Abstract
Residential drug treatment provides an opportunity to intervene with smokers substance use disorders (SUD). A randomized controlled clinical trial compared: (1) Contingent Vouchers (CV) for smoking abstinence to Noncontingent Vouchers (NCV), crossed with (2) Motivational Interviewing (MI) or Brief Advice (BA), for 184 smokers in SUD treatment. During the voucher period, 36% of carbon monoxide readings indicated smoking abstinence for those receiving CV versus 13% with NCV (p < .001). Post-treatment point-prevalence abstinence rates were low (3–4% at each follow up), with more abstinence when CV was combined with MI (6.6% on average) than with BA (0% on average). No differential effects on drug use or motivation to quit smoking occurred. Thus, CV had limited effects on long-term smoking abstinence in this population but effects were improved when CV was combined with MI. More effective methods are needed to increase motivation to quit smoking and quit rates in this high-risk population.
Keywords: contingent vouchers, contingency management, financial incentives, motivational interviewing, brief advice, smoking cessation’ substance use disorders, point-prevalence abstinence, nicotine dependence, motivation to quit smoking
1. Introduction
Smokers with substance use disorders (SUD) smoke more heavily than do smokers in general (e.g., Compton, Thomas, Stimson & Grant, 2007; Moliterno et al, 1994; Roll et al, 1996). Smokers with SUD have synergistic health risks (Castellsague et al., 1999; Pelucchi, Gallus, Garavello, Bosetti, & La Vecchia, 2006), and more alcohol dependent smokers die from tobacco than from the alcohol use disorder (AUD) (Hurt et al., 1996; Hurt & Patten, 2003; Zacny, 1990). Smoking cessation trials enrolling people with SUDs during substance treatment had very limited success in affecting smoking (e.g., Bien & Burge, 1991; Joseph, Willenbring, Nugent, & Nelson, 2005; Kalman et al., 2001; Monti, Rohsenow, Colby, & Abrams, 1995; Prochaska, Delucchi & Hall, 2004). Improving ways to help smokers with SUD to quit smoking is important for their health.
While SUD treatment provides a window of opportunity for smoking intervention, low motivation to quit smoking within 6 months is a major barrier to cessation in this population (Burling, Ramsey, Seidner, Kondo, 1997; Flach & Diener, 2004; Irving, Seidner, Burling, Thomas, & Brenner, 1994; Martin, Rohsenow, MacKinnon Abrams & Monti, 2006; Monti et al., 1995; Richter, Gibson, Ahluwalia & Schmelze, 2001; Sees & Clark, 1993; Seidner, Burling, Gaither, & Thomas, 1996). Since low pretreatment motivation predicts low success for smoking cessation in smokers with SUD (Rohsenow, Martin, Tidey, Monti, & Colby, 2013), treatments designed to enhance motivation may be particularly relevant for these smokers. Methods of motivating smoking abstinence include Motivational Interviewing (MI; Miller & Rollnick, 1991, 2002), Brief Advice (BA; Manley, Epps, Husten, Glynn, & Shopland, 1991), and contingent vouchers (CV) for smoking abstinence (Higgins et al., 2004).
MI uses an empathic nonconfrontational therapist style, emphasizes the client’s personal responsibility and choice about change, and provides objective feedback about the effects of the substance on the client (in most studies), advice to change, and advice about methods available for making change. Controlled studies of MI for smoking have had mixed results. MI or a similar motivational counseling in general practice settings resulted in more point-prevalence abstinence than did various types of BA (Butler et al., 1999; Soria, Legido, Escalano, Yeste, & Montoya, 2006). Meta-analyses concluded that MI is more effective for adult smoking cessation than other counseling methods but with a small effect (2.3% more smokers abstinent) (Heckman, Egleston, & Hofmann, 2010; Hettema & Hendricks, 2010). Among adults not seeking to quit smoking (thus similar to smokers with SUD), “motivational advice” produced more quit attempts compared to no treatment although it was not more effective than smoking reduction counseling with nicotine replacement (Carpenter, Hughes, Solomon, & Callas, 2004).
BA refers to a standardized procedure involving brief advice to quit smoking and brief coping skills training using methods designed to increase motivation (e.g., Glynn & Manly, 1990). BA is recommended for low motivation smokers in primary care settings in clinical practice guidelines (Coleman, 2004; Fiore et al., 2000; Hollis, Lichenstein, Vogt, Stevens, & Biglan, 1993; Katz, Muehlenbruch, Brown, Fiore, & Baker, 2004; Manley et al., 1991). Across studies, smokers in general show a 30% increase in odds of quitting with BA (Fiore et al., 2000; Rigotti, 2002). BA versus MI for methadone-maintained smokers showed about 5% abstinent at 6 months with either approach (Stein et al., 2006). Our previous clinical trial compared MI to BA for tobacco cessation among smokers in residential SUD treatment (Rohsenow et al., 2014). The smokers with more pretreatment drug use days who received BA were the only ones with abstinence at 12 months (7% versus 0%), perhaps because the strong direct message to quit smoking used in BA is more consistent with the strong messages about abstinence provided in SUD treatment than the MI message that change is up to them. Furthermore, BA increased post-treatment motivation to quit smoking more than did MI. Therefore, the next logical approach for this research program was to determine whether another method of increasing abstinence could enhance the effects of either BA or MI for smokers with SUD.
CV based smoking treatments provide vouchers for tangible incentives (money or merchandise) contingent on abstinence or reduction in smoking to a target level. CV methods have long been known to increase smoking abstinence in the general population (reviewed in Higgins, Tidey & Rogers, 2009; later study by Secades-Villa, García-Rodríguez, López-Núñes, Alonso-Pérez, & Hernández-Hermida, 2014), although most of these were laboratory analogue studies. Generally subjects return to regular smoking when CVs are withdrawn in studies relying on CV without counseling (Ledgerwood, Arfken, Petry & Alessi, 2014; Robles et al., 2005; Stitzer et al., 1986; Stitzer & Bigelow, 1982, 1983, 1985) so there is a need to incorporate CV into cessation counseling.
Ten published studies have investigated CV for smoking abstinence among smokers with SUD, mostly smokers receiving pharmacologic treatment for opiate dependence (reviewed by Sigmon & Patrick, 2012). In general, CV significantly increased smoking abstinence while the incentives were in place, but not for long after incentives were terminated (Alessi & Petry, 2014; Alessi, Petry & Urso, 2008; Dunn et al., 2008, 2010; Hunt et al., 2010; Robles et al., 2005; Shoptaw et al., 1996, 2002; Wiseman et al., 2005). Very few of these studies included behavioral counseling: these included brief smoking skills training (Robles et al., 2005), brief behavioral support (Alessi & Petry, 2014), and relapse prevention training (Shoptaw et al., 2002). Since CV is particularly effective at increasing abstinence early in treatment, it may have complementary effects with counseling approaches that build motivation during the treatment and thereby extend the effects of CV past the end of the incentive period (e.g., Carroll et al., 2006). Therefore, we conducted the first fully-powered randomized trial to investigate the effects of CV for smoking abstinence combined with counseling to increase motivation and coping skills among patients with SUD so as to see if effects of CV will persist after vouchers when combined with methods to increase intrinsic motivation.
The primary purpose of the study was to evaluate the hypothesis that CV, compared to non-contingent vouchers (NCV), when combined with either MI or BA, would increase the likelihood of tobacco abstinence among smokers with SUD, both while the contingencies are in place and during the 12-month follow-up period. While there is no theoretical reason for BA to be more or less effective than MI in increasing smoking abstinence, BA was more effective than MI for smokers with SUD in our previous study (Rohsenow et al., 2014). We also investigated several possible moderators of treatment effects: number of drug use days (per Rohsenow et al., 2014); initial motivation to quit smoking (Rohsenow et al., 2013); nicotine dependence (using minutes to first cigarette per Transdisciplinary Tobacco Research Center, 2007); and gender. No effects on substance use during follow up were expected but were investigated due to concerns in the treatment provider community about effects of smoking cessation on sobriety (Monti et al., 1995; Prochaska, 2010).
