Abstract
The canonical WNT-β-catenin pathway is essential for self-renewal, growth and survival of AML stem/blast progenitor cells (BPCs). Deregulated WNT signaling inhibits degradation of β-catenin, causing increased nuclear translocation and co-factor activity of β-catenin with the transcriptional regulator TCF4/LEF1 in AML BPCs. Here, we determined the pre-clinical anti-AML activity of the anthraquinone oxime-analog BC2059 (BC), known to attenuate β-catenin levels. BC treatment disrupted the binding of β-catenin with the scaffold protein TBL1 (transducin β-like 1) and proteasomal degradation and decline in the nuclear levels of β-catenin. This was associated with reduced transcriptional activity of TCF4 and expression of its target genes, cyclin D1, c-MYC and survivin. BC treatment dose-dependently induced apoptosis of cultured and primary AML BPCs. Treatment with BC also significantly improved the median survival of immune-depleted mice engrafted with either cultured or primary AML BPCs exhibiting nuclear expression of β-catenin. Co-treatment with the pan-histone deacetylase inhibitor panobinostat and BC synergistically induced apoptosis of cultured and primary AML BPCs, including those expressing FLT3-ITD, as well as further significantly improved the survival of immune-depleted mice engrafted with primary AML BPCs. These findings underscore the promising pre-clinical activity and warrant further testing of BC against human AML, especially those expressing FLT3-ITD.
Keywords: acute myeloid leukemia, Beta Catenin
INTRODUCTION
β-catenin acts as a co-activator for the T-cell factor (TCF) 4/lymphoid enhancer factor (LEF) 1 bipartite transcription factor at the promoters of the WNT-β-catenin target genes and is implicated in cancer transformation1. Deregulated canonical WNT-β-catenin pathway has also been documented to be essential for self-renewal, growth and survival of the AML stem and blast progenitor cells (BPCs)2–5. β-catenin is also required for the HOXA9 and MEIS1-mediated transformation of the hematopoietic stem cells, MLL-AF9-mediated transformation of the committed myeloid progenitor cells, as well as necessary for the development and growth of MLL fusion protein-transformed leukemia stem cells2–5. Cell intrinsic WNT-β-catenin activation in human AML stem cells makes them independent of the leukemia niche-derived WNT signals4. Consistent with this, aberrant expression of LEF1 in hematopoietic stem cells has also been shown to induce AML, with promiscuous expression of the myeloid and lymphoid factors5. As ligands, the binding of WNT proteins induces conformational change in the seven transmembrane domain receptor Frizzled (FZD) with its co-receptor LDL receptor-related protein 5/6 (LRP5/6)1,6. This is followed by the phosphorylation of the cytoplasmic tail of LRP6 by glycogen synthase kinase β (GSK3β) and Casein Kinase 1 γ (CK1γ), which promotes the binding of LRP6 to Axin, and of FZD to Dishevelled (DSH) protein1,6. In the absence of Wnt signaling, the levels of β-catenin are kept low through its degradation. Whereas CK1γ phosphorylates β-catenin on Ser45, GSK3β further phosphorylates β-catenin on Ser33, Ser37 and Thr41, creating a phospho-degron leading to polyubiquitylation and degradation by the 26S proteasome1,6. This occurs when the enzymes CK1γ and GSK3β along with β-catenin are bound to the SCF (Skp, Cullin and F-Box) containing cytoplasmic destruction complex, which includes the scaffolding proteins adenomatous polyposis coli (APC), Axin and TBL1 (transducin β like 1), as well as Siah-1, SIP (Siah-1 interacting protein) and Skp11,6–10. Lack of CK1γ and GSK3β-mediated phosphorylation stabilizes β-catenin in its hypo-phosphorylated form. This allows β-catenin to translocate to the nucleus even though it lacks a nuclear localization signal; although in a recent report FOXM1 was shown to promote the nuclear localization of β-catenin1,8,11. As a member of the Armadillo repeat (ARM) protein family, β-catenin contains central, 12 imperfect ARM repeats (R1–R12), as well as distinct N-terminal (NTD) and carboxy-terminal (CTD) domains12,13. Whereas the central ARM repeats (core TCF4 interaction region) are essential for β-catenin to act as a transcriptional co-regulator with TCF4 through WNT response elements (WREs) in the target gene promoters, the NTD and CTD recruit the other partner proteins involved in chromatin structure and RNA polymerase II regulation12–14. Thus, in the nucleus of AML stem/BPCs, the β-catenin-TCF4/LEF1 complex increases expression of the pro-growth and pro-survival genes, including cyclin D1, c-MYC and survivin, while decreasing Axin 2 levels1,3,15.
In AML stem/BPCs, multiple mechanisms are known to deregulate WNT signaling. Due to inhibition of the phosphorylation of β-catenin by GSK3β, the polyubiquitylation and proteasomal degradation of β-catenin is often abrogated in the AML BPCs1,16–19. This enables the preservation, nuclear translocation and transcriptional activity of β-catenin. In FLT3-ITD-expressing AML stem/BPCs and in chronic myeloid leukemia blast crisis myeloid progenitor (GMP) cells, deregulated Wnt-β-catenin signaling also leads to high nuclear expression of β-catenin, which has been correlated with a poor prognosis in both settings17,20. Additionally, FLT3 and BCR-ABL have also been documented to directly induce the tyrosine phosphorylation of β-catenin, which was shown to stabilize β-catenin, promote its nuclear localization and binding to TCF418,21. Furthermore, FLT3-ITD signaling is also associated with increased PI3K/AKT activity, which phosphorylates and inactivates GSK3β, thereby stabilizing and increasing the nuclear localization and transcriptional activity of β-catenin1,22–24. Overexpression of γ-catenin was also shown to stabilize and increase the nuclear localization of β-catenin19. Deregulation of WNT-β-catenin signaling in AML may also occur through promoter hyper-methylation and silencing of the secreted WNT antagonists, such as the sFRPs and DKK1&316. This has been correlated with a significantly lower relapse free survival16. Although inhibition of the deregulated WNT-β-catenin signaling at any level could potentially inhibit the growth and survival of AML BPCs, due to the several mechanisms involved and the possibility of the escape routes to a persistent β-catenin/TCF4-regualted transcription, the direct abrogation of β-catenin and/or disruption of the β-catenin/TCF4 target gene expressions is likely to be more effective against AML BPCs1,25–29. TBL1 is an adaptor protein which binds to, and is required for β-catenin transcriptional activity, thereby promoting the survival and self-renewing potential of the AML stem/BPCs30. Whereas TBL1 blocks the E3 ubiquitin ligase Siah-1-mediated degradation of β-catenin in the cytoplasm10, in the nucleus TBL1 reversibly binds to the co-repressor NCOR/SMRT, and through it with HDAC3, thereby regulating gene repression31–33. Upon nuclear localization and binding to β-catenin, TBL1 exchanges the NCOR/SMRT co-repressor complex with β-catenin, thereby promoting TCF/LEF-mediated transcription of the target genes cyclin D1, c-MYC and survivin30–34. Consistent with this, depletion of TBL1 was shown to inhibit the in vitro and in vivo oncogenic potential of WNT-β-catenin signaling32. WNT signaling has also been shown to induce the sumoylation of TBL131. This causes co-regulator switching, whereby TBL1-bound co-repressors are removed and cleared, leading to increased TBL1 binding to β-catenin that promotes the transcriptional activation of β-catenin/TCF4-targeted genes31. Conversely, the disruption of TBL1-β-catenin binding is sufficient to abrogate the pro-growth and oncogenic signaling of β-catenin30,31. Taken together, these findings support the rationale for determining the effects of targeted disruption of TBL1-β-catenin interaction in AML BPCs, especially those expressing FLT3-ITD. In the present studies, we have determined that BC2059, a small molecule, anthraquinone oxime-analog, disrupts the binding of β-catenin to TBL1 and promotes β-catenin degradation, thereby attenuating nuclear and cytoplasmic levels of β-catenin. Concomitantly, BC2059 treatment inhibited the expression of TCF4/LEF1 target genes and induced apoptosis of cultured and primary AML BPCs. Importantly, BC2059 treatment also exerted potent in vivo anti-AML activity against xenografts of cultured and primary AML BPCs in immunocompromised mice. Our findings also demonstrate that co-treatment with BC2059 and the pan-HDAC inhibitor panobinostat (PS) exerted synergistic in vitro lethality against cultured and patient-derived primary AML BPCs and significantly improved the survival of immune-depleted mice engrafted with primary AML BPCs.
Methods and Materials
Detailed experimental procedures are provided in the Supplemental Experimental Methods.
