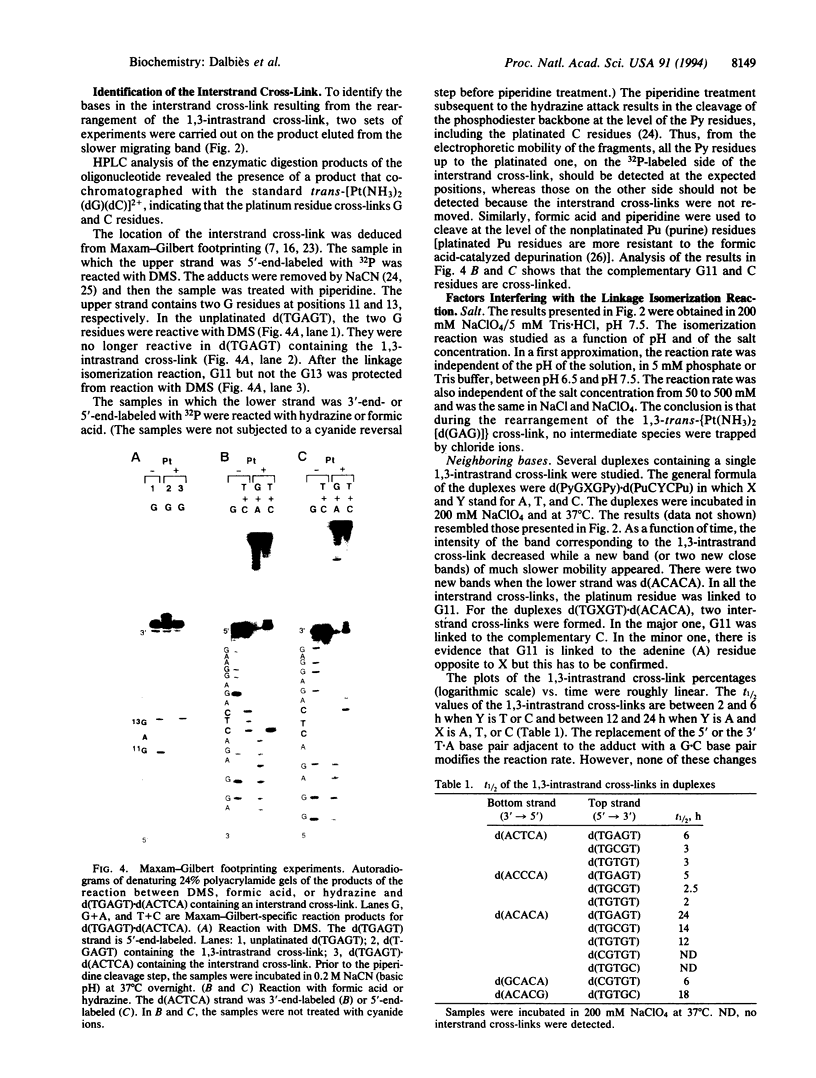
In the reaction between trans-diamminedichloroplatinum(II) and a single-stranded pyrimidin-rich oligodeoxyribonucleotide (22-mer) containing the central sequence TGAGT, the 1,3-trans-[Pt(NH3)2[d(GAG)]] cross-link is formed. The 1,3-intrastrand cross-link is inert within the single-stranded oligonucleotide. In contrast, it rearranges to an interstrand cross-link when the platinated oligonucleotide is paired with its complementary deoxyribo- or ribonucleotide strand. The half-life of the 1,3-intrastrand cross-link, approximately 6 h at 37 degrees C, is independent of the nature and concentration of the salt (NaCl or NaClO4). It is not dramatically affected when the intervening adenine residue between the chelated guanine residues is replaced by a cytosine or a thymine residue or when the T.A base pair adjacent to the 5' or 3' side of the adduct is replaced by a C.G base pair. On the other hand, a mismatch on the 3' or 5' side of the adduct prevents the rearrangement. We propose that the linkage isomerization reaction results from a direct nucleophilic attack of the cytosine residue complementary to the platinated 5' guanine residue on the platinum residue. Among others, the potential use of the DNA.RNA-promoted reaction is discussed in the context of the antisense strategy to irreversibly cross-link the antisense oligonucleotides to their targets.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anin M. F., Leng M. Distortions induced in double-stranded oligonucleotides by the binding of cis- or trans-diammine-dichloroplatinum(II) to the d(GTG) sequence. Nucleic Acids Res. 1990 Aug 11;18(15):4395–4400. doi: 10.1093/nar/18.15.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec V., Leng M. DNA interstrand cross-links of trans-diamminedichloroplatinum(II) are preferentially formed between guanine and complementary cytosine residues. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5345–5349. doi: 10.1073/pnas.90.11.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccia M., Nassi A., Loseto F., Boccarelli A., Mariggio M. A., Giordano D., Intini F. P., Caputo P., Natile G. A trans-platinum complex showing higher antitumor activity than the cis congeners. J Med Chem. 1993 Feb 19;36(4):510–512. doi: 10.1021/jm00056a012. [DOI] [PubMed] [Google Scholar]
- Comess K. M., Costello C. E., Lippard S. J. Identification and characterization of a novel linkage isomerization in the reaction of trans-diamminedichloroplatinum(II) with 5'-d(TCTACGCGTTCT). Biochemistry. 1990 Feb 27;29(8):2102–2110. doi: 10.1021/bi00460a020. [DOI] [PubMed] [Google Scholar]