2. Materials and Methods
2.1 Participants
2.1.1 Site
The clinical site was a state-funded inner-city 28-day residential substance abuse treatment program with state-wide catchment (The Providence Center, Residential Services program). The program was abstinence-oriented and provided SUD education in a group format based on 12-Step models, with outpatient aftercare available, with about 30 inpatients at a time. Smoking cessation was not addressed by the program and smoking was allowed outdoors at breaks. In-service training with clinical staff explained the benefits of smoking cessation for people with SUD. We had been conducting smoking treatment studies with SUD patients there for a number of years already. Any patient could receive our smoking self-help materials without participating in our research.
2.1.2 Eligibility Criteria
A trained research therapist determined eligibility and diagnosis. Patients were eligible if they met current DSM-IV SUD criteria (see 2.4.3), smoked at least 10 cigarettes per day for the past 6 months, and were not engaged in smoking treatment. Patients were excluded if they were psychotic, actively suicidal, terminally ill, cognitively impaired (unable to understand informed consent when tested on comprehension; none were excluded for this), or could not read. Recruitment occurred from June 2002 to June 2006. Recruits were told the study would provide “informational sessions about smoking” without requiring cessation and would offer payments either for reduced smoking followed by abstinence, or just for providing breath samples for 19 days, and that after the 19-day voucher period, they would receive free nicotine replacement for up to 8 weeks.
2.2 Overview of Procedures
The design was a 2 (CV vs. NCV) by 2 (MI vs. BA) randomized controlled clinical trial in which participants were randomized to one of four groups: CV + MI, CV + BA, NCV + MI, NCV + BA. Stratified random assignment, using urn randomization (Stout et al., 1994), was done on the first day of the voucher period while stratifying for gender, nicotine dependence severity (median split) based on the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and motivation to change smoking (median split) as assessed by the Smoking Contemplation Ladder (CL; Biener & Abrams, 1991) score. The median split for FTND and the Smoking Contemplation Ladder were based on medians from a previous study with participants from the same clinical site (Rohsenow et al., 2014). Follow ups were at 1, 3, 6 and 12 months with CO or cotinine confirmation. To ensure adequate follow-up rates detailed contact information was collected at baseline, the costs of transportation to an agreed upon interview location was covered by the study, some reminders were sent, and participants consented to designate a significant other as a locator if we lost contact with the participant.
2.3 Interventions
2.3.1 Procedures applying to both MI and BA
Both MI and BA were provided in four sessions: at baseline (the day before starting the voucher period) and 7, 14 and 19 days after the first session. Both interventions were fully manualized and audiotaped. All participants were informed of free access to nicotine replacement therapy (NRT; transdermal nicotine patch or nicotine gum; doses per product insert) for up to 12 weeks after the first 3 weeks if medically eligible, smoking cessation pamphlets, and hard candy (chewing gum not allowed on site). NRT was not offered during the 19-day active treatment period so as not to confound the assessment of CV vs. NCV, as those in the CV condition would more likely to use NRT, and so participants would not have any incentive to drop out of CV early to get access to NRT. To prevent diversion, participants were required to turn in their used gum or blister pack to receive the next day’s dose, and to put on the patch in staff presence.
2.3.2 Motivational Interviewing
MI used a motivational therapist style with assessment feedback, as recommended by Miller & Rollnick (1991), provided from a computer-generated personalized feedback report that we programmed. The initial session (45 min) involved discussing pros and cons of smoking, the health risks associated with their carbon monoxide (CO) level, the costs of smoking relative to their income, their smoking rate compared to state and national norms, the relationship of smoking to alcohol use and to sobriety, and their barriers to change (Asher et al., 2003) with corrective information (since more barriers are associated with lower motivation, Martin, Rohsenow, MacKinnon, Abrams, & Monti, 2006). Patients chose goals and methods from a menu of suggestions, and were provided with their choice of a variety of smoking cessation pamphlets. At additional sessions at 7, 14 and 19 days after the first session (15–30 min each), patients were asked about progress toward their own stated goals, barriers and ways to overcome barriers, successes (focusing on self-efficacy), and revised goal preferences. The last session discussed coping with the transition off of the contingencies.
2.3.3 Brief Advice
BA to promote motivation to quit used AHRQ-recommended methods (Manley et al., 1991; Hollis et al., 1993), adapted for SUD recovery issues. In the initial session (15 min), therapists assessed smoking rate and interest in quitting, directly advised patients to stop smoking now during SUD treatment for their health, assisted by giving advice about useful methods (quit date, nicotine replacement, support from family/friend, community resources, groups on site), and asked them to set a quit date within the next 2 weeks. If patients expressed concern about effects on sobriety, they were given corrective information. Patients were given a consumer guide for smoking cessation and were encouraged to select from a variety of nationally available published pamphlets on smoking cessation (e.g., effects on children, smoking and food, handling withdrawal, etc.). Additional sessions at 7, 14 and 19 days after the first session (10–15 min each) checked on progress toward smoking cessation, engaged in problem-solving around barriers (including concerns about effects on substance use, adapting the measure by Asher et al., 2003), noted successes in accomplishing goals in terms of methods they should continue using, repeated direct advice to quit smoking for their health, and reminded them of methods available including the pamphlets. The last session discussed coping with the transition off of the contingencies.
2.3.4 Therapists and monitoring
Interventions were provided by one of three research therapists (two masters’ level and one Ph.D.), with each conducting both types of treatment. Therapists received 30 hours of training in MI including supervised role-plays and practice with the treatment manual, conducted by the first author who had received 2-day MI training from Steven Rollnick (one of the original developers of MI). Training in BA involved 10 hours of training and role-played practice. Treatment session audiotapes (15% of initial sessions, 10% of additional sessions) were reviewed in weekly group supervision with the treatment coordinator and a psychologist trained in MI, and rated for MI style and adherence to the manual (see 2.4.4), with immediate feedback to therapists to prevent drift.
2.3.5 Contingent Voucher procedures
Vouchers were provided during a 5-day reduction phase plus a 14-day abstinent phase. CO monitoring used an EC50 Micro III Smokerlyzer® (Bedfont Scientific Ltd, Kent UK). We encouraged patients to use this opportunity as a way to start a lifetime of tobacco abstinence. Research staff explained the procedures in detail and provided a written handout explaining the contingencies.
Baseline phase
Breath CO was collected for two mornings between 8:00 and 8:15 AM, and the average of the two readings was used as the baseline CO level.
Reduction phase
Breath CO level was collected each morning between 8:00 and 8:30 a.m. for 5 days. Participants received a printed voucher with a monetary value of $2 per test for a 25% reduction from baseline CO level, $4 for 50% reduction, and $6 for a 75% or greater reduction.
Abstinence phase
Breath CO level was collected each morning between 8:00 and 8:30 a.m. and each afternoon between 5:30 and 6:00 p.m. for the next14 days. An escalating schedule of payments provided increasing levels of payments in vouchers for each successive CO level that showed abstinence (CO reading ≤6 ppm, per Cummings & Richard, 1988; Lamb et al., 2010) ranging from $3 for the first sample to $16.50 for the 28th consecutive abstinent breath sample, and with $10 bonuses provided every time three consecutive readings showed abstinence. Whenever a breath sample did not meet the criterion for abstinence, the participant earned no voucher and the payment schedule reverted to the initial $3 level, then after three consecutive abstinent samples the schedule returned to the payment level at which the reset occurred. This component was designed to support efforts to regain abstinence following a lapse. Participants who completed all 19 days of samples and missed no more than three of the scheduled breath tests earned a voucher for a $40 bonus (total possible = $433).
2.3.6 Noncontingent Voucher procedures
In NCV participants could earn the same payments per day for 19 days as those randomized to CV, simply for providing breath samples as scheduled. The NCV condition controls for the effects of receiving vouchers, providing daily breath samples to be analyzed for CO level, and degree of interaction between patient and research staff. Participants provided breath samples on the same schedule as those in the CV group, and the identical system of records, vouchers and merchandise certificates were used for payments.