Reagents and antibodies
β-Catenin antagonist, BC2059 was kindly provided by β-Cat Pharmaceuticals (Gaithersburg, MD). Panobinostat was kindly provided by Novartis Pharmaceuticals. Chemical structures are provided in Supplemental Figure 1. All antibodies were obtained from commercial sources.
Cell lines and cell culture
HL-60 and MV-411 cells were obtained from the American Type Culture Collection (ATCC) and maintained, as previously described35,36. OCI-AML3 and MOLM13 cells were obtained from the DSMZ and maintained as previously described37,38.
Primary AML blasts
Primary AML samples and normal CD34+ mononuclear cells were obtained with informed consent in accordance with the Declaration of Helsinki. Peripheral blood and/or bone marrow aspirate samples were collected and separated for mononuclear cells by Ficoll-Hypaque density gradient centrifugation as previously described37,38. Banked, de-linked and de-identified, normal or AML CD34+ or AML CD34+CD38−LIN-bone marrow progenitor/stem cells were purified, as previously described38,39.
Assessment of apoptosis
Apoptosis of BC2059-treated cells was assessed as previously described36, 37.
SDS-PAGE and immunoblot analyses
Seventy five micrograms of total cell lysate from the AML cells was used for SDS-PAGE following treatment with BC2059 and/or panobinostat. Western blot analyses were performed on total cell lysates using specific antisera or monoclonal antibodies. Immunoblot analyses were performed at least twice and representative blots were subjected to densitometric analysis. Densitometry was performed using ImageQuant 5.2 (GE Healthcare, Piscataway, NJ)35–38.
Immunoprecipitation
Immunoprecipitations were performed as previously described40.
In vivo model of AML
In vivo studies performed in the NOD/SCID and NSG mice41,42 are described in the Supplemental Experimental Methods.
Gene expression microarray analysis
Full description of processing and analysis of expression array data are described in the Supplemental Methods. All microarray data used in this manuscript are deposited in the Gene Expression Omnibus under GEO accession number (GSE61275).
Statistical Analysis
Significant differences between values obtained in a population of AML cells treated with different experimental conditions were determined using a two-tailed paired t-test or a one-way ANOVA analysis within Microsoft Excel 2010 software or using GraphPad Prism (GraphPad Software, Inc., CA). Statistical differences in the survival of the mice treated with BC2059 were determined by Log-rank (Mantel-Cox) test. P values of less than 0.05 were assigned significance.
Results
Treatment with BC2059 induces cell cycle growth arrest and apoptosis of cultured and primary AML cells with and without expression of FLT3-ITD
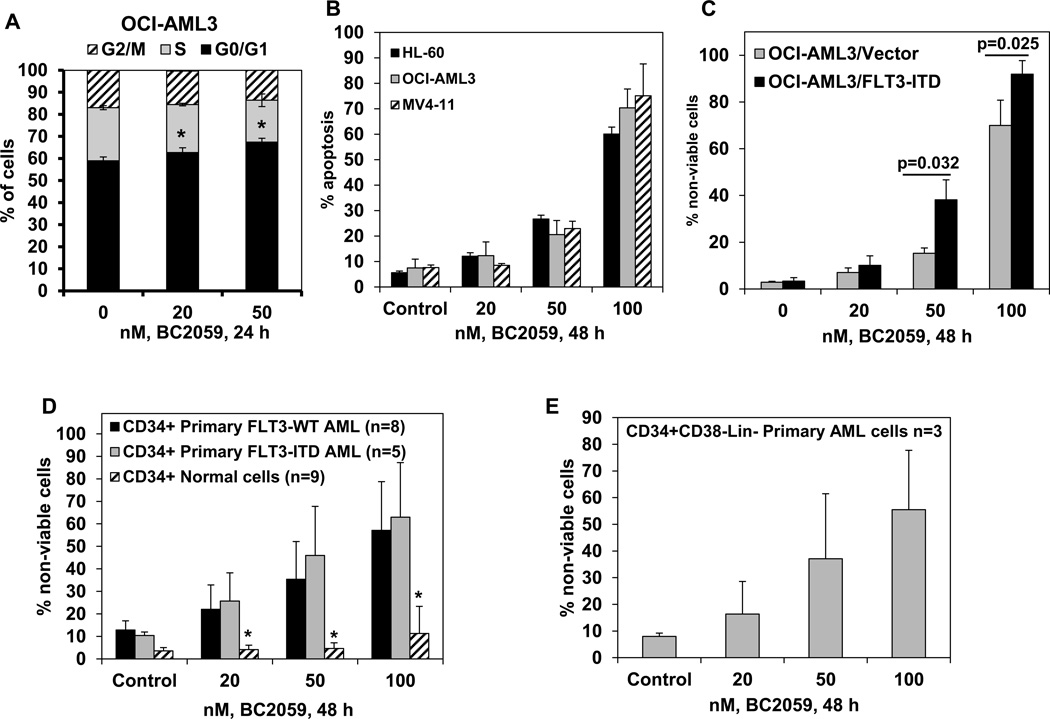
We first determined the effects of treatment with BC2059 on the cell cycle status in the cultured OCI-AML3 AML cells. As shown in Figure 1A and Supplemental Figure 2A, BC2059 induced a modest but significant accumulation of cells in the G0/G1 phase, with a concomitant decline in the % of cells in the G2/M phase of the cell cycle (P<0.05). Treatment with BC2059 also dose-dependently (20–100 nM) inhibited cell proliferation in suspension culture over 120 hours and induced apoptosis of cultured human AML HL-60, OCI-AML3 and MV4-11 cells (Figure 1B; and Supplemental Figure 2B. Representative flow cytometry plots are provided in Supplemental Figure 2C–2E). BC2059 induced apoptosis of FLT3-ITD expressing AML MV4-11 and MOLM13 cells (Figure 1B and Supplemental Figure 3A). We had previously reported the creation of OCI-AML3/FLT3-ITD cells with ectopic mRNA and protein expression of FLT3-ITD37. Here, we determined that treatment with BC2059 induced dose-dependently and significantly more apoptosis of OCI-AML3/FLT-ITD versus OCI-AML3 cells (Figure 1C). We also compared the efficacy of BC2059 in inducing loss of viability in the CD34+ primary AML blast progenitor cells (BPCs) versus the CD34+ normal bone marrow progenitor cells. As shown in Figure 1D and Supplemental Figure 2E, BC2059 dose-dependently induced significantly more lethality in the CD34+ primary AML BPCs, regardless of the expression of FLT3-ITD, than in the normal CD34+ normal bone marrow progenitor cells. We next determined the lethal effects of BC2059 on the primary purified AML stem/progenitor cells. As shown, treatment with BC2059 also dose-dependently induced loss of viability in three samples of primary CD34+ CD38−Lin- AML stem/progenitor cells of which two expressed FLT3-ITD (Figure 1E).
Figure 1. Treatment with the β-Catenin antagonist, BC2059, dose-dependently induces cell cycle growth arrest, apoptosis and loss of viability of cultured and primary AML cells.
A. OCI-AML3 cells were treated with the indicated concentrations of BC2059 for 24 hours. Following this, cell cycle status was determined by flow cytometry. Columns, mean of three experiments; Bars, standard deviation (S.D.). * indicates cells with significantly increased G0/G1 phase compared to untreated cells. B–C. AML cell lines HL-60, OCI-AML3, MV4-11 OCI-AML3/Vector and OCI-AML3/FLT3-ITD cells were treated with the indicated concentrations of BC2059 for 48 hours. At the end of treatment, cells were washed with 1X PBS and stained with annexin V and TO-PRO-3 iodide. Annexin V positive, apoptotic cells were determined by flow cytometry. Columns, mean of 3 independent experiments; Bars, (S.D.). D–E. Primary CD34+ AML cells with or without FLT3-ITD, normal CD34+ cells or CD34+CD38−Lin- AML cells were treated with the indicated concentrations of BC2059 for 48 hours. At the end of treatment, cells were washed with 1X PBS and stained with TO-PRO-3 iodide. The % of TO-PRO-3 positive, non-viable cells was determined by flow cytometry. Columns represent the mean % of non-viable cells; Bars represent (S.D.). * indicates values significantly less in normal CD34+ cells compared to CD34+ AML cells (p<0.05).