- Eastman A., Barry M. A. Interaction of trans-diamminedichloroplatinum(II) with DNA: formation of monofunctional adducts and their reaction with glutathione. Biochemistry. 1987 Jun 16;26(12):3303–3307. doi: 10.1021/bi00386a009. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A., Jennerwein M. M., Nagel D. L. Characterization of bifunctional adducts produced in DNA by trans-diamminedichloroplatinum(II). Chem Biol Interact. 1988;67(1-2):71–80. doi: 10.1016/0009-2797(88)90087-7. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Farrell N., Kelland L. R., Roberts J. D., Van Beusichem M. Activation of the trans geometry in platinum antitumor complexes: a survey of the cytotoxicity of trans complexes containing planar ligands in murine L1210 and human tumor panels and studies on their mechanism of action. Cancer Res. 1992 Sep 15;52(18):5065–5072. [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Gaucheron F., Malinge J. M., Blacker A. J., Lehn J. M., Leng M. Possible catalytic activity of DNA in the reaction between the antitumor drug cis-diamminedichloroplatinum(II) and the intercalator N-methyl-2,7-diazapyrenium. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3516–3519. doi: 10.1073/pnas.88.9.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Lemaire M. A., Schwartz A., Rahmouni A. R., Leng M. Interstrand cross-links are preferentially formed at the d(GC) sites in the reaction between cis-diamminedichloroplatinum (II) and DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1982–1985. doi: 10.1073/pnas.88.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepre C. A., Chassot L., Costello C. E., Lippard S. J. Synthesis and characterization of trans-[Pt(NH3)2Cl2] adducts of d(CCTCGAGTCTCC).d(GGAGACTCGAGG). Biochemistry. 1990 Jan 23;29(3):811–823. doi: 10.1021/bi00455a031. [DOI] [PubMed] [Google Scholar]
- Lepre C. A., Strothkamp K. G., Lippard S. J. Synthesis and 1H NMR spectroscopic characterization of trans-[Pt(NH3)2[d(ApGpGpCpCpT)-N7-A(1),N7-G(3)]]. Biochemistry. 1987 Sep 8;26(18):5651–5657. doi: 10.1021/bi00392a011. [DOI] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Caruthers M. H. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993 Mar 12;259(5101):1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet D., Gaucheron F., Sip M., Leng M. Instability of the monofunctional adducts in cis-[Pt(NH3)2(N7-N-methyl-2-diazapyrenium)Cl](2+)-modified DNA: rates of cross-linking reactions in cis-platinum-modified DNA. Nucleic Acids Res. 1993 Dec 25;21(25):5846–5851. doi: 10.1093/nar/21.25.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen W., Link C. J., Jr, O'Connor P. M., Reed E., Parker R., Howell S. B., Bohr V. A. Increased gene-specific repair of cisplatin interstrand cross-links in cisplatin-resistant human ovarian cancer cell lines. Mol Cell Biol. 1992 Sep;12(9):3689–3698. doi: 10.1128/mcb.12.9.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Van Houten B., Farrell N. Ligand effects on platinum binding to DNA. A comparison of DNA binding properties for cis- and trans-[PtCl2(amine)2] (amine = NH3, pyridine). Biochemistry. 1993 Sep 21;32(37):9632–9638. doi: 10.1021/bi00088a015. [DOI] [PubMed] [Google Scholar]