2.3.7 Vouchers and payments
In addition to printed vouchers participants received, staff maintained a log of vouchers earned in case of loss of vouchers. Vouchers were redeemed for merchandise certificates to popular area stores any day the participant requested them (usually when leaving on pass or providing them to family, both only available on weekends). Merchandise certificates were deposited in their accounts at the treatment agency weekly to protect them from theft while participants were in residence.
2.4 Assessments
2.4.1 Assessment procedures
Research interviewers blind to treatment condition conducted all assessments. Follow-up interviews, in person, were about 1, 3, 6 and 12 months after the initial session. Participants were given a breath alcohol test (per Sobell and Sobell, 1986) using Alco Sensor IV by Intoximeters; none had a breath alcohol reading > .02 g/dL. Follow-up interviews were conducted away from the clinical site after discharge, and all were assured that clinical staff would not be informed of the information provided, per Sobell and Sobell (1986). Participants received $35, $40, $45, and $50 in merchandise certificates for the 1, 3, 6, and 12 month interviews, respectively, and $15 in merchandise certificates at each follow-up for completing the interviews within 14 days of the due date. Significant others (family member or close friend) received $15 per assessment for their time and travel.
2.4.2 Outcome measures
A Timeline Followback interview (Brown et al., 1998; Ehrman & Robbins, 1994; Sobell & Sobell, 1980) was used at baseline (for 6 months pre-admission) and follow ups (for the period since the previous interview) to collect daily data on smoking, alcohol, and other drugs, scored for number of days of use and number of cigarettes and drinks per day. The primary smoking outcome measure during follow-up, 7-day point-prevalence abstinence, was confirmed with a CO level ≤4 ppm and salivary cotinine level ≤ 15 ng/ml (Cropsey et al., 2014; Hughes et al., 2003; Lamb et al., 2010). (CO was used within-treatment; cotinine was used at follow-ups except if the person was using nicotine replacement when CO needed to be used instead.) Urine drug screens (On Trak® test cups for screening confirmed with EMIT, gas chromatography and mass spectrometry) were conducted at follow-up. To count as abstinent from drugs, both self-report and urine drug screen must have been negative. A significant other was interviewed about the patient’s substance use to make participants believe that we could check the validity of their answers, but since such reports do not increase validity, they were not used as data (per Sobell & Sobell, 1986). Alcohol abstinence self-reports were accepted since there is no valid way to confirm them given the short half-life of alcohol and unreliability of significant other reports as confirmation. (See section 2.5.2 for handling missing data and data imputation methods.)
2.4.3 Individual difference measures
Current SUD diagnoses were made using the criteria of the Structured Clinical Interview for DSM-IV-Patient version (First, Spitzer, Gibbon, & Williams, 1995), administered by trained research interviewers. Other individual difference measures at baseline included a smoking history questionnaire, breath CO, and the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) from which minutes to first cigarette was obtained. Responses to the 5-question stage of change algorithm (Prochaska & DiClemente, 1992) were given to research therapists to help them tailor their motivational approach in the session. MI feedback forms included current number of cigarettes per day (with current annual cost to them), CO level at baseline (with health interpretations), and information from the following measures: Smoking Temptations Questionnaire (Velicer, DiClemente, Rossi, & Prochaska, 1990), Nicotine and Other Substance Interaction Expectancy Questionnaire (Rohsenow, Colby, Martin, & Monti, 2005), Barriers to Quitting Smoking in Substance Abuse Treatment (BQS-SAT; Asher et al., 2003), revised to change “alcohol” to “alcohol or drugs”.
2.4.4. Process and treatment delivery measures
At baseline and 1-month, participants completed the Smoking Contemplation Ladder (CL; Biener & Abrams, 1991), a single 10-point fully-anchored scale from 1 (no interest in quitting) to 10 (I have quit smoking and will never smoke again). During treatment we recorded use of NRT, the number of CO readings less than 6 ppm in the voucher period, and length of stay per agency records.
Treatment supervisors endorsed the adequacy of six MI adherence items (whether the treatment provider adequately discussed ambivalence [pros and cons, goal discrepancies], discussed the feedback about smoking effects, explored barriers to change, provided summaries, discussed various goals, and discussed methods for change). Treatment sessions were rated by the treatment supervisors on 1 (not at all) to 5 (extensively) scales for five motivational style measures (arguing, demonstrating empathy, reflective listening, supporting self-efficacy, emphasizing personal responsibility for change). All ratings were done for both conditions.
2.5 Data Analysis Approach
2.5.1 Preliminary analyses
Analyses were conducted using IBM SPSS Statistics® for PC except that multiple imputation analyses were run using MIANALYZE procedures (SAS/STAT, 2013) and moderation analyses were run using PROCESS (Hayes, 2012). All variables were checked for assumptions of normality and outliers, and other assumptions underlying regression. (Log transformation was required only for number of heavy drinking days, number of drug use days at follow-up, and minutes to first cigarette. Untransformed values are presented for ease of interpretation.) All other requirements for GEE, regression, and analysis of variance were met. Treatment group differences in baseline characteristics, in length of treatment (program, study counseling, and contingency period), in number of days of NRT use, and in follow-up rates were examined with 2 X 2 (contingency type by counseling type) one-way analyses of variance (ANOVA) for continuous variables, 2-group (contingency type or counseling type) chi-square tests for dichotomous variables, and 2-group (counseling type MI vs. BA) one-way ANOVAs for supervisor ratings of therapist style.
2.5.2 Handling missing data
At follow-up, people with a CO > 4 ppm, cotinine > 15 ng/ml (if not using NRT), or missing CO or cotinine data, or with self-reported smoking were coded as having smoked with the following exception: if the participant was in prison, self-report was accepted since biological verification equipment was not allowed so lack of verification was unrelated to participant decision. People claiming abstinence from drugs but who had a positive, missing or contaminated drug screen were coded as having used drugs for that follow-up interval with the following exceptions: 1) If the participant was in prison (N = 5 MI + CV, N = 5 BA + CV, N = 4 MI + NCV, N = 4 VA + NCV), self-report was accepted since urine samples were not allowed so lack of verification was unrelated to participant decision (Brown et al., 2009). (Number of prisoners claiming abstinence: N = 2 at 3 mo., N = 1 at 6 mo., N = 3 at 12 mo.) Participants who died during follow-up (n = 1 for all follow-ups and n = 3 at 12 months) were coded as missing for outcomes after death.
Since positive imputation, while the standard for smoking research since most likely to reflect the true values (e.g., Higgins & Green, 2011), could lead to false positives, analyses were re-run using multiple imputation methods (per Higgins & Green, 2011) to provide sensitivity analyses (Rosenbaum, 2005). We imputed data for those missing verified abstinence, average cigarettes per day, or number of days that any drugs were used at each follow up. Multiple imputation provides a method for handling missing data where missing values are imputed for each missing variable to complete the data (Rubin, 1987). Multiple imputation was performed for the outcome variables using multiple linear or logistic regression plus a random component to produce the imputed values. One-hundred-fifty imputed data sets were generated to yield estimates that were better than 95% efficient (Rubin, 1987; Schafer & Graham, 2002). Separate regression analyses were performed using all 150 of the imputed data sets, and the final results represent the effect sizes averaged across the 150 sets of estimates. Since none of these sensitivity analysis results differed in significance level from the analyses without multiple imputation, analyses with multiple imputation are not presented.
General estimating equations (GEE; Zeger and Liang, 1986) models tested the effects of intervention condition on primary and secondary outcomes over time (1, 3, 6 and 12 month follow-up). In these models, the main effects of contingency type and counseling type examined whether outcomes differed over the follow-up period. The two-way interaction of contingency type by counseling type was entered on the second step along with the two-way interactions of contingency type by time, and counseling type by time. The interactions with time examined whether the time slope, or rate of change, differed by these factors.