Treatment with BC2059 disrupts the binding of β-catenin and TBL1 in AML cells
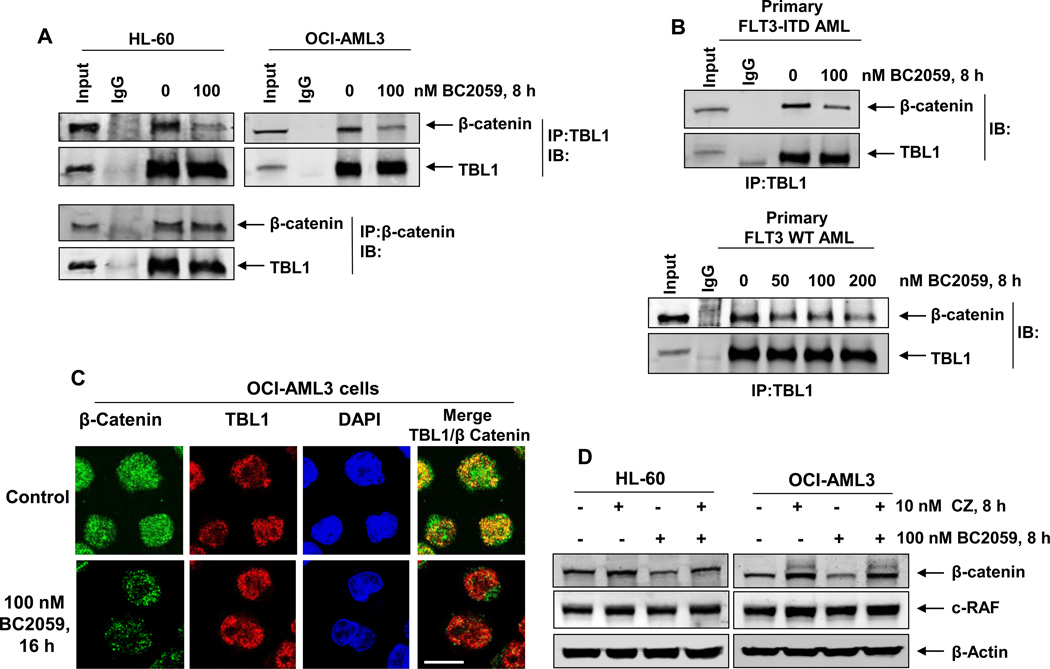
Binding of TBL1 to β-catenin and its subsequent co-recruitment with TCF4/LEF1 to the Wnt target-gene promoters is essential for activating their gene transcription. To elucidate the mechanism of activity of BC2059, we next determined the effects of BC2059 treatment on the association of β-catenin and TBL1 in AML cells. The co-immunoprecipitation data presented in Figure2A and 2B show that treatment with BC2059 inhibits the binding of β-catenin to TBL1 in cultured AML HL-60 and OCI-AML3 cells, as well as in the primary AML BPCs with or without expression of FLT3-ITD. Co-localization of β-catenin and TBL1 in the nucleus of OCI-AML3 cells was also determined by utilizing confocal immunofluorescence microscopy. Figure 2C shows that in untreated OCI-AML3 cells, β-catenin (green) and TBL1 (red) are co-localized in the nucleus, as demonstrated by the overlap in their fluorescent signals (orange). Similar effects were also observed in the FLT3-ITD expressing MV4-11 cells (Supplemental Figure 4A). Treatment with BC2059 also variably attenuated the levels of β-catenin and its co-localization with TBL1 in the nucleus of AML cells, whereas the expression levels and localization of TBL1 were not altered (Figure 2C and Supplemental Figure 4A). These findings are consistent with BC2059-mediated apoptosis shown in Figure 1B (cultured AML cells). BC2059-mediated inhibition of TBL1 binding to β-catenin and the attenuation in β-catenin levels occurred through proteasomal degradation, since co-treatment with the proteasome inhibitor carfilzomib restored (near complete) the levels of β-catenin in the AML cells (Figure 2D). Treatment with BC2059 selectively targeted β-catenin for degradation by the proteasome, since the levels of c-RAF, which is known to be degraded by the proteasome, was not depleted by treatment with BC2059 (Figure 2D).
Figure 2. Treatment with BC2059 disrupts binding of β-catenin to TBL1 leading to proteasomal degradation of β-catenin in AML cells.
A. HL-60 and OCI-AML3 cells were treated with the indicated concentrations of BC2059 for 8 hours. After treatment, cells were harvested, cell lysates were prepared and TBL1 or β-catenin was immunoprecipitated from the lysates. Immunoblot analyses were conducted for the expression levels of β-catenin and TBL1 in the immunoprecipitates. B. Primary FLT3-ITD-expressing and FLT3 wild-type AML cells were treated with the indicated concentrations of BC2059 for 8 hours. Following this, cells were harvested, cell lysates were prepared and TBL1 was immunoprecipitated from the lysates. Immunoblot analyses were conducted for the expression levels of β-catenin and TBL1 in the immunoprecipitates. C. OCI-AML3 cells were treated with the indicated concentration of BC2059 for 16 hours. At the end of treatment, cells were cytospun onto glass slides, fixed, permeabilized, blocked with 3% BSA/PBS, and incubated with anti- β-catenin and anti-TBL1 antibodies. Cells were washed and incubated with secondary antibodies conjugated to Alexa 488 (green) and Alexa 555 (red). Nuclei were stained with DAPI. Cells were imaged under oil at 63X using a Zeiss LSM 510 META confocal microscope. Images were processed utilizing LSM Browser (Zeiss). Scale bar indicates 10 µm. D. OCI-AML3 and HL-60 cells were treated with the indicated concentrations of BC2059 and/or the proteasome inhibitor, carfilzomib for 8 hours. Then, cells were harvested and total cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of β-catenin, c-RAF and β-actin in the lysates.
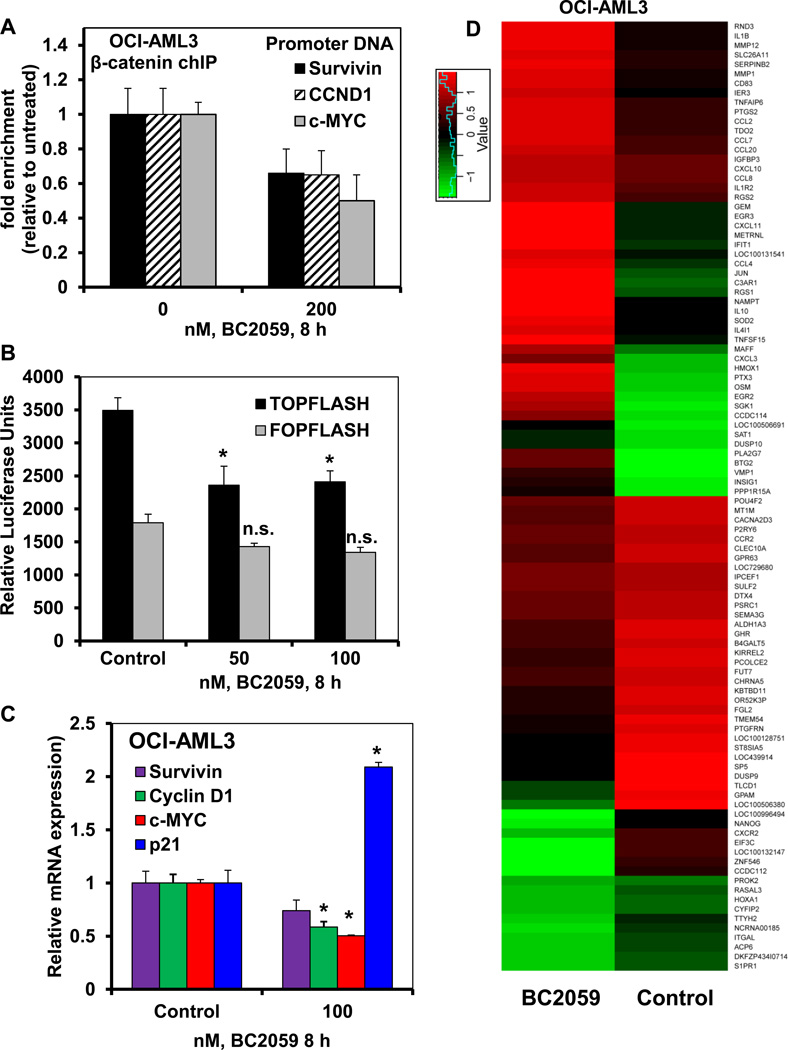
Treatment with BC2059 reduces β-catenin occupancy at the target gene promoters and inhibits the expression of genes with WNT response elements (WREs) in AML cells
As β-catenin is known to interact with TCF4/LEF1 at the promoters of Wnt-target genes to activate their transcription, we next determined the effects of BC2059 treatment on the binding of β-catenin to the chromatin at the Wnt-target gene promoters in AML cells. As shown in Figure 3A, treatment with BC2059 significantly reduced the binding of β-catenin to the promoter DNA of c-MYC, Cyclin D1, and survivin in OCI-AML3 cells (p<0.05). We also estimated the relative luciferase activity in AML cells transfected with either the TOP-FLASH or the FOP-FLASH construct to determine whether treatment with BC2059 selectively inhibited the expression of genes with the promoters containing WREs. As shown in Figure 3B, treatment with BC2059 significantly inhibited the TOP-FLASH associated luciferase but not of FOP-FLASH associated luciferase expression in AML cells (p<0.05). Consistent with this, the qPCR analyses of the Wnt-target gene expressions showed that BC2059 treatment attenuated the mRNA expression of c-MYC, Cyclin D1 and survivin in OCI-AML3 cells and (Figure 3C and Supplemental Figure 5A). The attenuating effect of BC2059 on c-MYC and survivin expression in MV4-11 cells is also shown in the Supplemental Figure 5B. Recently, β-catenin signaling has also been shown to directly regulate the expression of human telomerase (hTERT) and CD44 genes43,44. Treatment with BC2059 also reduced the mRNA levels of hTERT and CD44 in AML cells (Supplemental Figure5A and 5B). In contrast, BC2059 treatment concomitantly up regulated the mRNA expression of p21, which is known to be