2.5.3 Analyses of post-treatment outcome
Two post-treatment outcome measures for smoking were chosen: 7-day point-prevalence abstinence confirmed with CO and cotinine (per Hughes et al., 2003) and number of cigarettes per day (to detect reductions in smoking short of abstinence). Three substance use outcome variables were used: number of heavy drinking days (more than 6/4 standard drinks per day for men/women, per Flannery et al., 2002) log transformed during each follow-up period, number of drug use days log transformed during each follow-up period, and relapse to any heavy drinking or drug use over the 12 months. Analyses of the full intent-to-treat sample were conducted using 2 X 2 (contingency type by counseling type) GEE analyses. To follow-up significant interactions of contingency type and counseling type, we examined the effect of counseling type at each level of contingency type using the Wald statistic as the basis for significance testing (Tabachnick & Fidell, 2013). Results were also analyzed for each time period separately to compare with other studies.
2.5.4 Within-treatment and process analyses
One within-treatment outcome and two process measures were chosen: number of readings with CO abstinent during the 14-day abstinence induction phase of the contingency period, use of NRT during months 2–3 of follow up, and pre-post change in CL. CO levels were analyzed with 2 X 2 (contingency type by counseling type) ANOVA during the contingency period. The CL scores were analyzed with 2 X 2 X 2 repeated measures (pretreatment, 1 month) ANOVA; and 2 x 2 contingency type by counseling type logistic regression was used for use of NRT during months 2–3 of follow-up.
2.5.5 Moderator analyses
Moderation analyses (to inform patient-treatment matching) were conducted with pretreatment nicotine dependence (fewer minutes to first cigarette pretreatment), pretreatment frequency of drug use days, gender, and initial motivation level using the CL as the moderator variables. (Initial motivation level was predicted only to differentially affect CV versus NCV response, since both types of counseling are designed to induce motivation equally.) The outcome variables used for the moderator analyses were confirmed 7-day smoking abstinence and average cigarettes per day. In moderator analyses, the potential matching variable was entered with either contingency type or counseling type in path analysis-based moderation models (per Hayes, 2012). The model entered the matching variable, contingency type or counseling type in the first step, and the 2-way interaction of treatment and matching variable on the second step. Significant interaction effects were followed with simple slopes tests.
3. Results
3.1 Sample Size and Attrition
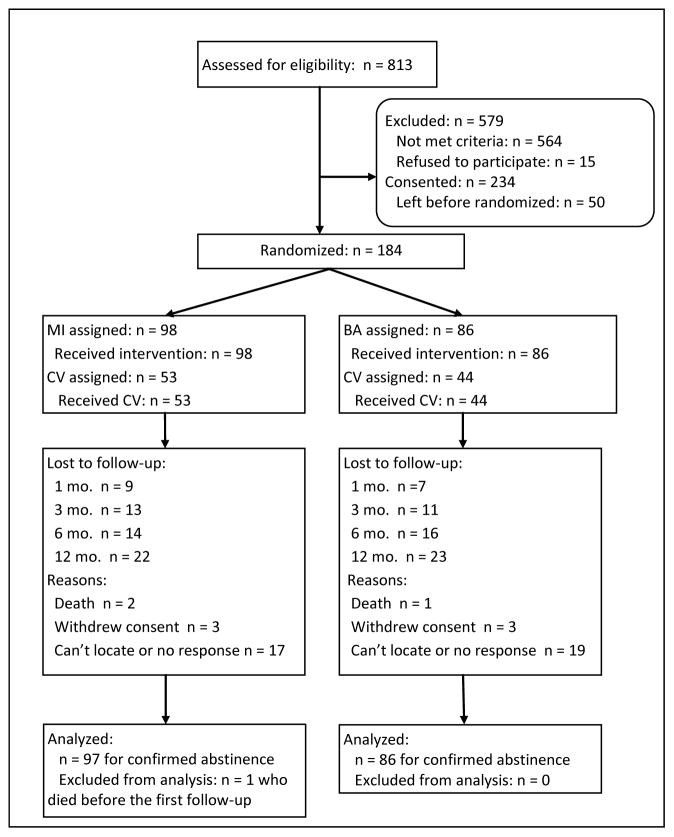
Of 249 eligible patients, 234 (94%) consented, and 184 (79% of the consented) stayed at the site long enough to be randomized to treatment (the intent-to-treat sample). (See Figure 1 for flow chart.) Of these, 98 (53.3%) were assigned to MI, 86 (46.7%) were assigned to BA, 97(52.7%) were assigned to CV, and 87 (47.3%) were assigned to NCV; all received their assigned treatment. Mean number of days in the residential program was 43.6 ± 23.9 [S.D.] with no significant difference between conditions. Of 184 randomized to treatment, 1-mo follow-up was completed by 168 (91.3%), 3-mo follow-up was completed by 160 (87%), 6-mo follow-up was completed by 154 (83.7%), and 12-mo follow-up was completed by 139 (75.5%), with no significant differences by condition. One person who died before the first follow-up was not included in any analyses, resulting in n = 183 for analyses. Two-group ANOVAs or χ2 analyses showed no differences between those who completed the follow-up versus those who did not complete the follow-up at each time on demographic variables (race, gender, age, education) or clinical variables (FTND, number of drinking days) except that those who completed the follow-up interview at 1 month were slightly younger (M = 34.1 ± 8.2 years) than those who did not (M = 38.6 ± 6.8; t(182) = 2.09, p = .04. Completion of the CO collection during the contingency period and number of study counseling sessions attended did not differ significantly by treatment condition. Of those with follow-up data collected, CO data at follow-up was missing for 16.7% (N=28) at 1 month, 33% (N=53) at 3 months, 17% (N=26) at 6 months, and 18.7% (N=26) at 12 months. Collection of CO data during follow-up did not differ significantly by treatment condition, except at 6 month follow-up where 90% (N = 63) of those in BA provided CO and 77.4% (N = 65) of those in MI provided CO, χ2 = 4.33, p = .04. Interviews were conducted at prison/jail for 4.4% (N = 7) at 3, 7.9% (N = 12) at 6, and 11.6% (N = 16) at 12 months where no CO data collection was allowed, as noted above (section 2.5.1). No serious adverse events related to the study occurred.
Figure 1.
Flow chart of recruitment and retention.
3.2 Participant Characteristics
Participants’ mean age was 34.5 ± 8.2 years; 83.2% (N = 153) were white, 9.2% (N = 17) were black, 7.5% (N = 14) were of other races; 6.6% (N = 12) were Hispanic; 44.6% (N = 82) were male; 10.9% (N = 20) were married or living with a romantic partner. In addition, participants’ mean education level was 12.2 ± 1.7 years; 81.9% (N = 164) were unemployed in the week before entering treatment, and their mean legal income was $9,487 ± 13,619 in the past year. At pretreatment, participants showed a CO level of M = 16.4 ± 7.4 ppm; smoked M = 22.3 ± 9.4 cigarettes/day; had a mean FTND score of 5.28 ± 2.29, and had a mean (median) minutes to first cigarette of 33.0 (15.0) ± 44.6 minutes (median provided due to skewness). They had been smoking daily on average for 18.3 ± 8.4 years. The current drug or alcohol diagnoses were: 71.2% (N = 131) alcohol abuse or dependence, 73.9% (N = 136) cocaine abuse or dependence, 52.8% (N = 97) opiate abuse or dependence, and 37% (N = 68) marijuana abuse or dependence. Participants drank 6.3 ± 14.3 drinks per day, had heavy drinking on 23.1 ± 30.7% of days, and had used drugs 43.6 ± 37.1% of days during the 6 months pretreatment. Contingency type by counseling type ANOVAs or χ2 analyses showed no differences between conditions for demographic variables or any pretreatment smoking or substance use variable. (See Table 1 for values by treatment condition. Untransformed values of the log-transformed variables are displayed to aid interpretation.)
Table 1.