repressed by c-MYC (Figure 3C and Supplemental Figure5C and 5D)38. We next determined the alterations in the mRNA levels in AML cells through gene expression microarray (GEM) analysis. Following treatment of OCI-AML3 cells with BC2059 for 8 hours, the heat map of the gene expression alterations is shown in Figure 3D. Treatment with BC2059 up regulated the mRNA expression of more genes, as compared to the number of genes whose mRNA expression was down regulated by BC2059 (Figure 3D). The fold-change in the most altered mRNA expressions is shown in Supplemental Table 1 and Table 2. Data sets of genes with the altered expression profile derived from the GEM analyses were imported into the Ingenuity Pathway Analysis (IPA) Tool (Ingenuity H Systems, Redwood City, CA). Within this gene list, IPA identified the top-four most perturbed gene networks in OCI-AML3 cells following treatment with BC2059 and assigned a score for these associated network functions (Supplemental Table 3). The score (e.g., a score of 31) assigned by the IPA indicates the probability (1 in 1031) that the focus-genes in the dataset are grouped together in a perturbed network due to random chance alone. Next, total RNA from the untreated and BC2059-treated OCI-AML3 and MV4-11 cells used for the GEM analysis was also reverse transcribed and the resulting cDNA was used for quantitative PCR analysis utilizing c-MYC, Cyclin D1, survivin and hTERT-specific TaqMan real-time PCR probes. QPCR analyses of the gene expressions showed that BC2059 treatment attenuated the mRNA expression of β-catenin target genes c-MYC, Cyclin D1, survivin and hTERT in the OCI-AML3 cells (Figure 3D and Supplemental Figure5A and 5B). In contrast, BC2059 treatment concomitantly up regulated the mRNA expression of p21 in AML cells (Supplemental Figure5C and 5D).
Figure 3. Treatment with BC2059 depletes β-catenin occupancy at target promoters and inhibits β-catenin-mediated transcriptional activity in AML cells.
A. OCI-AML3 cells were treated with the indicated concentration of BC2059 for 8 hours. Following treatment, cells were crosslinked with 2% formaldehyde, quenched with glycine and washed with 1X PBS. Chromatin was immunoprecipitated with anti-β-catenin antibody. Resulting chIP DNA and input DNA was used for qPCR analyses with primers directed against the promoters of c-MYC, Cyclin D1 and survivin. Fold enrichment was calculated using the Ct value of the chIP’ed DNA normalized to the Ct value of the input DNA. B. HEL cells transfected with a TCF/LEF luciferase reporter construct (TOPFLASH) and mutant TCF/LEF construct (FOPFLASH) were treated with the indicated concentrations of BC2059 for 8 hours. Following treatment, cells were harvested and lysed. Relative luciferase expression was measured with a dual luciferase assay kit and a plate reader. *indicates values significantly less in cells transfected with the TOPFLASH vector and treated with BC2059 compared to untreated cells transfected with TOPFLASH (p < 0.05). C. OCI-AML3 cells were treated with the indicated concentration of BC2059 for 8 hours. At the end of treatment, cells were harvested and total RNA was isolated and reverse transcribed. The resulting cDNAs were used for qPCR to determine the mRNA expression of c-MYC, survivin, Cyclin D1 and p21. The relative expression of each target was normalized to GAPDH and is reported as a percentage of untreated cells. * indicates expression values significantly less in cells treated with BC2059 compared to control cells (p< 0.005). D. OCI-AML3 cells were treated with the indicated concentration of BC2059 for 8 hours. At the end of treatment, cells were harvested and total RNA was isolated. Global mRNA expression alterations were analyzed by microarray analysis (Affymetrix). A heat map of the greatest expression changes (greater than 2 fold upregulated or downregulated) following treatment with BC2059 is shown.
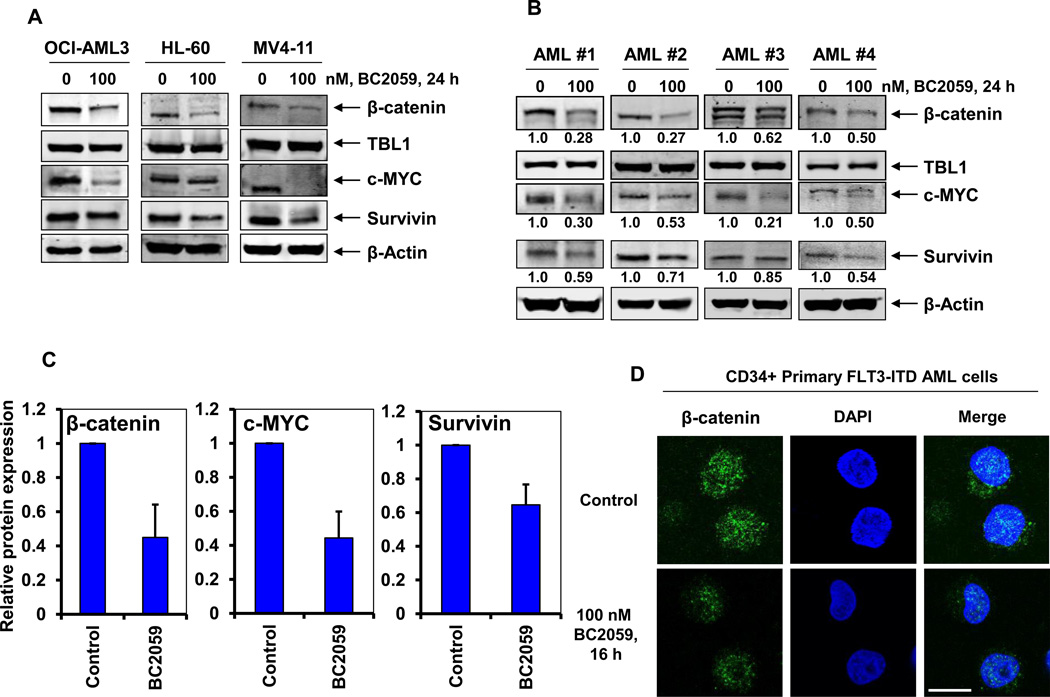
Treatment with BC2059 depletes β-Catenin expression levels and nuclear localization in cultured and primary AML cells
We next determined the effects of treatment with BC2059 on the protein expression of β-catenin in AML cells. As shown in Figure 4A, exposure to BC2059 depleted the levels of β-catenin and its target genes, including c-MYC and survivin without affecting the levels of the TBL1 in OCI-AML3, HL-60 and MV4-11 cells. Following BC2059 treatment, variable depletion of the protein levels of β-catenin and its target genes were also observed in the BPCs from four primary AML samples (Figure 4B) and a larger cohort of primary AML samples (Figure 4C). Treatment with BC2059 was also associated with the induction of all three isoforms of BIM and p27, with modest depletion in the levels of MCL1 in the AML cells (Supplemental Figure 3B–3D). As for the cultured OCI-AML3 cells (Figure 2C), we next determined whether treatment with BC2059 affected the nuclear localization of β-catenin in primary AML BPCs. Figure 4D and Supplemental Figure 4B–4D show that CD34+ primary AML BPCs exhibit appreciably high levels of β-catenin in the nucleus and cytoplasm. In addition, treatment with BC2059 markedly attenuated the nuclear and cytosolic levels of β-catenin in the representative primary AML BPCs (Figure 4C and Supplemental Figure 4B–4D). These findings are also consistent with BC2059-mediated apoptosis of primary AML progenitor cells shown in Figure 1D.