Participant Pretreatment Characteristics by Treatment Condition.
| MI Treatment | BA Treatment | CV | NCV | |
|---|---|---|---|---|
| Variable | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % |
| Age in years | 33.5 (7.3) | 35.7 (8.9) | 34.1 (8.4) | 34.9 (7.9) |
| Education (years) | 12.1 (1.7) | 12.4 (1.8) | 12.0 (1.5) | 12.4 (1.9) |
| Income yearly (legal) | $8,624 ($11,298) | $10,470 ($15,868) | $9,215 ($10,964) | $9,791 ($16,134) |
| Days in residential facility | 44.4 (23.8) | 42.6 (24.2) | 41.9 (25.0) | 45.4 (22.7) |
| CO level | 16.0 (7.4) | 16.9 (7.1) | 16.5 (7.2) | 16.4 (7.4) |
| Cigarettes/day | 23.6 (10.2) | 20.9 (8.2) | 22.8 (9.4) | 21.8 (9.3) |
| FTND | 5.54 (2.44) | 5.22 (2.10) | 5.55 (2.29) | 5.20 (2.30) |
| Minutes to first cigarette | 31.7 (37.5) | 34.4 (51.8) | 32.1 (35.0) | 34.0 (53.6) |
| Years smoked daily | 17.8 (7.9) | 19.0 (9.0) | 18.3 (8.5) | 18.4 (8.4) |
| Contemplation Ladder | 4.93 (1.54) | 5.19 (1.25) | 5.01 (1.37) | 5.09 (1.46) |
| % drinking days | 22.0 (31.8) | 29.6 (32.4) | 27.2 (33.6) | 23.8 (30.6) |
| % drug use days | 47.9 (36.7) | 38.8 (37.2) | 45.4 (37.8) | 41.8 (36.5) |
| Drinks per day | 6.2 (17.1) | 6.3 (10.4) | 6.2 (12.3) | 6.4 (16.4) |
| Race – white | 82.7% | 83.7% | 82.5% | 83.9% |
| Race – black | 10.2% | 8.1% | 9.3% | 9.2% |
| Race – other | 7.1% | 8.1% | 8.2% | 6.9% |
| Hispanic | 6.2% | 7.0% | 5.2% | 8.0% |
| Male | 50.0% | 38.4% | 43.3% | 46.0% |
| Married or living together | 12.2% | 9.3% | 9.3% | 12.6% |
| Unemployed pretreatment | 85.7% | 93.0% | 90.7% | 87.4% |
| Drug use disorder | 90.8% | 86.0% | 90.7% | 86.2% |
MI = motivational interviewing, BA = brief advice, CV = contingent vouchers, NCV = noncontingent vouchers.
FTND = Fagerström Test for Nicotine Dependence total score
CO = expired carbon monoxide in parts per million
3.3 Confirmation of Abstinence during Follow-up
Of people reporting 7-day abstinence from smoking at each follow-up, 8 out of 9 at 1 mo., 6 out of 6 at 3 mo., 5 out of 6 at 6 mo., and 8 out of 8 at 12 mo. were confirmed abstinent by cotinine or CO level. Of people reporting abstinence from drugs at each follow-up, 9.7% (13 of 134) at 1 mo., 11.3% (12 of 106) at 3 mo., 15% (18 of 122) at 6 mo., and 8.3% (9 of 109) at 12 mo. were identified as using drugs by urine screen.
3.4 Treatment Style and Adherence Ratings by Supervisor
Therapists were significantly more likely in MI vs. BA to discuss the following topics: increase ambivalence about smoking (93% of MI, 4% of BA sessions), provide assessment feedback (100% of MI, 0% of BA sessions), explore barriers to quitting smoking (100% of MI, 17% of BA sessions), provide summaries (100% of MI, 0% of BA sessions), discuss methods of quitting or preparing to quit (100% of MI, 58% of BA sessions), and discuss possible goals (100% of MI, 14.8% of BA sessions), all χ2s > 19.58, all ps < .001. Therapist style ratings did not differ significantly between treatment conditions for arguing, empathy, or reflective listening, but in MI therapists were more likely to support self-efficacy and emphasize personal responsibility (χ2s from 3.82 to 11.08, ps < .05).
3.5 Treatment Outcomes
3.5.1 Smoking abstinence during abstinence contingency period by condition
During the 14-day period when only abstinence earned vouchers in the CV condition, there was a main effect for contingency type on percent of CO readings indicating abstinence, F(1, 180) = 26.78, p < .001. There was no effect for counseling type or interaction between counseling and contingency type. In CV, on average 35.5% ± 35.3% (N = 10.0) of CO readings were abstinent compared to 12.9% ± 20.0% (N = 3.6) in NCV. In CV, 6.2% (N = 6) of participants were abstinent for all 28 readings compared to 1.0% (N = 1) in NCV, χ2 = 3.18, p = .07 (Fisher’s exact test, 1-tailed). In MI, 3.1% (N = 3) participants were abstinent for all 28 readings compared to 4.7% (N = 4) in BA, χ2 = .32, ns. Total amount earned within-treatment was $92.61 in CV, $280.80 in NCV.
3.5.2 Smoking abstinence at follow ups by condition
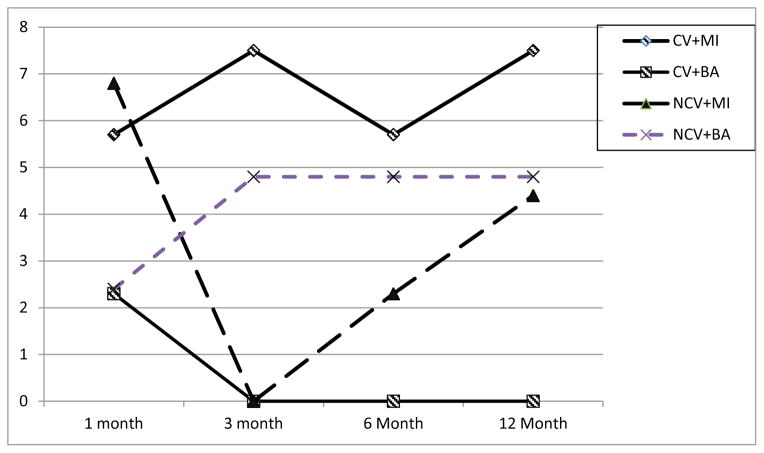
Confirmed 7-day point-prevalence smoking abstinence averaged 4.4% (N = 8 out of 183) at 1 mo., 3.3% (N = 6 out of 183) at 3 and 6 months, and 4.3% (N = 8 out of 181, excluding 2 more people who died) at 12 months. The 2 X 2 GEE model (N = 183) identified no main effects for contingency type or counseling type, and no interaction effects with time. There was a significant contingency type by counseling type interaction, B = −2.65 (SE = 1.33), Wald χ2 (df = 1) = 3.99, p < .05. Follow-up tests showed that within the CV condition there was a significant effect demonstrating higher overall 7-day point-prevalence smoking abstinence in those who received MI vs. BA, B = 2.49 (SE = 1.08), OR = 12.10, CI = 1.46 to 100.48, Wald χ2 (df = 1) = 5.33, p <.05. For those who received CV, MI increased the likelihood of abstinence by 12 times compared to BA. (See Table 2 for details, and Figure 2).
Table 2.
Number of participants with 7-day confirmed point-prevalence smoking abstinence* at each follow-up within each treatment condition (Total N=183)
| 1 Month | 3 Month | 6 Month | 12 Month | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Condition | N | N | % | N | % | N | % | N | % | |
| CV | MI | 53 | 3 | 5.7 | 4 | 7.5 | 3 | 5.7 | 4 | 7.5 |
| BA | 44 | 1 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
| ||||||||||
| NCV | MI | 44 | 3 | 6.8 | 0 | 0 | 1 | 2.3 | 2 | 4.5 |
| BA | 42 | 1 | 2.4 | 2 | 4.8 | 2 | 4.8 | 2 | 4.8 | |
CV = contingent vouchers; NCV = noncontingent vouchers; MI = motivational interviewing; BA = brief advice
or reporting abstinence while in prison
Figure 2.
Percent of participants confirmed abstinent from smoking at each follow-up period by assignment to Motivational Interviewing (MI) versus Brief Advice (BA) treatment and by CV (Contingent Vouchers) versus NCV (Noncontingent Vouchers) condition assignment. No main effects are significant. Standard errors are so small that standard error bars are not visible.
3.5.3 Cigarettes per day by condition
The GEE model identified no main effect for treatment, contingency by counseling interaction, or interactions with time but did indicate a significant overall time effect, B = 1.22 (SE = 0.24), Wald χ2 (df = 1) = 25.22, p < .01. Positive B-weights indicated a significant increase in cigarettes smoked per day over the 12 month follow-up period. Follow-up post-hoc Tukey tests found that 1-month cigarettes per day, M = 10.3 ± 6.4, differed from 3 month, M = 13.1 ± 7.6, 6 month, M = 13.1 ± 8.2, and 12 month, M = 14.4 ± 9.1 cigarettes per day (ps < .01). To test if cigarettes per day at follow-up were reduced compared to pretreatment, we added pretreatment cigarettes per day to the model. Collapsed across all treatments, average cigarettes per day at each follow-up were reduced when compared with pretreatment (ps < .001).
3.5.4 Moderator analyses
Moderation analyses on smoking abstinence during the post-treatment follow-up period entering pretreatment minutes to first cigarette, CL score, or number of drug use days as a factor were nonsignificant. Moderation analyses on cigarettes per day at follow-up entering pretreatment minutes to first cigarette, CL score or number of drug use days as a factor in the analyses were also nonsignificant. When minutes to first cigarette was replaced with FTND, models were still nonsignificant. In analyses adding gender as a factor, no significant main or interaction effects were found for gender.
3.5.5 Substance use outcomes
The GEE models predicting number of drug use days and number of heavy drinking days identified no main effect for treatment, contingency by counseling interaction, or interactions with time but did indicate a significant time effect for drug use days, B = .20 (SE = 0.02), Wald χ2 (df = 1) = 90.57, p < .001, and heavy drinking days, B = .14 (SE = 0.02), Wald χ2 (df = 1) = 60.50, p < .001, both indicating an increase over the whole period between 1 and 12 months. Post-hoc Tukey tests were performed on the log transformed variables to determine specific time points during which change occurred; the means and standard deviations of the untransformed variables are presented for easier interpretation. Results found 1-month number of drug use days, M = 1.00 ± 4.15, was lower than at 3, M = 4.33 ± 10.69, at 6, M = 9.13 ± 18.47, and at 12 months, M = 24.01 ± 38.52, and 12 month drug use days was significantly greater than all other follow-ups. Post-hoc Tukey tests found that 1 month number of heavy drinking days, M = 0.30 ± 2.28, was lower than at 3, M = 2.20 ± 7.40, at 6, M = 9.88 ± 12.45, and at 12 months, M = 12.33 ± 31.72, and 12-month heavy drinking days was significantly greater than all other follow-ups. Pretreatment drug use days and heavy drinking days at each follow-up were still reduced compared to pretreatment when pretreatment values were added to the model (ps < .001).
Analyses of number of participants with any relapse to drug or heavy alcohol use were also non-significant except for a significant time effect, B = −.81 (SE = 0.08), Wald χ2 (df = 1) = 102.13, p < .001. Follow-up post-hoc tests found that 1-month relapse to drug or heavy alcohol use, 10.1% of participants (N = 17 out of 168, 16 missing), differed from 3-month, 33.8% (N = 54 out of 160, 24 missing), 6-month, 44.2% (N = 68 out of 154, 30 missing), and 12-month, 64.0% (N = 89 out of 139, 45 missing) (ps <.01), and 12-month differed from 3-month and 6-month values (ps <.01).
3.6 Process Measures
3.6.1 Contemplation Ladder
There were no significant effects for counseling type, contingency type, time, or interactions with time on CL score in the repeated measures (pretreatment and 1 month follow-up) 2 X 2 X 2 ANOVA.
3.6.2 Use of NRT Months 2–3 of Follow-up
NRT patch or gum was used by 22.8% (n = 42) of patients on M = 10.60 ± 13.21 days during the 2–3 month follow-up period. Logistic regression found no significant differences by condition for percent of participants who used any NRT, and ANOVA found no effects of condition on number of days used NRT.
3.6.3 Multiple imputation analyses
Analyses re-run with multiple imputation methods for missing values on verified abstinence, average cigarettes per day, or number of drug use days did not differ in terms of significance level from the analyses without multiple imputation so details of multiple imputation analyses are not presented.
4.0 Discussion
CV, MI and BA each produced low rates of abstinence in this population, with only 3–4% of participants confirmed abstinent at each point of the one year follow up. However, the best outcomes across the year occurred when CV was combined with MI, resulting in 5.7 to 7.5% (mean of 6.6%) of participants being confirmed abstinent from smoking at each interval over the year of follow up. Most smoking treatments for smokers in SUD treatment do not present 1-year outcomes and/or show no significant effects of the smoking treatment (e.g., Stein et al., 2006). While Alessi and Petry (2014) showed 16% of smoking men treated in residential SUD treatment not smoking at 24 weeks, the trial was small, no differences by CV versus no CV were seen, and no 12-month results were reported. The 12-month smoking abstinence rates reported by Rohsenow et al. (2014) for alcohol dependent smokers in residential SUD treatment without CV were only 2% (0% after MI, 4% after BA, 7% after BA if pretreatment drug use was high). Compared to the very limited existing results from randomized trials of smokers recruited early in SUD treatment, even the relatively low abstinence rates in the present trial show some benefit for combining CV with MI.
Thus, contingent financial incentives when combined with MI do work better than each approach alone to promote smoking abstinence for many smokers with SUD, although with considerable room for improvement. Results were not differential for people with higher or lower pretreatment nicotine dependence, motivation to change, or number of drug use days. These results are encouraging about the value of combining financial incentives with MI but indicate that stronger combinations of smoking treatments are still needed for this population. During the voucher period, CV had significant short-term effects on number of abstinent readings, but even these rates were relatively low, with only 6% of participants having continuous abstinence throughout the contingent voucher condition. Thus, finding ways to increase abstinence during the voucher period are needed for this population.
Without voucher-based treatment (i.e., in the NCV condition), MI and BA had equivalent effects on abstinence. This is consistent with our previous study (Rohsenow et al., 2014) showing no advantage for MI overall (about 2% were abstinent in each counseling condition at each follow-up). The advantage for BA for a subset of individuals with heavier drug use that was found in our earlier study was not replicated in the present study despite the greater frequency of pretreatment drug use in the present study. Given that MI is more costly in therapist training and time, the present results indicate that BA is recommended in SUD programs that are not using voucher-based smoking treatment as a low-cost way to promote long-term smoking abstinence in 2–4% of individuals. While smoking cessation rates are still low, BA adapted for SUD programs is quick and easy to administer and, multiplied by the number of programs, could have a beneficial health effect in this population overall.
No harmful effects on substance use were found, consistent with most other controlled studies (reviewed by Monti et al., 1995; later studies by Burling, Burling, & Latini, 2001; Carmody et al., 2012; Hurt et al., 1994; Kalman et al., 2001; Nieva, Ortega, Mondon, Ballbè, & Gual, 2011; Reid et al., 2008; Rohsenow et al., 2014). Treatment providers are often reluctant to recommend smoking cessation to patients in SUD treatment for fear of harm to recovery (Prochaska, 2010). The present findings, combined with reviews or meta-analyses that showed increased probability of lasting alcohol and drug abstinence after smoking interventions were provided concurrent with SUD treatment (Baca & Yahne, 2009; Prochaska, Delucchi, & Hall, 2004), indicate that SUD treatment providers should be encouraged to incorporate smoking treatment into SUD programs. Providing smoking cessation treatment early in SUD treatment did not have any harmful effects on sobriety among patients recently abstinent from their substances of abuse across studies except in one study, and that involved a mandatory smoking cessation program imposed on patients (Joseph et al., 1993).