Figure 4. Treatment with BC2059 depletes β-catenin expression levels and nuclear localization in cultured and primary AML cells.
A. OCI-AML3, HL-60 and MV4-11 cells were treated with the indicated concentrations of BC2059 for 24 hours. Following this, cells were harvested and cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of β-catenin, TBL1, c-MYC, survivin and β-actin in the lysates. B. Primary FLT3-ITD expressing [AML#1, AML#2] and FLT3-WT [AML#3, AML#4] AML cells were treated with the indicated concentration of BC2059 for 24 hours. Cells were harvested and cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of β-catenin, TBL1, c-MYC, survivin, and β-actin in the lysates. Numbers beneath the bands represent densitometry analysis. C. Quantification of the relative protein expression of β-catenin, c-MYC and survivin from 8 primary AML cells treated with 100 nM of BC2059 compared to untreated controls. Values represent mean ± S.D. D. Primary CD34+ FLT3-ITD expressing AML cells were treated with the 100 nM of BC2059 for 16 hours. Following this, cells were cytospun onto glass slides, fixed, permeabilized, blocked with 3% BSA/PBS, and incubated with anti-β-catenin antibody. Cells were washed and incubated with an Alexa 488-conjugated secondary antibody. Nuclei were stained with DAPI. Cells were imaged under oil at 63X using a Zeiss LSM 510 META confocal microscope. Images were processed utilizing LSM Browser (Zeiss). Scale bar indicates 10 µm.
Co-treatment with BC2059 and a pan-HDAC inhibitor exerts synergistic anti-AML activity against cultured and primary AML cells
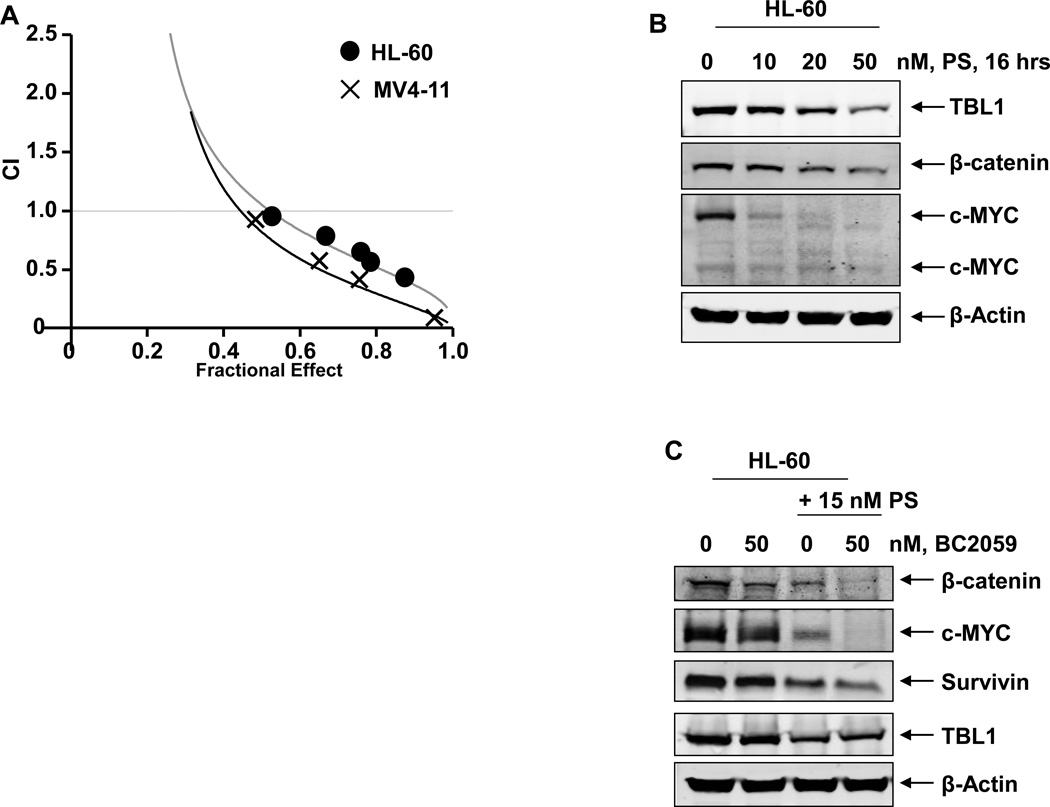
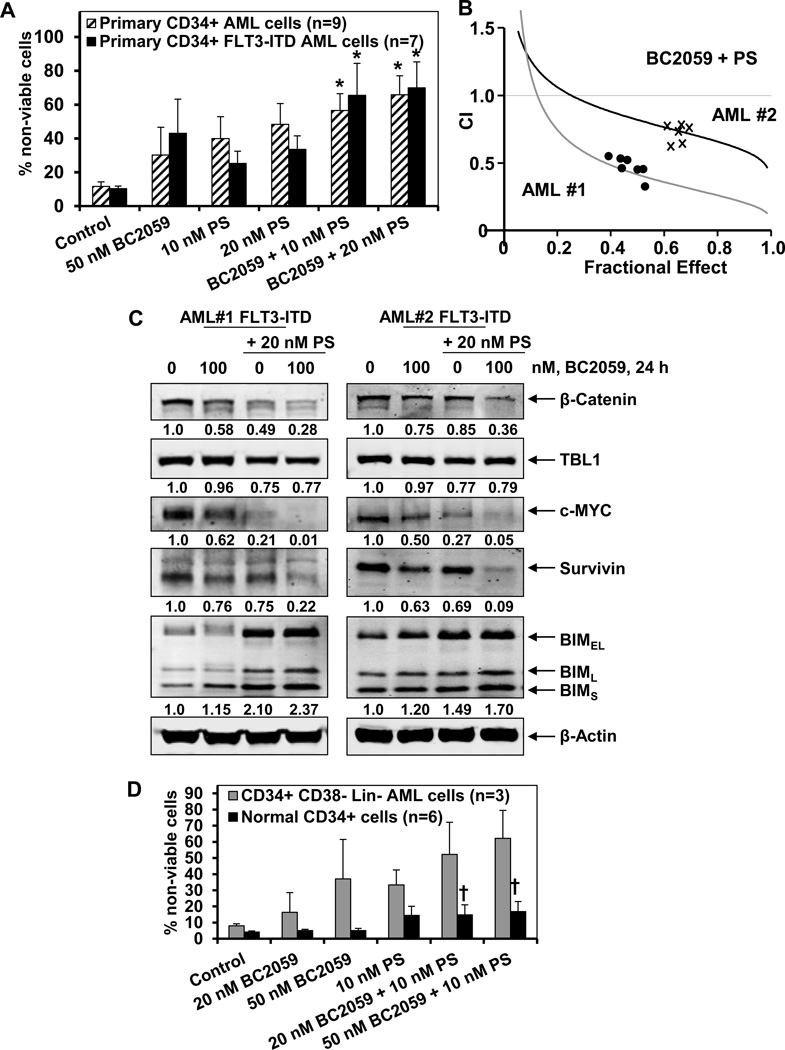
We have previously demonstrated the anti-AML activity of the pan-HDAC inhibitor panobinostat against cultured and primary AML cells35,36,38,39. Since TBL1 is bound to and involved with the core NCoR/SMRT complex that also includes HDAC activity, we next determined whether simultaneous inhibition of TBL1-β-catenin binding by BC2059 and of TBL1-SMRT/NCoR/HDAC complex by co-treatment with panobinostat would exert superior anti-AML activity. As shown in Supplemental Figure6A and 6B, co-treatment with 5 or 10 nM of panobinostat significantly enhanced apoptosis induced by BC2059 (20 or 50 nM) in OCI-AML3, HL-60, MOLM13, MV4-11 cells. Additionally, combined therapy with panobinostat and BC2059 synergistically induced apoptosis of HL-60, OCI-AML3 and MV4-11 cells, with combination indices less than 1.0, as determined by isobologram analysis (Figure 5A, and Supplemental Figure 6C). Utilizing both immunoblot and immunofluorescence analyses, we also noted that treatment with panobinostat also dose-dependently attenuated the levels of TBL1 in OCI-AML3 and HL-60 cells (Figure 5B and Supplemental Figure7A and 7B), which has been previously reported to independently induce lethality in the transformed cells30. Treatment with panobinostat also concomitantly reduced the levels of β-catenin in these cells, which was also associated with depletion of the levels of c-MYC and survivin (Figure5B and 5C). Notably, as compared to treatment with each agent alone, co-treatment with panobinostat and BC2059 caused greater depletion of TBL1, β-catenin, c-MYC and survivin levels in the AML cells (Figure 5C). We next determined whether co-treatment with panobinostat also enhanced the anti-AML activity of BC2059 against primary AML BPCs. As shown in Figure 6A, co-treatment with 10 or 20 nM of panobinostat significantly increased the apoptosis induced by BC2059 in the CD34+ primary AML BPCs, regardless of the expression of FLT3-ITD. Additionally, co-treatment with panobinostat and BC2059 synergistically induced apoptosis in two primary AML BPCs expressing FLT3-ITD, with combination indices less than 1.0, as determined by isobologram analysis (Figure 6B, and Supplemental Figure 8). Inexplicably, the combination indices were lower in AML#1 versus AML#2 (Supplemental Figure 8). The superior activity of the combination versus each drug alone was associated with greater depletion of β-catenin, c-MYC and survivin but greater induction of BIM in the two samples of primary AML progenitor cells (Figure 6C). We next determined the lethal effects of BC2059 and/or panobinostat against purified CD34+CD38−Lin- primary AML stem/progenitor cells versus CD34+ normal bone marrow progenitor cells. Figure 6D demonstrates that treatment with either BC2059 or panobinostat alone induced more loss of cell viability of the primary AML stem/progenitor versus normal progenitor cells (p<0.05). Furthermore, co-treatment with the combination was significantly more lethal against primary AML stem/progenitor versus normal progenitor cells (p<0.01) (Figure 6D).
Figure 5. Co-treatment with BC2059 and the pan-HDAC inhibitor, panobinostat (PS) induces synergistic apoptosis of cultured AML cells.
A. HL-60 and MV4-11 cells were treated with BC2059 (dose range 50–100 nM) and panobinostat (dose range 2.5–20 nM) at a fixed ratio of for 48 hours. At the end of treatment, the percentages of apoptotic cells (Fraction affected- Fa) were determined by flow cytometry. Median dose effect and isobologram analyses were performed utilizing the commercially available software CalcuSyn. Combination indices were calculated and plotted. CI values of ~1.0 indicate additive activity of the combination. CI values less than 1.0 indicate synergistic activity of the combination. B. HL-60 cells were treated with the indicated concentrations of PS for 16 hours. At the end of treatment, cells were harvested and total cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of TBL1, β-catenin, c-MYC, and β-actin in the cell lysates. C. HL-60 cells were treated with the indicated concentrations of BC2059 and/or PS for 24 hours. At the end of treatment, cells were harvested and total cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of β-catenin, c-MYC, survivin, TBL1 and β-actin in the lysates
Figure 6. Co-treatment with BC2059 and panobinostat exerts superior anti-AML activity against primary CD34+ and CD34+CD38−Lin- AML cells.
A. Primary CD34+ AML cells with or without FLT3-ITD were treated with the indicated concentrations of BC2059 and/or panobinostat for 48 hours. At the end of treatment, cells were stained with propidium iodide and the percentages of non-viable cells were determined by flow cytometry. Values represent mean ± S.D. B. Primary CD34+ AML cells were treated with BC2059 (dose range 50–100 nM) and panobinostat (dose range 5–20 nM) at a fixed ratio of for 48 hours. At the end of treatment, the percentages of apoptotic cells (Fraction affected- Fa) were determined by flow cytometry. Median dose effect and isobologram analyses were performed utilizing CalcuSyn. CI values less than 1.0 indicate synergistic activity of the combination. C. Primary FLT3-ITD AML cells were treated with the indicated concentrations of BC2059 and/or panobinostat for 24 hours. At the end of treatment, cells were harvested and total cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of β-catenin, c-MYC, survivin, TBL1, BIM and β-actin in the lysates. Numbers beneath the bands represent densitometry analysis. D. Primary CD34+CD38−Lin- AML and normal human CD34+ bone marrow progenitor cells were treated with the indicated concentrations of BC2059 and/or panobinostat for 48 hours. At the end of treatment, cells were stained with propidium iodide and the percentages of non-viable cells were determined by flow cytometry. Columns, mean loss of viability; Bars, S.D. † indicated values significantly less in normal CD34+ cells treated with the combination of BC2059 and panobinostat compared to the Lin- AML cells (p < 0.05).
Treatment with BC2059 exerts significant in vivo activity against established AML xenografts in immunocompromised mice
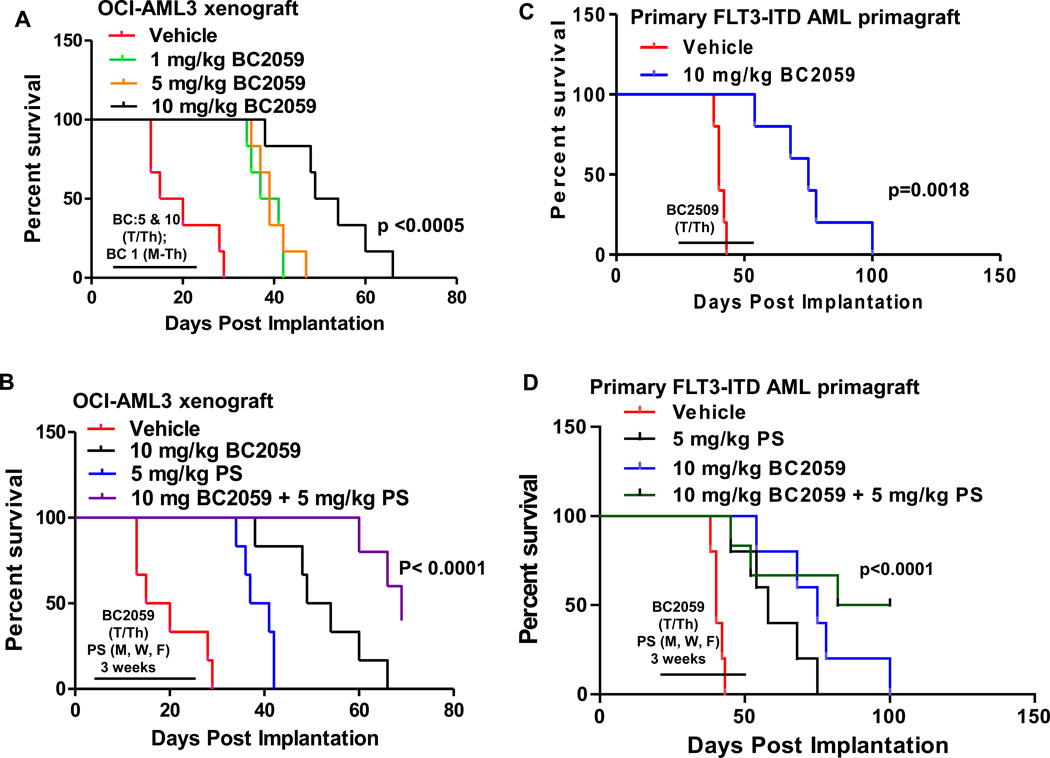
We next determined the in vivo anti-AML activity of BC2059 against the OCI-AML3 AML xenografts engrafted in the bone marrow of the NOD/SCID mice. Following the tail vein infusion and engraftment of OCI-AML3 cells in the bone marrow of the NOD/SCID mice, the anti-AML effects and the survival improvement due to treatment with 10, 5.0 or 10.0 mg/kg dose level of BC2059 administered for 3 weeks was compared to the effects of the treatment with the vehicle alone. The Kaplan Meier plot depicting the survival of mice demonstrated that, as compared to treatment with the vehicle alone, treatment with BC2059 at 1.0 or 5.0 mg/kg/day significantly improved the median survival of the mice from approximately 17.5 to 39 days (p<0.001), whereas treatment with 10 mg/kg/day of BC2059 alone further improved the median survival to 51.5 days (p<0.0001) (Figure 7A). Additionally, whereas treatment with 5.0 mg/kg/day of panobinostat alone versus treatment with vehicle control also improved the median survival to 39 days (p<0.001), co-treatment with BC2059 and panobinostat versus treatment with vehicle control alone yielded the most improvement in the survival of the mice (median survival of 69 days) (p<0.0005) (Figure 7B). In a separate model, we tested the in vivo activity of BC2059 against FLT3-ITD-expressing primary AML BPCs engrafted into NSG mice. Following engraftment, mice were treated with 10 mg/kg/day of BC2059, as noted above for 3 weeks. Figure 7C shows that compared to the treatment with vehicle control, BC2059 treatment significantly improved the median survival of NSG mice infused with the primary AML BPCs (p=0.0018). As shown in Figure 7D, either treatment with BC2059 or panobinostat alone for 3 weeks significantly improved the median survival of the mice, as compared to mice treated with vehicle control (p<0.01). Notably, combined therapy with BC2059 and panobinostat was superior to the treatment with BC2059 or panobinostat alone in improving the median survival of the mice (91 versus 75 and 56 days, respectively) (p<0.01), with 50% of the mice surviving more than 100 days after the engraftment of AML BPCs in the mice. This plateau in the survival curve suggests a potentially curative impact of the combination on the survival of the mice (Figure 7D).