Future directions for developing treatments that combine financial incentives with motivational counseling methods may include comparing the efficacy and cost-effectiveness of prize-based CV versus standard voucher methods (e.g., Alessi & Petry, 2014). Furthermore, given that higher levels of withdrawal discomfort reported by smokers with SUD predict less smoking abstinence during and after CV (Rohsenow, Tidey, Kahler, Martin, Colby, & Sirota, 2015), combining CV treatments with pharmacotherapies that reduce craving or withdrawal discomfort may boost the effectiveness of CV, as has been shown in smokers with serious mental illness (Tidey, Rohsenow, Kaplan, Swift, & Reid, 2011).
Limitations
Participants did not need to be motivated for smoking cessation to engage in this program so the current results may underestimate the effectiveness of these treatments among smokers who are ready to quit smoking. However, the purpose was to use SUD treatment as a the window of opportunity to find ways to motivate these smokers to try to quit smoking. Given the low levels of motivation to quit smoking in this population in general (Flach & Diener, 2004; Monti et al., 1995), including less-motivated smokers in this study best mirrored the motivation characteristics of this population. The study was conducted at one low-income inner city SUD treatment agency, and results might be different with higher-income populations and agencies requiring private funding. No adjunctive pharmacotherapy was permitted during the voucher period due to concerns about confounding the investigation of CV versus NCV, so the current results may underestimate the effects of these treatments when combined with nicotine replacement or other pharmacotherapies for smoking cessation. Smokers with missing biological confirmation of smoking abstinence were considered to be smoking, but analyses using multiple imputation indicated that the method of imputation did not affect any results.
Conclusions
Although voucher-based smoking treatment combined with MI has modest effects on smoking abstinence over the year after contingencies are withdrawn in this population, abstinence rates were significantly better when combining CV with MI compared to CV with BA or compared to BA or MI without CV. Thus, CV plus MI may be worth implementing in SUD programs to promote smoking abstinence. For SUD programs that do not have the resources for CV, BA and MI have equivalent effects on abstinence so BA is recommended as less costly in terms of time and training. These behavioral treatments need to be investigated in combination with pharmacotherapies for smoking cessation, since reducing the aversiveness of smoking cessation might make smokers with SUD more likely to engage in cessation attempts.
Highlights.
We provided contingent vouchers for smoking abstinence for smokers in residential drug treatment.
We also provided motivational interviewing or brief advice to quit smoking.
More smoking abstinence occurred in treatment with contingent vouchers than without.
In the following year, smoking abstinence was low, 3 to 4% of patients.
Smoking abstinence was greater for people who got vouchers and motivational interviewing.
Acknowledgments
Supported by 1 RO1 DA13616; two Senior Career Research Scientist Awards from the Department of Veterans Affairs (DJR and PMM); and K05AA019681. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Grateful appreciation is expressed to Suzanne Sales for her data analyses and to the staff of The Providence Center and of Gateway Healthcare Systems, Inc.
Footnotes
Work was performed at: Brown University Center for Alcohol and Addiction Studies, Gateway Healthcare Systems, Inc., and The Providence Center in Providence, RI.
Preliminary results were presented at the annual meeting of the Research Society on Alcoholism, Santa Barbara, CA, June 25, 2005, at the annual meeting of the Research Society on Alcoholism, San Francisco, CA, June 26, 2012, and at the annual meeting of the College on Problems in Drug Dependence, San Diego, June 17, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi SM, Petry NM. Smoking reductions and increased self-efficacy in a RCT of smoking abstinence-contingent incentives in residential substance abuse treatment patients. Nicotine and Tobacco Research. 2014;16:1436–1445. doi: 10.1093/ntr/ntu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. Journal of Applied Behavioral Analysis. 2008;41:617–622. doi: 10.1901/jaba.2008.41-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher MK, Martin RA, Rohsenow DJ, MacKinnon SV, Traficante R, Monti PM. Perceived barriers to quitting smoking among alcohol dependent patients in treatment. Journal of Substance Abuse Treatment. 2003;24:169–174. doi: 10.1016/s0740-5472(02)00354-9. [DOI] [PubMed] [Google Scholar]
- Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: What you need to know. Journal of Substance Abuse Treatment. 2009;36:205–219. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Battjes RJ. Smoking as an issue in alcohol and drug abuse treatment. Addictive Behaviors. 1988;13:225–230. doi: 10.1016/0306-4603(88)90049-4. [DOI] [PubMed] [Google Scholar]
- Bien TH, Burge R. Smoking and drinking: A review of the literature. International Journal of Addictions. 1991;25:1429–1454. doi: 10.3109/10826089009056229. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Brown RA, Strong DR, Abrantes AM, Myers MG, Ramsey SE, Kahler CW. Effects on substance use outcomes in adolescents receiving motivational interviewing for smoking cessation during psychiatric hospitalization. Addictive Behaviors. 2009;34:887–891. doi: 10.1016/j.addbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance-dependent inpatients. Journal of Consulting and Clinical Psychology. 2001;69:295–304. doi: 10.1037//0022-006x.69.2.295. [DOI] [PubMed] [Google Scholar]
- Burling TA, Ramsey TG, Seidner AL, Kondo CS. Issues related to smoking cessation among substance abusers. Journal of Substance Abuse. 1997;9:27–40. doi: 10.1016/s0899-3289(97)90004-3. [DOI] [PubMed] [Google Scholar]
- Butler CC, Rollnick S, Cohen D, Bachman M, Russell I, Stott N. Motivational consulting versus brief advice for smokers in general practice: A randomized trial. British Journal of General Practice. 1999;49:611–616. [Google Scholar]
- Carmody T, Delucchi K, Duncan CL, Banys P, Simon JL, Hall SM. Intensive intervention for alcohol-dependent smokers in early recovery: A randomized trial. Drug and Alcohol Dependence. 2012;122:186–194. doi: 10.1016/j.drugalcdep.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction and motivational advice increase future cessation among smokers not currently planning to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology. 2006;74:955–66. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Munoz N, De Stefani E, Victora CG, Catelletto R, Rolon PA, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. International Journal of Cancer. 1999;82:657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Coleman T. ABC of smoking cessation: Use of simple advice and behavioural support. BMJ: British Medical Journal. 2004;328:397–399. doi: 10.1136/bmj.328.7436.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence using cotinine as reference. Nicotine & Tobacco Research. 2014;16:1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Richard RJ. Optimum cutoff points for biochemical validation of smoking status. American Journal of Public Health. 1988;78:574–575. doi: 10.2105/ajph.78.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavioral Analysis. 2008;41:527–538. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Experimental and Clinical Psychopharmacology. 2010;18:37–50. doi: 10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology. 1994;62:843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Public Health Service. USDHHS; Washington, DC: 2000. Treating Tobacco Use and Dependence: Clinical Practice Guideline. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Flach SD, Diener A. Eliciting patients’ preferences for cigarette and alcohol cessation: An application of conjoint analysis. Addictive Behaviors. 2004;29:791–799. doi: 10.1016/j.addbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Allen JP, Pettinati HM, Rohsenow DJ, Cisler RA, Litten RZ. Using acquired knowledge and new technologies in alcoholism treatment trials. Alcoholism: Clinical and Experimental Research. 2002;26:423–429. [PubMed] [Google Scholar]
- Glynn TJ, Manley MW. How to Help Your Patient Stop Smoking: A National Cancer Institute Manual for Physicians. Washington, D.C: National Cancer Institute; 1991. DHHS Publication #89–3064. [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. White paper. 2012 Retrieved from http://www.afhayes.com/public/process2012.pdf.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: A systematic review and meta-analysis. Tobacco Control: An International Journal. 2010;19:410–416. doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:868–884. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. Guilford Press; New York, NY: 2008. [Google Scholar]
- Higgins ST, Tidey JW, Rogers RE. Contingency management and the community reinforcement approach. In: Ries R, Fiellin D, Miller S, Saitz R, editors. ASAM Principles of Addiction Medicine. 4th. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. [Google Scholar]
- Hollis JF, Lichenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Annals of Internal Medicine. 1993;118:521–525. doi: 10.7326/0003-4819-118-7-199304010-00006. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: Comparing two different cognitive behavioral treatments. Addictive Disorders Treatment. 2010;9:64–74. [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcoholism: Clinical and Experimental Research. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJI. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. Journal of the American Medical Association. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Patten CA. Treatment of tobacco dependence in alcoholics. Recent Developments in Alcoholism. 2003;16:335–359. doi: 10.1007/0-306-47939-7_23. [DOI] [PubMed] [Google Scholar]
- Irving LM, Seidner AL, Burling TA, Thomas RG, Brenner GF. Drug and alcohol inpatients’ attitudes about smoking cessation. Journal of Substance Abuse. 1994;6:267–278. doi: 10.1016/s0899-3289(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Nichol KL, Anderson H. Effect of treatment for nicotine dependence on alcohol and drug treatment outcomes. Addictive Behaviors. 1993;18:635–644. doi: 10.1016/0306-4603(93)90017-4. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking interventions for patients in alcohol dependence treatment. Journal of Studies on Alcohol. 2005;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for alcoholics: A pilot study. Journal of Substance Abuse Treatment. 2001;20:233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. AHRQ Smoking Cessation Guideline Study Group. Effectiveness of implementing the agency for healthcare research and quality smoking cessation clinical practice guideline: a randomized, controlled trial. Journal of the National Cancer Institute. 2004;96:594–603. doi: 10.1093/jnci/djh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral R, Galbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology. 2010;78:62–71. doi: 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Arfken CL, Petry NM, Alessi SM. Prize contingency management for smoking cessation: A randomized trial. Drug and Alcohol Dependence. 2014;140:208–212. doi: 10.1016/j.drugalcdep.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley M, Epps RP, Husten C, Glynn T, Shopland D. Clinical interventions in tobacco control. A National Cancer Institute training program for physicians. Journal of the American Medical Association. 1991;266:3172–3173. [PubMed] [Google Scholar]
- Martin RA, Rohsenow DJ, MacKinnon SV, Abrams DA, Monti PM. Correlates of motivation to quit smoking among alcohol dependent patients in residential treatment. Drug and Alcohol Dependence. 2006;83:73–78. doi: 10.1016/j.drugalcdep.2005.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]
- Moliterno DJ, Willard JE, Lange RA, Negus BH, Boehrer JD, Glamann DB, Landau C, Rossen JD, Winniford MD, Hillis LD. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. New England Journal of Medicine. 1994;330:454–459. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies and policy. In: Fertig JB, Allen JP, editors. Alcohol and tobacco: From basic science to clinical practice. National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 187–206. Research Monograph 30. [Google Scholar]
- Nieva G, Ortega LL, Mondon S, Ballbè M, Gual A. Simultaneous versus delayed treatment of tobacco dependence in alcohol-dependent outpatients. European Addiction Research. 2011;17:1–9. doi: 10.1159/000321256. [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol Research and Health. 2006;29:193–198. [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ. Failure to treat tobacco use in mental health and addiction treatment settings: A form of harm reduction? Drug and Alcohol Dependence. 2010;110:177–182. doi: 10.1016/j.drugalcdep.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. In: Hersen M, Eisler RM, Miller PM, editors. Progress in Behavioral Modification. Sycamore IL: Sycamore Press; 1992. [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Rotrosen J. Smoking cessation treatment in community-based substance abuse rehabilitation programs. Journal of Substance Abuse Treatment. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91:296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti N. Treatment of tobacco use and dependence. The New England Journal of Medicine. 2002;346:506–512. doi: 10.1056/NEJMcp012279. [DOI] [PubMed] [Google Scholar]
- Robles E, Crone CC, Whiteside-Mansell L, Conners NA, Bokony PA, Worley LLM, McMillan DE. Voucher-based incentives for cigarette smoking reduction in a women’s residential treatment program. Nicotine & Tobacco Research. 2005;7:111–117. doi: 10.1080/14622200412331328448. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Colby SM, Martin RA, Monti PM. Nicotine and Other Substance Interaction Expectancies questionnaire: Relationship of expectancies to substance use. Addictive Behaviors. 2005;30:629–641. doi: 10.1016/j.addbeh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Monti PM, Colby SM, Day AM, Abrams DB, Sirota AD, Swift RM. Motivational interviewing versus brief advice for cigarette smokers in residential alcohol treatment. Journal of Substance Abuse Treatment. 2014;46:346–355. doi: 10.1016/j.jsat.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Tidey JW, Monti PM, Colby SM. Comparison of the Cigarette Dependence Scale with four other measures of nicotine involvement: Correlations with smoking history and smoking treatment outcome in smokers with substance use disorders. Addictive Behaviors. 2013;38:2409–2413. doi: 10.1016/j.addbeh.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Kahler CW, Martin RA, Colby SM, Sirota AD. Intolerance for withdrawal discomfort and motivation predict voucher-based smoking treatment outcomes for smoker with substance use disorders. Addictive Behaviors. 2015;43:18–24. doi: 10.1016/j.addbeh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: A feasibility study. Experimental Clinical Psychopharmacology. 1998;6:157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR. Sensitivity analysis in observational studies. Encyclopedia of Statistics in Behavioral Science. 2005;4:1809–1814. [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- SAS/STAT User’s Guide: Version 12.3. Cary, NC: SAS Institute; 2013. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Secades-Villa R, García-Rodríguez O, López-Núñez C, Alonso-Pérez F, Fernández-Hermida JR. Contingency management for smoking cessation among treatment-seeking patients in a community setting. Drug and Alcohol Dependence. 2014;140:63–68. doi: 10.1016/j.drugalcdep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. Journal of Substance Abuse Treatment. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Seidner AL, Burling TA, Gaither DE, Thomas RG. Substance-dependent inpatients who accept smoking treatment. Journal of Substance Abuse. 1996;8:33–44. doi: 10.1016/s0899-3289(96)90067-x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Preventive Medicine. 2012;55:S24–S32. doi: 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Can we do without alcohol abusers’ self-reports? The Behavior Therapist. 1986;7:141–146. [Google Scholar]
- Sobell LC, Sobell MB. Convergent validity: An approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. Elmsford, NY: Pergamon Press; 1980. pp. 177–183. [Google Scholar]
- Soria R, Legido A, Escalano C, Yeste AL, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. British Journal of General Practice. 2006 Oct;:768–774. [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;10:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: Effects of pay amount. Behavior Therapy. 1983;14:647–656. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavior Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced breath carbon monoxide levels: Target-specific effects on cigarette smoking. Addictive Behaviors. 1985;10:345–349. doi: 10.1016/0306-4603(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addictive Behaviors. 1982;7:403–412. doi: 10.1016/0306-4603(82)90010-7. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz P, Carbonari JP, Boca FD. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;(Supplement No. 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 6. Boston, MA: Pearson; 2013. [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology. 2011;217:179–287. doi: 10.1007/s00213-011-2282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transdisciplinary Tobacco Use Research Center, Tobacco Dependence Phenotype Workgroup. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:272–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Wiseman EJ, Williams DK, McMillen DE. Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine-abusing outpatients wearing nicotine or placebo patches. Experimental and Clinical Psychopharmacology. 2005;13:102–110. doi: 10.1037/1064-1297.13.2.102. [DOI] [PubMed] [Google Scholar]
- Zacny JP. Behavioral aspects of alcohol-tobacco interactions. In: Galanter M, editor. Recent Developments in Alcoholism: Volume 8. Combined Alcohol and Other Drug Dependence. New York: Plenum Press; 1990. pp. 205–219. [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]