Figure 7. Treatment with BC2059 and/or panobinostat significantly improves survival of NOD/SCID mice bearing OCI-AML3 xenografts or NSG mice engrafted with primary AML blasts.
A. NOD/SCID mice (n=6 per cohort) were injected with 2 million OCI-AML3 cells via the lateral tail vein. Treatment was started five days after implantation. Mice were treated with 1 mg/kg daily 4 days per week or 5 mg/kg or 10 mg/kg of BC2059 twice per week (Tuesday and Thursday) for 3 weeks. Survival of the mice is represented by a Kaplan Meier plot. p= 0.0005 for mice treated with 5 or 10 mg/kg of BC2059 compared to the vehicle control mice. B. NOD/SCID were treated as above with 10 mg/kg of BC2059 and/or panobinostat (PS) 5 mg/kg of panobinostat three times per week (Monday, Wednesday, Friday) for 3 weeks. Survival of the mice is represented by a Kaplan Meier plot. C–D. NSG mice (n=5 per cohort) were injected with 10 million primary FLT3-ITD expressing AML cells. Treatment was started when 1% CD45+ cells were detected in the peripheral blood of the mice. (C) Mice were treated with 10 mg/kg of BC2059 twice per week (Tuesday and Thursday). Survival of the mice is represented by a Kaplan Meier plot. p= 0.0018 for mice treated with 10 mg/kg of BC2059 compared to the vehicle control mice. (D) NSG mice were treated as above with 10 mg/kg of BC2059 and/or panobinostat (PS) 5 mg/kg of panobinostat three times per week (Monday, Wednesday, Friday) for 3 weeks. Survival of the mice is represented by a Kaplan Meier plot.
Discussion
Multiple mechanisms, noted above, are known to potentially either inhibit its phospho-degron or directly increase the stability of β-catenin in AML BPCs16–19, 21–24. This is associated with increased nuclear localization and transcriptional co-factor activity of β-catenin in AML stem/BPCs. Therefore, targeted depletion of β-catenin remains an important therapeutic goal for eliminating AML stem/BPCs. We show here for the first time that the novel agent BC2059 disrupts the binding of β-catenin with the scaffold protein TBL1 and promotes the proteasomal degradation and attenuation of the nuclear and cytoplasmic levels of β-catenin. This was confirmed both by immunofluorescence analyses and the specific antibody pull-down assays. Our findings also confirmed that targeted depletion of the nuclear levels β-catenin effectively attenuated transcriptional activity of TCF4, thereby depleting the levels of the TCF4 target genes, including c-MYC, cyclin D1 and survivin, while concomitantly up regulating the growth inhibitory p21 in the AML BPCs. Additionally, BC2059 treatment up regulated the levels of the pro-apoptotic proteins p27 and BIM in the cultured and primary BPCs. Accordingly, following BC2059 treatment more growth inhibition and significantly greater loss of viability of the cultured and primary AML BPCs versus the normal CD34+ bone marrow progenitor cells was observed. This relative selectivity of action against AML stem/BPCs highlights the greater dependency of BPCs in myeloid leukemia on the β-catenin-TCF4 regulated transcriptome and possible differential effect on the nuclear β-catenin levels2–6, 20,43–46. In this context it is also important to note that it is the fold-change, not absolute amount, in β-catenin that determines the intensity of Wnt signaling47. Although not the focus of the present studies, β-catenin is known to associate with Hes1, NICD (Notch intracellular domain) and YAP1 (YES associated protein 1), as well as stabilize Gli1, which points to a potentially central role for β-catenin in regulating the stem cell transcriptome through Wnt, Hedgehog, Notch and Hippo pathways48–51. This transcriptome, among other gene expressions, also includes c-MYC, cyclin D1 and survivin, which were down regulated following treatment of AML BPCs with BC2059. The GEM analysis and IPA demonstrated that BC2059 treatment especially perturbed the expression of the genes involved in the network for cellular movement, hematological system development and function and of immune cell trafficking. Interestingly, among the gene-expressions induced several-fold were NAMPT (nicotinamide phosphoribosyltransferase), which is an essential enzyme mediating granulocyte colony-stimulating factor (G-CSF)-triggered granulopoiesis52, as well as RGS1 (regulator of G protein signaling 1). RGS1 is a GTPase activator protein (GAP) that dramatically increases the intrinsic Gα subunit’s GTPase activity, thereby promoting hetrotrimeric G-protein complex formation and inhibition of G-protein coupled receptor (GPCR) signaling53. Whether abrogation of the induction of NAMPT and RGS1 would significantly attenuate the anti-AML BPC activity of BC2059, thereby establishing their mechanistic role in mediating BC2059 lethality, remains to be determined.
AML stem/BPCs that express gain-of-function FLT3-(ITD mutations and/or FLT3-TK domain (TKD) mutations attain through multiple mechanisms increased nuclear localization of β-catenin and dependency on β-catenin-TCF4-regulated transcriptome17,18,22. Our findings demonstrate that BC2059 dose-dependently induced significantly more apoptosis of the cultured OCI-AML3/FLT3-ITD cells, with ectopic overexpression of FLT3-ITD, versus the OCI-AML3 cells lacking FLT3-ITD expression. Consistent with this, BC2059 dose dependently also induced slightly more apoptosis in samples of CD34+ AML BPCs expressing FLT3-ITD, as compared to those lacking FLT3-ITD expression. This may be due to the expression and function of genes other than mutant FLT3 that govern growth and survival of AML blast progenitor cells. Importantly, BC2059 also exerted lethal activity against the CD34+ CD38− Lin- primary AML stem/progenitor cells expressing FLT3-ITD. Together these in vitro data are also consistent with and support our in vivo findings demonstrating that even a short-term treatment over three weeks with BC2059 significantly improved the survival of immune depleted mice engrafted with either cultured AML cells or with primary AML BPCs expressing FLT3-ITD. Recently, an activating mutation and nuclear localization of β-catenin was shown to be associated with increased transcriptional upregulation of the Notch ligand Jagged1 in the mouse bone marrow osteoblasts, which was pathogenetically involved with the activated Notch signaling and leukemic transformation in the hematopoietic stem/progenitor cells54. These observations support and highlight the potential therapeutic implications of the observed pre-clinical in vivo anti-AML efficacy of BC2059 in the mouse model of primary AML BPCs.
Clinically achievable low nanomolar levels of panobinostat are known to inhibit the class I HDACs55. We have previously reported on the pre-clinical efficacy of panobinostat against AML BPCs, including those expressing FLT-ITD35,36,38. Extending this, findings presented here also validate that simultaneous inhibition of TBL1-β-catenin binding by BC2059 and of TBL1-SMRT/NCoR/HDAC complex by co-treatment with panobinostat results in superior anti-AML efficacy of the combination of panobinostat and BC2059. Data presented also show that panobinostat treatment attenuates the levels of TBL1, which may further assist in abrogating the association of TBL1 with β-catenin in the cultured and primary AML BPCs. Notably, co-treatment with BC2059 and panobinostat was associated with greater depletion of β-catenin, c-MYC and survivin, while concomitantly inducing higher levels of BIM. Taken together, these findings could potentially account for the synergistic lethal effects of co-treatment with BC2059 and panobinostat against cultured and primary AML BPCs. The selective in vitro lethality of the combination was especially evident against the immunophenotypically characterized AML stem versus normal bone marrow progenitor cells. Notably, and consistent with this, the combination was significantly superior than treatment with each agent alone in improving not only the median survival of the mice engrafted with primary AML BPCs expressing FLT3-ITD, with 50% surviving more than 100 days after AML engraftment, but also in producing a plateau in the survival curve. This highlights the possibility that, if the treatment with the combination of BC2059 and panobinostat was administered for longer intervals, it could potentially result in a long-term disease-free survival of the mice engrafted with an aggressive subtype of AML expressing FLT3-ITD. Collectively, combined with the reported observations, our findings presented here underscore the rationale for pre-clinically testing the combination of BC2059 and the novel and effective FLT3 antagonists such as quizartinib and crenolinib against AML stem/BPCs expressing FLT3-ITD56,57.
Supplementary Material
Acknowledgements
This work was supported by a grant from the National Institutes of Health, National Cancer Institute (R01 CA173877) (K.N.B.).
Footnotes
Conflict of interest: Stephan Horrigan is Chief Scientific Officer of β-Cat Pharma, Inc. All other authors have no relevant financial interests to disclose.
Supplementary information is available at Leukemia's website.
REFERENCES
- 1.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, et al. beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer cell. 2010;18:606–618. doi: 10.1016/j.ccr.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118:2849–2856. doi: 10.1182/blood-2011-03-345165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petropoulos K, Arseni N, Schessl C, Stadler CR, Rawat VP, Deshpande AJ, et al. A novel role for Lef-1, a central transcription mediator of Wnt signaling, in leukemogenesis. J Exp Med. 2008;205:515–522. doi: 10.1084/jem.20071875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niehrs C. The complex world of WNT receptor signaling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Hou Y, Ming M, Yu L, Seba A, Qian Z. Apc regulates the function of hematopoietic stem cells largely through beta-catenin-dependent mechanisms. Blood. 2013;121:4063–4072. doi: 10.1182/blood-2012-12-473470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nature Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitrova YN, Li J, Lee YT, Rios-Esteves J, Friedman DB, Choi HJ, et al. Direct ubiquitination of beta-catenin by Siah-1 and regulation by the exchange factor TBL1. J Biol Chem. 2010;285:13507–13516. doi: 10.1074/jbc.M109.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and gliomatumorigenesis. Cancer Cell. 2011;20:427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, et al. Crystal structure of a full-length beta-catenin. Structure. 2008;16:478–487. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Mol Cell. 2006;24:293–300. doi: 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Mo R, Chew TL, Maher MT, Bellipanni G, Weinberg ES, Gottardi CJ. The terminal region of beta-catenin promotes stability by shielding the Armadillo repeats from the axin-scaffold destruction complex. J Biol Chem. 2009;284:28222–28231. doi: 10.1074/jbc.M109.045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 16.Valencia A, Roman-Gomez J, Cervera J, Such E, Barragan E, Bolufer P, et al. Wnt signaling pathway is epigenetically regulated by methylation of Wnt antagonists in acute myeloid leukemia. Leukemia. 2009;23:1658–1666. doi: 10.1038/leu.2009.86. [DOI] [PubMed] [Google Scholar]
- 17.Kajiguchi T, Chung EJ, Lee S, Stine A, Kiyoi H, Naoe T, et al. FLT3 regulates beta-catenin tyrosine phosphorylation, nuclear localization, and transcriptional activity in acute myeloid leukemia cells. Leukemia. 2007;21:2476–2484. doi: 10.1038/sj.leu.2404923. [DOI] [PubMed] [Google Scholar]
- 18.Kajiguchi T, Katsumi A, Tanizaki R, Kiyoi H, Naoe T. Y654 of beta-catenin is essential for FLT3/ITD-related tyrosine phosphorylation and nuclear localization of beta-catenin. Eur J Haematol. 2012;88:314–320. doi: 10.1111/j.1600-0609.2011.01738.x. [DOI] [PubMed] [Google Scholar]
- 19.Morgan RG, Pearn L, Liddiard K, Pumford SL, Burnett AK, Tonks A, et al. γ-Catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of β-catenin. Leukemia. 2013;27:336–343. doi: 10.1038/leu.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 21.Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. The EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, Griffin JD. Wnt/beta-catenin Pathway Modulates the Sensitivity of the Mutant FLT3 Receptor Kinase Inhibitors in a GSK-3beta Dependent Manner. Genes & Cancer. 2010;1:164–176. doi: 10.1177/1947601910362446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S, et al. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25:1928–1942. doi: 10.1101/gad.17421911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, et al. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 25.Gehrke I, Gandhirajan RK, Kreuzer KA. Targeting the WNT/beta-catenin/TCF/LEF1 axis in solid and haematological cancers: Multiplicity of therapeutic options. Eur J Cancer. 2009;45:2759–2767. doi: 10.1016/j.ejca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Minke KS, Staib P, Puetter A, Gehrke I, Gandhirajan RK, Schlosser A, et al. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol. 2009;82:165–175. doi: 10.1111/j.1600-0609.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 27.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nature Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 28.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1:552–566. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Wang CY. TBL1-TBLR1 and beta-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10:160–169. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- 31.Choi HK, Choi KC, Yoo JY, Song M, Ko SJ, Kim CH, et al. Reversible SUMOylation of TBL1-TBLR1 regulates beta-catenin-mediated Wnt signaling. Molecular Cell. 2011;43:203–216. doi: 10.1016/j.molcel.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Perissi V, Scafoglio C, Zhang J, Ohgi KA, Rose DW, Glass CK, et al. TBL1 and TBLR1 phosphorylation on regulated gene promoters overcomes dual CtBP and NCoR/SMRT transcriptional repression checkpoints. Mol Cell. 2008;29:755–766. doi: 10.1016/j.molcel.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberoi J, Fairall L, Watson PJ, Yang JC, Czimmerer Z, Kampmann T, et al. Structural basis for the assembly of the SMRT/NCoR core transcriptional repression machinery. Nat Struct Mol Biol. 2011;18:177–184. doi: 10.1038/nsmb.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramadoss S, Li J, Ding X, Al Hezaimi K, Wang CY. Transducin beta-like protein 1 recruits nuclear factor kappaB to the target gene promoter for transcriptional activation. Mol Cell Biol. 2011;31:924–934. doi: 10.1128/MCB.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, et al. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 36.Fiskus W, Wang Y, Sreekumar A, Buckley KM, Shi H, Jillella A, et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood. 2009;114:2733–2743. doi: 10.1182/blood-2009-03-213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balusu R, Fiskus W, Rao R, Chong DG, Nalluri S, Mudunuru U, et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood. 2011;118:3096–3106. doi: 10.1182/blood-2010-09-309674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiskus W, Sharma S, Qi J, Valenta JA, Schaub LJ, Shah B, et al. Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells. Mol Cancer Ther. 2014;13:1142–1154. doi: 10.1158/1535-7163.MCT-13-0770. [DOI] [PubMed] [Google Scholar]
- 39.Fiskus W, Sharma S, Shah B, Portier BP, Devaraj SGT, et al. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014 Apr 4; doi: 10.1038/leu.2014.119. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiskus W, Saba N, Shen M, Ghias M, Das Gupta S, Chauhan L, et al. Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res. 2014;74:2520–2532. doi: 10.1158/0008-5472.CAN-13-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez PV, Perry RL, Sarry JE, Perl AE, Murphy K, Swider CR, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009;23:2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, et al. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Toh L, Lau P, Wang X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/beta-catenin pathway in human cancer. J Biol Chem. 2012;287:32494–32511. doi: 10.1074/jbc.M112.368282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siapati EK, Papadaki M, Kozaou Z, Rouka E, Michali E, Savvidou I, et al. Proliferation and bone marrow engraftment of AML blasts is dependent on β-catenin signalling. Br J Haematol. 2011;152:164–174. doi: 10.1111/j.1365-2141.2010.08471.x. [DOI] [PubMed] [Google Scholar]
- 46.Abrahamsson AE, Geron I, Gotlib J, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci. 2009;106:3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell. 2009;36:872–884. doi: 10.1016/j.molcel.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noubissi FK, Goswami S, Sanek NA, Kawakami K, Minamoto T, Moser A, et al. Wnt signaling stimulates transcriptional outcome of the Hedgehog pathway by stabilizing GLI1 mRNA. Cancer Res. 2009;69:8572–8578. doi: 10.1158/0008-5472.CAN-09-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbluh J, Nijhawan D, Cox AJ, Li X, Neal JT, Schafer EJ, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, et al. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai BP, Hoverter NP, Waterman ML. Blending hippo and WNT: sharing messengers and regulation. Cell. 2012;151:1401–1403. doi: 10.1016/j.cell.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- 53.Tran T, Paz P, Velichko S, Cifrese J, Belur P, Yamaguchi KD, et al. Interferon β-1b induces the expression of RGS1 a negative regulator of G-protein signaling. Int J Cell Biol. 2010 doi: 10.1155/2010/529376. Article ID 529376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CC, Lasater EA, Lin KC, Wang Q, McCreery MQ, Stewart WK, et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci U S A. 2014;111:5319–5324. doi: 10.1073/pnas.1320661111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.